Abstract
Enteric Escherichia coli (E. coli) are both natural flora of humans and important pathogens causing significant morbidity and mortality worldwide. Traditionally enteric E. coli have been divided into 6 pathotypes, with further pathotypes often proposed. In this review we suggest expansion of the enteric E. coli into 8 pathotypes to include the emerging pathotypes of adherent invasive E. coli (AIEC) and Shiga-toxin producing enteroaggregative E. coli (STEAEC). The molecular mechanisms that allow enteric E. coli to colonize and cause disease in the human host are examined and for two of the pathotypes that express a type 3 secretion system (T3SS) we discuss the complex interplay between translocated effectors and manipulation of host cell signaling pathways that occurs during infection.
Keywords: enteric E. coli, gut microbes, host-pathogen interactions, molecular mechanisms of pathogenesis, T3SS
Since its identification in 1885, Escherichia coli has become one of the most comprehensively studied bacterial species. E. coli strains are comparatively easy to grow and manipulate in the laboratory, are amenable to genetic manipulation, and naturally acquire mobile genetic elements. While E. coli isolates form part of the beneficial normal flora of the intestine, some strains have evolved pathogenic mechanisms to cause disease in humans and animals. E. coli strains can cause enteric/diarrhogenic or extraintestinal (ExPEC) infections in humans. ExPEC infections are primarily urinary tract (caused by uropathogenic E. coli, UPEC) and sepsis/meningitis (caused by neonatal meningitis E. coli, NMEC). Only the enteric E. coli will be covered in this review.
Enteric E. coli infections are traditionally divided into 6 pathotypes based on their pathogenicity profiles (virulence factors, clinical disease and phylogenetic profile): Enteropathogenic E. coli (EPEC), Enterohamerrhagic E. coli (EHEC), Enteroinvasive E. coli (EIEC, including Shigella sp), Enteroaggregative E. coli (EAEC), Enterotoxigenic E. coli (ETEC) and Diffusely Adherent E. coli (DAEC).1,2 Characteristic features of these pathotypes are shown in Table 1. Two further pathotypes have recently emerged; Adherent Invasive E. coli (AIEC) which is thought to be associated with Crohn disease but does not cause diarrhogenic infection3 and the Shiga Toxin (Stx) producing Enteroaggregative E. coli (STEAEC) responsible for the 2011 Germany E. coli outbreak. E. coli strains can also be categorized by their serogroup, e.g., E. coli O157 where O refers to the LPS O-antigen or serotype e.g., E. coli O157:H7 where H refers to the flagellar antigen. However, as each pathotype contains many serotypes (117 ETEC serotypes have been identified4) and some serotypes can belong to more than one pathotype (e.g., O26:H11 can be either EPEC or EHEC), serotyping strains may not provide definitive identification of pathotypes.
Table 1. Summary of enteric E. coli pathotypes.
| Pathotype | Adhesin | Toxin | T3SS | SPATE | Disease |
|---|---|---|---|---|---|
|
ETEC |
Colonization factors (CF) Porcine A/E associated adhesin (Paa) |
Heat-labile enterotoxin (LT) Heat-stable enterotoxin (ST) Cytolysin A (ClyA) |
- |
ETEC autotransporter A (EatA) |
Acute watery diarrhea (< 5yo) Travelers’ diarrhea |
|
EAEC |
Aggregative adherence fimbriae (AAF) (I, II, III, Hda) Toxigenic invasion loci A (Tia) |
EAEC heat-stable enterotoxin 1 (EAST1) Shigella enterotoxin (ShET)1 Hemolysin E (HlyE) |
+/−* |
Plasmid-encoded toxin (Pet) Protein involved in intestinal colonization (Pic) Secreted autotransporter toxin (Sat) Shigella IgA-like protease homology (SigA) E. coli secreted protein (Esp)P |
Travelers’ diarrhea Infant diarrhea |
|
STEAEC |
AAF IrgA homolog adhesin (Iha) |
Shiga toxin (Stx) |
- |
Pic Pet |
Food poisoning |
|
DAEC |
afimbrial (Afa) or fimbrial (Dr) adhesins |
- |
- |
Sat |
Acute diarrhea (< 5yo) |
|
AIEC |
Type 1 pili Long polar fimbriae (LPF) |
- |
- |
- |
Crohn disease |
|
EHEC |
Intimin Paa Toxin B (ToxB) E. coli factor for adherence (Efa)-1 LPF STEC autoagglutinating adhesin (Saa) E. coli immunoglobulin-binding protein (EibG) EHEC autotransporter encoding gene A (EhaA) Outer membrane protein A (OmpA) Iha |
Stx |
LEE encoded |
EspP |
Food poisoning |
|
EPEC |
Intimin Bundle forming pili (BFP) Paa LPF Iha EhaA |
- |
LEE encoded |
EspC |
Infant diarrhea |
| EIEC (Shigella) | - | ShET1/2 | pINV encoded | Shigella extracellular protein (Sep)A SigA |
Shigellosis |
One potentially functional but as yet uncharacterized T3SS (ETT2) was found in the genome sequence of EAEC O42 (and remnants of a second).
Epidemiology
Enteric E. coli are part of the natural flora of many animals. Human infections occur through consumption of contaminated food products (undercooked meat, or contaminated fresh produce such as salad leaves), drinking water contaminated with animal or human waste, or through direct person-to-person spread from poor hygiene.5 Accurate figures of the incidence of enteric E. coli infections worldwide are difficult to determine, as the causative agents of diarrhogenic infections are often not identified. In the developing world ETEC, EPEC and EAEC appear to be major causes of infantile diarrhea with potentially fatal consequences when untreated, while in the developed world these infections are mild and self-limiting. EHEC and more recently EAEC and STEAEC are the main E. coli pathotypes associated with food poisoning outbreaks in the developed world.
ETEC is reported to be the most commonly isolated bacterial enteropathogen in children under 5 y of age in developing countries, accounting for approximately 20% of cases, equivalent to several hundred million cases of diarrhea and several tens of thousands of deaths each year.6 ETEC is also the most common cause of travelers’ diarrhea accounting for 10–60% of infections depending on the region visited.7,8 Extrapolation of these figures suggests there may be 10 million cases of travelers’ diarrhea caused by ETEC per year.9 ETEC also causes disease in animals including cattle and neonatal and post-weaning pigs10 with host specificity occurring through acquisition of colonization factors (CF) rather than emergence of animal specific lineages.
EAEC is the second most common cause of travelers’ diarrhea after ETEC11 and its prevalence in endemic and epidemic disease is becoming well recognized. It causes persistent diarrhea in children in developing countries12,13 and has been implicated as an important enteric pathogen affecting AIDS patients.14 No animal reservoir has been described for EAEC suggesting that it is persisting in the human population. The 2011 German E. coli foodborne outbreak was caused by an EAEC strain (O104:H4) that had acquired typical EHEC phenotypes, most notably Stx production. Infection with STEAEC O104:H4 resulted in a high percentage of patients developing hemolytic uremic syndrome (HUS) and a mortality rate of 1%;15 852 cases of HUS, resulting in 32 deaths and 3469 cases of non-HUS STEAEC, resulting in 18 deaths.16 Taking into account this large outbreak and the previous outbreaks of Stx2-positive O104:H4,17,18 STEAEC could now be considered an emerging pathotype of enteric E. coli. Confirmation of STEAEC as an emerging pathotype will require continued detection of this distinct population of hybrid EAEC/EHEC strains.
The importance of DAEC to enteric disease remains uncertain. Some studies suggest DAEC may be an important contributor to diarrhogenic disease in children, however problems of cross-reactivity of one of the standard detection probes raises questions about this.19 A correlation with disease may occur in specific age demographics (children aged 18 mo–5 y20 or 13–24 mo21) although further epidemiological studies are required if DAEC is to remain a distinct enteric E. coli pathotype.
It is still under debate whether the association of AIEC with Crohn disease (CD) is causative or symptomatic. A combination of the two is likely with a genetic predisposition to developing CD exacerbated by microbial infection (including AIEC) into active CD. AIEC strains have been found associated with CD lesions in ileal and neo-terminal ileal and colonic specimens.22 An increased immune response to E. coli in CD patients also suggests an involvement of E. coli in the pathology of CD.23
In the developed world epidemiological data for enteric E. coli infections is generally collected based on toxin production rather than pathotypes or serotypes and infections are therefore commonly referred to as Stx-producing E. coli (STEC) or Verotoxigenic E. coli (VTEC). These classifications can include all of a pathotype (all EHEC strains are STECs) or part of a pathotype (STEAEC strains are also STECs) and may be further categorized as STEC/VTEC O157 referring to the most prevalent EHEC serogroup. 2011 estimates from the United States suggest 9.4 million foodborne illnesses occur annually, resulting in 55,961 hospitalizations and 1,351 deaths.24 While STEC O157 infections accounted for only 4% of laboratory confirmed foodborne infections in the US from 1996–2005, STEC O157 had the highest case fatality rate across the population and the highest annual population mortality rate in children 0–4 y.25 Data from the European Union for 2009 suggests 1% of laboratory confirmed zoonotic infections were attributable to VTEC with 7% of those developing HUS.26 Therefore while incidence rates of STEC/VTEC are relatively low compared with Campylobacter and Salmonella infection rates, the severity of the disease and high case fatality rates means these infections are of major health concern.
While EPEC was the first strain of E. coli generally accepted to cause diarrhogenic outbreaks in the developed world,27,28 its incidence has declined and EPEC outbreaks are now rare in developed countries. However it does remain an important cause of infant diarrhea in the developing world with recent estimates of EPEC prevalence among children with diarrhea ranging from 6–54%, although high carriage rates among healthy controls makes the contribution of EPEC to disease difficult to assess.29 Atypical EPEC [i.e., those lacking the EAF plasmid that encodes bundle-forming pili (BFP)] appear to have a propensity to cause persistent diarrhea.30,31
EIEC and Shigella can be distinguished by minor biochemical tests but in general have the same virulence mechanisms and disease symptoms. Strains of EIEC and Shigella appear to have evolved independently to share many characteristics,32 and EIEC strains seen today may simply be intermediates between E. coli and Shigella. We therefore direct the reader to the excellent review on Shigella species contained in this issue for in depth analysis of this pathotype.
Molecular Mechanisms of Virulence
Three pathotypes of E. coli (EHEC, EPEC and EIEC) employ a T3SS to translocate bacterial proteins, known as effectors, directly into the eukaryotic host cell in order to subvert host cell processes. For these pathotypes the T3SS is a major, but not the only, contributor to virulence. For convenience the pathotypes have been divided into non-T3SS dependent pathotypes (ETEC, EAEC, STEAEC, DAEC and AIEC) and T3SS dependent pathotypes (EHEC, EPEC and EIEC). The non-T3SS dependent pathotypes of enteric E. coli have comparatively simple and efficient molecular mechanisms of virulence requiring effective colonization factors followed by secretion of toxins that subsequently enter the host cell (for ETEC, EAEC and STEAEC).
Non-T3SS dependent pathotypes
ETEC
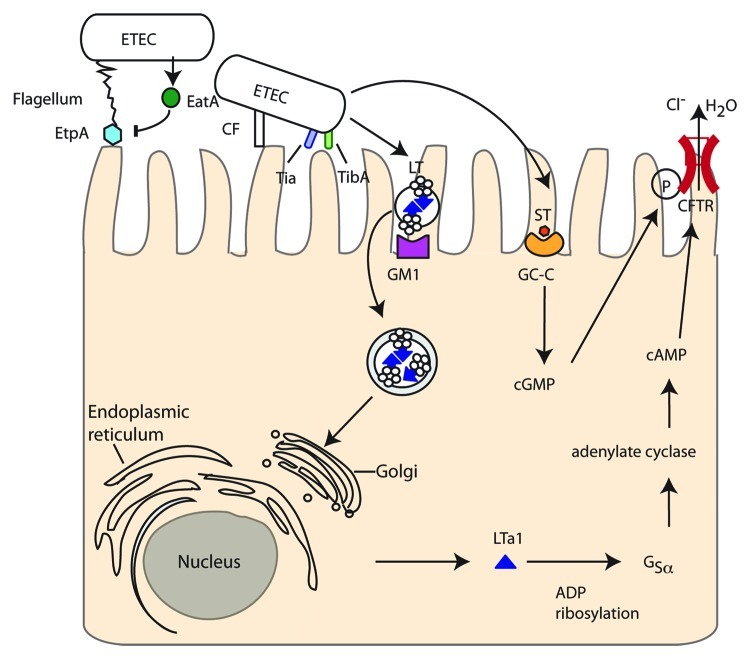
At least 25 distinct proteinaceous colonization factors (CFs) have been identified in ETEC strains33 which mediate adhesion to epithelial cells (Fig. 1). Although 30–50% of ETEC isolates have no characterized CF by phenotypic screening,6 novel CFs are constantly being identified genetically thus reducing the number of isolates with no apparent CF.34 Two further proteins, the outer membrane protein Tia and the glycosylated autotransporter TibA, have been reported to mediate intimate cell attachment and to induce ETEC invasion into epithelial cells, for the prototype ETEC strain H10407.35 While ETEC binds to leaf surfaces through the flagellum shaft,36 a novel adhesin, EtpA, located on the tip of ETEC flagella mediates attachment to mammalian host cells.37 EtpA is degraded by the serine protease autotransporter of Enterobacteriaceae (SPATE), EatA, thereby modulating bacterial adhesion and accelerating delivery of heat labile (LT) toxin into host cells.38 A model of sequential attachment is proposed whereby the long-range flagella-EtpA first anchors the bacterium to the host cell and allows shorter CFs to interact. EatA then degrades EtpA and finally intimate attachment is mediated by Tia and TibB.
