Abstract
Cone photoreceptor cyclic nucleotide-gated (CNG) channel is essential for central and color vision and visual acuity. Mutations in the cone channel subunits CNGA3 and CNGB3 are linked to achromatopsia and progressive cone dystrophy in humans. Over 50 mutations have been identified in the CNGA3 subunit. The R277C and R283W substitutions are among the most frequently occurring mutations. This study investigated the defects of these two mutations using a heterologous expression system. The wild type and mutant CNGA3 were expressed in HEK293 cells, the channel’s expression and cellular localization were examined by immunoblotting and immunofluorecences labeling, and activity of the channel was evaluated by ratiometric [Ca2+]i measurements and by electrophysiological recordings. By using this model system we observed dysfunction of the mutant channels. Co-expression of the mutant channel with the wild type subunit did not affect the wild type channel’s activity. Immunoflurescence labeling showed apparent cytosol aggregation of the immunoreactivity in cells expressing the mutants. Thus these disease-causing mutations appear to induce loss of function by impairing the channel cellular trafficking and plasma membrane targeting. Therapeutic supplementation of the wild type transgene may help correct the visual disorders caused by these two mutations.
1. INTRODUCTION
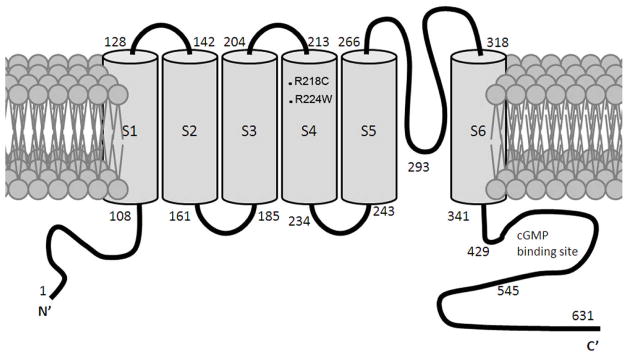
Photoreceptor cyclic nucleotide-gated (CNG) channels play a central role in phototransduction. Rod and cone CNG channels comprise two structurally related subunit types; CNGA1 and CNGB1 for the rod channel, and CNGA3 and CNGB3 for the cone channel. Rod CNG channel complex has a stoichiometry of three CNGA1 subunits and one CNGB1 subunit (Weitz et al., 2002) while cone CNG channel is thought to contain two CNGA3 and two CNGB3 subunits (Peng et al., 2004). Like other members of this ion channel family, CNG channel A and B subunits contain six putative membrane-spanning segments, cytoplasmic amino- and carboxyl-termini, a cyclic nucleotide-binding domain, and a conserved pore region. Figure 1 shows membrane topology of the mouse CNGA3 subunit.
Figure 1.
Membrane topology of the mouse CNGA3 with locations of the R218C and R224W mutations indicated. cGMP binding site is between the residue 429 and 545.
Cone vision mediated by CNG channel activation is essential for central and color vision and visual acuity. Naturally occurring mutations in CNGA3 and CNGB3 are associated with achromatopsia and progressive cone dystrophy (Kohl et al., 1998). Mutations in CNGA3 and CNGB3 account for 70% of all mutations found in achromatopsia patients. Nearly 50 mutations have been identified in the CNGA3 subunit. Among these mutations, the R277C and R283W substitutions are identified as the two most frequently occurring mutations (Wissinger et al., 2001). This work investigated the effects of the R277C and R283W mutations on the channel activity and cellular localization using a heterologous expression system. We found that both mutations abolish the channel activity and interfere with the channel plasma membrane localization. Thus the R277C and R283W mutations appear to cause loss of channel function by impairing the channel cellular trafficking and plasma membrane targeting. This work provides experimental evidence for understanding the pathogenesis of R277C and R283W mutations in the CNGA3 subunit.
2. MATERIALS AND METHODS
2.1. Constructs, cell culture and transfection
Construct encoding the full-length mouse CNGA3 was generated as we described previously (Ding et al., 2008). The R218C and R224W mutations in the mouse CNGA3, equivalent to the R277C and R283W mutations in the human CNGA3 (Figure 1), respectively, were obtained by site-directed mutagenesis using the Quickchange Site-directed Mutagenesis Kit (Stratagene, La Jolla, CA). Cell culture and transfection was performed as described previously (Ding et al., 2008).
