Abstract
Cone vision mediated by photoreceptor cyclic nucleotide-gated (CNG) channel is essential for central and color vision and visual acuity. Cone CNG channel is composed of two structurally related subunit types, CNGA3 and CNGB3. Naturally occurring mutations in cone CNG channel are associated with a variety of cone diseases including achromatopsia, progressive cone dystrophy, and some maculopathies. Nevertheless, our understanding of the structure of cone CNG channel is quite limited. This is, in part, due to the challenge of studying cones in a rod-dominant mammalian retina. We have demonstrated a robust expression of cone CNG channel and lack of rod CNG channel in the cone-dominant Nrl−/− retina and shown that the Nrl−/− mouse line is a valuable model to study cone CNG channel. This work examined the complex structure of cone CNG channel using infrared fluorescence Western detection combined with chemical cross-linking and blue native-PAGE. Our results suggest that the native cone CNG channel is a heterotetrameric complex likely at a stoichiometry of three CNGA3 and one CNGB3.
Keywords: retina, photoreceptor, cone, CNG channel, achromatopsia
98.1 Introduction
Photoreceptor cyclic nucleotide-gated (CNG) channels play a pivotal role in phototransduction (Kaupp and Seifert, 2002). Structurally, CNG channels belong to thesuperfamily of voltage-gated ion channels. The channel comprises two structurally related subunit types, the A and B subunits. Rod CNG channel is composed of CNGA1 and CNGB1 while cone CNG channelis composed of CNGA3 and CNGB3. It is known that the proper subunit interaction and complex formation is critical for the channel function (Trudeau and Zagotta, 2002). The structure of the native rod CNG channel has been well studied using bovine retina, showing a stoichiometry of three CNGA1 and one CNGB1 (Weitz et al., 2002; Zhong et al., 2002). In contrast, our understanding of the cone CNG channel structure is quite limited. This is due to the difficulty of studying cone specific protein in a rod-dominant retina and the structural feature of the cone CNG channel subunits. The molecular masses of CNGA3 and CNGB3 are similar to each other (72 vs. 80 kDa), which makes the study of composition and stoichiometry of cone CNG channel quite challenging using the conventional approaches. We have shown that the cone-dominant Nrl−/− retina is a valuable tool to study cone CNG channel (Matveev et al., 2008). This work examined the complex structure of cone CNG channel prepared from Nrl−/− retina using the infrared fluorescence Western detection combined with chemical cross-linking and blue native-PAGE and the findings improved our understandings of the cone CNG channel structure.
98.2 Materials and Methods
98.2.1 Infrared Fluorescence Western Detection of Cone CNG Channel
Mouse retinal membrane protein extracts were prepared as described previously (Ding et al., 2009). The membrane proteins were separated by 10% SDS-PAGE, followed by transferring onto polyvinylidene fluoride (PVDF) membrane for infrared fluorescence Western Detection. The rabbit anti-CNGA3 and anti-CNGB3 were labeled with the IRDye-800CW and IRDye-680 fluorescence dyes (LI-COR Biosciences UK Ltd). The Odyssey® Infrared Imaging System (LI-COR Biosciences UK Ltd) was used for the imaging quantitative analysis. Peptide competition experiments were performed as described previously (Matveev et al., 2008).
98.2.2 Chemical Cross-linking
Chemical cross-linking experiments were performed using Nrl−/− retinal membrane preparations as described previously (Matveev et al., 2008). The amino-specific cross-linker BS3 at varying concentrations was used; the cross-linking reactions were terminated by addition of 5.0 mM DTT; and cross-linking products were resolved onto 3–8% Nu-PAGE, followed by infrared fluorescence detection.
98.2.3 Blue Native-PAGE
Blue native-PAGE was performed as described by Wittig et al (Wittig et al., 2006). Several types of detergents, including digitonin, Brij 96, Triton X100 and dodecylmaltoside (DDM), were tested to solubilize the membranes and DDM was shown to produce an optimal solubilization and was used in this study.
