Abstract
Elevated pulse pressure (PP) is associated with cognitive decline and increased risk of Alzheimer’s disease (AD) in older adults, although the mechanisms behind these associations remain unclear. To address this question, we examined whether antemortem late-life PP elevation predicted vascular or AD pathology in autopsy-confirmed AD patients. Sixty-five elderly patients (mean age 74.2 years) clinically diagnosed with possible or probable AD underwent neuropsychological testing and blood pressure examinations. Postmortem histopathological measures of cerebrovascular disease (CVD) and AD neuropathology were later obtained on these same patients. We expected that antemortem PP elevation, but not standard blood pressure measures such as systolic or diastolic blood pressure, would predict the autopsy-based presence of CVD, and possibly AD pathology, in elderly AD patients. Results demonstrated that antemortem PP elevation was associated with the presence and severity of CVD at autopsy. For every 5 mmHg increase in antemortem PP there was an estimated 36% increase in the odds of having CVD at autopsy. Additionally, PP accounted for 12% of variance in CVD severity. No significant associations were present for cerebral amyloid angiopathy or Braak and Braak staging of the severity of AD pathology. Other standard blood pressure measures also did not significantly predict neuropathology. The association between antemortem PP and CVD at autopsy suggests that in older adults with AD, PP elevation may increase the risk of CVD. These findings may have treatment implications since some antihypertensive medications specifically address the pulsatile component of blood pressure (e.g., renin-angiotensin system inhibitors, calcium channel blockers).
Keywords: Alzheimer’s disease, blood pressure, cerebrovascular disease, pulse pressure
INTRODUCTION
Hypertension is the most common modifiable risk factor for Alzheimer’s disease (AD) [1], but the exact mechanisms involved remain unclear [2] and clinical trials examining the ability of antihypertensive medications to prevent dementia have yielded mixed results [3, 4]. One line of research indicates that the various components of the blood pressure curve may have differential effects on the risk of dementia in older adults [5]. Blood pressure is typically measured at two points within the cardiac cycle, systole and diastole, which are used to calculate the steady component of blood pressure, mean arterial pressure (MAP), or its pulsatile component, pulse pressure (PP) [6]. The steady component, MAP, is conceptualized as the product of cardiac output and total peripheral resistance. The pulsatile component, PP, is determined by a more complex combination of factors, including stroke volume, arterial compliance, and wave reflection [7]. Most studies examining the effects of blood pressure on the risk of AD have focused on standard measures of blood pressure taken during peak (systole) and trough (diastole) [8]. Measures representing the pulsatile component of blood pressure, such as PP (SBP–DBP), [6, 9] have been less extensively investigated, [5] yet prospective studies have specifically linked PP elevation to increased risk of AD [10–12]. It has been hypothesized that PP elevation may impair amyloid-β (Aβ) clearance from the brain [13], which could increase the risk of AD and cerebral amyloid angiopathy (CAA). Other studies have suggested that PP elevation may contribute to AD risk indirectly by increasing the risk of cerebrovascular disease (CVD) [10, 14]. To our knowledge, no studies have examined whether antemortem measures of the pulsatile component of blood pressure in late-life predict postmortem CVD or AD pathology in patients with autopsy-confirmed AD. Thus, in order to further our understanding of the mechanisms underlying the increased risk of AD associated with blood pressure elevation in older adults, the current study sought to evaluate whether antemortem blood pressure elevation was related to CVD, CAA, and AD severity in AD patients at autopsy. We hypothesized that PP elevation would predict the presence of comorbid CVD in AD patients, and possibly AD pathology, and that standard blood pressure measures would show no association with neuropathology.
MATERIALS AND METHODS
Participants
Blood pressure and autopsy-based neuropathological data from 138 dementia patients recruited through the University of California San Diego (UCSD) Alzheimer’s Disease Research Center (ADRC) were initially screened. From this initial sample, patients were included if they met NINDS-ADRDA criteria for probable or possible AD [15] at the time of blood pressure assessment and were later confirmed to meet National Institute on Aging (NIA) Reagan and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) criteria for probable or definite AD at autopsy [16]. Cases were specifically excluded if autopsy revealed any significant pathological process other than AD or CVD (e.g., hippocampal sclerosis, Lewy Body pathology, Pick’s disease). Participants with more extensive CVD, meeting criteria for vascular dementia or mixed dementia, were also excluded. After application of inclusion and exclusion criteria, 65 AD patients remained for examination of relationships among blood pressure and neuropathologic markers. All procedures were approved by the UCSD IRB and all participants and/or their caregivers were provided informed consent prior to being enrolled.