2.2. Ratiometric measurement of intracellular Ca2+ concentration
The fluorescent indicator indo-1/AM was used to monitor Ca2+ influx through CNGA3 channels in cell suspensions. The assays were performed as described previously (Ding et al., 2008) using a PTI QuantaMaster spectrofluorometer (Photon Technology International). Briefly, cells (~48 h post-transfection) were harvested, washed, and loaded with 2 μM Indo-1/AM (Sigma-Aldrich) for 40 minutes at room temperature. After loading, cells were washed and resuspended in ECS buffer (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 10 mM glucose, and 15 mM HEPES, pH 7.4) (2 × 106) for the assay. Ca2+ entry in response to 8-pCPT-cGMP (100 μM) was determined by ratiometric measurement which represents changes of free intracellular Ca2+ concentrations (expressed as a Δ340/380 ratio). Data were analyzed and graphed using GraphPad Prism software (GraphPad software, San Diego, CA).
2.3. Electrophysiological recordings
Standard whole-cell patch clamp recordings on cells grown on 35 mm culture dishes were performed as described previously (Malykhina et al., 2006; Fitzgerald et al., 2008). The pipette solution consisted of (in mM): K+ aspartate 100, KCl 30, NaCl 5, MgCl2 2, Na-ATP 2, EGTA 1, HEPES 5 with pH 7.2 adjusted with KOH. Patch electrodes had resistances of 3–5 MΩ when filled with internal solution. Whole cell currents were recorded by using gap-free protocol with a holding potential at – 50 mV. All experiments were performed at room temperature (23°C) and recorded using an Axopatch 200B amplifier (Axon Instrument, Foster City, CA). pCLAMP software (Axon Instruments) was used for data acquisition and analysis.
2.4. SDS-PAGE and Western blot analysis
SDS-PAGE and Western blot analysis was performed to detect expression of the wild type and mutant CNGA3 subunits using the rabbit polyclonal anti-CNGA3 as described previously (Ding et al., 2008; Matveev et al., 2008).
2.5. Immunofluorescence labeling and confocal microscopy
Immunofluorescence labeling was performed as described previously (Ding et al., 2008; Matveev et al., 2008). Briefly cells were grown in DMEM medium on coverslips pre-coated with fibronectin (Sigma-Aldrich). Cells were washed, fixed with 4% (w/v) paraformaldehyde for 10 min at room temperature, and blocked for 1 h at room temperature in 5% BSA. Cells were then incubated with the rat monoclonal anti-CNGA3 (kindly provided by Dr. Benjamin Kaupp at the Institute of Neurosciences and Biophysics, Forschungszentrum, Jülich, Germany) (1:50) overnight at 4°C, followed by incubation with Alexa-conjugated goat anti-rat secondary antibody (1:1000) for 1 h at room temperature. Vectashield™ containing DAPI stain (Vector Laboratories) was used to mount the coverslips onto the slides.
The fluorescent signals were visualized using a 40X water immersion objective lens on an Olympus IX81-FV500 confocal laser scanning microscope (Olympus, Melville, NY) and analyzed with FluoView imaging software (Olympus) as described previously (Ding et al., 2008). Quantification of fluorescent labeling intensity was performed using FluoView to evaluate cellular distribution of the channel subunits. The plasma membrane localization of the wild type and mutant subunits was determined and expressed in terms of percent of total cellular fluorescence intensity. Data were analyzed and graphed using GraphPad Prism software (GraphPad software, San Diego, CA).
