Abstract
The past three decades have seen a global wine glut. So far, well-intended but wasteful and expensive market-intervention has failed to drag the wine industry out of a chronic annual oversupply of roughly 15%. Can yeast research succeed where these approaches have failed by providing a means of improving wine quality, thereby making wine more appealing to consumers? To molecular biologists Saccharomyces cerevisiae is as intriguing as it is tractable. A simple unicellular eukaryote, it is an ideal model organism, enabling scientists to shed new light on some of the biggest scientific challenges such as the biology of cancer and aging. It is amenable to almost any modification that modern biology can throw at a cell, making it an ideal host for genetic manipulation, whether by the application of traditional or modern genetic techniques. To the winemaker, this yeast is integral to crafting wonderful, complex wines from simple, sugar-rich grape juice. Thus any improvements that we can make to wine, yeast fermentation performance or the sensory properties it imparts to wine will benefit winemakers and consumers. With this in mind, the application of frontier technologies, particularly the burgeoning fields of systems and synthetic biology, have much to offer in their pursuit of “novel” yeast strains to produce high quality wine. This paper discusses the nexus between yeast research and winemaking. It also addresses how winemakers and scientists face up to the challenges of consumer perceptions and opinions regarding the intervention of science and technology; the greater this intervention, the stronger the criticism that wine is no longer “natural.” How can wine researchers respond to the growing number of wine commentators and consumers who feel that scientific endeavors favor wine quantity over quality and “technical sophistication, fermentation reliability and product consistency” over “artisanal variation”? This paper seeks to present yeast research in a new light and a new context, and it raises important questions about the direction of yeast research, its contribution to science and the future of winemaking.
Keywords: Saccharomyces cerevisiae, wine, yeast
Accidental Beginnings
The winemaker’s bug, Saccharomyces cerevisiae, is so closely associated with humans it is rarely found in environs removed from human habitation.1 In fact, its evolutionary success can probably be explained by its relationship with humans, particularly in the production of alcoholic beverages, an activity that has been with us for at least 7,000 years.2 Because of us, S. cerevisiae enjoys phenomenal reproductive success with, for example, an estimated 600,000 tons of baker’s yeast being produced every year.3 But how did this close relationship get started?
It is likely that the first alcoholic fermentations were “happy accidents”: harvested grapes were not eaten quickly enough and began to rot, Saccharomyces spp “moved in” and took advantage of the free sugary meal and the first wines were made (Fig. 1). These early wines presumably tasted good and had an interesting, pleasing, psychotropic effect. One can only assume that early farmers learned from this experience and repeated the “accidents” of previous “vintages.” Winemaking was born and wine yeast had a secure future in the hands of its human guardians.
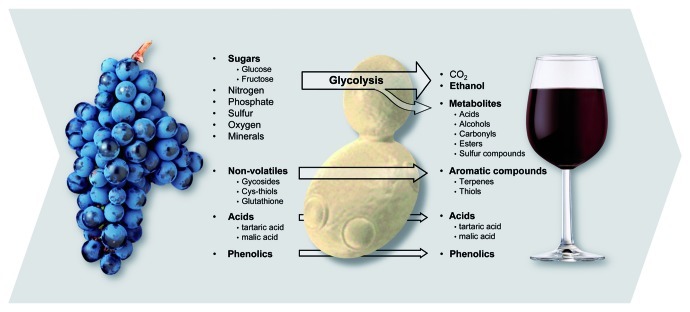
Figure 1. To the winemaker, yeast is integral to crafting wonderful, complex wines from simple, sugar-rich grape juice. Grape juice is converted into wine by the action of wine yeast. Some wine components are wholly generated by yeast as part of metabolism while others are essentially created by the grapevine. The large number of compounds synthesized or modified by wine yeast have a major impact on wine quality and style.
Modern Winemaking: To Intervene or Let Nature Take its Course
It has probably been known since the earliest of times that wine is susceptible to spoilage. The discovery that it is largely microbes that are responsible for this has led, in more recent times, to a debate on whether wine should be “natural” or whether we should protect it from undesirable microorganisms by “pasteurization” or the addition of sulfur dioxide. And this debate has broadened in the past decade to include questions on whether wine should be the fermentation product of its “natural” microflora or of a controlled inoculated wine yeast (Fig. 1).
Some winemakers and commentators believe that the ambient yeast population in the vineyard and winery constitute part of the characteristics of a “natural” wine. They believe that the unique contributions of diverse yeast species confer a complexity upon wine not seen in inoculated ferments. This might be true,4 but it comes with the risk of spoilage. There’s also an increased risk that the fermentation will become “stuck”1—i.e., the ferment will stop and be difficult to restart. In addition, spontaneous—“natural,” “wild” or “feral”—ferments also tend to take longer.
The debate continues and sets the backdrop for wine yeast research, but over the past two decades, active yeast strain development programs have been launched the world over to generate strains that can improve wine quality when used as inocula at the start of fermentation. This is a very fertile research field where advances in wine yeast strain development and fundamental yeast science have leveraged from one another.
This new era in wine yeast research, embracing cross-disciplinary expertise, is worthy of review. It started following the revelation that genes are made of DNA; the stage was set for an explosive growth of knowledge, driven by a convergence of genetics, biochemistry, cell biology, microbiology and computing. And work on yeast was often at the forefront of developments.
There were compelling reasons for molecular biologists from all fields to look on this simple single-celled fungus as the ideal “guinea pig” for fundamental research. Our close relationship with S. cerevisiae in food and beverage production over millennia tells us that it is safe to work with; for example, it is designated “Generally Recognized as Safe” (GRAS) by the United States’ Food and Drug Administration.5 In addition, it is inexpensive and easy to grow and can be stored for long periods in suspended animation. But, perhaps, its best asset is an accessible genetic system that can be followed through asexual and sexual cycles. The three basic cell types—a, α and a/α cells—can undergo mitosis and reproduce through an asexual budding process. The a and α haploid cells are also able to undergo mating, a sexual process that culminates in nuclear fusion and creation of a/α diploid cells, which can be induced to undergo meiosis to produce asci carrying four haploid spores.
