Abstract
The effects of As4O6 as adjuvant on photodynamic therapy (PDT) were studied. As4O6 is considered to have anticancer activity via several biological actions, such as free radical production and inhibition of VEGF expression. PDT or As4O6 significantly inhibited TC-1 cell proliferation in a dose-dependent manner (P<0.05) by MTT assay. The anti-proliferative effect of the combination treatment was significantly higher than in TC-1 cells treated with either photodynamic therapy or As4O6 alone (62.4 and 52.5% decrease compared to vehicle-only treated TC-1 cells, respectively, P<0.05). In addition, cell proliferation in combination of photodynamic therapy and As4O6 treatment significantly decreased by 77.4% (P<0.05). Cell survival pathway (Naip1, Tert and Aip1) and p53-dependent pathway (Bax, p21Cip1, Fas, Gadd45, IGFBP-3 and Mdm-2) were markedly increased by combination treatment of photodynamic therapy and As4O6. In addition, the immune response in the NEAT pathway (Ly-12, CD178 and IL-2) was also modulated after combination treatment, suggesting improved antitumor effects by controlling unwanted growth-stimulatory pathways. The combination effect apparently reflected concordance with in vitro data, in restricting tumor growth in vivo and in relation to some common signaling pathways to those observed in vitro. These findings suggest the benefit of combinatory treatment with photodynamic therapy and As4O6 for inhibition of cervical cancer cell growth.
Introduction
Photodynamic therapy (PDT) involves the combination of non-toxic dyes known as photosensitizers (PSs) and visible light of the correct wavelength to be absorbed by the PSs. In the presence of oxygen, this leads to generation of reactive oxygen species (ROS) that can damage cellular constituents, leading to cell death [1], [2]. The discovery of programmed cell death, or apoptosis, has revolutionized the field of cytotoxic therapy in general, and PDT in particular [3]–[5]. However, a complete eradication of tumor cells by PDT alone has not been guaranteed [6]. Further study of controlling unwanted growth-stimulatory pathways after PDT is desirable to minimize the risk of harmful adverse effects.
Arsenical compounds have been demonstrated to possess life-preserving qualities in cancer treatment [7]–[10]. Promising results were reported, showing that diarsenic oxide (As2O3) treatment could offer an alternative to chemotherapy for acute promyelocytic leukemia (APL). Cytopathological studies also showed induction of apoptosis in APL cells. Recent reports suggested that arsenical compounds inhibit the proliferation of human umbilical vein endothelial cells (HUVEC) via G1 and G2/M phase arrest of the cell cycle [8]. In addition, the inhibitory effects on bFGF- or VEGF-stimulated cell proliferation suggest the antiangiogenic potential of arsenical compounds [11]. Furthermore, tetra-arsenic oxide (As4O6) was reported to have antiangiogenic effects on the NGF-induced formation of new vessels in the rat cornea, compared to control group and As2O3 treated group [9]. It has been therefore suggested that As4O6 might be a new arsenic compound, as it induced apoptosis in cancer cells at much lower concentration than As2O3 [10]. Arsenic compounds can increase reactive oxygen species (ROS) in cells and thereby increase apoptosis inducing factor (AIF) secretion through activation of PARP-1, and finally induce cell apoptosis. The release of cytochrome c and apoptosis inducing factor (AIF) is finely tuned by Bcl-2 family proteins in either of the following two ways: according to one possible mechanism, Bax present in the cytoplasm is translocated to the mitochondrial membrane, where it undergoes conformational changes assisted by Bid. The binding of Bax to the outer membrane (OM) causes its in situ multimerization, whereby PTP is gated and cytochrome c is released. Bcl-2 and Bcl-xL inhibit conformational changes in Bax and therefore inhibit release of cytochrome c and apoptosis. High level of ROS production is the main cause of apoptosis by arsenic compound. As free radical, ROS can react with most biological macromolecules, and therefore not only can induce oxidative damage on DNA, but also change structure and function of protein [12]–[14].