Demographic characteristics and clinical data
Patient age, gender, years of education, and use of antihypertensive medications were recorded on the same day as neuropsychological and blood pressure assessments. Due to difficulty measuring and controlling for the date of dementia onset in this population, we used patient “time-to-death” (i.e., number of years between blood pressure evaluation and death) and the Mattis Dementia Rating Scale (DRS) score to control for the severity of dementia at the time of blood pressure evaluation [17]. Apolipoprotein E (APOE) genotype was determined for all participants using a polymerase chain reaction-based method [18]. Participants were grouped into those with and without at least one copy of the APOE ε4 allele for data analysis.
Vascular risk factors
The presence or absence of the following vascular risk factors derived from the Framingham Stroke Risk Profile [19] were identified from patient interview, chart review, and physical assessment on the date of neuropsychological testing and blood pressure assessment: history of cardiovascular disease (coronary artery disease [myocardial infarction, angina pectoris, coronary insufficiency], intermittent claudication, cardiac failure), diabetes, atrial fibrillation, left ventricular hypertrophy, and transient ischemic attack (TIA) or stroke.
Neuropathology
Autopsy was performed according to established UCSD ADRC protocols [20]. Briefly, the left hemi-brain was fixed in 10% formalin for 5–7 days. Following fixation the brain was examined externally and serially sectioned into 1-cm thick slices in the coronal plane. Tissue blocks were taken from all gross lesions, as well as from the midfrontal cortex, inferior parietal cortex, superior temporal gyrus, inferior temporal gyrus, anterior cingulate gyrus, posterior cingulate gyrus, anterior hippocampus, posterior hippocampus, amygdala, basal ganglia including substantia innominata and adjacent insular cortex, mesencephalon, rostral pons, and cerebellar vermis. Paraffin-embedded sections from tissue blocks were made and stained with hematoxylin and eosin and thioflavin-S for visual evaluation in 10-μm thick sections [21]. For select cases, some tissue blocks were also stained with antibodies against ubiquitin or α-synuclein and a phosphorylated form of tau to rule out other forms of neurodegeneration. Total plaque, neuritic plaque, and neurofibrillary tangle counts were then performed on midfrontal, rostral superior temporal, inferior parietal, entorhinal, and hippocampal regions. Brains were then staged for the degree of neurofibrillary tangle pathology by a modification [22] of the method of Braak and Braak [23] by one neuropathologist (L.A.H.). All AD participant brains (n = 65) were examined for CAA and CVD (i.e., hemorrhage, large artery infarction, lacunes, cortical microinfarcts, arteriosclerosis, and atherosclerosis in the Circle of Willis). The severity of CAA was assessed semiquantitatively by the study neuropathologist (L.A.H.) on a scale of 1 to 3 (mild, moderate, severe) on thioflavin-S stained preparations of the midfrontal cortex, superior temporal gyrus, inferior parietal cortex, and posterior hippocampus using a method described previously [24]. Capillary CAA was not evaluated. A semi-quantitative measure of CVD was calculated for a subset of participants with CVD (n = 45) using the following method. The severity of both arteriosclerosis and atherosclerosis was scored separately on a 3 point scale (mild, moderate, severe) by the study neuropathologist and other forms of vascular neuropathology (lacunar infarction, large artery infarction, hemorrhage, cortical microinfarcts) were assigned 1 point each. The total points for each form of vascular pathology were then summed to yield a semi-quantitative measure of CVD severity from 0 to a maximum of 10 possible points.
Participant groupings
For categorical analysis of neuropathological variables, participants were classified in three different ways based on the presence or severity of various forms of neuropathology: 1) Participants were divided into AD with CVD (AD+CVD; n = 49) or AD without CVD (AD−CVD; n = 16) groups based on the presence or absence of CVD at autopsy. CAA was not included in the CVD category and was analyzed separately. 2) Participants were classified as having AD with CAA (AD+CAA; n = 52) or AD without CAA (AD−CAA; n = 13). 3) Participants were classified as having less severe AD (low Braak and Braak stage) or more severe AD (high Braak and Braak stage). All participants were diagnosed with probable or definite AD at autopsy, consequently, patients with Braak and Braak scores less than VI were defined as having “less severe” AD (low Braak and Braak; n = 26) and those with a Braak and Braak score of VI were defined as having “more severe” AD (high Braak and Braak; n = 39).