Figure 1. Enterotoxigenic E. coli (ETEC). EtpA located on the tip of flagella attaches to host cells but is then degraded by the SPATE EatA. Adherence is maintained by colonization factors (CF) and intimate attachment achieved with Tia and the autotransporter TibB. Heat stable toxin (ST) is secreted by ETEC and binds to guanylate cyclase-C receptor increasing cGMP and cGMP-dependent protein kinase II. Heat labile toxin (LT) is contained in outer membrane vesicles, which are endocytosed after interaction with ganglioside receptors (GM1). Retrograde transport through the Golgi and ER leads to the A1 subunit being released in the cytosol where it can ADP ribosylate mammalian guanine nucleotide binding protein α-subunit (Gsα) inhibiting the GTPase activity of Gsα and activating adenylate cyclase resulting in uncontrolled cAMP levels. cAMP and cGMP both contribute to phosphorylation of the cystic fibrosis transmembrane regulator (CFTR) chloride channel and modulation of other ion channels leading to osmotic diarrhea.
The main pathology of ETEC occurs through secretion of heat stable (ST) and/or heat labile (LT) toxins. Two small (2,000 Da) distinct heat-stable toxins, STa/STI and STb/STII, exist although only the former is thought to contribute to human disease. STa/STI mimics the native intestinal hormone guanylin, binding to and activating the intestinal brush border guanylate-cyclase-C (GC-C) receptor, increasing intracellular messenger cyclic GMP (cGMP). This activates cGMP-dependent protein kinase II leading to phosphorylation of the cystic fibrosis transmembrane regulator (CFTR) and deregulated ion absorption/secretion and hence diarrhea.39-41
The LT toxins can be divided into Type I (LT-I), generally in human isolates and closely related to cholera toxin, and Type II (LT-II), which are mainly from non-human isolates.6 LT toxins are AB5 toxins (one A subunit linked to a pentameric B subunit) and are transported across the bacterial outer membrane by the type 2 secretion system.42 LT remains membrane-associated by binding lipopolysaccharide (LPS)43 and is secreted in outer membrane vesicles (OMVs) that bind to ganglioside receptors on the mammalian cell (GM1a for LT-I or GD1a/b for LT-II) via the LT-B subunit. The OMVs are then actively endocytosed and the LT transported via the Golgi and endoplasmic reticulum (ER) to the cytosol44 where the A1 subunit then ADP-ribosylates mammalian guanine nucleotide binding protein α-subunit (Gsα). This inhibits the GTPase activity of Gsα and constitutively activates adenylate cyclase leading to uncontrolled elevation of the intracellular cAMP concentration.45 This has pleotropic effects within the cell including phosphorylation of the CFTR chloride channel by protein kinase A. The combination of LT and CT ultimately leads to secretion of electrolytes and water resulting in osmotic diarrhea.
Several other putative toxins have been described for ETEC including the pore-forming cytotoxin ClyA,46 however details of their mechanism of action, frequency among isolates, and importance during infection is unclear.
EAEC
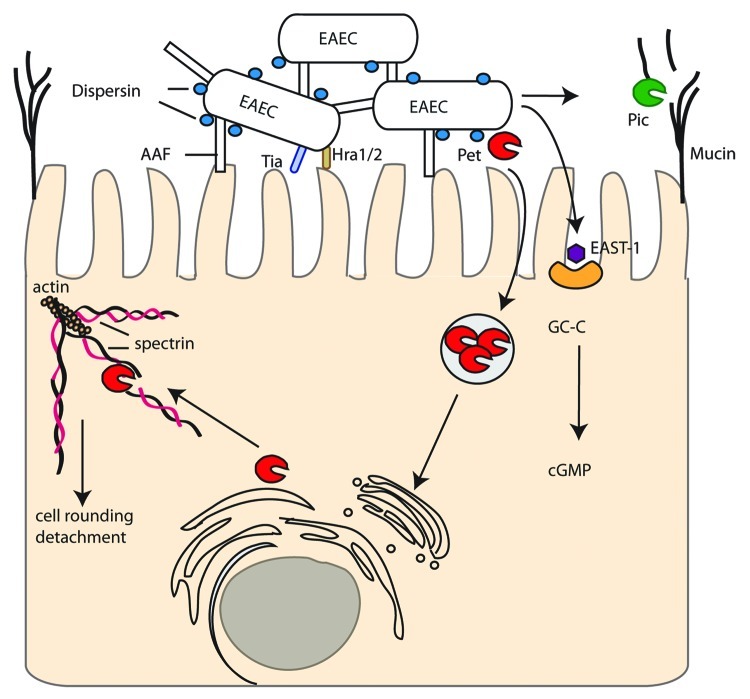
EAEC strains are defined by their aggregative adherence or “stacked brick” phenotype on HEp-2 cells while in the intestinal mucosa EAEC forms a biofilm with bacteria incased in a thick mucus layer. Colonization requires the AAF (Fig. 2) and the regulator AggR both encoded on a large virulence plasmid pAA. AAF, of which four variants have been described, mediates attachment of EAEC to salad leaves (in combination with flagella),47 host cells48 and human intestine ex vivo49 but were not shown to confer a colonization advantage in a mouse model.50 Other than AAF and AggR, there is a great deal of genomic diversity among EAEC strains with corresponding heterogeneity in virulence and few conserved virulence factors. Further colonization factors found in some EAEC isolates include Heat-resistant agglutinin (Hra) 1 and 2 and Tia (also found in ETEC).51 The secreted small hydrophilic protein dispersin (encoded by aap) aids colonization by attaching noncovalently to the bacterial cell surface potentially neutralizing the negative charge of the LPS and allowing the positively charged AAF to extend away from the cell.52
Figure 2. Enteroaggregative E. coli (EAEC). EAEC attach to host cells and each other by Aggregative Adherence Fimbriae (AAF) that are kept extended from the bacterial cell by dispersin. Adhesins Tia and Hra1/2 also contribute to intimate attachment. SPATEs include Pic, which digests mucin on host cells, and Pet which is endocytosed, undergoes retrograde trafficking and cleaves spectrin disrupting the actin cytoskeleton and inducing cell rounding and detachment. EAST-1 binds to and activates GC-C resulting in increased cGMP.
EAEC strains produce a variety of SPATEs of either class I (cytotoxic) or class II (non-cytotoxic). Pic (protease involved in colonization, also found in Shigella flexneri and UPEC) is a class II SPATE with hemagglutinin and mucinolytic activity which may help to penetrate the mucus layer in which EAEC resides on enterocytes.53 Conversely, Pic can induce mucus hypersecretion and an increase in the number of mucus-producing goblet cells.54 Pic has also been implicated in immunomodulation by cleaving leukocyte surface glycoproteins and inducing both activation and apoptosis in T cells, but impaired migration, of polymorphonuclear leukocytes (PMNs).55 Pet is a well characterized class I SPATE that is endocytosed by host cells, undergoes retrograde trafficking and utilizes the ER-associated degradation (ERAD) pathway to be released into the cytosol. Pet then cleaves the actin binding protein spectrin in the host cytosol, disrupting the actin cytoskeleton and causing cell rounding and detachment.56 Recent evidence has also suggested a role for Pet in disrupting focal adhesions.57 Pet is only present in a small minority of strains58 and alternative class I SPATES (Sat, SigA, EspP) may have similar roles. Sat in particular has 52% amino acid identity with Pet and is discussed further under DAEC.
Non-SPATE toxins include the EAEC heat-stable enterotoxin 1 (EAST-1), which is encoded on pAA and is 50% identical to, but antigenetically distinct from, the enterotoxic domain of STa. Like STa, EAST-1 activates guanylate cyclase leading to increased cGMP, although the toxigenic effect appears milder than for STa.59 The prevalence of EAST-1 among EAEC strains and its contribution to virulence remains unclear.60,61 Further toxins include ShET1 (characterized as an AB5 toxin in Shigella flexneri)62 and HlyE (a pore-forming toxin)63 have also been proposed to contribute to EAEC virulence.
Recent sequencing of the prototype EAEC strain 042 has revealed some interesting potential virulence factors including two T3SS and potential effector proteins as well as a locus encoding a polysaccharide capsule, but these remain to be tested and their frequency among clinical isolates determined.64 EAEC 042 also encodes three type 6 secretion systems and, like many EAEC clinical isolates, has multiple antibiotic resistance genes,65 making treatment and eradication difficult.
STEAEC
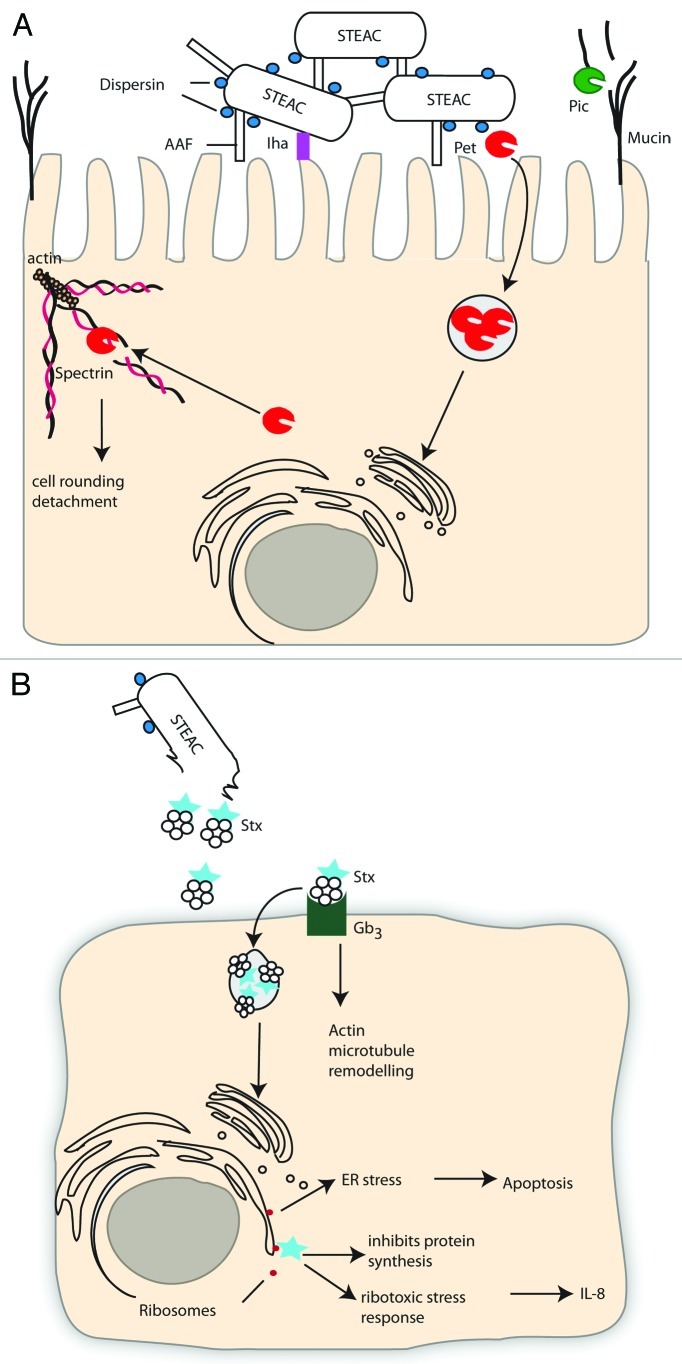
The chromosome of STEAEC 2011 outbreak strain O104:H4 is most similar to EAEC strain 55989.15,66 This STEAEC outbreak strain carries Pic on the chromosome and a pAA-like virulence plasmid encoding AAF, AggR, Pet, ShET1 and dispersin (Fig. 3). A second virulence plasmid encodes multiple antibiotic resistances. In addition to these standard EAEC virulence determinants STEAEC O104:H4 has an stx2-harbouring prophage integrated into the wrbA locus, and therefore can produce Stx, a defining characteristic of the EHEC pathotype (discussed under EPEC/EHEC—Non T3SS virulence mechanisms—Toxins). The outbreak strain has also acquired the IrgA homolog adhesin (Iha)67 and a tellurite resistance cluster, which are common features of EHEC strains.68 Therefore, there do not appear to be new virulence determinants in this strain, rather a combination of known virulence determinants from two pathotypes. The high morbidity and mortality associated with this strain may reflect the stronger adherence of EAEC compared with EHEC allowing more Stx to be transferred and more resultant pathology.