On the other hand, ROS can modulate genetic expression by acting as a second messenger. Several studies have suggested that oxidative damage might be involved in initiating events in cancer, and could help induce the initiation of apoptosis after an increase in cell proliferation [15], [16]. Arsenic compound could also reportedly regulate the immune response to involve anti-cancer function, through decrease of VEGF expression [12]–[14].
In this study, we firstly showed the enhanced anti-tumor effect of PDT using Radachlorin with As4O6 in mice bearing tumors caused by human papillomavirus (HPV) 16 E6/E7 oncogene expressed TC-1 tumor cells. The present study showed that the combination therapy of PDT plus As4O6 was much more effective on the suppression of tumor growth, compared with PDT or As4O6 alone.
Results
In vitro cell growth inhibitory effect of As4O6 plus Radachlorin/PDT on TC-1 cells
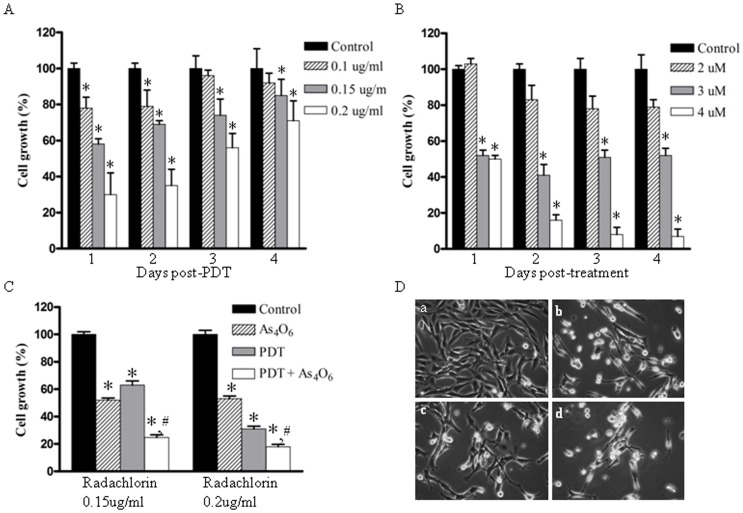
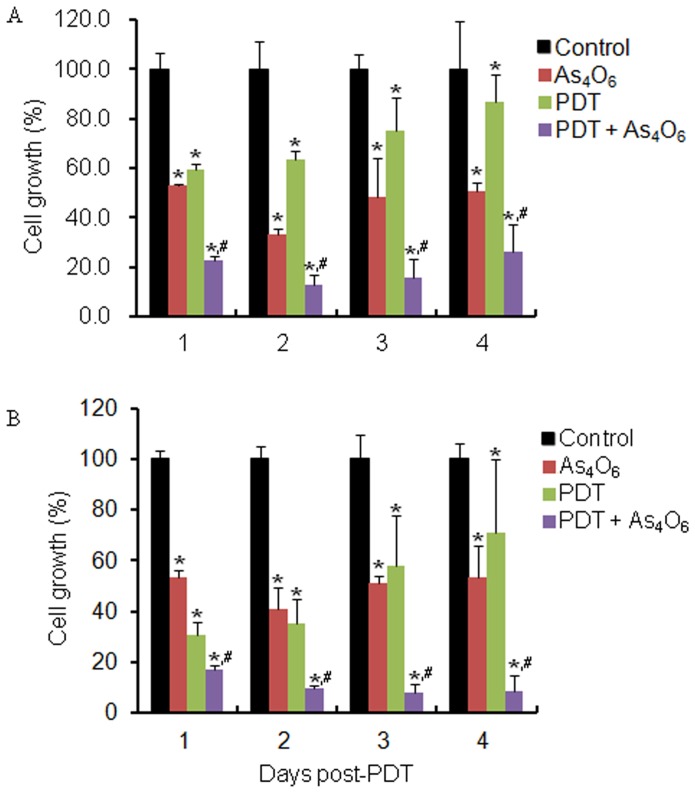
To see the growth inhibition effect of PDT on TC-1 cell, the light of 6.25J/cm2 was exposed at 12 hr after Radachlorin treatment on the cells, and then the cell growth was measured for a predetermined time. Viability of cells treated with various doses of Radachlorin followed by light irradiation was reduced in a dose dependent manner compared to control, respectively (Figure 1A). To see the growth inhibition effect of As4O6 on TC-1 cell, the cell growth was measured for a predetermined time after As4O6 treatment. Viability of cells treated with various doses of As4O6 was reduced in a dose dependent manner compared to control, respectively (Figure 1B). Using these data, the viability of cells was determined after treating the cultured TC-1 cells with 3 uM of As4O6 and different doses of Radachlorin/PDT per day. The combination treatment showed synergistic effect, decreasing viability in a dose dependent manner as compared to control, as shown in Figure 1C. Cell viability was found to be 62.4% for PDT alone and 52.5% after As4O6 alone treatment at a low dose. In contrast, after PDT plus As4O6 treatment, the percentage of cell growth was found less than 23%. We also observed the combined effect vs. single doses over time to elucidate whether the combinatory approach can result in longer-lived restriction of cell proliferation compared to individual