Blood pressure
Brachial artery blood pressure measures were obtained using a sphygmomanometer and a stethoscope on the same day as neuropsychological testing procedures, but before the beginning of testing. Participants were seated comfortably for a few minutes prior to taking blood pressure measures. SBP was recorded at the first Korotkoff sound and DBP was recorded at the cessation Korotkoff sounds (i.e., fifth Korotkoff sound). Two recordings were taken from each arm while the participant was seated. These measures were averaged to obtain an estimate of resting blood pressure. PP and MAP were calculated as follows: PP = SBP − DBP; MAP = DBP + 1/3(PP).
Statistical analyses
Independent samples t-tests were used to examine differences between the AD+CVD and AD−CVD groups in age, education, time-to-death, and DRS score. Chi-square analyses were used to test group differences in gender, use of antihypertensive medications, presence of the APOE ε4 allele, and presence of two or more vascular risk factors. Univariate Pearson product moment correlations and logistic regression models were initially examined to determine whether blood pressure measures significantly predicted the presence and severity of different forms of neuropathology. For each logistic regression analysis, the predictor variable was the blood pressure measure of interest (i.e., SBP, DBP, MAP, PP) and the dependent variable was the neuropathological group (i.e., AD+CVD versus AD−CVD; AD+CAA versus AD−CAA; low versus high Braak and Braak). For correlational analyses, the dependent variable was a measure of the severity of neuropathology (i.e., semi-quantitative CVD measure; neuropathologist rating of CAA severity; Braak and Braak stage). Blood pressure measures that significantly predicted or correlated with neuropathology after Bonferonni correction for multiple comparisons (3 neuropathology groups: p < 0.05/3 or 0.017), were further examined in binary logistic and multiple linear regression models.
Binary logistic regression analysis was used to determine whether remaining blood pressure measures significantly predicted neuropathology beyond the model containing all demographic, clinical, and vascular risk factor covariates. For each analysis, the first block contained all covariates, including age, education, gender, DRS score, time-to-death, use of antihypertensive medication, presence of the APOE ε4 allele, and the presence of two or more vascular risk factors. The second block contained the blood pressure measure of interest, with the neuropathologic group as the dependent variable.
Multiple linear regression was also used to examine the relationship between remaining blood pressure measures and the severity of neuropathology using the same covariates listed above. All statistical tests were two-tailed with a significance cutoff of p < 0.05.
RESULTS
Participant groups
Forty-nine patients (77.5%) displayed CVD at autopsy, which included in order of frequency: atherosclerosis in the Circle of Willis (70.8%), arteriosclerosis (15%), lacunar infarction (12.3%), cortical microinfarcts (6.2%), large artery infarction (3.1%), and hemorrhage (1.5%). CAA was present in 80.0% of participant brains. Among participants with CVD, the average semi-quantitative score for CVD severity was 2.1 with a standard deviation (SD) of 1.2.
All participant brains showed AD pathology at Braak and Braak stage IV or greater (IV, 6.2%; V, 33.8%; VI, 60.0%). Braak and Braak scores were divided into lower (stages IV or V; 40.0%) and higher (VI; 60.0%) groups for logistic regression analyses.
Demographic characteristics and clinical data
For the total sample, the average age during the ante-mortem assessment was 74.2 years (SD = 7.0), average educational attainment was 12.8 years (SD = 3.2), average DRS score was 101.8 points (SD = 24.5), and average time from blood pressure and cognitive assessments until death (i.e., “time-to-death”) was 6.0 years (SD = 3.2). The male to female ratio was 31/34 (47.7% men) and 27.7% of patients were on antihypertensive medication. At the time of blood pressure and cognitive assessment, 55 (84.6%) participants were diagnosed with probable AD and 10 (15.4%) were diagnosed with possible AD. At the time of death, all participants had been diagnosed with probable AD.
Comparison of AD+CVD and AD−CVD groups on demographic and clinical characteristics indicated a significant difference in time-to-death, such that the AD+CVD group lived significantly longer than the AD−CVD group, t (63) = −2.26, p = 0.03. There was a marginally significant trend toward the AD+CVD group performing better than the AD−CVD group on the DRS, t (63) = −1.98, p = 0.05. There were no differences in age, t (63) < 1, p = 0.60; education, t (63) < 1, p = 0.81; gender, χ2 < 1, p = 0.83; or use of antihypertensive medication, χ2 < 1, p = 0.78, between those with and without CVD. At least one copy of the APOE ε4 allele was present in 70.8% of participants in the sample. There was no significant difference between AD+CVD and AD−CVD groups in frequency of the APOE ε4 allele, χ2 = < 1, p = 0.40 (Table 1).