98.3 Results
98.3.1 Simultaneous Detection of CNGA3 and CNGB3 in the Mouse Retina by Infrared Fluorescence Western Detection
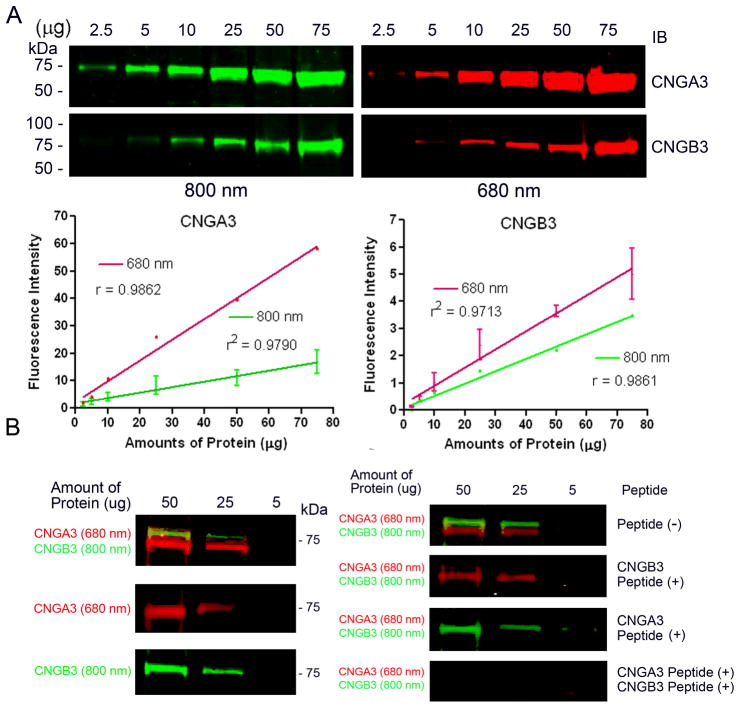
CNGA3 and CNGB3 in the mouse retina were detected by the respective antibodies labeled with both IRDye-800CW (green) and IRDye-680 (red) dyes with a linear range (r2 ≥ 0.97) (Figure 1A). Simultaneous detection of CNGA3 and CNGB3 on the same blots was achieved (Figure 1B, left panel) and the specific detection was shown by peptide competition experiments (Figure 1B, right panel).
Fig. 98.1.
Simultaneous detection of CNGA3 and CNGB3 in the mouse retina by infrared fluorescence Western detection. Increasing amounts (2.5–75 μg) of Nrl−/− retinal membranes were resolved by 10% SDS-PAGE, followed by immunoblotting using anti-CNGA3 and anti-CNGB3 labeled with IRDye-680 and IRDye-800CW. A. Quantitative detection of CNGA3 and CNGB3 in the mouse retina. B. left panel, Simultaneous detection of CNGA3 and CNGB3 in the mouse retina using infrared fluoresecence detection. Right panel, Peptide competition experiments showed specificity of the detection. The blots were incubated with antibodies in the absence and presence of the specific peptides (150 μg/ml) followed by fluorescence scanning and imaging.
98.3.2 Analysis of Cone CNG Channel Complexes Using Chemical Cross-linking and Infrared Fluorescence Detection
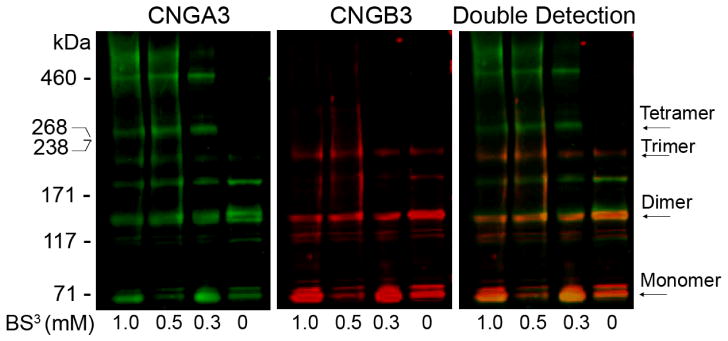
Chemical cross-linking is a useful approach to evaluate the composition of a protein complex and the spatial proximity between two or more macromolecules (Matveev et al., 2008; Schwarzer et al., 2000). We analyzed the cone CNG channel complexes by chemical cross-linking combined with infrared fluorescence detection. Figure 2 shows a typical gel separation of the cross-linked products with varying concentrations of BS3, analyzed by infrared fluorescence detection. The cross-linked products equivalent to the dimer (~150 kDa), trimer (~240 kDa), and tetramer (~320 kDa) of the channel complexes, respectively, were detected by both anti-CNGA3 and anti-CNGB3 antibodies. The overlay image shows the co-detections of CNGA3 and CNGB3 subunits at varying sizes of the complexes. The relative signals of CNGB3 were more abundant in the dimmeric and trimeric complexes but less abundant in the tetrameric complexes. Quantification of the fluorescence intensities of the tetrameric complexes shows that the CNGA3 signal was nearly 3-fold higher than the CNGB3 signal.