3. RESULTS
3.1. The R218C and R224W mutations cause loss of channel function
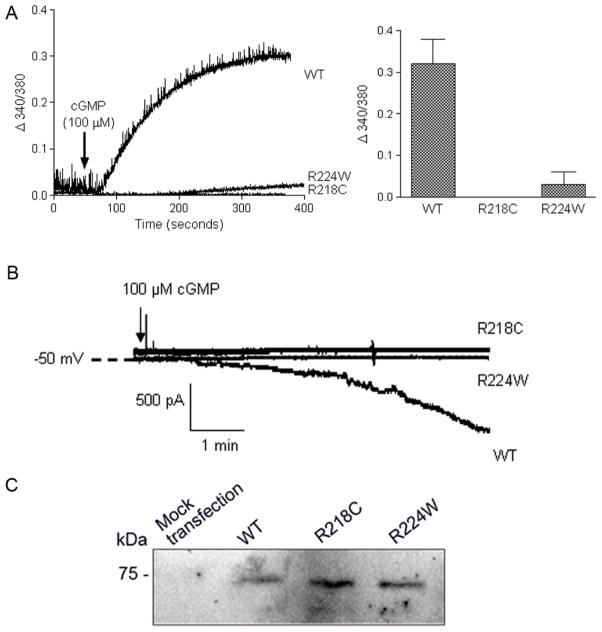
The concentration-dependent response of the wild type CNGA3 to cGMP stimulation has been characterized in our previous studies (Ding et al., 2008; Fitzgerald et al., 2008). The channel subunits were expressed in HEK293 cells and the mutation effects on the channel activity were investigated. With the functional assays we found that the two mutations had profound negative effects on the channel activity. As shown in Figure 2A, the intracellular calcium response to cGMP (100 μM) stimulation was completely abolished in cells expressing the R218C mutant. The response in R224W mutant was lowered to less than 10% (as analyzed at 300 sec after cGMP stimulation) of the wild type response (Figure 2A). The deficiency of the mutant channels was also confirmed by electrophysiological recordings. Figure 2B shows representative patch clamp recording profiles of the wild type, R218C and R224W mutants in response to 100 μM cGMP stimulation. No current was recorded in cells expressing the mutants, leading to the conclusion that the R218C and R224W mutations abolished the channel activity. While the physiological assays indicated abolished or severely reduced responses, Western blot analysis revealed similar levels of mutant expression when compared to wild type expression (Figure 2C). These data together indicate channel deficiency, not expression levels, as that which contributes to loss of cone function in achromatopsia patients.
Figure 2.
The R218C and R224W mutations cause loss of channel function. A. Intracellular calcium response to 8-pCPT-cGMP (100 μM) stimulation in HEK293 cells expressing the wild type, R218C and R224W mutants. The left panel shows the representative response curves and the right panel is the bar graph showing the quantitative analysis of the calcium measurement (at 300 s after cGMP stimulation) from 3–5 independently performed experiments. B. Representative patch-clamp recording profiles from HEK293 cells expressing the wild type (WT), R218C and R224W mutants in response to 8-pCPT-cGMP (100 μM) stimulation. C. Western blot detection of the wild type, R218C and R224W mutant expression in HEK293 cells that had been transfected with the respective cDNAs.
3.2. The R218C and R224W mutations cause channel mis-localization
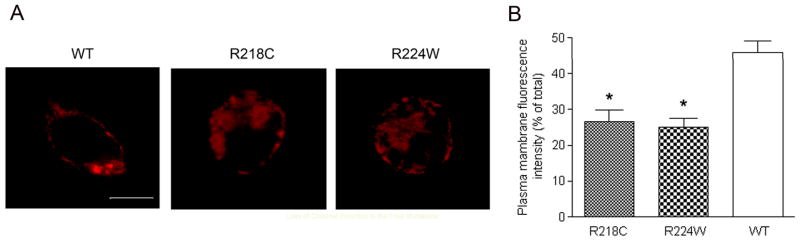
Immunofluorescence labeling was performed to determine cellular localization of the wild type and mutant channel subunits in cells. From these assays we observed that cells expressing the R218C and R224W mutants showed apparent cytosol aggregation of the immunofluorescent signal (Figure 3A). Quantification immunofluorescence intensity analysis showed that cells expressing the mutants displayed the decreased plasma membrane labeling compared to cells expressing the wild type subunit (Figure 3B). This data indicates the mislocalization of mutants is a contributing factor to decreased cone function.
Figure 3.
The R218C and R224W mutations cause channel mis-localization. A. Representative confocal images showing cellular localization of the wild type, R218C and R224W mutants in HEK293 cells. Scale bar: 10 μm. B. The bar graph shows the quantitative analysis results of the plasma membrane fluorescence labeling intensities. Bars represent the means ± SEM of the number of cells (12 for the wild type, 15 for the R218C transfected, and 15 for the R224W transfected) from 3 independently performed experiments. Unpaired Student’s t test was used for determination of the significance. *, p < 0.05.