Table 1.
Comparison of Alzheimer’s disease groups with cerebrovascular disease (AD+CVD) and without cerebrovascular disease (AD−CVD) on demographic characteristics, and clinical and vascular risk factors
| AD+CVD | AD−CVD | |
|---|---|---|
| Age (years) | 74.4 ± 7.4 | 73.4 ± 5.9 |
| Men/women ratio (% Men) | 23/26 (46.9%) | 8/8 (50.0%) |
| Education (years) | 12.8 ± 3.2 | 13.0 ± 3.1 |
| Antihypertensives (% on meds) | 14 (28.6%) | 4 (25.0%) |
| DRS score (raw) | 105.2 ± 22.7 | 91.6 ± 27.3 |
| Time-to-death (years) | 6.4 ± 3.3 | 4.4 ± 2.3* |
| APOE4 (% with ε4 allele) | 36 (73.5%) | 10 (62.5%) |
| APOE genotype | ||
| 33 | 13 (26.5%) | 6 (37.5%) |
| 34 | 24 (49.0%) | 9 (56.3%) |
| 44 | 10 (20.4%) | 1 (6.3%) |
| 42 | 2 (4.1%) | 0 (0%) |
| Cardiovascular disease | 20 (40.8%) | 5 (31.3%) |
| TIA/Stroke | 7 (14.3%) | 0 (0%) |
| Diabetes | 6 (12.2%) | 0 (0%) |
| Atrial fibrillation | 5 (10.2%) | 0 (0%) |
| Left ventricular hypertrophy | 0 (0%) | 0 (0%) |
| ≥ 2 Vascular risk factors | 10 (20.4%) | 0%* |
Values indicate mean ± standard deviation or number of cases (% of cases).
p < 0.05.
Patients with higher Braak and Braak scores were younger than those with lower Braak and Braak scores, t (63) = 3.40, p = 0.001, and exhibited greater cognitive impairment on the DRS, t (53.1) = 3.13, p = 0.01. There was no significant difference between Braak and Braak groups in the frequency of the APOE4 allele χ2 < 1, p = 0.37 or the time-to-death, t (63) −1.05, p = 0.30.
Vascular risk factors
At least one vascular risk factor was present in 44.6% of participants in the total sample. A subset (n = 5) of participants in the AD−CVD group had a known history of cardiovascular disease, but none of the other vascular risk factors were present in this group, whereas a subset (n = 10) of participants in the AD+CVD group had a history of two or more vascular risk factors. Chi-square analyses indicated that significantly more AD+CVD patients had two or more vascular risk factors relative to those in the AD−CVD group, χ2 = 3.86, p = 0.05 (Table 1).
Blood pressure and neuropathology
Logistic regression analyses revealed that PP elevation significantly predicted the presence of CVD, odds ratio = 1.06, p = 0.016. This relationship remained significant after controlling for age, gender, education, DRS score, time-to-death, use of antihypertensive medication, APOE ε4 allele, and the presence of two or more vascular risk factors, odds ratio = 1.07, p = 0.04. Thus, for every 5 mmHg increase in antemortem PP, there is an estimated increase in the odds of having CVD at autopsy of approximately 36%. PP elevation was not significantly associated with Braak and Braak stage after controlling for all covariates, β = −0.04, p = 0.08. None of the standard blood pressure measures (SBP, DBP, MAP) significantly predicted any form of neuropathology after controlling for all covariates (p-values = 0.12 to 0.95). No blood pressure measures significantly predicted the presence of CAA (p-values = 0.36 to 0.84).