therapies (Figure 2). Cell viability was found to be less than 10% for 0.2 ug/ml of Radachlorin/PDT plus As4O6 treatment for three days, as compared to individual treatment. For the evaluation of synergism between Radachlorin/PDT and As4O6 treatment, we used a combination index that calculated by Chou and Talalay's method (Table 1). Among the several combinations of treatment, 0.2 ug/ml Radachlorin/PDT plus 3 uM of As4O6 on day 3 and 4 led to the highest cell death rate and it showed synergism. A few more cell lines such as HaCaT, HeLa, and SiHa cells were included for evaluating the inhibition of cell growth (Supplement Figure S1). While the results of HaCaT and SiHa were consistent with earlier estimates of MTT assay, HeLa showed Radachlorine/PDT-resistant trend compared to the other cells. The cell viability of the two cell line was found to be less than 25% for 0.15 ug/ml of Radachlorin/PDT plus As4O6 treatment for four days, as compared to individual treatment. We characterized cell death by staining the TC-1 cells treated with 0.15 ug/ml Radachlorin/PDT or/and 3 uM of As4O6 for 1 day. As expected, the effect of the combination treatment was larger than each single treatment. In the absence of Radachlorin/PDT or As4O6, the cells achieved a completely confluent, dense monolayer after 48 h of culture (Figure 1D). The cells remained attached to the tissue culture substrate and they adopted an elongated morphology. In contrast, the majority of the cells treated with Radachlorin/PDT plus As4O6 was detached from the plate and was rounded; characteristic of cells undergoing death by apoptosis. The cells treated with Radachlorin/PDT or As4O6, however, adopted morphologies that were intermediate in nature. We counted different apoptotic cell populations induced by 0.15 ug/ml Radachlorin/PDT or/and 3 uM of As4O6 for 1 day. As shown in Supplement Figure S2, the cell death significantly increased after Radachlorin/PDT plus As4O6 treatment. Early apoptotic population was 9.9% at Radachlorin/PDT plus As4O6 treatment. In contrast, early apoptotic cell populations were 4.3% and 4.1% at Radachlorin/PDT and As4O6 treatment, respectively. This shows that the combination treatment induced more early apoptotic cells compared to individual therapies.
Figure 1. Cell growth inhibition effects of photodynamic therapy and As4O6.