3.3. Co-expression of the R218C and R224W mutants with the wild type channel does not affect the channel activity
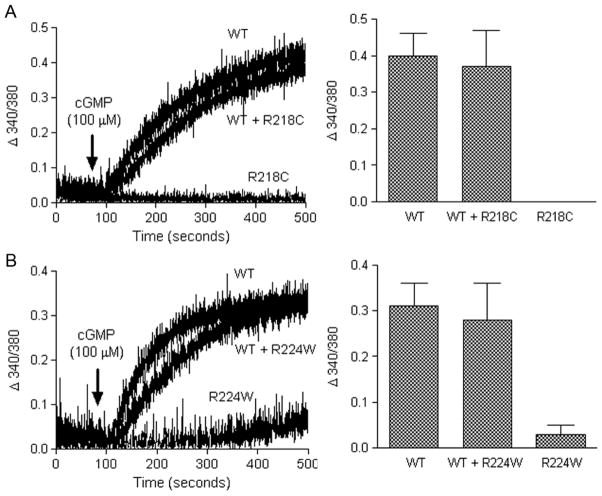
We examined the effects of co-expression of the mutant channel subunits with the wild type channel on the channel activity. Cells were co-transfected with equal amounts of the wild type cDNA and the mutant cDNA or pcDNA3.1 (the channel harboring plasmid) (10 μg of each per 100 mm dish) and assayed for intracellular calcium response to cGMP stimulation ~48 h post-transfection. From these assays we found that co-expression of the mutant channel with the wild type channel did not affect the wild type channel’s activity. As shown in Figure 4 the calcium responses to cGMP stimulation in cells co-expressing the wild type with R218C (Figure 4A) or R224W (Figure 4B) mutants were almost indistinguishable from those in cells expressing the wild type channel alone. This data indicates that the wild type channels do not form heterodimers with mutant channels. Or it indicates that the wild type/mutant heterodimers do form and that the wild type channels are dominant in the complex
Figure 4.
Co-expression of the R218C or R224W mutants with the wild type subunit does not affect the channel activity. HEK293 cells were co-transfected with equal amounts of the wild type and R218C (A) or R224W (B) mutants and assayed for calcium response to 8-pCPT-cGMP (100 μM) stimulation ~48 h post-transfection. Shown in the left panels are the representative response curves and the right panels are the bar graphs showing the quantitative analysis of the calcium measurement at 400 s after 8-pCPT-cGMP stimulation from 3–6 independently performed experiments.
4. DISCUSSION
Achromatopsia is an inherited disorder that affects approximately 1 in every 33,000 Americans. The condition is associated with color blindness, visual acuity loss, extreme light sensitivity and nystagmus. To date, mutations in three genes, CNGA3, CNGB3 and Gnat2, have been identified in achromatopsia patients. Indeed mutations in cone CNG channel are highly linked to various forms of achromatopsia and other types of cone degenerative diseases including early onset macular degeneration and progressive cone dystrophy. There are nearly 70 disease-causing mutations in CNGA3 and CNGB3 and these mutations account for 70% of the achromatopsia patients (Kohl et al., 1998; Wissinger et al., 2001; Nishiguchi et al., 2005).
Efforts are being made to explore the mechanism underlying the disease-causing mutations in cone CNG channel. Patel et al. (Patel et al., 2005) studied 4 mutations (Y181C, N182Y, L186F, and C191Y) in the S1 segment of human CNGA3. They showed that the mutations resulted in loss of channel function and that the full-length mutant channel subunits were synthesized but were retained in the endoplasmic reticulum. They proposed a role of the S1 segment in both maturation and function of CNG channels. Faillace et al. (Faillace et al., 2004) studied 4 mutations (F294L, R296Q, N295Q, R302W; sequences in bovine CNGA3) in the S4 segment and demonstrated that the mutant channel failed to become glycosylated and arrive at the plasma membrane. This work implicated a role of the S4 segment in the channel proper intracellular processing and trafficking. Liu et al. (Liu & Varnum, 2005) described an alteration in the channel membrane localization and gating properties in the two C-terminal mutants (N471S and R563H). Thus these studies suggest that an impaired cellular processing and membrane targeting is a common mechanism for a variety of mutations located at distinct regions of the channel subunit. The present work investigated the effects of the R218C and R224W mutations on the channel activity and cellular localization and showed that both mutations abolished the channel activity and interfered with the channel plasma membrane localization. These results are consistent with the previous findings by Faillace et al. (Faillace et al., 2004), supporting the view that S4 segment is critical for the channel cellular processing. With co-expression experiments we observed that co-expression of the mutant channel with the wild type subunit did not affect the wild type channel’s activity. This observation is consistent with the clinical findings in which individuals with the heterozygous mutations show normal cone function and only individuals with homozygous mutations or compound heterozygous mutations experience cone defect symptoms. The channel subunit encoded by one wild type allele appears sufficient to maintain the normal channel activity. This observation may also suggest that the mutant channel subunits lack the ability to be recruited into the channel complexes.