Figure 3. (See opposite page). Shiga Toxin producing Enteroaggregative E. coli (STEAEC). (A) STEAEC attach to each other and to enterocytes by AAF and dispersin, as for EAEC. STEAEC also encodes the Iha adhesin and SPATES Pet and Pic, although their contribution to infection is unknown. The action of shiga toxin (Stx) on an endothelial, toxin sensitive cell is shown in (B). The B subunit of Stx interacts with Gb3 on the host cell and Stx is endocytosed and undergoes retrograde trafficking through the Golgi and ER, the A subunit then cleaves an adenine residue from the 28S rRNA of eukaryotic ribosomes inhibiting protein synthesis and leading to cell death. Stx can also cause cells to undergo a ribotoxic stress response, which leads to release of IL-8, or to undergo ER-dependent apoptosis.
DAEC
Grouping of DAEC strains is due to their diffusely adherent phenotype on HEp2 cells, which for most strains is due to the action of afimbrial (Afa) or fimbrial (Dr) adhesins (Afa-Dr adhesins) (Fig. 4). All Afa/Dr adhesins bind the decay-accelerating factor (DAF), while a subfamily of Afa/Dr adhesins (AfaE-III, Dr and F1845 adhesins) can also bind carcinoembryonic antigen-related molecules (CEACAMs)69,70 and the Dr adhesin can also bind type IV collagen.71 A small number of DAEC strains may also express the CS20 colonization factor from ETEC.72
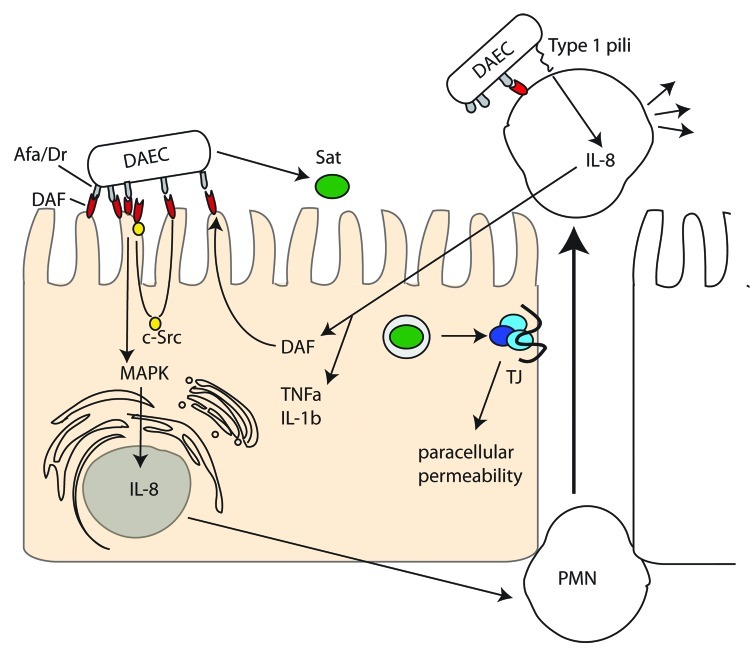
Figure 4. Diffusely Adherent E. coli (DAEC). AFA/Dr adhesins interact with the decay-accelerating factor (DAF) on host cells. Src kinase activation mobilizes DAF around the attachment site mediating stronger attachment and MAPK and PI3K pathway activation culminating in IL-8 synthesis, which induces transmigration of PMNs. PMN transmigration stimulates upregulation of DAF and TNFα and Il-1β synthesis. DAEC Type 1 pili induces IL-8 release from PMNs and apoptosis. Sat induces rearrangement of the tight junction proteins ZO-1, ZO-2 and occludin leading to paracellular permeability.
Once the Afa/Dr adhesins bind their cellular target on enterocytes (DAF or CEACAMs) they relocalize the target around the site of bacterial attachment. For the Dr adhesin this relocalization has been shown to be dependent on Src kinase activation.73 Following target mobilization, enterocyte signaling pathways (e.g., MAPK and PI3K) are activated and IL-8 is synthesized (for the F1845 adhesin this requires HIF-1α74) inducing transepithelial migration of human polymorphonuclear neutrophils (PMN). This stimulates the enterocytes to synthesize TNFα and IL-1β and upregulate DAF to strengthen bacterial adhesion.75 DAEC can interact with the transmigrating PMNs and induce type I pili-dependent IL-8 release.76 Transmigrated PMNs are also induced to undergo apoptosis after interaction with DAEC and have a diminished phagocytic capacity, prolonging bacterial persistence in the gut.77
The only documented secreted factor associated with DAEC infection is the SPATE Sat. Sat can induce rearrangement of the tight junction proteins ZO-1, ZO-3 and occludin increasing paracellular permeability but not transepithelial resistance78 and can also bind spectrin,79 rearrange focal adhesion associated proteins vinculin and paxillin, and cause cell detachment and caspase-independent cell death.80
AIEC
While AIEC strains are genetically related to ExPEC strains they appear to have acquired novel virulence-specific features which can be characterized phenotypically (adhesion, invasion and intramacrophage replication) but the genetic basis of which is still largely undetermined.81 This has hampered identification and hence research into the prevalence and importance of AIEC. The majority of AIEC research has used a single strain, LF82, and extrapolation of these results to other AIEC strains is vital as the field progresses.
AIEC infection requires both a susceptible host as well as bacterial virulence determinants. The first step in AIEC infection is abnormal colonization of the intestinal epithelium via type I pili binding to the CEACAM6 receptor, which is overexpressed in the ileal mucosa of CD patients.82,83 In addition to type I pili, long polar fimbriae were recently shown to also be required for AIEC to bind to M cells in Peyers patches.84 This suggests that AIEC may utilize the transcytic characteristics of M cells to cross the intestinal barrier, as is the case for many other intestinal pathogens (e.g., Yersinia sp).
AIEC secrete OMVs that appear to be required for AIEC to invade intestinal epithelial cells (IECs),85 potentially by delivering effector proteins into the host cell. The OMVs contain OmpA which interacts with the ER-localized stress response protein Gp96 which has been shown to be overexpressed on the apical surface of ileal epithelial cells in Crohn disease patients.86 Other than OmpF/C and OmpA the composition of these OMVs and potential effector proteins delivered by this mechanism are unknown. While AIEC invasion into epithelial cells requires actin and microtubule involvement87 the molecular mechanisms remain unknown. AIEC can survive and replicate in phagolysomes of infected macrophages in the lamina propria, resulting in increased TNFα secretion88 which may lead to the inflammation associated with Crohn disease.
T3SS-dependent pathotypes
Of the T3SS-dependent pathotypes EHEC and EPEC primarily remain extracellular during infection while EIEC are found intracellular. Despite these very different lifestyles and the different T3SS origins (LEE encoded or pINV encoded, respectively) EPEC/EHEC and EIEC/Shigella actually share a number of T3SS translocated proteins, e.g., EspG/VirA, EspO/OspE, NleE/OspZ reflecting similar infection strategies.
EPEC/EHEC
The EPEC and EHEC T3SS is encoded on a pathogenicity island termed the locus of enterocyte effacement (LEE), a region highly conserved between the attaching/effacing (A/E) pathogens EPEC, EHEC, rabbit enteropathogenic E. coli (REPEC) and murine pathogen Citrobacter rodentium.89 The LEE encodes gene regulators, structural components of the T3SS, chaperones, the bacterial surface protein intimin and a number of translocated proteins.90
EHEC O157:H7 appears to have evolved from EPEC O55:H791 and non-O157:H7 EHEC strains to have evolved by parallel evolution.92 Defining characteristics of EHEC are the presence of stx genes, leading to more serious disease pathology and complications (HUS), and absence of BFP, leading to different adherence mechanisms. EPEC and EHEC are primarily human pathogens; although a range of ruminants carry EHEC it is generally asymptomatic in these animals. This host restriction makes modeling EPEC/EHEC infections difficult and so the related A/E pathogens REPEC and C. rodentium are commonly used as infection models.
Non T3SS virulence mechanisms
Adherence
EPEC/EHEC encode several well-characterized fimbrial (pili) adhesins. Human EPEC strains are divided into typical or atypical strains according to the presence or absence of the EPEC adherence factor (EAF) plasmid93 which encodes the BFP. BFP is responsible for the formation of microcolonies through bacterial-bacterial interactions and a binding pattern known as localized adherence.94,95 Long polar fimbria (LPF) play a significant role in EHEC adherence to epithelial cells, although they are not present in all strains.96
A variety of non-fimbrial adhesins have been identified for both EPEC and EHEC (Table 1). EHEC factor for adherence (Efa-1), found in some EHEC strains, contributes to in vitro adherence.97 The major outer membrane protein OmpA has been reported to interact with cultured human intestinal cells in O157 EHEC infections.98 Porcine attaching and effacing associated adhesin (Paa), found in EHEC, EPEC and ETEC strains, contributes to A/E lesions in pig ileal explants.99 Additionally, there is a growing list of autotransporters involved in adherence in some EPEC/EHEC strains including STEC autoagglutinating adhesin (Saa),100 E. coli immunoglobulin-binding protein (EibG)101 and EhaA.102 Intimate attachment of the bacteria to the host cell is T3SS-dependent and is described in detail below (Intimate attachment and A/E lesion).
Toxins
The major toxin produced by EHEC is the phage encoded Stx of which there are two subgroups, Stx1 and Stx2 (and variants thereof), with Stx2 more common among human isolates. Antibiotic treatment of Stx-producing bacteria is not recommended as it induces both expression of the toxin and allows release and dissemination (Stx does not appear to be actively transported from the cell).103 For a review of Stx activity and intracellular trafficking see Johannes et al.104
Stx are AB5 toxins with the B subunit mediating binding to the membrane glycolipid globotriaosylceramide (Gb3). The B subunit induces endocytic plasma membrane invaginations105and vesicles traffic to early endosomes where, in toxin sensitive cells, Stx leaves the endocytic pathway106 and travels through the Golgi apparatus and ER by retrograde transport. The catalytic A-subunit is translocated to the cytosol to reach its molecular target, rRNA, where it cleaves an adenine residue from the 28S rRNA of eukaryotic ribosomes, inhibiting protein synthesis and eventually leading to cell death.107 In toxin resistant cells (e.g., Monocytes, macrophages) Stx does not leave the endocytic pathway but is degraded by lysosomes.108 In these cells Stx activates the MAPK pathway and produces IL-6 and TNFα, which can in turn increase Gb3 expression on endothelial cells.