Fig. 98.2.
Analysis of cone CNG channel complexes using chemical cross-linking and infrared fluorescence detection. The reactions were performed using Nrl−/− retinal membranes and the amino-specific cross-linker BS3 at varying concentrations as indicated at room temperature for 30 min. The cross-linking products were separated by 3–8% Nu-PAGE, followed by infrared fluorescence Western detection.
98.3.3 Analysis of Cone CNG Channel by Blue Native-PAGE
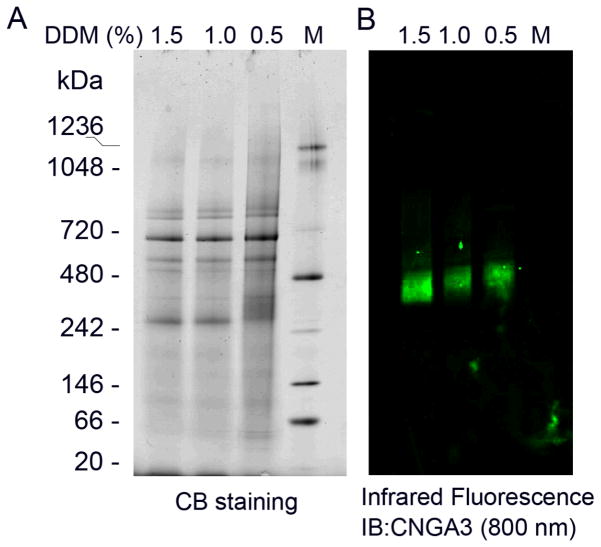
Blue native-PAGE has been widely used to study the structure of a protein complex (Chavan et al., 2006; Pyndiah et al., 2007). We applied this technique to evaluate the structure of cone CNG channel under native conditions. Figure 3 shows a typical blue native-PAGE separation of Nrl−/− retinal membranes visualized by Commassie blue staining (Figure 3A) and by infrared fluorescence Western detection (Figure 3B). The channel complexes migrated to an area between 300 and 480 kDa, which is close to the results obtained from the chemical cross-linking experiments.
Fig. 98.3.
Analysis of cone CNG channel by blue native-PAGE. A. Blue native-PAGE separation of Nrl−/− retinal membranes solubilized by various concentrations of DDM. The membrane proteins were resolved by 3–12% native gel using a blue native gel electrophoresis system. CB: Commassie blue. B. Proteins resolved on blue native gel were transferred onto PVDF membranes, followed by infrared fluorescence Western detection using anti-CNGA3 labeled with IRDye-800CW.
98.4 Discussion
Unlike that of the rod CNG channel our understanding of the cone CNG channel structure is quite limited. Two reports so far have described the stoichiometry of cone CNG channel using heterologous expression systems. The study by Zhong et al. proposed a three CNGA3 and one CNGB3 stoichiometry based on the identification of a C-terminal leucine zipper homology domain in all the A subunits of CNG channel (Zhong et al., 2002). In contrast, a 2A:2B stoichiometry was proposed by Peng et al. based on their electrophysiological recordings and biochemical studies using a Xenopus oocytes expression system (Peng et al., 2004). With co-immunoprecipitation and chemical cross-linking we have shown that the native cone CNG channel is a heterotetrameric complex. This work utilizes the advantages of infrared fluorescence detection to analyze the complex structure of the native cone CNG channel. The infrared fluorescence Western detection has been shown to provide a valuable tool for quantitative, two-color detection of proteins and analysis of components of a protein complex (Yashiro et al., 2005). This especially gives advantages in detecting proteins that migrate on a gel at a similar position. By using this technique combined with chemical cross-linking we detected more abundant CNGA3 in the tetrameric complex. Taken together with the findings of the specific interactions between CNGA3 and CNGB3 and between two CNGA3 but not between two CNGB3 (Matveev et al., 2010), this work provided an experimental evidence supporting a three CNGA3 and one CNGB3 stoichiometry.