Since the mid 1970s, when recombinant DNA technologies revolutionized the way research in biological sciences is conducted, S. cerevisiae has been one of the most important model organisms in molecular biology and emerging fields. For example, a haploid laboratory strain (S288c) was the first eukaryote to have its genome sequenced, a feat achieved through a collaborative international effort involving more than 600 scientists under the able leadership of Andre´ Goffeau and Stephen Oliver.6 This paved the way for the first chip-based gene array experiments.7
S. cerevisiae was also the first organism to be used to build a systematic collection of bar-coded gene deletion mutants enabling high throughput functional genomics experiments.8. But the most important resource available to the yeast scientific community is the Saccharomyces Genome Database (SGD; www.yeastgenome.org), which provides, free of charge, access or links to the most comprehensive data sets (genomic, transcriptomic, proteomic, metabolomics, etc.) available to a molecular biologist. All of this has been achieved by international collaborations on a grand scale.
What does all of this mean for wine research? The above international efforts have put the winemaker’s bug center stage in thousands of laboratories worldwide. And our knowledge is no longer limited to the S288c laboratory version of S. cerevisiae whose genome sequence was announced in 1996. Recently, the genomes of several industrial yeast strains were sequenced, including two ale strains (Foster’s O and Foster’s B) and five wine yeast, AWRI 696, QA23, VIN7, VIN13 and VL3,9,10 enabling us to conduct comparative genomic analyses. This means we can better understand what makes wine yeasts “tick” and why there is such variation in S. cerevisiae “winemaking phenotypes”.
Engineering Yeast to Make Better Wine
Wine research and wine yeast strain development are certainly well placed to benefit from the privileged place that S. cerevisiae occupies in the life sciences. The following includes some examples that demonstrate this.
Getting control of alcohol levels in wine
Without question the greatest challenge faced by the wine industry is rapidly mounting concerns over alcohol consumption; excess consumption creates problems for society and human health. In addition, too much alcohol in wine can overwhelm flavor and make the wine “hot” on the palate. The technical challenges associated with reducing the alcohol content of wine, however, are substantial.11
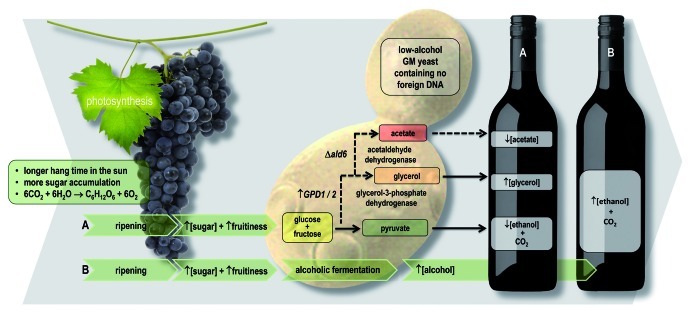
Several genetic modification (GM) based metabolic engineering strategies have been explored to generate wine yeasts that partially divert carbon metabolism away from ethanol production, with the aim of decreasing ethanol yields during vinification. Two glycerol-3-phosphate dehydrogenase isozymes, GPD1 and GPD2, which divert carbon from glycolysis to glycerol production, have proven to be the best candidates to date (Fig. 2).
Figure 2. Reducing alcohol levels in wine: several GM-based strategies have been explored to generate wine yeasts that partially divert sugar metabolism away from ethanol production. (A) Two glycerol-3-phosphate dehydrogenase isozymes, GPD1 and GPD2, can be harnessed to divert carbon from glycolysis to glycerol production. However, increased glycerol production was accompanied by undesirable increased concentrations of acetic acid. This problem was alleviated by knocking out ALD6. (B) Wild-type yeast convert most of the sugar they consume into ethanol and CO2.
Enhanced expression of either GPD paralog achieved the desired outcome with regard to ethanol yields;12-14 however, increased glycerol production was accompanied by undesirable increased concentrations of acetic acid. This was probably due to a perturbation in redox balance in the engineered strain, requiring the action of one or more of the five aldehyde dehydrogenase (Ald) isozymes; these enzymes help maintain redox balance by reducing coenzymes NAD+ or NADP+, when they oxidize acetaldehyde to acetic acid. The problem, however, was alleviated quite simply by knocking out ALD6.15,16
Similar approaches11 have targeted S. cerevisiae pyruvate decarboxylase isozymes, alcohol dehydrogenase isozymes and glycerol transporters, mostly leading to increased glycerol yields and accompanying reduced ethanol production. Alternative approaches have included expression of the Aspergillus niger glucose oxidase encoding gene (GOX) in S. cerevisiae, which redirects glucose to gluconic acid, and extensive modification of S. cerevisiae hexose transporters, which forces the yeast to respire rather than ferment, regardless of the concentration of glucose and fructose it encounters. Arising from these research efforts are several promising candidate “low-ethanol” wine yeast strains awaiting widespread acceptance of their use in commercial winemaking. These strains could immediately enable production of wines that contain 12% alcohol instead of 15%, from optimally ripened grapes.
Enhancing varietal wine flavor during fermentation
To casual wine drinkers it may seem fanciful, even pretentious, when a wine enthusiast states that Shiraz offers impressions of “black pepper;” Pinot Noir displays overtones of “earthy strawberries;” or that Sauvignon Blanc is characterized by traces of “asparagus” and “passionfruit.” But these descriptors of wine flavors may become clearer to casual wine drinkers if they are informed that, for example, strawberry flavor is considered to be relatively complex among fruits and contributed to by a large number of aroma compounds;17 and that wine flavor is really the sum of complex interactions between more than a thousand volatile compounds, many of which overlap with those found in strawberry, and some impact compounds that are, in fact, found in black pepper (e.g., rotundone18) and passionfruit (e.g., polyfunctional thiols19). Therefore, perceiving the aromas of these aforementioned fruits, vegetables and spices in wine is not surprising. The relative amounts of each compound, and the resultant flavor profile, ultimately define differences among the vast array of wines and wine styles produced throughout the world.