Once Stx is released into the gut lumen it is translocated across the intestinal epithelium into the underlying tissues and bloodstream and then targets host cells expressing Gb3. In humans, high concentrations of Gb3 are found in renal tubular cells and microvascular endothelial cells, particularly those in the kidney, gut and brain, explaining the clinical manifestations of HUS. Stx has also been shown to induce apoptosis in various cell types which may contribute to disease pathology.109,110
Some EHEC and EPEC strains also produce SPATEs, the two best characterized being EspP from EHEC and EspC from EPEC. EspC shares 70% amino acid similarity with Pet (EAEC) and also cleaves spectrin, although at a different site than Pet. Unlike Pet which is internalized by receptor-mediated endocytosis EspC internalization requires EPEC contact with the host cell and production of a T3SS.111 Four subtypes of EspP have been described with subtype α associated with highly pathogenic strains.112 EspP has recently been described to cleave complement factors C3/C3b and C5 and impair complement function in vitro.113
The T3SS and delivered effectors
The general T3SS is comprised of a cytosolic ATPase, inner and outer membrane rings, a periplasmic shaft and an extracellular needle protein (reviewed in ref. 114). The EPEC and EHEC systems have an additional filament of ~260 nm protruding from the T3SS needle which is generated by polymerization of EspA subunits.115 This filament functions in initial attachment to the host cell before secretion of the translocators, EspB and EspD, which form the translocation pore in the host cell membrane.116,117 EHEC and EPEC use the T3SS to inject dozens of effector proteins into the eukaryotic cell cytoplasm. Once translocated, these effector proteins are targeted to different subcellular compartments and affect diverse signaling pathways and physiological processes. In addition to the seven effectors encoded in the LEE, there are other effectors encoded within prophages and other integrative elements.118 Although EPEC and EHEC show high levels of conservation between the LEE encoded effectors, there is significant diversity in their non-LEE effector (NLE) repertoire; EPEC E2348/69 has been shown to have 21 intact effector genes whereas the EHEC O157 strain Sakai is estimated to have closer to 50 T3SS effectors.118,119 For a recent detailed review of EPEC/EHEC effectors see Wong et al.120
Intimate attachment and A/E lesion
EPEC/EHEC colonization results in the formation of A/E lesions on the apical surfaces of enterocytes (Fig. 5A). These characteristic T3SS-dependent lesions describe the effacement of microvilli and intimate bacterial attachment with actin accumulation at the bacterial host cell interface; in vitro the actin polymerization activity results in formation of raised pedestal-like structures underneath the attached bacteria.121 The outer membrane adhesin, intimin was the first bacterial gene product found to be essential for the intimate attachment of bacteria to epithelial cells.122 The intimin encoding eae gene was later found on the LEE and its product was shown to be secreted via the general secretory pathway and inserted into the bacterial outer membrane.123,124 Four distinct intimin types were originally reported (α, β, γ and δ),125 although more than 20 types are now recognized.126 Intimin shares homology (31% identity) with invasin from Yersinia pseudotuberculosis and further studies on the intimin/invasin family have shown that the C-terminal 280 amino acids (Int280/Inv280) are required for binding β1-chain integrins.127,128 The transmembrane intimin receptor (Tir) is a LEE-encoded effector translocated via the T3SS,129 which resides in the host cell plasma membrane and interacts with intimin to allow intimate attachment of the bacteria with the host cell. EPEC/EHEC therefore delivers its own adhesin into the host to ensure intimate attachment is achieved. Intimin can also interact with the host protein nucleolin, which is upregulated by Stx.130
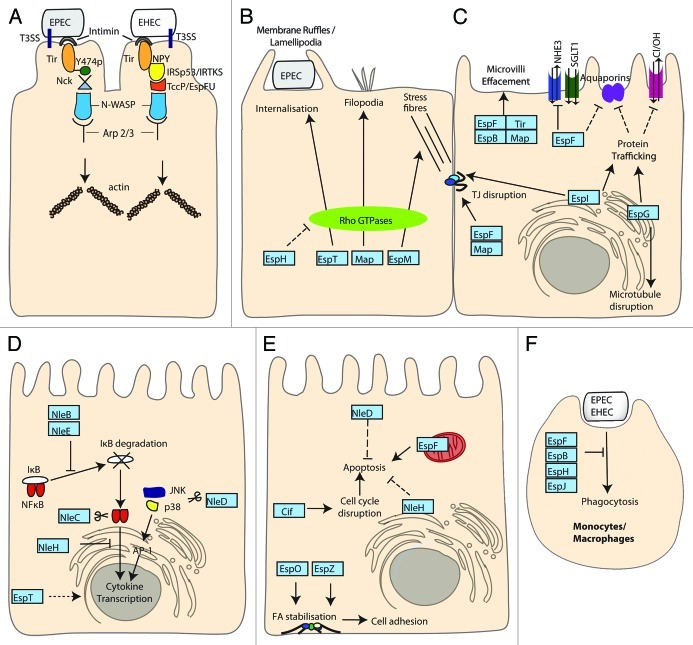
Figure 5. Enteropathogenic E. coli and Enterohamerrhagic E. coli (EPEC and EHEC). EPEC/EHEC inject an array of T3SS effector proteins to mediate intimate attachment and subvert host cell processes. In addition EHEC produces Stx, the action of which is described in Figure 3B. (A) Intimate adherence. The translocated intimin receptor (Tir) binds intimin on the bacterial surface to initiate intimate attachment, actin accumulation and pedestal formation. TirEPEC is phosphorylated at Y474 resulting in Nck and N-WASP recruitment. TirEHEC signaling proceeds independently of Nck via the T3SS effector TccP/EspFU that interacts with IRTKS/IRSp53 and N-WASP. Both pathways lead to Arp2/3 mediated initiation of actin polymerization. (B) Actin remodeling. Map, EspM and EspT activate Rho GTPases leading to filopodia, stress fibers and ruffles/lamellipodia respectively. Additionally, EspT can induce internalization of EPEC. EspH disrupts Rho GTPase signaling by targeting host DH-PH GEFs. (C) Disruption of gut integrity. Tir, Map, EspF and EspB contribute to effacement of the normal absorptive microvilli. Map, EspF and EspI disrupt tight junction (TJ) integrity and epithelial barrier function. EspG disrupts microtubules while EspI and EspG both modulate protein trafficking and affect TJs and the DRA Cl−/OH− exchanger respectively. EspG and EspF alter aquaporin levels disrupting water and ion absorption. EspF reduces the activity of the NHE3 Na+/H+ exchanger and multiple effectors target the SGLT1 Na+/glucose co-transporter. (D) Manipulating immune responses. NleB, NleC, NleD NleE and NleH inhibit inflammatory responses through targeting NFκB, JNK and p38 pathways. NleB and NleE inhibit IκB degradation and subsequent nuclear translocation of NFκB. NleH can also block NFκB nuclear translocation. NleC and NleD function as metalloproteases acting on NFκB and JNK/p38 respectively blocking transcription of pro-inflammatory genes initiated by NFκB and AP-1 transcription factors. EspT promotes expression of inflammatory genes through Erk, JNK and NFκB pathways. (E) Balancing apoptosis and survival. Pro-apoptotic EspF causes mitochondrial dysfunction leading to activation of apoptotic pathways while Cif causes cell cycle disruption. Anti-apoptotic NleH interacts with BI-1 at the ER and NleD inhibition of AP-1 dependent gene expression (shown in C) reduces pro-apoptotic gene expression. EspO and EspZ promote integrin mediated cell adhesion and survival through interacting with ILK and CD98 respectively. (F) Inhibiting phagocytosis. EspF, EspB, EspH and EspJ inhibit phagocytosis by macrophages through disruption of PI3K signaling, myosin-actin interactions, Rho GTPase signaling and an unknown mechanism respectively.
In addition to the role of Tir as a receptor for intimin, it is also an important mediator of protein signaling within epithelial cells. In EPEC, Tir is phosphorylated at tyrosine 474 (Y474p) to promote its interaction with the adaptor protein Nck leading to the recruitment of neural Wiskott-Aldrich syndrome protein (N-WASP).131,132 This initiates actin polymerization mediated by the actin-related protein 2/3 (Arp 2/3) complex.131,133 However, EHEC Tir lacks a tyrosine 474 equivalent and the process of actin polymerization is mediated via the T3SS translocated effector protein, TccP (Tir-cytoskeleton coupling protein)134 also known as EspFU (E. coli secreted protein F in prophage U).135 TccP/EspFU interacts with the IRSp53/MIM proteins, IRTKS and IRSp53, which also bind Tir at an Asn-Pro-Tyr (NPY458) tripeptide in the Tir C-terminal domain thereby linking TccP/EspFU indirectly to Tir.136-138 TccP/EspFU interacts with and activates N-WASP preventing its autoinhibition fold and thus, initiating an Nck-independent actin polymerization pathway.139 The NPY motif is conserved in EPEC Tir (NPY454) but in EPEC belonging to lineage 1 this pathway accounts only for low levels of pedestal formation in the absence of TccP/EspFU.138 Previous studies have demonstrated the conservation of the Nck and TccP/EspFU pathways in EPEC lineage 1 and EHEC O157 respectively.140 Interestingly, both pathways are utilized simultaneously in vitro for most non-O157 EHEC strains, EPEC O119:H6141 and EPEC lineage 2 strains.140 Unexpectedly neither pathway appears to be necessary for A/E lesion formation in vivo in C. rodentium infections, in EPEC and EHEC infection of human intestinal in vitro organ cultures (IVOC),142,143 or for EHEC colonization in the infant rabbits and gnotobiotic piglets models,144 indicating the molecular mechanisms of A/E formation and their functions are far from understood.
Actin remodeling
Several EPEC T3SS effectors disrupt Rho GTPase signaling and hence subvert actin dynamics (Fig. 5B).145 The Rho family small G proteins are crucial in the regulation of key cellular functions and the best characterized are Cdc42, Rac1 and RhoA, which trigger filopodia, lamellipodia/ruffles and stress fibers respectively.146 T3SS effectors have been shown to regulate Rho GTPases by functioning as guanine exchange factors (GEFs), which control the switch from GDP-bound (inactive) to the GTP-bound (active) state,147 or GTPase-activating proteins (GAPs), which increase the hydrolysis of GTP leading to the inactive state of the Rho GTPases.148
Alto et al. grouped 24 bacterial effector proteins in the WxxxE family based on a conserved motif of two invariant amino acids, Trp and Glu separated by three variable amino acids.149 Members of the family include Map, EspM, EspT from EPEC/EHEC, IpgB1 and IpgB2 from Shigella spp, SopE and SifA from Salmonella spp Map and EspM function as GEFs on Cdc42 and RhoA respectively, leading to filopodia150 and stress fibers formation.151 In the case of EspT, Rac1 and Cdc42 are both activated which results in the formation of membrane ruffles and lamellipodia and bacterial internalization.152 Map and SopE have been shown to have similar crystal structures when resolved in complex with Cdc42.147 Despite the lack of sequence or structural similarity bacterial WxxxE effectors and eukaryotic GEFs appear to form the same conformational complex with Cdc42. The T3SS effector EspH was shown to subvert actin dynamics affecting filopodia and pedestal formation.153 EspH inactivates mammalian Dbl-homology and pleckstrin-homology (DH-PH) Rho GEFs,154 but not the bacterial Rho GEFs. This suggests that EspH might clear the cell of endogenous Rho GEFs allowing the bacterial Rho GEFs (WxxxE effectors) to take over cell signaling to fulfill the bacterial infection strategy.
Another T3SS effector, EspV present in EPEC strains E110019 and E22 is able to cause nuclear condensation, cell rounding and actin-rich dendrite-like projections upon overexpression in mammalian cells155 although the mechanisms involved in this actin rearrangement are not yet known.
Disrupting gut integrity
EPEC induces diarrhea through a variety of mechanisms (Fig. 5C). First, the effacement of the microvilli, a defining feature of the A/E lesion, results in a reduced surface area for normal absorptive processes. EspB, Map, EspF, Tir and intimin have all been shown to be involved in microvillus effacement.156,157 EPEC also inhibits ion and water exchange through more targeted mechanisms. For example, Cl- absorption is reduced by targeting the DRA Cl-/OH- exchanger through reducing the exchanger cell surface levels in an EspG and EspG2 dependent manner.158 While EspG was originally described to degrade microtubules it can also bind the Golgi matrix protein GM130, p21-activated kinases (PAKs) and ADP-ribosylation factors (ARFs)159,160 acting as a molecular scaffold to regulate host signaling cascades, leading to decreased protein secretion and receptor trafficking. Consequences of this manipulation of protein trafficking within the host cell include altering the paracellular permeability and membrane channel expression in enterocytes.161,162
In addition, the effector EspF is responsible for reducing the activity of Na+/H+ exchanger 3163 and EPEC infection leads to a significant reduction in the activity of the SGLT1 Na+/glucose cotransporter.156 EspF and EspG have both been implicated in affecting water transport by altering aquaporin levels on apical and lateral membranes.164 EPEC infections also affect the integrity of the epithelial monolayer and so disrupt barrier function. EspF, Map and intimin have all been shown to contribute to diarrhea through the disruption of tight junction integrity.165 EspI (NleA) also disrupts intestinal tight junctions166 and like EspG is able to decrease protein transport. EspI does so by binding to SEC24 through a PDZ binding motif and inhibiting COPII vesicle fusion167,168 indicating EPEC/EHEC have developed multiple mechanisms to modulate the intestinal barrier function and integrity during infection.
Manipulating the immune response
Manipulation of the host immune system is a common theme in bacterial infections and a requirement for successful colonization and dissemination. EPEC and EHEC infections lead to an inflammatory response predominantly initiated by recognition of the bacterial flagella169. The production of pro-inflammatory cytokines is subsequently dampened by the bacteria with increased bacterial loads leading to reduced IL-8 production170 with several effector proteins acting in concert to illicit this effect (Fig. 5D). The transcription factor NFκB is central to the initiation of inflammatory responses; NFκB dimers are held inactive in the cytoplasm by IκB until IκB is phosphorylated and degraded allowing NFκB to translocate to the nucleus and initiate transcription. The effector proteins NleB and NleE inhibit IκB degradation and therefore the nuclear translocation of NFκB subunits.171,172 Additionally, the effectors NleC and NleD have been shown to function as metalloproteases downregulating inflammatory responses by targeting NFκB (NleC) and the mitogen activated protein kinases c-Jun N-terminal Kinase (JNK) and p38 (NleD).173,174 NleH effectors have an uncertain role in immune modulation as they have been shown to enhance inflammatory responses175 and conversely attenuate NFκB activation176 with NleH1 able to block nuclear translocation of the RPS3 subunit of NFκB while NleH2 can induce expression of RPS3 dependent genes.177,178 Interestingly the WxxxE effector EspT, triggers the expression of pro-inflammatory genes through Erk, JNK and NFκB pathways179 suggesting a careful balance between pro- and anti-inflammatory actions exists.