(A) TC-1 cells (3×103) were cultured in 12-well plates in triplicate overnight and treated with different concentrations of Radachlorin and PDT (6.25 J/cm2) as described in Materials and Methods. After PDT, the cells were cultured for a predetermined time. (B) Inhibition effects of cell growth of As4O6 on TC-1 cells. TC-1 cells were cultured and treated as described in Materials and Methods. After As4O6 treatment, the cells were cultured for a predetermined time. Cell viability was determined based on the MTT assay. (C) In vitro cell growth inhibitory effects of As4O6 plus Radachlorin/PDT on TC-1 cells. TC-1 cells were cultured with 3 uM of As4O6 and different doses of Radachlorin/PDT for 1 day, as described above. Cell viability was determined based on the MTT assay. Each bar represents a mean [± SD (vertical line)] of three replicates per dose (n = 3). * and #: significantly different (P<0.05) from the control and the PDT by the student’s t-test. (D) Morphological changes of TC-1 cells. TC-1 cells were treated with 0.15 ug/ml Radachlorin/PDT or/and 3 uM of As4O6 for 1 day. Cells were then viewed under microscope. Pictures were taken with phase contrast microscopy at X300. (a) non-treated; (b) 0.15 ug/ml Radachlorin/PDT alone; (c) 3 uM As4O6 alone; (d) 3 uM As4O6 plus 0.15 ug/ml Radachlorin/PDT.
Figure 2. In vitro cell growth inhibitory effects of As4O6 plus Radachlorin/PDT on TC-1 cells.
TC-1 cells were cultured with 3 uM of As4O6 and (A) 0.15 ug/ml and (B) 0.2 ug/ml of Radachlorin/PDT, respectively. Cell viability was determined based on the MTT assay. Each bar represents a mean [± SD (vertical line)] of three replicates (n = 3). * and #: significantly different (P<0.05) from the control and the PDT by the student's t-test.
Table 1. Combination index (CI) values for TC-1 cells treated with Radachlorin/PDT and As4O6.
| Day 1 | Day 2 | Day 3 | Day 4 | ||||||
| As4O6 | PDT | f*(%) | CI** | f(%) | CI | f(%) | CI | f(%) | CI |