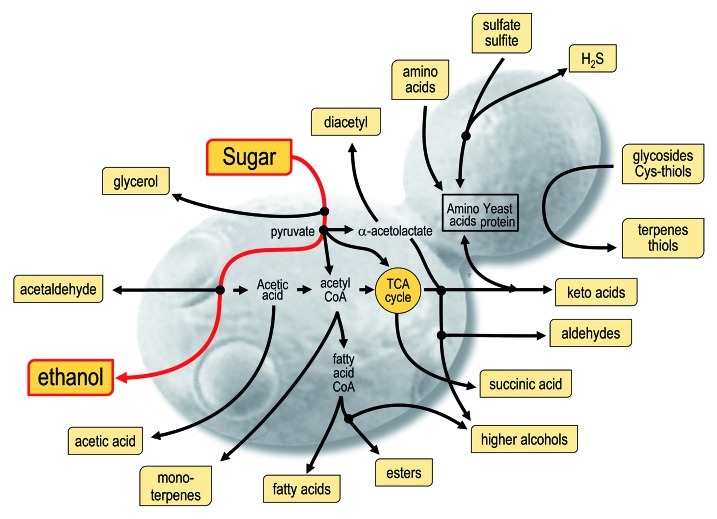
Grape variety is the starting point for differentiation—many volatile compounds provide varietal distinction in addition to giving wine its basic structure. The concentrations of many volatile compounds are, however, dependent upon an almost infinite number of variations in production, whether in the vineyard or the winery. It is known, for example, that commercial yeast strains possess different abilities to form and modulate volatile compounds during alcoholic fermentation (Fig. 3) that significantly affect the flavor and overall quality of wines.20 Therefore, while the proportion of wine volatiles modulated by yeast may be relatively low,21 the choice of yeast strain controlling fermentation is an effective method for shaping wine aroma according to the preferences of consumers in target markets.22
Figure 3. Commercial yeast strains possess different abilities to form and modulate compounds that impact on wine sensory properties. These compounds are produced as a result of yeast metabolic processes.
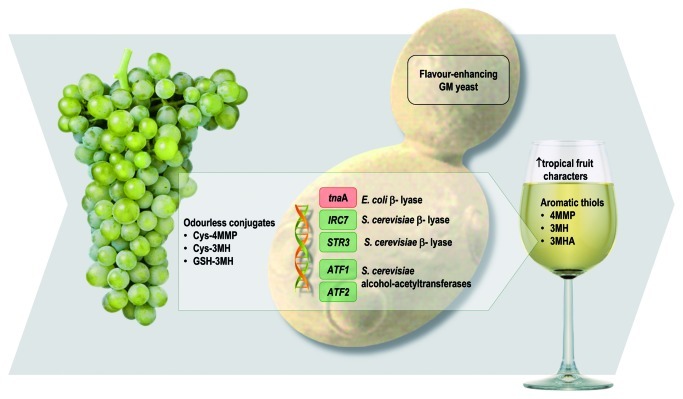
A case in point is the incidence of powerful synergies between Sauvignon Blanc grapes and yeast strains in formation of the compounds responsible for “box-hedge” and “tropical fruit” flavors—the polyfunctional thiols 4-mercapto-4-methylpentan-2-one (4MMP), 3-mercaptohexan-1-ol (3MH) and 3-mercaptohexyl acetate (3MHA). Odorless cysteine and glutathione conjugates of 3MH and 4MMP form in the grape berry and during crushing and can be found at higher concentrations in Sauvignon Blanc juice in comparison to other white varieties.23 Due to the potency of the free thiols, with perception thresholds in the ng/l range, only a fraction of the available conjugated precursors need to be released to impart strong “passionfruit,” “grapefruit,” “gooseberry” and “guava” flavors to wine. Yeast carbon-sulfur-lyase enzymes are responsible for the release of 4MMP and 3MH from their cysteine conjugates, while 3MHA is produced by yeast metabolism through the esterification of 3MH during fermentation (Fig. 4).
Figure 4. There are powerful synergies between Sauvignon Blanc grapes and yeast strains in formation of the compounds responsible for tropical fruit flavors: 4-mercapto-4-methylpentan-2-one (4MMP), 3-mercaptohexan-1-ol (3MH) and 3-mercaptohexyl acetate (3MHA). Odorless cysteine and glutathione conjugates are converted to aromatic thiols by carbon-sulfur-lyase enzymes. Alcohol acetyl transferase further modifies 3MH, converting it to the more potent 3MHA.
By mining the S. cerevisiae genome for putative carbon-sulfur-lyase encoding genes, we identified four candidates (BNA3, CYS3, GLO1 and IRC7) that when deleted decreased the ability of yeast to release 4MMP.24 Subsequent studies have narrowed this list to one gene, the β-lyase encoding IRC7,25 and in fact, a particular allele of this gene26 as the main determinant of 4MMP formation during winemaking. This line of research has, therefore, provided a clear quantitative trait locus (QTL) for molecular breeding of wine yeast. 3MH release, on the other hand, is not monogenetically determined;25,26 therefore, optimization of its release through non-GM approaches remains an empirical exercise.
Early research into precursors for the polyfunctional thiols utilized a column-immobilized Escherichia coli carbon-sulfur lyase enzyme, apo-tryptophanase, in a method designed to measure aromatic potential.27 We engineered a wine yeast, VIN13, to constitutively express the gene encoding this enzyme, tnaA.28 Wine made from warm-climate Sauvignon Blanc grapes with this yeast exhibited intense tropical characters, while in model ferments the VIN13-tnaA strain released up to 20-fold more 3MH and 4MMP.