Balancing apoptosis and maintaining cell survival
Like many enteric pathogens EPEC and EHEC are able to induce apoptosis upon infection.180,181 However, apoptosis induction needs to be modulated in order to maintain an infective niche within a host, by balancing pro and anti-apoptotic effectors (Fig. 5E). In EPEC and EHEC, two T3SS effectors are currently known to act as inducers of apoptosis, EspF182 and Cycle inhibitory factor (Cif).183 EspF is targeted to the mitochondria via an N-terminal mitochondrion-targeting sequence and interferes with the mitochondrial membrane potential.184 EspF has specifically been shown to induce the release of cytochrome c and cleavage of caspases 9 and 3182 while Cif acts mainly as a bacterial cyclomodulin subverting the eukaryotic cell cycle by blocking both the G1/S and G2/M transitions resulting in apoptosis.182 The action of these pro-apoptotic effectors is balanced by the translocation of anti-apoptotic effectors such as NleH and NleD. NleH inhibits intrinsic apoptotic pathways via its direct interaction with the anti-apoptotic protein Bax inhibitor-1 (BI-1)185 while NleD inactivates JNK leading to the suppression of the downstream transcription factor AP-1 which activates several pro-apoptotic proteins.186
Additionally, other T3SS effectors indirectly contribute to the inhibition of apoptosis through their ability to promote host cell survival mechanisms. The LEE encoded effector EspZ interacts with CD98, a host protein involved in integrin mediated cell adhesion and cell survival187 while the non-LEE encoded effector EspO increases cell adhesion and survival through its direct binding with integrin-linked kinase (ILK).188 EPEC and EHEC also activate several other signaling pathways known to inhibit apoptosis including the protein kinase C, tyrosine kinases and phosphatidylinositol 3-kinase189-191 suggesting other effectors might also be involved in manipulation of apoptosis.
Inhibiting phagocytosis
During infection EPEC and EHEC primarily survive extracellularly and have developed mechanisms to inhibit internalization by phagocytic cells (Fig. 5F). Phagocytosis involves rearrangement of the actin cytoskeleton and additional membrane recruitment and is initiated by the engagement of surface receptors, which recognize a variety of ligands including pathogen-associated lipids and sugars as well as immunoglobulin (Ig) and components of the complement cascade. EPEC and EHEC inhibit internalization of unopsonized (cis-phagocytosis) and opsonized bacteria (trans-phagocytosis) through the actions of a number of effector proteins. EspF targets PI3K signaling, inhibiting its activation rather than recruitment to the site of attachment, preventing cis-phagocytosis and internalization of IgG opsonized particles.192 EspB has been shown to bind several members of the myosin superfamily preventing the interaction of Myosin -1c and actin, inhibiting cis-phagocytosis.193 Recently the effector EspH, which inactivates Rho GTPase signaling, was also shown to inhibit both cis-phagocytosis and trans-phagocytosis,154 while, EspJ has been shown to inhibit trans-phagocytosis through an unknown mechanism. EspJ is capable of preventing phagocytosis of both IgG and complement opsonized particles despite significant differences between these FcγR and CR3 mediated phagocytic pathways.194
The T3SS is obviously of fundamental importance to EPEC and EHEC infection. Some effectors appear redundant and others to have multiple functions. The challenge is now to move away from studying effector proteins in isolation and to apply more holistic approaches to study the function of individual effectors in the context of the whole effector repertoire and to determine the significance of the many different effector phenotypes in vivo with respect to the temporal-spatial kinetics of infection.
Conclusions
E. coli are a remarkably versatile and diverse genus of bacteria, which includes both commensals and pathogens. Despite more than 100 years of research we still do not understand all of the pathogenic mechanisms utilized by the different E. coli pathotypes. Making research on E. coli more challenging is the fact that the species is constantly evolving, as is evident by the recent emergence of a new pathotype (STEAEC). Emergence of novel pathotypes is likely due to selective pressure, changes to human behavior and demographics and to the environment, allowing these strains to present within the human population (e.g., changes in food growing, harvesting and consumption). The rapid growth and easy acquisition of new traits by these pathotypes means researchers and clinicians are forced to play ‘catch-up’ in treatments and preventative methods. However better understanding of the emergence and changes in pathotypes may help us to predict and potentially prevent the next round of adaptations. The role of E. coli and other bacteria in complex disease processes such as Crohn disease is only beginning to be studied and requires much further research to unravel the contributions and interplay between the host, microbiota and pathogen.
Acknowledgments
We would like to thank Dr James Collins for critical reading of the manuscript. A.C. is a Marie Curie International Incoming Fellow. J.C.Y. and N.C. are MRC and BBSRC PhD candidates respectively.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/19182
References
- 1.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–40. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 3.Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 2010;8:26–38. doi: 10.1038/nrmicro2265. [DOI] [PubMed] [Google Scholar]
- 4.Wolf MK. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin Microbiol Rev. 1997;10:569–84. doi: 10.1128/cmr.10.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger CN, Sodha SV, Shaw RK, Griffin PM, Pink D, Hand P, et al. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ Microbiol. 2010;12:2385–97. doi: 10.1111/j.1462-2920.2010.02297.x. [DOI] [PubMed] [Google Scholar]
- 6.Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465–83. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasco´n J, Vargas M, Quinto´ L, Corachán M, Jimenez de Anta MT, Vila J. Enteroaggregative Escherichia coli strains as a cause of traveler’s diarrhea: a case-control study. J Infect Dis. 1998;177:1409–12. doi: 10.1086/517826. [DOI] [PubMed] [Google Scholar]
- 8.Black RE. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev Infect Dis. 1990;12(Suppl 1):S73–9. doi: 10.1093/clinids/12.Supplement_1.S73. [DOI] [PubMed] [Google Scholar]
- 9.Steffen R, Castelli F, Dieter Nothdurft H, Rombo L, Jane Zuckerman N. Vaccination against enterotoxigenic Escherichia coli, a cause of travelers’ diarrhea. J Travel Med. 2005;12:102–7. doi: 10.2310/7060.2005.12207. [DOI] [PubMed] [Google Scholar]
- 10.Nagy B, Fekete PZ. Enterotoxigenic Escherichia coli in veterinary medicine. Int J Med Microbiol. 2005;295:443–54. doi: 10.1016/j.ijmm.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80:609–14. [PubMed] [Google Scholar]
- 12.Sarantuya J, Nishi J, Wakimoto N, Erdene S, Nataro JP, Sheikh J, et al. Typical enteroaggregative Escherichia coli is the most prevalent pathotype among E. coli strains causing diarrhea in Mongolian children. J Clin Microbiol. 2004;42:133–9. doi: 10.1128/JCM.42.1.133-139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta S, Pal S, Chakrabarti S, Dutta P, Manna B. Use of PCR to identify enteroaggregative Escherichia coli as an important cause of acute diarrhoea among children living in Calcutta, India. J Med Microbiol. 1999;48:1011–6. doi: 10.1099/00222615-48-11-1011. [DOI] [PubMed] [Google Scholar]
- 14.Wanke CA, Mayer H, Weber R, Zbinden R, Watson DA, Acheson D. Enteroaggregative Escherichia coli as a potential cause of diarrheal disease in adults infected with human immunodeficiency virus. J Infect Dis. 1998;178:185–90. doi: 10.1086/314443. [DOI] [PubMed] [Google Scholar]
- 15.Bielaszewska M, Mellmann A, Zhang W, Köck R, Fruth A, Bauwens A, et al. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis. 2011;11:671–6. doi: 10.1016/S1473-3099(11)70165-7. [DOI] [PubMed] [Google Scholar]
- 16.RKI. EHEC/HUS O104:H4 – The outbreak is considered to be over. Robert Koch Institute, 2011. [Google Scholar]
- 17.Mellmann A, Bielaszewska M, Köck R, Friedrich AW, Fruth A, Middendorf B, et al. Analysis of collection of hemolytic uremic syndrome-associated enterohemorrhagic Escherichia coli. Emerg Infect Dis. 2008;14:1287–90. doi: 10.3201/eid1408.071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae WK, Lee YK, Cho MS, Ma SK, Kim SW, Kim NH, et al. A case of hemolytic uremic syndrome caused by Escherichia coli O104:H4. Yonsei Med J. 2006;47:437–9. doi: 10.3349/ymj.2006.47.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snelling AM, Macfarlane-Smith LR, Fletcher JN, Okeke IN. The commonly-used DNA probe for diffusely-adherent Escherichia coli cross-reacts with a subset of enteroaggregative E. coli. BMC Microbiol. 2009;9:269. doi: 10.1186/1471-2180-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunzburg ST, Chang BJ, Elliott SJ, Burke V, Gracey M. Diffuse and enteroaggregative patterns of adherence of enteric Escherichia coli isolated from aboriginal children from the Kimberley region of Western Australia. J Infect Dis. 1993;167:755–8. doi: 10.1093/infdis/167.3.755. [DOI] [PubMed] [Google Scholar]
- 21.Scaletsky IC, Fabbricotti SH, Carvalho RL, Nunes CR, Maranhão HS, Morais MB, et al. Diffusely adherent Escherichia coli as a cause of acute diarrhea in young children in Northeast Brazil: a case-control study. J Clin Microbiol. 2002;40:645–8. doi: 10.1128/JCM.40.2.645-648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–21. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 23.Rolhion N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1277–83. doi: 10.1002/ibd.20176. [DOI] [PubMed] [Google Scholar]
- 24.Scallan E, Hoekstra RM, Widdowson MA, Hall AJ, Griffin PM. Foodborne illness acquired in the United States. Emerg Infect Dis. 2011;17:1339–40. doi: 10.3201/eid1707.110572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barton Behravesh C, Jones TF, Vugia DJ, Long C, Marcus R, Smith K, et al. FoodNet Working Group Deaths associated with bacterial pathogens transmitted commonly through food: foodborne diseases active surveillance network (FoodNet), 1996-2005. J Infect Dis. 2011;204:263–7. doi: 10.1093/infdis/jir263. [DOI] [PubMed] [Google Scholar]
- 26.European Food Safety Authority. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2009. EFSA Journal. 2011;9:2090–468. [Google Scholar]
- 27.Neter E, Westphal O, Luderitz O, Gino RM, Gorzynski EA. Demonstration of antibodies against enteropathogenic Escherichia coli in sera of children of various ages. Pediatrics. 1955;16:801–8. [PubMed] [Google Scholar]
- 28.Bray J. Isolation of antigenically homogenous strains of Bact. coli neopolitanum from summer diarrhoea of infants. J Pathol Bacteriol. 1945;57:239–47. doi: 10.1002/path.1700570210. [DOI] [Google Scholar]
- 29.Ochoa TJ, Barletta F, Contreras C, Mercado E. New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans R Soc Trop Med Hyg. 2008;102:852–6. doi: 10.1016/j.trstmh.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen RN, Taylor LS, Tauschek M, Robins-Browne RM. Atypical enteropathogenic Escherichia coli infection and prolonged diarrhea in children. Emerg Infect Dis. 2006;12:597–603. doi: 10.3201/eid1204.051112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afset JE, Bevanger L, Romundstad P, Bergh K. Association of atypical enteropathogenic Escherichia coli (EPEC) with prolonged diarrhoea. J Med Microbiol. 2004;53:1137–44. doi: 10.1099/jmm.0.45719-0. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Nie H, Chen L, Zhang X, Yang F, Xu X, et al. Revisiting the molecular evolutionary history of Shigella spp. J Mol Evol. 2007;64:71–9. doi: 10.1007/s00239-006-0052-8. [DOI] [PubMed] [Google Scholar]
- 33.Gaastra W, Svennerholm AM. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–52. doi: 10.1016/0966-842X(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 34.Nada RA, Shaheen HI, Khalil SB, Mansour A, El-Sayed N, Touni I, et al. Discovery and phylogenetic analysis of novel members of class b enterotoxigenic Escherichia coli adhesive fimbriae. J Clin Microbiol. 2011;49:1403–10. doi: 10.1128/JCM.02006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elsinghorst EA, Kopecko DJ. Molecular cloning of epithelial cell invasion determinants from enterotoxigenic Escherichia coli. Infect Immun. 1992;60:2409–17. doi: 10.1128/iai.60.6.2409-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw RK, Berger CN, Pallen MJ, Sjöling Å, Frankel G. Flagella mediate attachment of enterotoxigenic Escherichia coli to fresh salad leaves. Env Microbiol Rep. 2011;3:112–7. doi: 10.1111/j.1758-2229.2010.00195.x. [DOI] [PubMed] [Google Scholar]