We also analyzed the channel complexes that were resolved under native conditions using blue native-PAGE. This techniquepermits a high-resolution separation of multi-protein complexes under native conditions and is specially designed for membrane proteins. We successfully resolved the channel complexes on a blue native-PAGE. Combined with a subsequent SDS-PAGE in the second dimension, protein complexes separated by blue native-PAGE can be dissected into their subunits. Success in the blue native-PAGE enables us to further analyze the channel complexes with a blue native-PAGE/SDS-PAGE 2D system.
In summary, we analyzed native cone CNG channel complexes using infrared fluorescence detection combined with chemical cross-linking and blue native-PAGE. The experimental results suggest that the cone CNG channel is a heterotetrameric complex likely at a stoichiometry of three CNGA3 and one CNGB3.
Acknowledgments
This work was supported by grants from the National Center for Research Resources (P20RR017703), the National Eye Institute (P30EY12190, R01EY019490, and R21EY17888), the American Health Assistance Foundation, and the Oklahoma Center for the Advancement of Science & Technology (OCAST). We thank Micaela J. Langevin for technical assistance.
References
- Chavan M, Chen Z, Li G, Schindelin H, Lennarz WJ, Li H. Dimeric organization of the yeast oligosaccharyl transferase complex. Proc Natl Acad Sci USA. 2006;103:8947–52. doi: 10.1073/pnas.0603262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XQ, Harry S, Umino Y, Matveev AV, Fliesler SJ, Barlow RB. Impaired cone function and cone degeneration resulting from CNGB3 deficiency: down-regulation of CNGA3 biosynthesis as a potential mechanism. Hum Mol Genet. 2009;18:4770–80. doi: 10.1093/hmg/ddp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- Matveev AV, Fitzgerald JB, Xu JH, Malykhina AP, Rodgers KK, Ding XQ. The disease-causing mutations in the carboxyl terminus of the cone cyclic nucleotide-gated channel CNGA3 subunit alter the local secondary structure and interfere with the channel active conformational change. Biochemistry. 2010;49:1628–39. doi: 10.1021/bi901960u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveev AV, Quiambao AB, Browning Fitzgerald J, Ding XQ. Native cone photoreceptor cyclic nucleotide-gated channel is a heterotetrameric complex comprising both CNGA3 and CNGB3: a study using the cone-dominant retina of Nrl−/− mice. J Neurochem. 2008;106:2042–55. doi: 10.1111/j.1471-4159.2008.05548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Rich ED, Varnum MD. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron. 2004;42:401–10. doi: 10.1016/s0896-6273(04)00225-9. [DOI] [PubMed] [Google Scholar]
- Pyndiah S, Lasserre JP, Menard A, Claverol S, Prouzet-Mauleon V, Megraud F, Zerbib F, Bonneu M. Two-dimensional blue native/SDS gel electrophoresis of multiprotein complexes from Helicobacter pylori. Mol Cell Proteomics. 2007;6:193–206. doi: 10.1074/mcp.M600363-MCP200. [DOI] [PubMed] [Google Scholar]
- Schwarzer A, Schauf H, Bauer PJ. Binding of the cGMP-gated channel to the Na/Ca-K exchanger in rod photoreceptors. J Biol Chem. 2000;275:13448–54. doi: 10.1074/jbc.275.18.13448. [DOI] [PubMed] [Google Scholar]
- Trudeau MC, Zagotta WN. An intersubunit interaction regulates trafficking of rod cyclic nucleotide-gated channels and is disrupted in an inherited form of blindness. Neuron. 2002;34:197–207. doi: 10.1016/s0896-6273(02)00647-5. [DOI] [PubMed] [Google Scholar]
- Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron. 2002;36:881–9. doi: 10.1016/s0896-6273(02)01098-x. [DOI] [PubMed] [Google Scholar]
- Wittig I, Braun HP, Schagger H. Blue native PAGE. Nat Protoc. 2006;1:418–28. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Corlew R, Philpot BD. Visual deprivation modifies both presynaptic glutamate release and the composition of perisynaptic/extrasynaptic NMDA receptors in adult visual cortex. J Neurosci. 2005;25:11684–92. doi: 10.1523/JNEUROSCI.4362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Molday LL, Molday RS, Yau KW. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature. 2002;420:193–8. doi: 10.1038/nature01201. [DOI] [PMC free article] [PubMed] [Google Scholar]