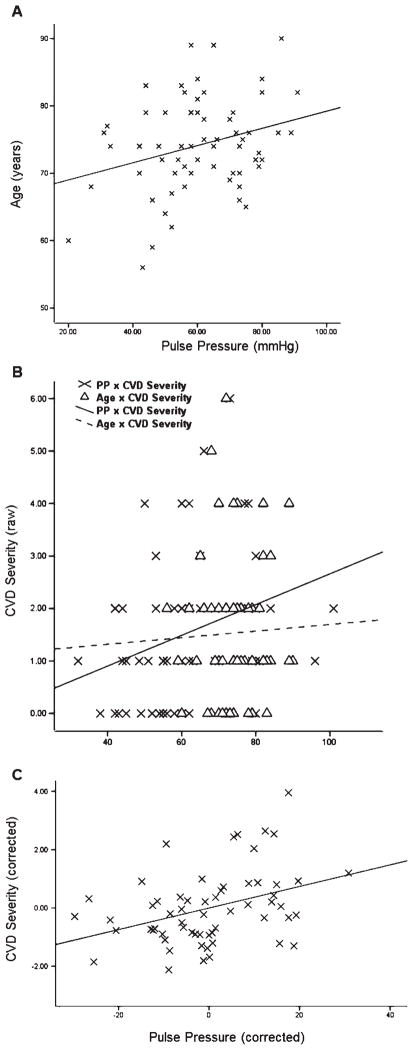
Linear regression analyses indicated that PP was positively correlated with age, r = 0.31, p = 0.01 (Fig. 1A) and CVD severity, r = 0.31, p = 0.016 (Fig. 1B). There was no significant relationship between age and CVD severity, r = 0.03, p = 0.80 (Fig. 1B). PP remained a significant predictor of CVD severity after controlling for age and all other covariates, ΔR2 = 0.12, β = 0.39, p = 0.006 (Fig. 1C). No other significant correlational associations were observed between blood pressure measures and neuropathology severity after correcting for multiple comparisons (all p-values >0.15).
Fig. 1.
Scatterplots and regression lines are shown for A) pulse pressure (PP) significantly predicting age (p < 0.05) and B) cerebrovascular disease (CVD) severity (p < 0.05). Age was not significantly related to CVD. C) The partial scatterplot depicts regression line for PP significantly predicting the severity of CVD in AD patients after correcting for all covariates (p < 0.01).
DISCUSSION
Our results indicate that AD patients with elevated PP are more likely to exhibit CVD at autopsy. Elevation in the pulsatile component of blood pressure was also associated with more severe CVD. This relationship is consistent with prior observations from neuroimaging studies indicating a possible association between PP and CVD in patients with AD [10, 14]. Importantly, the association between PP and CVD was independent of dementia severity and the presence of other vascular risk factors, as well as other clinical and demographic confounds. Interestingly, standard measures of blood pressure, including SBP, DBP, and MAP, were not significantly associated with the presence of CVD. These findings are consistent with our recent study showing that indices of the pulsatile component of blood pressure, but not the steady or point measures, predicted cognitive impairment characteristic of the earliest stages of AD in nondemented older adults [5]. Together these findings underscore the potential importance of the pulsatile component of blood pressure relative to the more commonly assessed mean and point blood pressure measures in assessing the risk of a vascular contribution to cognitive impairment in older adults with AD.
Although PP elevation is known to increase the risk of AD in older adults, the mechanism behind this relationship has remained unclear [11]. Weller and colleagues have suggested that increased pulsatile strain may accelerate AD pathophysiology, including CAA and Aβ deposition, by interfering with Aβ clearance mechanisms [13]. In contrast, our data showed that PP was not related to the presence of CAA or the severity of AD. Due to sample size limitations within the AD−CAA group (n = 13), we also examined the relationship between blood pressure measures and the severity of CAA at autopsy. Findings indicated no significant relationship between CAA severity and any of the blood pressure measures examined in this study. These results could suggest that blood pressure elevation is not associated with CAA in older adults with AD; however, further studies utilizing a larger sample of patients both with and without CAA are needed to clarify this relationship. It should also be noted that the relationship between blood pressure and CAA may differ in younger adults or in nondemented older adults. Additionally, none of the blood pressure measures examined in the current study were associated with AD severity after controlling for all potential confounds.
Collectively these findings suggest that, in older adults with AD, blood pressure elevation is not related to AD pathology (i.e., plaques, tangles, and CAA). Our results contrast with findings indicating that elevated blood pressure in mid-life is predictive of AD neuropathology (e.g., higher density of hippocampal neurofibrillary tangles) in late-life [25]. However, the current study examined blood pressure levels in older adults already diagnosed with possible or probable AD and was restricted to those diagnosed with probable or definite AD at autopsy. It is possible that blood pressure exerts a different influence on vascular and AD neuropathology over a more extended period of time, or for different age groups, or for individuals with more extensive CVD. This possibility is consistent with findings indicating an age-dependent relationship between blood pressure and cognition, with mid-life blood pressure elevation predicting late-life dementia but both high and low blood pressure predicting dementia in late-life [8]. Thus, the findings of the present study support the hypothesis that the pulsatile component of blood pressure remains an important determinant of co-morbid CVD in older adults with AD.