- 37.Roy K, Hilliard GM, Hamilton DJ, Luo J, Ostmann MM, Fleckenstein JM. Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells. Nature. 2009;457:594–8. doi: 10.1038/nature07568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy K, Kansal R, Bartels SR, Hamilton DJ, Shaaban S, Fleckenstein JM. Adhesin degradation accelerates delivery of heat-labile toxin by enterotoxigenic Escherichia coli. J Biol Chem. 2011;286:29771–9. doi: 10.1074/jbc.M111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz S, Green CK, Yuen PS, Garbers DL. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990;63:941–8. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- 40.Vaandrager AB. Structure and function of the heat-stable enterotoxin receptor/guanylyl cyclase C. Mol Cell Biochem. 2002;230:73–83. doi: 10.1023/A:1014231722696. [DOI] [PubMed] [Google Scholar]
- 41.Chao AC, de Sauvage FJ, Dong YJ, Wagner JA, Goeddel DV, Gardner P. Activation of intestinal CFTR Cl- channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J. 1994;13:1065–72. doi: 10.1002/j.1460-2075.1994.tb06355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tauschek M, Gorrell RJ, Strugnell RA, Robins-Browne RM. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc Natl Acad Sci U S A. 2002;99:7066–71. doi: 10.1073/pnas.092152899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horstman AL, Bauman SJ, Kuehn MJ. Lipopolysaccharide 3-deoxy-D-manno-octulosonic acid (Kdo) core determines bacterial association of secreted toxins. J Biol Chem. 2004;279:8070–5. doi: 10.1074/jbc.M308633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004;23:4538–49. doi: 10.1038/sj.emboj.7600471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–47. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ludwig A, Bauer S, Benz R, Bergmann B, Goebel W. Analysis of the SlyA-controlled expression, subcellular localization and pore-forming activity of a 34 kDa haemolysin (ClyA) from Escherichia coli K-12. Mol Microbiol. 1999;31:557–67. doi: 10.1046/j.1365-2958.1999.01196.x. [DOI] [PubMed] [Google Scholar]
- 47.Berger CN, Shaw RK, Ruiz-Perez F, Nataro JP, Henderson IR, Pallen MJ, et al. Interaction of enteroaggregative Escherichia coli with salad leaves. Env Microbiol Rep. 2009;1:234–9. doi: 10.1111/j.1758-2229.2009.00037.x. [DOI] [PubMed] [Google Scholar]
- 48.Nataro JP, Deng Y, Maneval DR, German AL, Martin WC, Levine MM. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect Immun. 1992;60:2297–304. doi: 10.1128/iai.60.6.2297-2304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Czeczulin JR, Balepur S, Hicks S, Phillips A, Hall R, Kothary MH, et al. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect Immun. 1997;65:4135–45. doi: 10.1128/iai.65.10.4135-4145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrington SM, Sheikh J, Henderson IR, Ruiz-Perez F, Cohen PS, Nataro JP. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect Immun. 2009;77:2465–73. doi: 10.1128/IAI.01494-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mancini J, Weckselblatt B, Chung YK, Durante JC, Andelman S, Glaubman J, et al. The heat-resistant agglutinin family includes a novel adhesin from enteroaggregative Escherichia coli strain 60A. J Bacteriol. 2011;193:4813–20. doi: 10.1128/JB.05142-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheikh J, Czeczulin JR, Harrington S, Hicks S, Henderson IR, Le Bougue´nec C, et al. A novel dispersin protein in enteroaggregative Escherichia coli. J Clin Invest. 2002;110:1329–37. doi: 10.1172/JCI16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henderson IR, Czeczulin J, Eslava C, Noriega F, Nataro JP. Characterization of pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect Immun. 1999;67:5587–96. doi: 10.1128/iai.67.11.5587-5596.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navarro-Garcia F, Gutierrez-Jimenez J, Garcia-Tovar C, Castro LA, Salazar-Gonzalez H, Cordova V. Pic, an autotransporter protein secreted by different pathogens in the Enterobacteriaceae family, is a potent mucus secretagogue. Infect Immun. 2010;78:4101–9. doi: 10.1128/IAI.00523-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiz-Perez F, Wahid R, Faherty CS, Kolappaswamy K, Rodriguez L, Santiago A, et al. Serine protease autotransporters from Shigella flexneri and pathogenic Escherichia coli target a broad range of leukocyte glycoproteins. Proc Natl Acad Sci U S A. 2011;108:12881–6. doi: 10.1073/pnas.1101006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eslava C, Navarro-Garci´a F, Czeczulin JR, Henderson IR, Cravioto A, Nataro JP. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3155–63. doi: 10.1128/iai.66.7.3155-3163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cappello RE, Estrada-Gutierrez G, Irles C, Giono-Cerezo S, Bloch RJ, Nataro JP. Effects of the plasmid-encoded toxin of enteroaggregative Escherichia coli on focal adhesion complexes. FEMS Immunol Med Microbiol. 2011;61:301–14. doi: 10.1111/j.1574-695X.2010.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Czeczulin JR, Whittam TS, Henderson IR, Navarro-Garcia F, Nataro JP. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect Immun. 1999;67:2692–9. doi: 10.1128/iai.67.6.2692-2699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savarino SJ, Fasano A, Watson J, Martin BM, Levine MM, Guandalini S, et al. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc Natl Acad Sci U S A. 1993;90:3093–7. doi: 10.1073/pnas.90.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rich C, Favre-Bonte S, Sapena F, Joly B, Forestier C. Characterization of enteroaggregative Escherichia coli isolates. FEMS Microbiol Lett. 1999;173:55–61. doi: 10.1111/j.1574-6968.1999.tb13484.x. [DOI] [PubMed] [Google Scholar]
- 61.Zamboni A, Fabbricotti SH, Fagundes-Neto U, Scaletsky IC. Enteroaggregative Escherichia coli virulence factors are found to be associated with infantile diarrhea in Brazil. J Clin Microbiol. 2004;42:1058–63. doi: 10.1128/JCM.42.3.1058-1063.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fasano A, Noriega FR, Maneval DR, Jr., Chanasongcram S, Russell R, Guandalini S, et al. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J Clin Invest. 1995;95:2853–61. doi: 10.1172/JCI117991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mueller M, Grauschopf U, Maier T, Glockshuber R, Ban N. The structure of a cytolytic α-helical toxin pore reveals its assembly mechanism. Nature. 2009;459:726–30. doi: 10.1038/nature08026. [DOI] [PubMed] [Google Scholar]
- 64.Chaudhuri RR, Sebaihia M, Hobman JL, Webber MA, Leyton DL, Goldberg MD, et al. Complete genome sequence and comparative metabolic profiling of the prototypical enteroaggregative Escherichia coli strain 042. PLoS One. 2010;5:e8801. doi: 10.1371/journal.pone.0008801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aslani MM, Alikhani MY, Zavari A, Yousefi R, Zamani AR. Characterization of enteroaggregative Escherichia coli (EAEC) clinical isolates and their antibiotic resistance pattern. Int J Infect Dis. 2011;15:e136–9. doi: 10.1016/j.ijid.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Mellmann A, Harmsen D, Cummings CA, Zentz EB, Leopold SR, Rico A, et al. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One. 2011;6:e22751. doi: 10.1371/journal.pone.0022751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tarr PI, Bilge SS, Vary JC, Jr., Jelacic S, Habeeb RL, Ward TR, et al. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect Immun. 2000;68:1400–7. doi: 10.1128/IAI.68.3.1400-1407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bielaszewska M, Middendorf B, Tarr PI, Zhang W, Prager R, Aldick T, et al. Chromosomal instability in enterohaemorrhagic Escherichia coli O157:H7: impact on adherence, tellurite resistance and colony phenotype. Mol Microbiol. 2011;79:1024–44. doi: 10.1111/j.1365-2958.2010.07499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guignot J, Peiffer I, Bernet-Camard MF, Lublin DM, Carnoy C, Moseley SL, et al. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect Immun. 2000;68:3554–63. doi: 10.1128/IAI.68.6.3554-3563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berger CN, Billker O, Meyer TF, Servin AL, Kansau I. Differential recognition of members of the carcinoembryonic antigen family by Afa/Dr adhesins of diffusely adhering Escherichia coli (Afa/Dr DAEC) Mol Microbiol. 2004;52:963–83. doi: 10.1111/j.1365-2958.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- 71.Nowicki B, Moulds J, Hull R, Hull S. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect Immun. 1988;56:1057–60. doi: 10.1128/iai.56.5.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ochoa TJ, Rivera FP, Bernal M, Meza R, Ecker L, Gil AI, et al. Detection of the CS20 colonization factor antigen in diffuse-adhering Escherichia coli strains. FEMS Immunol Med Microbiol. 2010;60:186–9. doi: 10.1111/j.1574-695X.2010.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Queval CJ, Nicolas V, Beau I. Role of Src kinases in mobilization of glycosylphosphatidylinositol-anchored decay-accelerating factor by Dr fimbria-positive adhering bacteria. Infect Immun. 2011;79:2519–34. doi: 10.1128/IAI.01052-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cane G, Ginouvès A, Marchetti S, Buscà R, Pouysse´gur J, Berra E, et al. HIF-1alpha mediates the induction of IL-8 and VEGF expression on infection with Afa/Dr diffusely adhering E. coli and promotes EMT-like behaviour. Cell Microbiol. 2010;12:640–53. doi: 10.1111/j.1462-5822.2009.01422.x. [DOI] [PubMed] [Google Scholar]
- 75.Be´tis F, Brest P, Hofman V, Guignot J, Kansau I, Rossi B, et al. Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesins. Infect Immun. 2003;71:1774–83. doi: 10.1128/IAI.71.4.1774-1783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Se´miramoth N, Gleizes A, Turbica I, Sandre´ C, Gorges R, Kansau I, et al. Escherichia coli type 1 pili trigger late IL-8 production by neutrophil-like differentiated PLB-985 cells through a Src family kinase- and MAPK-dependent mechanism. J Leukoc Biol. 2009;85:310–21. doi: 10.1189/jlb.0608350. [DOI] [PubMed] [Google Scholar]
- 77.Brest P, Be´tis F, Cuburu N, Selva E, Herrant M, Servin A, et al. Increased rate of apoptosis and diminished phagocytic ability of human neutrophils infected with Afa/Dr diffusely adhering Escherichia coli strains. Infect Immun. 2004;72:5741–9. doi: 10.1128/IAI.72.10.5741-5749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guignot J, Chaplais C, Coconnier-Polter MH, Servin AL. The secreted autotransporter toxin, Sat, functions as a virulence factor in Afa/Dr diffusely adhering Escherichia coli by promoting lesions in tight junction of polarized epithelial cells. Cell Microbiol. 2007;9:204–21. doi: 10.1111/j.1462-5822.2006.00782.x. [DOI] [PubMed] [Google Scholar]
- 79.Maroncle NM, Sivick KE, Brady R, Stokes FE, Mobley HL. Protease activity, secretion, cell entry, cytotoxicity, and cellular targets of secreted autotransporter toxin of uropathogenic Escherichia coli. Infect Immun. 2006;74:6124–34. doi: 10.1128/IAI.01086-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lie´vin-Le Moal V, Comenge Y, Ruby V, Amsellem R, Nicolas V, Servin AL. Secreted autotransporter toxin (Sat) triggers autophagy in epithelial cells that relies on cell detachment. Cell Microbiol. 2011;13:992–1013. doi: 10.1111/j.1462-5822.2011.01595.x. [DOI] [PubMed] [Google Scholar]
- 81.Martinez-Medina M, Mora A, Blanco M, Lo´pez C, Alonso MP, Bonacorsi S, et al. Similarity and divergence among adherent-invasive Escherichia coli and extraintestinal pathogenic E. coli strains. J Clin Microbiol. 2009;47:3968–79. doi: 10.1128/JCM.01484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117:1566–74. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carvalho FA, Barnich N, Sivignon A, Darcha C, Chan CH, Stanners CP, et al. Crohn’s disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J Exp Med. 2009;206:2179–89. doi: 10.1084/jem.20090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chassaing B, Rolhion N, de Valle´e A, Salim SY, Prorok-Hamon M, Neut C, et al. Crohn disease--associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. J Clin Invest. 2011;121:966–75. doi: 10.1172/JCI44632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rolhion N, Barnich N, Claret L, Darfeuille-Michaud A. Strong decrease in invasive ability and outer membrane vesicle release in Crohn’s disease-associated adherent-invasive Escherichia coli strain LF82 with the yfgL gene deleted. J Bacteriol. 2005;187:2286–96. doi: 10.1128/JB.187.7.2286-2296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rolhion N, Barnich N, Bringer MA, Glasser AL, Ranc J, He´buterne X, et al. Abnormally expressed ER stress response chaperone Gp96 in CD favours adherent-invasive Escherichia coli invasion. Gut. 2010;59:1355–62. doi: 10.1136/gut.2010.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn’s disease. Infect Immun. 1999;67:4499–509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glasser AL, Boudeau J, Barnich N, Perruchot MH, Colombel JF, Darfeuille-Michaud A. Adherent invasive Escherichia coli strains from patients with Crohn’s disease survive and replicate within macrophages without inducing host cell death. Infect Immun. 2001;69:5529–37. doi: 10.1128/IAI.69.9.5529-5537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci U S A. 1995;92:1664–8. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elliott SJ, Wainwright LA, McDaniel TK, Jarvis KG, Deng YK, Lai LC, et al. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 91.Feng P, Lampel KA, Karch H, Whittam TS. Genotypic and phenotypic changes in the emergence of Escherichia coli O157:H7. J Infect Dis. 1998;177:1750–3. doi: 10.1086/517438. [DOI] [PubMed] [Google Scholar]