The same wine yeast, VIN13, engineered to overexpress S. cerevisiae alcohol-acetyltransferase encoding genes (ATF1 and ATF2), was able to produce high concentrations of acetate esters.29 In neutral-tasting grape varieties, such as Colombard, this results in lifted “banana” characters. Overexpression of ATF1 in VIN13 increased conversion of 3MH into its acetate ester,30 3MHA, which, due to its lower perception threshold, is significantly more potent. Taken together, it is therefore possible to specifically manipulate 4MMP production by controlling the expression of S. cerevisiae's own IRC7; and to release high concentrations of both 3MH and 4MMP through the expression of E. coli’s tnaA gene in yeast and increase the potency of “tropical fruit” aromas by boosting the conversion of 3MH into 3MHA by overexpression of S. cerevisiae’s own ATF1 gene. While significantly less powerful than the tnaA gene product, modest enhancement of 3MH release is also possible through expression of the S. cerevisiae cystathionine β-lyase encoding gene, STR3,31 thereby paving the way for a range of “self-cloned” thiol-modulating wine yeast.
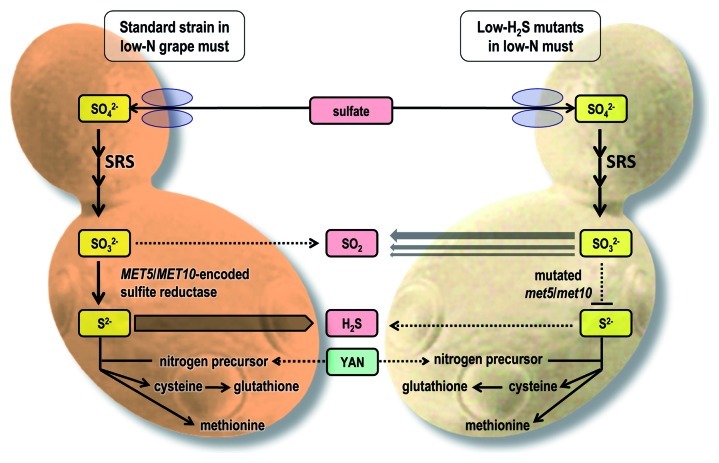
Polyfunctional thiols are not the only sulfur-containing aroma compounds that contribute to wine style. One need not be a wine expert to know that “reductive” aromas with descriptors such as “rotten egg,” “burnt rubber” and “sewage” are not going to appeal to wine consumers. While there are several chemical and biological mechanisms that contribute to “reductive” aromas in wine, “rotten egg” gas, also known as hydrogen sulfide (H2S), is largely a by-product of yeast metabolism. Under certain fermentation conditions, most wine strains of S. cerevisiae produce H2S hile incorporating inorganic sulfur into the amino acids methionine and cysteine—a process known as the sulfate reduction sequence (SRS) pathway.
Several GM strategies in the laboratory have been successful in limiting H2S production by S. cerevisiae and these are generally based on overexpression or inactivation of one or more genes involved in the SRS pathway.32,33 One of the targets has been sulfite reductase, which comprises two α- and two β-subunits (α2β2) encoded by yeast’s MET10 and MET5 genes, respectively. This knowledge informed a classical, non-GM, mutagenesis approach to develop three “low-H2S” strains, derived from the widely-used commercial wine yeast Maurivin PDM (Fig. 5).34 These strains, commercialized under the names Maurivin Advantage, Platinum and Distinction, provide winemakers with new strategies to manage “reductive” aromas, especially in grape musts low in assimilable nitrogen.
Figure 5. Building on knowledge from work utilizing GM strategies, a classical, non-GM mutagenesis approach was used to develop three “low-H2S” strains. These strains have impaired sulfite reductase activity due to mutations in their MET10 and MET5 genes.
Making the first modest moves with GM yeast strains
Australia, New Zealand and many European countries have effectively banned the use of genetically-modified organisms (GMOs) in commercial wine production. A multitude of interconnected agronomic, business, regulatory, cultural and social factors have led to these bans, but consumer sentiment is clearly one of the main drivers. While it is unlikely that the situation will change in the near future, the first modest move to release a commercialized GM yeast to market was made in 2005; a transgenic wine yeast, ML01, was given the green light from regulatory authorities in the USA, Canada and Moldova.
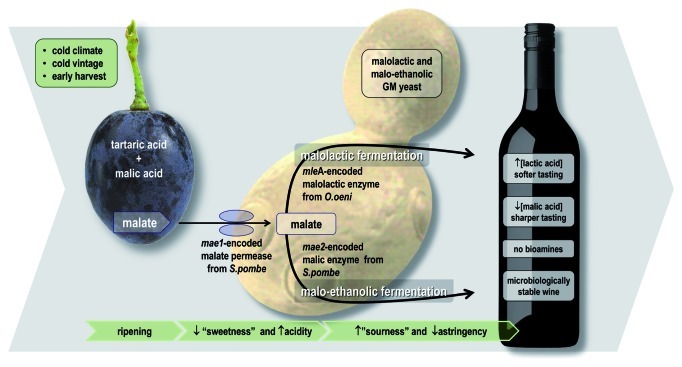
ML01 carries genes that enable it to perform malolactic fermentation (MLF), in which grape-derived malic acid is deacidified (decarboxylated) to lactic acid. MLF is performed by lactic acid bacteria, particularly Oenococcus oeni, following alcoholic fermentation. However, O. oeni is rather fastidious, being inhibited by a range of conditions typical of fermented grape juice (e.g., low pH, high alcohol content and poor nutrient availability) and can become “stuck” or sluggish.35 In addition, some lactic acid bacteria produce biogenic amines that impose health risks. Clearly, a wine yeast that performs MLF should be of great interest to both winemakers and consumers.