Acknowledgments
This work was supported by grants from the National Center For Research Resources (P20RR017703), the National Eye Institute (P30EY12190), the American Health Assistance Foundation, and the Presbyterian Health Foundation. We thank Dr. Benjamin Kaupp for providing the monoclonal anti-CNGA3 antibody.
References
- Ding XQ, Fitzgerald JB, Matveev AV, McClellan ME, Elliott MH. Functional activity of photoreceptor cyclic nucleotide-gated channels is dependent on the integrity of cholesterol- and sphingolipid-enriched membrane domains. Biochemistry. 2008;47:3677–3687. doi: 10.1021/bi7019645. [DOI] [PubMed] [Google Scholar]
- Faillace MP, Bernabeu RO, Korenbrot JI. Cellular processing of cone photoreceptor cyclic GMP-gated ion channels: a role for the S4 structural motif. J Biol Chem. 2004;279:22643–22653. doi: 10.1074/jbc.M400035200. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JB, Malykhina AP, Al-Ubaidi MR, Ding XQ. Functional expression of cone cyclic nucleotide-gated channel in cone photoreceptor-derived 661W cells. Adv Exp Med Biol. 2008;613:327–334. doi: 10.1007/978-0-387-74904-4_38. [DOI] [PubMed] [Google Scholar]
- Kohl S, Marx T, Giddings I, Jagle H, Jacobson SG, Apfelstedt-Sylla E, Zrenner E, Sharpe LT, Wissinger B. Total colourblindness is caused by mutations in the gene encoding the alpha-subunit of the cone photoreceptor cGMP-gated cation channel. Nat Genet. 1998;19:257–259. doi: 10.1038/935. [DOI] [PubMed] [Google Scholar]
- Liu C, Varnum MD. Functional consequences of progressive cone dystrophy-associated mutations in the human cone photoreceptor cyclic nucleotide-gated channel CNGA3 subunit. Am J Physiol Cell Physiol. 2005;289:C187–198. doi: 10.1152/ajpcell.00490.2004. [DOI] [PubMed] [Google Scholar]
- Malykhina AP, Qin C, Greenwood-van Meerveld B, Foreman RD, Lupu F, Akbarali HI. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil. 2006;18:936–948. doi: 10.1111/j.1365-2982.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- Matveev AV, Quiambao AB, Fitzgerald JB, Ding X-Q. Native Cone Photoreceptor Cyclic Nucleotide-Gated Channel Is A Heterotetrameric Complex Comprising both CNGA3 and CNGB3: A Study Using the Cone-Dominant Retina of Nrl−/− Mice. Journal of neurochemistry. 2008 doi: 10.1111/j.1471-4159.2008.05548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi KM, Sandberg MA, Gorji N, Berson EL, Dryja TP. Cone cGMP-gated channel mutations and clinical findings in patients with achromatopsia, macular degeneration, and other hereditary cone diseases. Hum Mutat. 2005;25:248–258. doi: 10.1002/humu.20142. [DOI] [PubMed] [Google Scholar]
- Patel KA, Bartoli KM, Fandino RA, Ngatchou AN, Woch G, Carey J, Tanaka JC. Transmembrane S1 mutations in CNGA3 from achromatopsia 2 patients cause loss of function and impaired cellular trafficking of the cone CNG channel. Invest Ophthalmol Vis Sci. 2005;46:2282–2290. doi: 10.1167/iovs.05-0179. [DOI] [PubMed] [Google Scholar]
- Peng C, Rich ED, Varnum MD. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron. 2004;42:401–410. doi: 10.1016/s0896-6273(04)00225-9. [DOI] [PubMed] [Google Scholar]
- Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron. 2002;36:881–889. doi: 10.1016/s0896-6273(02)01098-x. [DOI] [PubMed] [Google Scholar]
- Wissinger B, Gamer D, Jagle H, Giorda R, Marx T, Mayer S, Tippmann S, Broghammer M, Jurklies B, Rosenberg T, Jacobson SG, Sener EC, Tatlipinar S, Hoyng CB, Castellan C, Bitoun P, Andreasson S, Rudolph G, Kellner U, Lorenz B, Wolff G, Verellen-Dumoulin C, Schwartz M, Cremers FP, Apfelstedt-Sylla E, Zrenner E, Salati R, Sharpe LT, Kohl S. CNGA3 mutations in hereditary cone photoreceptor disorders. Am J Hum Genet. 2001;69:722–737. doi: 10.1086/323613. [DOI] [PMC free article] [PubMed] [Google Scholar]