- 92.Ogura Y, Ooka T, Iguchi A, Toh H, Asadulghani M, Oshima K, et al. Comparative genomics reveal the mechanism of the parallel evolution of O157 and non-O157 enterohemorrhagic Escherichia coli. Proc Natl Acad Sci U S A. 2009;106:17939–44. doi: 10.1073/pnas.0903585106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trabulsi LR, Keller R, Tardelli Gomes TA. Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis. 2002;8:508–13. doi: 10.3201/eid0805.010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giro´n JA, Ho AS, Schoolnik GK. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254:710–3. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 95.Scaletsky IC, Silva ML, Trabulsi LR. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–6. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fitzhenry R, Dahan S, Torres AG, Chong Y, Heuschkel R, Murch SH, et al. Long polar fimbriae and tissue tropism in Escherichia coli O157:H7. Microbes Infect. 2006;8:1741–9. doi: 10.1016/j.micinf.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 97.Nicholls L, Grant TH, Robins-Browne RM. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol Microbiol. 2000;35:275–88. doi: 10.1046/j.1365-2958.2000.01690.x. [DOI] [PubMed] [Google Scholar]
- 98.Torres AG, Li Y, Tutt CB, Xin L, Eaves-Pyles T, Soong L. Outer membrane protein A of Escherichia coli O157:H7 stimulates dendritic cell activation. Infect Immun. 2006;74:2676–85. doi: 10.1128/IAI.74.5.2676-2685.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Batisson I, Guimond MP, Girard F, An H, Zhu C, Oswald E, et al. Characterization of the novel factor paa involved in the early steps of the adhesion mechanism of attaching and effacing Escherichia coli. Infect Immun. 2003;71:4516–25. doi: 10.1128/IAI.71.8.4516-4525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paton AW, Srimanote P, Woodrow MC, Paton JC. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect Immun. 2001;69:6999–7009. doi: 10.1128/IAI.69.11.6999-7009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu Y, Iyoda S, Satou H, Satou H, Itoh K, Saitoh T, et al. A new immunoglobulin-binding protein, EibG, is responsible for the chain-like adhesion phenotype of locus of enterocyte effacement-negative, shiga toxin-producing Escherichia coli. Infect Immun. 2006;74:5747–55. doi: 10.1128/IAI.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wells TJ, Sherlock O, Rivas L, Mahajan A, Beatson SA, Torpdahl M, et al. EhaA is a novel autotransporter protein of enterohemorrhagic Escherichia coli O157:H7 that contributes to adhesion and biofilm formation. Environ Microbiol. 2008;10:589–604. doi: 10.1111/j.1462-2920.2007.01479.x. [DOI] [PubMed] [Google Scholar]
- 103.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med. 2000;342:1930–6. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johannes L, Römer W. Shiga toxins--from cell biology to biomedical applications. Nat Rev Microbiol. 2010;8:105–16. doi: 10.1038/nrmicro2279. [DOI] [PubMed] [Google Scholar]
- 105.Römer W, Berland L, Chambon V, Gaus K, Windschiegl B, Tenza D, et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–5. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 106.Mallard F, Antony C, Tenza D, Salamero J, Goud B, Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J Cell Biol. 1998;143:973–90. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988;171:45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- 108.Falguières T, Mallard F, Baron C, Hanau D, Lingwood C, Goud B, et al. Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol Biol Cell. 2001;12:2453–68. doi: 10.1091/mbc.12.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee SY, Lee MS, Cherla RP, Tesh VL. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell Microbiol. 2008;10:770–80. doi: 10.1111/j.1462-5822.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- 110.Fujii J, Wood K, Matsuda F, Carneiro-Filho BA, Schlegel KH, Yutsudo T, et al. Shiga toxin 2 causes apoptosis in human brain microvascular endothelial cells via C/EBP homologous protein. Infect Immun. 2008;76:3679–89. doi: 10.1128/IAI.01581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vidal JE, Navarro-Garci´a F. EspC translocation into epithelial cells by enteropathogenic Escherichia coli requires a concerted participation of type V and III secretion systems. Cell Microbiol. 2008;10:1975–86. doi: 10.1111/j.1462-5822.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- 112.Khan AB, Naim A, Orth D, Grif K, Mohsin M, Prager R, et al. Serine protease espP subtype alpha, but not beta or gamma, of Shiga toxin-producing Escherichia coli is associated with highly pathogenic serogroups. Int J Med Microbiol. 2009;299:247–54. doi: 10.1016/j.ijmm.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 113.Orth D, Ehrlenbach S, Brockmeyer J, Khan AB, Huber G, Karch H, et al. EspP, a serine protease of enterohemorrhagic Escherichia coli, impairs complement activation by cleaving complement factors C3/C3b and C5. Infect Immun. 2010;78:4294–301. doi: 10.1128/IAI.00488-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghosh P. Process of protein transport by the type III secretion system. Microbiol Mol Biol Rev. 2004;68:771–95. doi: 10.1128/MMBR.68.4.771-795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Knutton S, Rosenshine I, Pallen MJ, Nisan I, Neves BC, Bain C, et al. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–76. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wachter C, Beinke C, Mattes M, Schmidt MA. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1999;31:1695–707. doi: 10.1046/j.1365-2958.1999.01303.x. [DOI] [PubMed] [Google Scholar]
- 117.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–55. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]
- 118.Tobe T, Beatson SA, Taniguchi H, Abe H, Bailey CM, Fivian A, et al. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc Natl Acad Sci U S A. 2006;103:14941–6. doi: 10.1073/pnas.0604891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Iguchi A, Thomson NR, Ogura Y, Saunders D, Ooka T, Henderson IR, et al. Complete genome sequence and comparative genome analysis of enteropathogenic Escherichia coli O127:H6 strain E2348/69. J Bacteriol. 2009;191:347–54. doi: 10.1128/JB.01238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wong AR, Pearson JS, Bright MD, Munera D, Robinson KS, Lee SF, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol Microbiol. 2011;80:1420–38. doi: 10.1111/j.1365-2958.2011.07661.x. [DOI] [PubMed] [Google Scholar]
- 121.Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol. 1998;30:911–21. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 122.Jerse AE, Yu J, Tall BD, Kaper JB. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990;87:7839–43. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci U S A. 1995;92:1664–8. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Touze´ T, Hayward RD, Eswaran J, Leong JM, Koronakis V. Self-association of EPEC intimin mediated by the beta-barrel-containing anchor domain: a role in clustering of the Tir receptor. Mol Microbiol. 2004;51:73–87. doi: 10.1046/j.1365-2958.2003.03830.x. [DOI] [PubMed] [Google Scholar]
- 125.Adu-Bobie J, Frankel G, Bain C, Goncalves AG, Trabulsi LR, Douce G, et al. Detection of intimins alpha, beta, gamma, and delta, four intimin derivatives expressed by attaching and effacing microbial pathogens. J Clin Microbiol. 1998;36:662–8. doi: 10.1128/jcm.36.3.662-668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lacher DW, Steinsland H, Whittam TS. Allelic subtyping of the intimin locus (eae) of pathogenic Escherichia coli by fluorescent RFLP. FEMS Microbiol Lett. 2006;261:80–7. doi: 10.1111/j.1574-6968.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- 127.Frankel G, Lider O, Hershkoviz R, Mould AP, Kachalsky SG, Candy DC, et al. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to beta1 integrins. J Biol Chem. 1996;271:20359–64. doi: 10.1074/jbc.271.34.20359. [DOI] [PubMed] [Google Scholar]
- 128.Isberg RR, Leong JM. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–71. doi: 10.1016/0092-8674(90)90099-Z. [DOI] [PubMed] [Google Scholar]
- 129.Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–20. doi: 10.1016/S0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 130.Robinson CM, Sinclair JF, Smith MJ, O’Brien AD. Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc Natl Acad Sci U S A. 2006;103:9667–72. doi: 10.1073/pnas.0602359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gruenheid S, DeVinney R, Bladt F, Goosney D, Gelkop S, Gish GD, et al. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat Cell Biol. 2001;3:856–9. doi: 10.1038/ncb0901-856. [DOI] [PubMed] [Google Scholar]
- 132.Phillips N, Hayward RD, Koronakis V. Phosphorylation of the enteropathogenic E. coli receptor by the Src-family kinase c-Fyn triggers actin pedestal formation. Nat Cell Biol. 2004;6:618–25. doi: 10.1038/ncb1148. [DOI] [PubMed] [Google Scholar]
- 133.Campellone KG, Giese A, Tipper DJ, Leong JM. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol Microbiol. 2002;43:1227–41. doi: 10.1046/j.1365-2958.2002.02817.x. [DOI] [PubMed] [Google Scholar]
- 134.Garmendia J, Phillips AD, Carlier MF, Chong Y, Schüller S, Marches O, et al. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell Microbiol. 2004;6:1167–83. doi: 10.1111/j.1462-5822.2004.00459.x. [DOI] [PubMed] [Google Scholar]
- 135.Campellone KG, Robbins D, Leong JM. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev Cell. 2004;7:217–28. doi: 10.1016/j.devcel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 136.Brady MJ, Campellone KG, Ghildiyal M, Leong JM. Enterohaemorrhagic and enteropathogenic Escherichia coli Tir proteins trigger a common Nck-independent actin assembly pathway. Cell Microbiol. 2007;9:2242–53. doi: 10.1111/j.1462-5822.2007.00954.x. [DOI] [PubMed] [Google Scholar]
- 137.Vingadassalom D, Kazlauskas A, Skehan B, Cheng HC, Magoun L, Robbins D, et al. Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspF(U) during pedestal formation. Proc Natl Acad Sci U S A. 2009;106:6754–9. doi: 10.1073/pnas.0809131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Campellone KG, Leong JM. Nck-independent actin assembly is mediated by two phosphorylated tyrosines within enteropathogenic Escherichia coli Tir. Mol Microbiol. 2005;56:416–32. doi: 10.1111/j.1365-2958.2005.04558.x. [DOI] [PubMed] [Google Scholar]
- 139.Cheng HC, Skehan BM, Campellone KG, Leong JM, Rosen MK. Structural mechanism of WASP activation by the enterohaemorrhagic E. coli effector EspF(U) Nature. 2008;454:1009–13. doi: 10.1038/nature07160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ogura Y, Ooka T, Whale A, Garmendia J, Beutin L, Tennant S, et al. TccP2 of O157:H7 and non-O157 enterohemorrhagic Escherichia coli (EHEC): challenging the dogma of EHEC-induced actin polymerization. Infect Immun. 2007;75:604–12. doi: 10.1128/IAI.01491-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Whale AD, Garmendia J, Gomes TA, Frankel G. A novel category of enteropathogenic Escherichia coli simultaneously utilizes the Nck and TccP pathways to induce actin remodelling. Cell Microbiol. 2006;8:999–1008. doi: 10.1111/j.1462-5822.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 142.Crepin VF, Girard F, Schüller S, Phillips AD, Mousnier A, Frankel G. Dissecting the role of the Tir:Nck and Tir:IRTKS/IRSp53 signalling pathways in vivo. Mol Microbiol. 2010;75:308–23. doi: 10.1111/j.1365-2958.2009.06938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schüller S, Chong Y, Lewin J, Kenny B, Frankel G, Phillips AD. Tir phosphorylation and Nck/N-WASP recruitment by enteropathogenic and enterohaemorrhagic Escherichia coli during ex vivo colonization of human intestinal mucosa is different to cell culture models. Cell Microbiol. 2007;9:1352–64. doi: 10.1111/j.1462-5822.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- 144.Ritchie JM, Brady MJ, Riley KN, Ho TD, Campellone KG, Herman IM, et al. EspFU, a type III-translocated effector of actin assembly, fosters epithelial association and late-stage intestinal colonization by E. coli O157:H7. Cell Microbiol. 2008;10:836–47. doi: 10.1111/j.1462-5822.2007.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bulgin R, Raymond B, Garnett JA, Frankel G, Crepin VF, Berger CN, et al. Bacterial guanine nucleotide exchange factors SopE-like and WxxxE effectors. Infect Immun. 2010;78:1417–25. doi: 10.1128/IAI.01250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 147.Huang Z, Sutton SE, Wallenfang AJ, Orchard RC, Wu X, Feng Y, et al. Structural insights into host GTPase isoform selection by a family of bacterial GEF mimics. Nat Struct Mol Biol. 2009;16:853–60. doi: 10.1038/nsmb.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 149.Alto NM, Shao F, Lazar CS, Brost RL, Chua G, Mattoo S, et al. Identification of a bacterial type III effector family with G protein mimicry functions. Cell. 2006;124:133–45. doi: 10.1016/j.cell.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 150.Kenny B, Ellis S, Leard AD, Warawa J, Mellor H, Jepson MA. Co-ordinate regulation of distinct host cell signalling pathways by multifunctional enteropathogenic Escherichia coli effector molecules. Mol Microbiol. 2002;44:1095–107. doi: 10.1046/j.1365-2958.2002.02952.x. [DOI] [PubMed] [Google Scholar]