ML01 carries the Schizosaccharomyces pombe malate transporter gene (mae1) and the O. oeni malolactic enzyme gene (mleA);36 both are chromosomally integrated and regulated by the S. cerevisiae PGK1 promoter and terminator. This enables the ML01 to perform MLF in parallel with alcoholic fermentation (Fig. 6). In fermentation trials it was shown that 5 g/l of malic acid was decarboxylated to lactic acid within 5 days, without negative impacts on the sensory aspects on wine. Further analyses of the phenotype, genotype, transcriptome and proteome revealed that ML01 is “substantially equivalent” to its parental industrial wine yeast.
Figure 6. There are two options to genetically engineer extraneous malate utilization in order to deacidify wine. One approach utilizes the Schizosaccharomyces pombe malate transporter gene (mae1) and the O. oeni malolactic enzyme gene (mleA), enabling yeast to perform malolactic fermentation in parallel with alcoholic fermentation. Alternatively, Saccharomyces cerevisiae can be modified by the introduction of mae1 and the S. pombe malic enzyme gene (mae2), thereby enabling the conversion of malate into ethanol.
An alternative GM approach to lowering malic acid levels in wine has been to engineer a wine yeast that is able to conduct malo-ethanolic fermentation. In this case, malate is decarboxylated to pyruvate, which is then converted to ethanol. S. cerevisiae requires two heterologous genes for this, a malate transporter gene (mae1) and a malic enzyme gene (mae2), both of which come from S. pombe. While this strategy appears to be successful,37 there has not been a commercially available version of a malo-ethanolic wine yeast released to market.
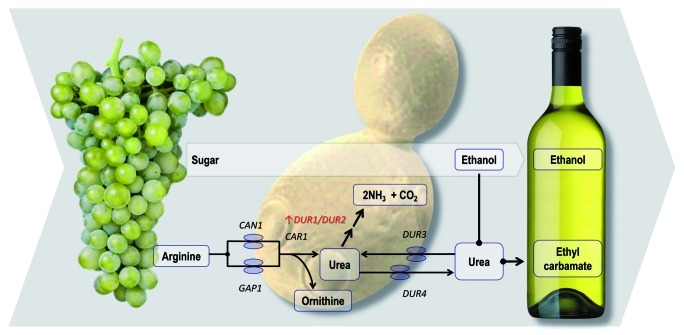
A second commercially-available GM wine yeast, ECMo01, received clearance from the American and Canadian regulatory bodies in 2006. ECMo01 was engineered to reduce the risk of ethyl carbamate production during fermentation. Ethyl carbamate, a potential carcinogen, is the product of urea reacting with ethanol, but is typically produced at such low levels (if at all) in winemaking that it is generally not a concern. Nonetheless, in some fortified wines and in some wine-producing regions, it can make an appearance.
ECMo01 has an extra copy of the S. cerevisiae DUR1,2 gene (Fig. 7) under the control of the yeast PGK1 regulatory sequences.38 DUR1,2 encodes urea amidolyase, which converts urea into ammonia and carbon dioxide, thereby removing substrate for ethyl carbamate production. The ammonia that is produced is consumed as a preferred nitrogen source by yeast. ECMo01 has been shown to reduce ethyl carbamate in Chardonnay wine by almost 90%, and analyses of ECMo01’s phenotype and transcriptome also revealed that the ECMo01 yeast is “substantially equivalent” to its parental strain.
Figure 7. A wine yeast has been genetically engineered to reduce ethyl carbamate production during fermentation. Through increased expression of DUR1/DUR2, this yeast breaks down urea to ammonia and CO2 before it is able to react with ethanol.
Interestingly, this yeast is cis (or “self”) cloned; it carries no foreign DNA and, therefore, is not transgenic. Nevertheless, because it was generated using techniques that involve manipulation of DNA in vitro, the regulations of many countries require it to be classed as a GMO.
Because wine yeasts are classified as “processing aids” by American and Canadian regulators, wines made with GM yeasts are not required to be labeled as such. While no winemakers from these two countries have admitted to using ML01 or ECMo01, it is common knowledge that these GM yeasts have been used, albeit on a very limited scale; for understandable reasons, in the current anti-GMO climate, users prefer to keep this confidential.
For wine yeast researchers and many grapegrowers and winemakers, it is frustrating that we cannot take full advantage of the many beneficial outcomes arising from the application of GM technologies in the food and beverage sector. It is to be hoped that, in the near future, consumers will see through the misrepresentations and scaremongering of anti-GM lobby groups and be more accepting of what GM science has to offer. To hasten this, scientists who bioengineer bugs for industrial applications must be prepared to communicate their views to the wider community and ensure that the debate is not so one-sided. After all, there is no intrinsic fear of the technologies; indeed the pharmaceutical industry has been very successful in developing GM therapeutics, which the vast majority of us have welcomed because of their efficacy and safety.
Opening New Vistas with Frontier Yeast Omics
With the advent of omics approaches we are seeing great advances in understanding wine yeast biology and what makes wine yeast so different from other strains of S. cerevisiae. Comparing the genomes of a wine yeast (AWRI1631), a laboratory strain (S288c) and a clinical isolate (YJM789), we uncovered a 0.6% difference in nucleotide sequence, but, perhaps more importantly, there was 100 kb additional genome sequence—enough to carry at least 27 genes.39 Open reading frames (ORFs) in the additional sequences do not resemble anything found in other species of Saccharomyces but appear to be similar to genes found in distant fungal relatives. Blast searches indicated that some of the wine yeast-specific genes have similarities to genes encoding cell wall proteins, perhaps contributing to the greater robustness of wine yeast compared with laboratory strains. Others may encode proteins associated with amino acid uptake, which is significant in the context of wine sensory attributes; amino acid metabolism is central to the production of many sensorially-important volatile aroma compounds.