We have previously shown that elevation of the pulsatile component of blood pressure continues to be associated with cognitive decline in older adults despite the lack of a consistent relationship with standard blood pressure measures [5, 11]. The present study findings suggest that in older adults with AD, PP elevation may be influencing cognition through effects on CVD. This supports the hypothesis that PP elevation increases mechanical strain on cerebral vessels that in turn leads to atherosclerosis and arteriosclerosis, the most common forms of vascular pathology in our sample [27]. Additionally, our findings are consistent with animal studies indicating that the pulsatile component of blood pressure is a particularly important causal factor in the development of intracranial vascular disease [27]. Although the causal explanation of these results has merit, it is also possible that pulsatile measures are simply more sensitive to underlying cardiovascular disease that is in turn associated with vascular disease in the brain. Nevertheless, antemortem PP elevation continued to be associated with CVD at autopsy even after controlling for other vascular risk factors.
The majority of the patients in our sample demonstrated some vascular lesions at autopsy, mostly atherosclerosis within the circle of Willis and arteriosclerosis. These findings are consistent with results from large epidemiological studies indicating that the “pure” form of AD represents a relative minority of patients [28]. Patients with AD+CVD had more vascular risk factors than those with “pure” AD, particularly stroke risk factors beyond cardiovascular disease (i.e., diabetes, TIA/minor stroke, atrial fibrillation). These findings support the continued use of stroke risk factors other than cardiovascular disease to assess the risk of co-morbid CVD in AD patients.
Our data further showed that patients with AD+CVD lived longer and displayed a trend toward being less cognitively impaired than those with “pure” AD, despite being close in age. The reason for these differences is not immediately clear. These findings may seem counterintuitive given the presence of two forms of pathology in the AD+CVD group, which may be thought to convey increased cognitive impairment. This assumption may apply when considering cases with more extensive or severe CVD, such as those with mixed vascular and AD, but our sample was restricted to patients with autopsy-confirmed AD and did not include mixed or vascular dementia patients. This sample was deliberately chosen to specifically assess the mechanism underlying the association between PP elevation and CVD in AD, rather than mixed or vascular dementia. We speculate on at least two possible explanations for the observed group differences in time-to-death and cognition. First, the “pure” AD group may represent a more aggressive form of the disease, potentially associated with increased genetic risk or different age of onset. However, our data did not indicate any differences in the presence of APOE ε4 allele or age between the AD+CVD and AD−CVD groups. The second possibility is that patients with AD+CVD may have a less severe form of AD because less AD pathology is required to cause dementia in individuals who also have some degree of cognitive impairment related to underlying CVD.
We caution that these data do not indicate a “protective effect” of CVD on AD. Indeed we found that CVD is a common comorbidity and is present in the majority of cases at all Braak and Braak stages observed in this sample (IV thru VI). Nevertheless, the differences in time-to-death and dementia severity between AD+CVD and AD−CVD groups may be partly explained by their additive effect on cognition and independent functioning. This hypothesis has been previously described [1] and has significant treatment implications since it implies that dementia may have possibly been delayed, attenuated, or prevented in the majority of our sample (over 75%) by prevention of CVD [1, 29]. PP elevation and the presence of stroke risk factors beyond cardiovascular disease were the only variables that predicted the presence of CVD in this sample, suggesting that treatments targeting these risk factors may prevent or delay the onset of dementia in some patients. Taken together these data underscore the importance of co-morbid CVD in AD and the potential value of the pulsatile component of blood pressure in the diagnosis and treatment of this common comorbidity.
The fact that patients with more severe AD pathology were younger and exhibited more severe cognitive impairment is consistent with prior studies demonstrating that very old adults require less severe AD pathology to express clinical dementia and tend to perform better on age-corrected cognitive testing [30]. Interestingly, we did not find any such relationship between age and CVD. Thus, age appeared to modify the expression of AD pathology in our sample, but not comorbid CVD.