- 151.Arbeloa A, Bulgin RR, MacKenzie G, Shaw RK, Pallen MJ, Crepin VF, et al. Subversion of actin dynamics by EspM effectors of attaching and effacing bacterial pathogens. Cell Microbiol. 2008;10:1429–41. doi: 10.1111/j.1462-5822.2008.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bulgin R, Arbeloa A, Goulding D, Dougan G, Crepin VF, Raymond B, et al. The T3SS effector EspT defines a new category of invasive enteropathogenic E. coli (EPEC) which form intracellular actin pedestals. PLoS Pathog. 2009;5:e1000683. doi: 10.1371/journal.ppat.1000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tu X, Nisan I, Yona C, Hanski E, Rosenshine I. EspH, a new cytoskeleton-modulating effector of enterohaemorrhagic and enteropathogenic Escherichia coli. Mol Microbiol. 2003;47:595–606. doi: 10.1046/j.1365-2958.2003.03329.x. [DOI] [PubMed] [Google Scholar]
- 154.Dong N, Liu L, Shao F. A bacterial effector targets host DH-PH domain RhoGEFs and antagonizes macrophage phagocytosis. EMBO J. 2010;29:1363–76. doi: 10.1038/emboj.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Arbeloa A, Oates CV, Marchès O, Hartland EL, Frankel G. Enteropathogenic and enterohemorrhagic Escherichia coli type III secretion effector EspV induces radical morphological changes in eukaryotic cells. Infect Immun. 2011;79:1067–76. doi: 10.1128/IAI.01003-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dean P, Maresca M, Schüller S, Phillips AD, Kenny B. Potent diarrheagenic mechanism mediated by the cooperative action of three enteropathogenic Escherichia coli-injected effector proteins. Proc Natl Acad Sci U S A. 2006;103:1876–81. doi: 10.1073/pnas.0509451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Iizumi Y, Sagara H, Kabe Y, Azuma M, Kume K, Ogawa M, et al. The enteropathogenic E. coli effector EspB facilitates microvillus effacing and antiphagocytosis by inhibiting myosin function. Cell Host Microbe. 2007;2:383–92. doi: 10.1016/j.chom.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 158.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, et al. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest. 2007;117:428–37. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Clements A, Smollett K, Lee SF, Hartland EL, Lowe M, Frankel G. EspG of enteropathogenic and enterohemorrhagic E. coli binds the Golgi matrix protein GM130 and disrupts the Golgi structure and function. Cell Microbiol. 2011;13:1429–39. doi: 10.1111/j.1462-5822.2011.01631.x. [DOI] [PubMed] [Google Scholar]
- 160.Selyunin AS, Sutton SE, Weigele BA, Reddick LE, Orchard RC, Bresson SM, et al. The assembly of a GTPase-kinase signalling complex by a bacterial catalytic scaffold. Nature. 2011;469:107–11. doi: 10.1038/nature09593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tomson FL, Viswanathan VK, Kanack KJ, Kanteti RP, Straub KV, Menet M, et al. Enteropathogenic Escherichia coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier. Mol Microbiol. 2005;56:447–64. doi: 10.1111/j.1365-2958.2005.04571.x. [DOI] [PubMed] [Google Scholar]
- 162.Matsuzawa T, Kuwae A, Abe A. Enteropathogenic Escherichia coli type III effectors EspG and EspG2 alter epithelial paracellular permeability. Infect Immun. 2005;73:6283–9. doi: 10.1128/IAI.73.10.6283-6289.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hodges K, Alto NM, Ramaswamy K, Dudeja PK, Hecht G. The enteropathogenic Escherichia coli effector protein EspF decreases sodium hydrogen exchanger 3 activity. Cell Microbiol. 2008;10:1735–45. doi: 10.1111/j.1462-5822.2008.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guttman JA, Samji FN, Li Y, Deng W, Lin A, Finlay BB. Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cell Microbiol. 2007;9:131–41. doi: 10.1111/j.1462-5822.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- 165.Dean P, Kenny B. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol Microbiol. 2004;54:665–75. doi: 10.1111/j.1365-2958.2004.04308.x. [DOI] [PubMed] [Google Scholar]
- 166.Thanabalasuriar A, Koutsouris A, Weflen A, Mimee M, Hecht G, Gruenheid S. The bacterial virulence factor NleA is required for the disruption of intestinal tight junctions by enteropathogenic Escherichia coli. Cell Microbiol. 2010;12:31–41. doi: 10.1111/j.1462-5822.2009.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim J, Thanabalasuriar A, Chaworth-Musters T, Fromme JC, Frey EA, Lario PI, et al. The bacterial virulence factor NleA inhibits cellular protein secretion by disrupting mammalian COPII function. Cell Host Microbe. 2007;2:160–71. doi: 10.1016/j.chom.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 168.Lee SF, Kelly M, McAlister A, Luck SN, Garcia EL, Hall RA, et al. A C-terminal class I PDZ binding motif of EspI/NleA modulates the virulence of attaching and effacing Escherichia coli and Citrobacter rodentium. Cell Microbiol. 2008;10:499–513. doi: 10.1111/j.1462-5822.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- 169.Schüller S, Lucas M, Kaper JB, Giro´n JA, Phillips AD. The ex vivo response of human intestinal mucosa to enteropathogenic Escherichia coli infection. Cell Microbiol. 2009;11:521–30. doi: 10.1111/j.1462-5822.2008.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ruchaud-Sparagano MH, Maresca M, Kenny B. Enteropathogenic Escherichia coli (EPEC) inactivate innate immune responses prior to compromising epithelial barrier function. Cell Microbiol. 2007;9:1909–21. doi: 10.1111/j.1462-5822.2007.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nadler C, Baruch K, Kobi S, Mills E, Haviv G, Farago M, et al. The type III secretion effector NleE inhibits NF-kappaB activation. PLoS Pathog. 2010;6:e1000743. doi: 10.1371/journal.ppat.1000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Newton HJ, Pearson JS, Badea L, Kelly M, Lucas M, Holloway G, et al. The type III effectors NleE and NleB from enteropathogenic E. coli and OspZ from Shigella block nuclear translocation of NF-kappaB p65. PLoS Pathog. 2010;6:e1000898. doi: 10.1371/journal.ppat.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pearson JS, Riedmaier P, Marchès O, Frankel G, Hartland EL. A type III effector protease NleC from enteropathogenic Escherichia coli targets NF-κB for degradation. Mol Microbiol. 2011;80:219–30. doi: 10.1111/j.1365-2958.2011.07568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Baruch K, Gur-Arie L, Nadler C, Koby S, Yerushalmi G, Ben-Neriah Y, et al. Metalloprotease type III effectors that specifically cleave JNK and NF-κB. EMBO J. 2011;30:221–31. doi: 10.1038/emboj.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hemrajani C, Marches O, Wiles S, Girard F, Dennis A, Dziva F, et al. Role of NleH, a type III secreted effector from attaching and effacing pathogens, in colonization of the bovine, ovine, and murine gut. Infect Immun. 2008;76:4804–13. doi: 10.1128/IAI.00742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Royan SV, Jones RM, Koutsouris A, Roxas JL, Falzari K, Weflen AW, et al. Enteropathogenic E. coli non-LEE encoded effectors NleH1 and NleH2 attenuate NF-κB activation. Mol Microbiol. 2010;78:1232–45. doi: 10.1111/j.1365-2958.2010.07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gao X, Wan F, Mateo K, Callegari E, Wang D, Deng W, et al. Bacterial effector binding to ribosomal protein s3 subverts NF-kappaB function. PLoS Pathog. 2009;5:e1000708. doi: 10.1371/journal.ppat.1000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wan F, Weaver A, Gao X, Bern M, Hardwidge PR, Lenardo MJ. IKKβ phosphorylation regulates RPS3 nuclear translocation and NF-κB function during infection with Escherichia coli strain O157:H7. Nat Immunol. 2011;12:335–43. doi: 10.1038/ni.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Raymond B, Crepin VF, Collins JW, Frankel G. The WxxxE effector EspT triggers expression of immune mediators in an Erk/JNK and NF-κB-dependent manner. Cell Microbiol. 2011;13:1881–93. doi: 10.1111/j.1462-5822.2011.01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Crane JK, Majumdar S, Pickhardt DF., 3rd Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun. 1999;67:2575–84. doi: 10.1128/iai.67.5.2575-2584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Barnett Foster D, Abul-Milh M, Huesca M, Lingwood CA. Enterohemorrhagic Escherichia coli induces apoptosis which augments bacterial binding and phosphatidylethanolamine exposure on the plasma membrane outer leaflet. Infect Immun. 2000;68:3108–15. doi: 10.1128/IAI.68.6.3108-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nougayrède JP, Donnenberg MS. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell Microbiol. 2004;6:1097–111. doi: 10.1111/j.1462-5822.2004.00421.x. [DOI] [PubMed] [Google Scholar]
- 183.Samba-Louaka A, Nougayrède JP, Watrin C, Oswald E, Taieb F. The enteropathogenic Escherichia coli effector Cif induces delayed apoptosis in epithelial cells. Infect Immun. 2009;77:5471–7. doi: 10.1128/IAI.00860-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Crane JK, McNamara BP, Donnenberg MS. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell Microbiol. 2001;3:197–211. doi: 10.1046/j.1462-5822.2001.00103.x. [DOI] [PubMed] [Google Scholar]
- 185.Hemrajani C, Berger CN, Robinson KS, Marchès O, Mousnier A, Frankel G. NleH effectors interact with Bax inhibitor-1 to block apoptosis during enteropathogenic Escherichia coli infection. Proc Natl Acad Sci U S A. 2010;107:3129–34. doi: 10.1073/pnas.0911609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Baruch K, Gur-Arie L, Nadler C, Koby S, Yerushalmi G, Ben-Neriah Y, et al. Metalloprotease type III effectors that specifically cleave JNK and NF-κB. EMBO J. 2011;30:221–31. doi: 10.1038/emboj.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shames SR, Deng W, Guttman JA, de Hoog CL, Li Y, Hardwidge PR, et al. The pathogenic E. coli type III effector EspZ interacts with host CD98 and facilitates host cell prosurvival signalling. Cell Microbiol. 2010;12:1322–39. doi: 10.1111/j.1462-5822.2010.01470.x. [DOI] [PubMed] [Google Scholar]
- 188.Kim M, Ogawa M, Fujita Y, Yoshikawa Y, Nagai T, Koyama T, et al. Bacteria hijack integrin-linked kinase to stabilize focal adhesions and block cell detachment. Nature. 2009;459:578–82. doi: 10.1038/nature07952. [DOI] [PubMed] [Google Scholar]
- 189.Crane JK, Oh JS. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect Immun. 1997;65:3277–85. doi: 10.1128/iai.65.8.3277-3285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rosenshine I, Donnenberg MS, Kaper JB, Finlay BB. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–60. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sason H, Milgrom M, Weiss AM, Melamed-Book N, Balla T, Grinstein S, et al. Enteropathogenic Escherichia coli subverts phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate upon epithelial cell infection. Mol Biol Cell. 2009;20:544–55. doi: 10.1091/mbc.E08-05-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Quitard S, Dean P, Maresca M, Kenny B. The enteropathogenic Escherichia coli EspF effector molecule inhibits PI-3 kinase-mediated uptake independently of mitochondrial targeting. Cell Microbiol. 2006;8:972–81. doi: 10.1111/j.1462-5822.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- 193.Iizumi Y, Sagara H, Kabe Y, Azuma M, Kume K, Ogawa M, et al. The enteropathogenic E. coli effector EspB facilitates microvillus effacing and antiphagocytosis by inhibiting myosin function. Cell Host Microbe. 2007;2:383–92. doi: 10.1016/j.chom.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 194.Marchès O, Covarelli V, Dahan S, Cougoule C, Bhatta P, Frankel G, et al. EspJ of enteropathogenic and enterohaemorrhagic Escherichia coli inhibits opsono-phagocytosis. Cell Microbiol. 2008;10:1104–15. doi: 10.1111/j.1462-5822.2007.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]