In a subsequent study, analysis of the genome sequence of another related wine yeast, EC 1118, revealed an additional sequence that might be the result of horizontal gene transfer from a different yeast, Zygosaccharomyces.40 A limitation of the whole-genome sequencing studies, however, was that haploid representations of diploid, and often heterozygous, commercial and environmental strains were used to expedite sequence assembly. In more recent studies,9,10 five wine strains (AWRI 796, QA23, VL3, VIN7 and VIN13) and two brewing strains (Foster’s O and B ale strains) were sequenced in their industrially-used forms. The genomes of these strains were compared with one another and previously sequenced strains, S288c (the reference laboratory strain), YJM789 (a clinical isolate), RM11–1a (a strain derived from a vineyard isolate) and JAY291 (a biofuel strain).
We found that these industrial yeasts displayed significant genotypic heterogeneity both between strains but also between alleles present within strains (i.e., heterozygosity). This variation manifested as single nucleotide polymorphisms (SNPs), small insertions and deletions, and as novel, strain and allele-specific ORFs. None had been found previously in the S. cerevisiae genome and may provide the basis for novel phenotypic characteristics.
Interestingly, several strain-specific ORFs form a gene cluster, which has been found in multiple copies and at a variety of genomic loci in a strain-dependent manner, but which is entirely lacking from the S288c laboratory strain. Furthermore, this cluster of sequences appears to have integrated into genomic locations by a novel circular intermediate, but without employing classical transposition or homologous recombination,9,41 which represents the first time such an element has been characterized in S. cerevisiae. Overall, this work suggests that, despite the scrutiny that has been directed at the yeast genome, there remains a significant reservoir of ORFs and novel modes of genetic transmission that may have significant phenotypic impact in this important model and industrial species.
There have also been numerous studies describing transcriptomic and metabolomic analysis of wine yeast fermentations. This work is beginning to provide insights into wine yeast fermentations, but it is still early days. Looking to the future, as the various omics fields progress, it should be possible to build systems-based mathematical models of metabolism that will facilitate the in silico design of new wine yeast strains. In parallel with this, we see the emergence of synthetic biology where, yet again, S. cerevisiae is a key player. It should not be too long before there are synthetically-customized S. cerevisiae genomic components (e.g., regulatory elements to control expression of targeted genes; cassettes carrying genes encoding metabolic pathways to shape wine relevant traits, etc.) available “off the shelf” for designing, building and refining metabolic processes in wine yeast. But the key question remains: are consumers ready for this brave and exciting new world?
Looking to the Future
Truly great wines are born from great marriages between grape variety, climate, soil and landscape, on the one hand, and technology, innovation and craftsmanship on the other. Thus, the art in the science of winemaking lies in the choices made regarding which technological tools and innovations are selected and how they are applied to craft the infinite diversity of wine styles. Put differently, if we gave the same tools, i.e., paint, brushes and canvasses, to different artists—Da Vinci, Monet, Picasso—and all were asked to paint the same thing, they will invariably come up with very different masterpieces.
There is a fear that technological innovation—including the tailoring of wine yeast strains—could result in wine homogeneity and uniformity. Such fear is unfounded. The reality is that technology creates diversity by offering more options to grapegrowers and winemakers to respond to market needs and consumer preferences. These are options that the wine industry desperately needs as it faces so many challenges: an endemic oversupply of wine globally; prohibitionist-like propaganda campaigns from some anti-alcohol lobbyists; outbreaks of new diseases and pests in vineyards; climate change and environmental concerns.
The story of yeast research raises some important questions, therefore. It leads us to question public perception of the terms “natural” and “unnatural”; “technological” and “traditional”. Where is the dividing line between “natural” and “unnatural” in the context of yeast and wine research, and indeed, science as a whole?
The story of yeast also highlights the importance of cross-disciplinary research. We may be wine scientists, microbiologists, molecular geneticists or researchers engaged in the new omics technologies—genomics, transcriptomics, proteomics or metabolomics—but we all share a common goal. We all seek greater understanding of yeast as a simple, model organism: a microorganism that has the potential to shed new light on disease as well as processes such as fermentation. Wine science has made a significant contribution to understanding in this area of inquiry.
Finally, the story of yeast research raises questions about the future of omics technologies and their perception by society. Does yeast research bring into question, perhaps, the way that GMOs and synthetic genomes are perceived? We imagine that anyone reading this article holds his/her own views on this important and highly controversial subject. And this is the story of the journey of the winemaker’s bug—7,000 years and counting...
Acknowledgments
Research at the Australian Wine Research Institute (AWRI) is financially supported by Australia’s grapegrowers and winemakers through their investment body the Grape and Wine Research and Development Corporation, with matching funds from the Australian Government. Systems biology research at the AWRI uses resources provided as part of the National Collaborative Research Infrastructure Strategy (NCRIS), an initiative of the Australian Government, in addition to funds from the South Australian State Government. AWRI's collaborating partners within this NCRIS-funded initiative—which is overseen by Bioplatforms Australia—are Genomics Australia, Proteomics Australia, Metabolomics Australia (of which the Microbial Metabolomics unit is housed at the AWRI) and Bioinformatics Australia. Various results discussed in this paper stems from research projects that were funded by yeast supplier companies, Anchor Yeast, AB Mauri, Laffort and Lallemand. The AWRI is part of the Wine Innovation Cluster in Adelaide. This paper draws upon results from several AWRI research projects and publications; therefore, we gratefully acknowledge the contributions from several past and present colleagues, particularly, Paul Henschke, Anthony Borneman, Toni Cordente, Simon Schmidt, Hentie Swiegers, Cristian Varela, Jenny Bellon and Robyn Kievit. We also acknowledge the numerous yeast researchers, wine scientists and wine commentators from around the world on whose publications we have drawn to put this paper together.