The current study findings may have substantial clinical implications because they suggest that antihypertensive treatments targeting the pulsatile component of blood pressure may reduce the vascular contribution to cognitive impairment in AD patients or individuals at risk of AD. Certain classes of antihypertensive medications specifically affect the pulsatile component of blood pressure through their effects on arterial compliance. These include drugs impacting the renin-angiotensin system and calcium channel blockers. Other antihypertensive medications, such as diuretics and β-blockers, may have little to no effect on pulsatile forces [31]. Interestingly, a recent review of clinical trials for antihypertensive agents in the prevention of dementia concluded that drugs inhibiting the renin-angiotensin system and calcium channel blockers are superior to diuretics and β-blockers [3, 32]. These drugs may be reducing the risk of dementia through their specific effects on the pulsatile component of blood pressure. Prior studies have associated antihypertensive medication use with a lesser degree of AD pathology, but sample size has typically precluded the investigation of specific medication classes and AD pathology has been the focus more than comorbid CVD [33]. The current study found no significant differences in antihypertensive medication use between AD patients with and without CVD. The sample included participants taking a wide range of antihypertensive medications, including those with and without destiffening effects, in various combinations and monotherapy. Consequently, the study was not adequately powered to address different medication classes. Future studies examining the effects of destiffening medications on PP and CVD may inform treatment decisions in older adults with blood pressure elevation.
The limitations of the current study include the relatively small sample size within the “pure” AD group that may have limited power to detect group differences. This was partially corrected by the use of supplemental correlational and linear regression analyses to support the main study findings. The use of neuropathologic measures is always limited by our inability to determine the pathology at the time of antemortem assessment, making all postmortem data cross-sectional in nature. The timing of the antemortem blood pressure assessment is another important consideration in the interpretation of these results, as late-life blood pressure values may not reflect mid-life exposure, which has been more strongly associated with cognitive impairment and neuropathology in prior studies. The current study was specifically designed to address contribution of late-life PP elevation to neuropathology in AD. The average time-to-death of patients in the current study was 6.4 years for those with CVD and 4.4 years for those without CVD. Blood pressure measures examined for longer or shorter intervals from death may be differentially related to neuropathological measures. Future studies investigating multiple antemortem time intervals may clarify how the relationship between blood pressure and neuropathology varies with exposure. Finally, the relationship between blood pressure and CVD may differ in a sample of patients with more extensive CVD (i.e., vascular dementia, mixed dementia) or with more vascular risk factors. However, the fact that PP elevation was associated with CVD even in a group with relatively few vascular risk factors and mild CVD only further supports the potential usefulness of this measure as a sensitive index of vascular risk. On balance the utilization of a well-characterized patient group with extensive neuropathologic data is a major strength of the study. Future studies examining the relationship between medications impacting pulsatile forces and neuropathologic measures of AD and CVD may further clarify the clinical significance of the current study findings.
Acknowledgments
The current study was supported by the Alzheimer’s Association (IIRG 07-59343, NIRG 09-131856) and the National Institute of Health (R01 AG012674, K24 AG026431, and P50 AG05131). The authors thank the staff and participants of the ADRC for their important contributions.
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=1190).
References
- 1.Knopman DS, Roberts R. Vascular risk factors: Imaging and neuropathologic correlates. J Alzheimers Dis. 2010;20:699–709. doi: 10.3233/JAD-2010-091555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manolio TA, Olson J, Longstreth WT. Hypertension and cognitive function: Pathophysiologic effects of hypertension on the brain. Curr Hypertens Rep. 2003;5:255–261. doi: 10.1007/s11906-003-0029-6. [DOI] [PubMed] [Google Scholar]
- 3.Duron E, Hanon O. Antihypertensive treatments, cognitive decline, and dementia. J Alzheimers Dis. 2010;20:903–914. doi: 10.3233/JAD-2010-091552. [DOI] [PubMed] [Google Scholar]
- 4.Staessen JA, Thijs L, Richart T, Odili AN, Birkenhager WH. Placebo-controlled trials of blood pressure-lowering therapies for primary prevention of dementia. Hypertension. 2011;57:e6–e7. doi: 10.1161/HYPERTENSIONAHA.110.165142. [DOI] [PubMed] [Google Scholar]