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/19687
References
- 1.Martini A. Origin and domestication of the wine yeast Saccharomyces cerevisiae. J Wine Res. 1993;4:165–76. doi: 10.1080/09571269308717966. [DOI] [Google Scholar]
- 2.McGovern PE, Zhang J, Tang J, Zhang Z, Hall GR, Moreau RA, et al. Fermented beverages of pre- and proto-historic China. Proc Natl Acad Sci U S A. 2004;101:17593–8. doi: 10.1073/pnas.0407921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pretorius IS, Toit MD, van Rensburg P. Designer yeasts for the fermentation industry of the 21st Century. Food Technol Biotechnol. 2003;41:3–10. [Google Scholar]
- 4.Varela C, Siebert TE, Cozzolino D, Rose L, McLean H, Henschke PA. Discovering a chemical basis for differentiating wines made by fermentation with “wild” indigenous and inoculated yeasts: role of yeast volatile compounds. Aust J Grape Wine Res. 2009;15:238–48. doi: 10.1111/j.1755-0238.2009.00054.x. [DOI] [Google Scholar]
- 5.Verstrepen KJ, Chambers PJ, Pretorius IS. The development of superior yeast strains for the food and beverage industries: Challenges, opportunities and potential benefits. The Yeast Handbook: Yeasts in Food and Beverages. 2006:399–444.
- 6.Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, Feldmann H, et al. Life with 6000 genes. Science. 1996;274:546–, 563-7. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- 7.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–70. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 8.Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–6. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 9.Borneman AR, Desany BA, Riches D, Affourtit JP, Forgan AH, Pretorius IS, et al. Whole-genome comparison reveals novel genetic elements that characterize the genome of industrial strains of Saccharomyces cerevisiae. PLoS Genet. 2011;7:e1001287. doi: 10.1371/journal.pgen.1001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borneman AR, Desany BA, Riches D, Affourtit JP, Forgan AH, Pretorius IS, et al. The genome sequence of the wine yeast VIN7 reveals an allotriploid hybrid genome with Saccharomyces cerevisiae and Saccharomyces kudriavzevii origins. FEMS Yeast Res. 2012;12:88–96. doi: 10.1111/j.1567-1364.2011.00773.x. [DOI] [PubMed] [Google Scholar]
- 11.Kutyna DR, Varela C, Henschke PA, Chambers PJ, Stanley GA. Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci Technol. 2010;21:293–302. doi: 10.1016/j.tifs.2010.03.004. [DOI] [Google Scholar]
- 12.Michnick S, Roustan J-L, Remize F, Barre P, Dequin S. Modulation of glycerol and ethanol yields during alcoholic fermentation in Saccharomyces cerevisiae strains overexpressed or disrupted for GPD1 encoding glycerol 3-phosphate dehydrogenase. Yeast. 1997;13:783–93. doi: 10.1002/(SICI)1097-0061(199707)13:9<783::AID-YEA128>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Remize F, Roustan JL, Sablayrolles JM, Barre P, Dequin S. Glycerol overproduction by engineered saccharomyces cerevisiae wine yeast strains leads to substantial changes in By-product formation and to a stimulation of fermentation rate in stationary phase. Appl Environ Microbiol. 1999;65:143–9. doi: 10.1128/aem.65.1.143-149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Barros Lopes MA. Ata-ur-Rehman, Gockowiak H, Heinrich AJ, Langridge P, Henschke PA. Fermentation properties of a wine yeast over-expressing the Saccharomyces cerevisiae glycerol 3-phosphate dehydrogenase gene (GPD2) Aust J Grape Wine Res. 2000;6:208–15. doi: 10.1111/j.1755-0238.2000.tb00181.x. [DOI] [Google Scholar]
- 15.Eglinton JM, Heinrich AJ, Pollnitz AP, Langridge P, Henschke PA, de Barros Lopes MA. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeast. 2002;19:295–301. doi: 10.1002/yea.834. [DOI] [PubMed] [Google Scholar]
- 16.Cambon B, Monteil V, Remize F, Camarasa C, Dequin S. Effects of GPD1 overexpression in Saccharomyces cerevisiae commercial wine yeast strains lacking ALD6 genes. Appl Environ Microbiol. 2006;72:4688–94. doi: 10.1128/AEM.02975-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zabetakis I, Holden MA. Strawberry Flavor: Analysis and Biosynthesis. J Sci Food Agric. 1997;74:421–34. doi: 10.1002/(SICI)1097-0010(199708)74:4<421::AID-JSFA817>3.0.CO;2-6. [DOI] [Google Scholar]
- 18.Wood C, Siebert TE, Parker M, Capone DL, Elsey GM, Pollnitz AP, et al. From wine to pepper: rotundone, an obscure sesquiterpene, is a potent spicy aroma compound. J Agric Food Chem. 2008;56:3738–44. doi: 10.1021/jf800183k. [DOI] [PubMed] [Google Scholar]
- 19.Engel KH, Tressl R. Identification of new sulfur-containing volatiles in yellow passionfruit (Passiflora edulis f. flavicarpa) J Agric Food Chem. 1991;39:2249–52. doi: 10.1021/jf00012a030. [DOI] [Google Scholar]
- 20.Swiegers JH, Kievit RL, Siebert T, Lattey KA, Bramley BR, Francis IL, et al. The influence of yeast on the aroma of Sauvignon Blanc wine. Food Microbiol. 2009;26:204–11. doi: 10.1016/j.fm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Robinson AL, Boss PK, Heymann H, Solomon PS, Trengove RD. Influence of yeast strain, canopy management, and site on the volatile composition and sensory attributes of cabernet sauvignon wines from Western Australia. J Agric Food Chem. 2011;59:3273–84. doi: 10.1021/jf104324d. [DOI] [PubMed] [Google Scholar]
- 22.King E, Osidacz P, Curtin C, Bastian S, Francis I. Assessing desirable levels of sensory properties in Sauvignon Blanc wines–consumer preferences and contribution of key aroma compounds. Aust J Grape Wine Res. 2011;17:169–80. doi: 10.1111/j.1755-0238.2011.00133.x. [DOI] [Google Scholar]
- 23.Peña-Gallego A, Hern´ndez-Orte P, Cacho J, Ferreira V. S-cysteinylated and S-glutathionylated thiol precursors in grapes. A review. Food Chem. 2012;131:1–13. doi: 10.1016/j.foodchem.2011.07.079. [DOI] [Google Scholar]
- 24.Howell KS, Klein M, Swiegers JH, Hayasaka Y, Elsey GM, Fleet GH, et al. Genetic determinants of volatile-thiol release by Saccharomyces cerevisiae during wine fermentation. Appl Environ Microbiol. 2005;71:5420–6. doi: 10.1128/AEM.71.9.5420-5426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thibon C, Marullo P, Claisse O, Cullin C, Dubourdieu D, Tominaga T. Nitrogen catabolic repression controls the release of volatile thiols by Saccharomyces cerevisiae during wine fermentation. FEMS Yeast Res. 2008;8:1076–86. doi: 10.1111/j.1567-1364.2008.00381.x. [DOI] [PubMed] [Google Scholar]
- 26.Roncoroni M, Santiago M, Hooks DO, Moroney S, Harsch MJ, Lee SA, et al. The yeast IRC7 gene encodes a β-lyase responsible for production of the varietal thiol 4-mercapto-4-methylpentan-2-one in wine. Food Microbiol. 2011;28:926–35. doi: 10.1016/j.fm.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Peyrot Des Gachons C, Tominaga T, Dubourdieu D. Measuring the aromatic potential of Vitis vinifera L. Cv. Sauvignon blanc grapes by assaying S-cysteine conjugates, precursors of the volatile thiols responsible for their varietal aroma. J Agric Food Chem. 2000;48:3387–91. doi: 10.1021/jf990979b. [DOI] [PubMed] [Google Scholar]
- 28.Swiegers JH, Capone DL, Pardon KH, Elsey GM, Sefton MA, Francis IL, et al. Engineering volatile thiol release in Saccharomyces cerevisiae for improved wine aroma. Yeast. 2007;24:561–74. doi: 10.1002/yea.1493. [DOI] [PubMed] [Google Scholar]
- 29.Lilly M, Bauer FF, Lambrechts MG, Swiegers JH, Cozzolino D, Pretorius IS. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavor profiles of wine and distillates. Yeast. 2006;23:641–59. doi: 10.1002/yea.1382. [DOI] [PubMed] [Google Scholar]
- 30.Swiegers JH, Pretorius IS. Modulation of volatile sulfur compounds by wine yeast. Appl Microbiol Biotechnol. 2007;74:954–60. doi: 10.1007/s00253-006-0828-1. [DOI] [PubMed] [Google Scholar]
- 31.Holt S, Cordente AG, Williams SJ, Capone DL, Jitjaroen W, Menz IR, et al. Engineering Saccharomyces cerevisiae to release 3-Mercaptohexan-1-ol during fermentation through overexpression of an S. cerevisiae Gene, STR3, for improvement of wine aroma. Appl Environ Microbiol. 2011;77:3626–32. doi: 10.1128/AEM.03009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omura F, Shibano Y, Fukui N, Nakatani K. Reduction of hydrogen sulfide production in brewing yeast by constitutive expression of MET25 gene. J Am Soc Brew Chem. 1995;53:58–62. [Google Scholar]
- 33.Tezuka H, Mori T, Okumura Y, Kitabatake K, Tsumura Y. Cloning of a gene suppressing hydrogen sulfide production by Saccharomyces cerevisiae and its expression in a brewing yeast. J Am Soc Brew Chem. 1992;50:130–3. [Google Scholar]
- 34.Cordente AG, Heinrich AJ, Pretorius IS, Swiegers JH. Isolation of sulfite reductase variants of a commercial wine yeast with significantly reduced hydrogen sulfide production. FEMS Yeast Res. 2009;9:446–59. doi: 10.1111/j.1567-1364.2009.00489.x. [DOI] [PubMed] [Google Scholar]
- 35.Davis CR, Wibowo D, Eschenbruch R, Lee TH, Fleet GH. Practical Implications of Malolactic Fermentation: A Review. Am J Enol Vitic. 1985;36:290–301. [Google Scholar]
- 36.Husnik JI, Volschenk H, Bauer J, Colavizza D, Luo Z, van Vuuren HJ. Metabolic engineering of malolactic wine yeast. Metab Eng. 2006;8:315–23. doi: 10.1016/j.ymben.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Volschenk H, Viljoen-Bloom M, Van Staden J, Husnik J, Van Vuuren HJJ. Genetic engineering of an industrial strain of Saccharomyces cerevisiae for L-malic acid degradation via an efficient malo-ethanolic pathway. S Afr J Enol Vitic. 2004;25:63–73. [Google Scholar]
- 38.Coulon J, Husnik JI, Inglis DL, van der Merwe GK, Lonvaud A, Erasmus DJ, et al. Metabolic engineering of Saccharomyces cerevisiae to minimize the production of ethyl carbamate in wine. Am J Enol Vitic. 2006;57:113–24. [Google Scholar]
- 39.Borneman AR, Forgan AH, Pretorius IS, Chambers PJ. Comparative genome analysis of a Saccharomyces cerevisiae wine strain. FEMS Yeast Res. 2008;8:1185–95. doi: 10.1111/j.1567-1364.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 40.Novo M, Bigey F, Beyne E, Galeote V, Gavory F, Mallet S, et al. Eukaryote-to-eukaryote gene transfer events revealed by the genome sequence of the wine yeast Saccharomyces cerevisiae EC1118. Proc Natl Acad Sci U S A. 2009;106:16333–8. doi: 10.1073/pnas.0904673106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galeote V, Bigey F, Beyne E, Novo M, Legras J-L, Casaregola S, et al. Amplification of a Zygosaccharomyces bailii DNA segment in wine yeast genomes by extrachromosomal circular DNA formation. PLoS One. 2011;6:e17872. doi: 10.1371/journal.pone.0017872. [DOI] [PMC free article] [PubMed] [Google Scholar]