- 5.Nation DA, Wierenga CE, Delano-Wood L, Jak AJ, Delis DC, Salmon DP, Bondi MW. Elevated pulse pressure is associated with age-related decline in language ability. J Int Neuropsychol Soc. 2010;16:933–938. doi: 10.1017/S1355617710000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols WW, O’Rourke MF. McDonald’s blood flow in arteries. Theoretical, experimental and clinical principles. 4. Edward Arnold; London: 2006. [Google Scholar]
- 7.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 8.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 9.Jankowski P, Bilo G, Kawecka-Jaszcz K. The pulsatile component of blood pressure: Its role in the pathogenesis of atherosclerosis. Blood Press. 2007;16:238–245. doi: 10.1080/08037050701428166. [DOI] [PubMed] [Google Scholar]
- 10.Lee AY, Jeong SH, Choi BH, Sohn EH, Chui H. Pulse pressure correlates with leukoaraiosis in Alzheimer disease. Arch Gerontol Geriatr. 2006;42:157–166. doi: 10.1016/j.archger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: A community-based, longitudinal study. Stroke. 2003;34:594–599. doi: 10.1161/01.STR.0000060127.96986.F4. [DOI] [PubMed] [Google Scholar]
- 12.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the baltimore longitudinal study of aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 13.Weller RO, Boche D, Nicoll JA. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer’s disease and their potential impact on therapy. Acta Neuropathol. 2009;118:87–102. doi: 10.1007/s00401-009-0498-z. [DOI] [PubMed] [Google Scholar]
- 14.Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, Lecompte T, Lacolley P, Benetos A, Zannad F. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke. 2009;40:1229–1236. doi: 10.1161/STROKEAHA.108.532853. [DOI] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 17.Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellack L, Karasu TB, editors. Geriatric psychiatry: A handbook for psychiatrists and primary care physicians. Grune & Stratton; New York: 1976. pp. 77–121. [Google Scholar]
- 18.Saunders NB, Zollinger WD, Rao VB. A rapid and sensitive PCR strategy employed for amplification and sequencing of porA from a single colony-forming unit of Neisseria meningitidis. Gene. 1993;137:153–162. doi: 10.1016/0378-1119(93)90001-j. [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: Adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25(1):40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 20.Hansen LA, Samuel W. Criteria for Alzheimer’s disease and the nosology of dementia with Lewy bodies. Neurology. 1997;48:126–132. doi: 10.1212/wnl.48.1.126. [DOI] [PubMed] [Google Scholar]
- 21.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: Report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 22.Samuel W, Galasko D, Masliah E, Hansen LA. Neo-cortical Lewy body counts correlate with dementia in the Lewy body variant of Alzheimer’s disease. J Neuropathol Exp Neurol. 1996;55:44–52. doi: 10.1097/00005072-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 24.Olichney JM, Hansen LA, Galasko D, Saitoh T, Hofstetter CR, Katzman R, Thal LJ. The apolipoprotein E ε4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer’s disease and Lewy body variant. Neurology. 1996;47:190–196. doi: 10.1212/wnl.47.1.190. [DOI] [PubMed] [Google Scholar]
- 25.Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: The HAAS. Honolulu-Asia Aging Study. Neurobiol Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. J Appl Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumbach GL, Heistad DD. Drug-induced changes in mechanics and structure of cerebral arterioles. J Hypertens Suppl. 1992;10:S137–S140. [PubMed] [Google Scholar]
- 28.Etiene D, Kraft J, Ganju N, Gomez-Isla T, Gemelli B, Hyman BT, Hedley-Whyte ET, Wands JR, De La Monte SM. Cerebrovascular pathology contributes to the heterogeneity of Alzheimer’s disease. J Alzheimers Dis. 1998;1:119–134. doi: 10.3233/jad-1998-1205. [DOI] [PubMed] [Google Scholar]
- 29.Honig LS, Tang MX, Albert S, Costa R, Luchsinger J, Manly J, Stern Y, Mayeux R. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707–1712. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- 30.Stricker NH, Chang YL, Fennema-Notestine C, Delano-Wood L, Salmon DP, Bondi MW, Dale AM Alzheimer’s Disease Neuroimaging, Initiative . Distinct profiles of brain and cognitive changes in the very old with Alzheimer disease. Neurology. 2011;77:713–721. doi: 10.1212/WNL.0b013e31822b0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safar ME, Jankowski P. Antihypertensive therapy and de-stiffening of the arteries. Expert Opin Pharmacother. 2010;11:2625–2634. doi: 10.1517/14656566.2010.496452. [DOI] [PubMed] [Google Scholar]
- 32.Amenta F, Mignini F, Rabbia F, Tomassoni D, Veglio F. Protective effect of anti-hypertensive treatment on cognitive function in essential hypertension: Analysis of published clinical data. J Neurol Sci. 2002;203:147–151. doi: 10.1016/s0022-510x(02)00281-2. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman LB, Schmeidler J, Lesser GT, Beeri MS, Purohit DP, Grossman HT, Haroutunian V. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72:1720–1726. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]