| 3uM | 0.2ug/ml | 17.3 | 1.61 | 9.7 | 1.16 | 8.2 | 0.98 | 8.7 | 0.67 |
| 3uM | 0.15ug/ml | 22.6 | 1.52 | 12.7 | 1.13 | 15.7 | 1.02 | 26.4 | 1.19 |
The combination index (CI) values were calculated using the Chou and Talalay mathematical model for drug interaction on Calcusyn software. A CI was equal to 1 denotes additivity; antagonism if the CI>1; CI values between 1 and 0.7 indicate slight synergism; 0.7 to 0.3, synergism; <0.3, strong synergism. *Viability.
Gene expression profile of TC-1 cells treated with As4O6 plus Radachlorin/PDT
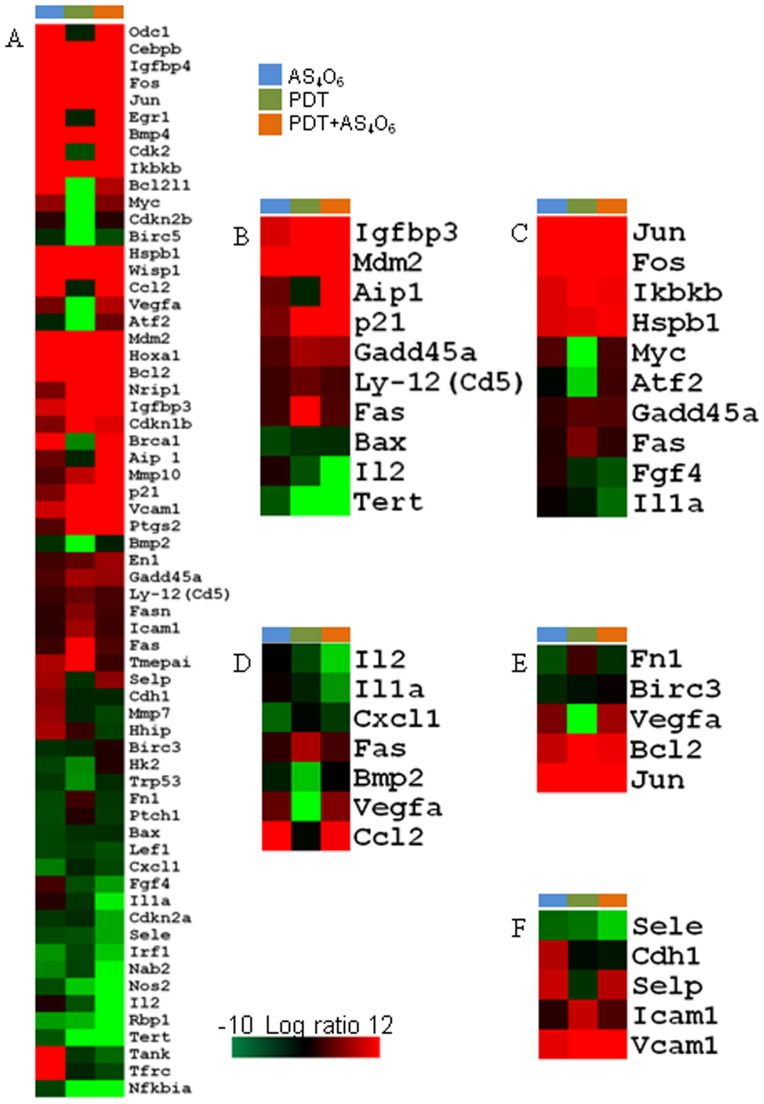
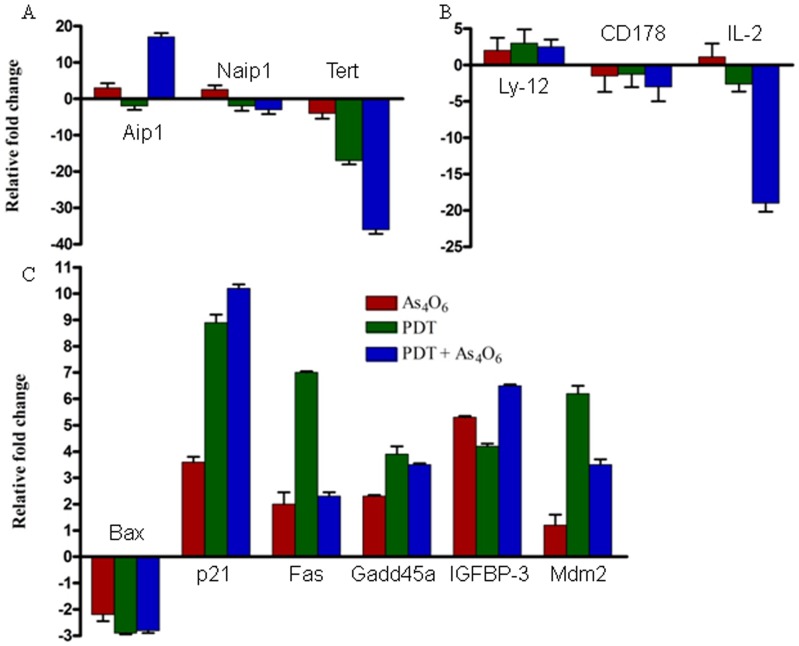
We used RT2 Profiler PCR Array System to understand the cellular process changes through which TC-1 cells could be influenced by the combination treatment with As4O6 plus Radachlorin/PDT. We found 63 genes which were at least two fold up- or downregulated (each gene is detailed in Table 2) and used hierarchical clustering to show differentially expressed genes (Figure 3A). To detect the differences in the functional profiles, we placed differentially expressed genes in the context of present interactome knowledge, using the Ingenuity Pathways Analysis tools (P for all <0.05), showing that the enhanced cell growth inhibition and antitumor effects were significantly related with gene expression levels of genes related with cell death, i.e. p53 and NFAT pathway (Figure 3B). Each gene in the pathway was tested by quantitative PCR (Figure 4) and the results of the PCR array and qRT-PCR were similar. These included genes coding for Tert, Aip1, Bax, p21, Fas, Gadd45, IGFBP-3, Mdm-2, Ly-12, and IL-2, as compared to slightly expressed genes such as Naip1 and CD178. Significantly up-regulated molecular functions for the combination treatment group was p53 pathway. The down-regulated molecular function was NEAT pathway. MAPK, cytokine-cytokine receptor interaction, focal adhesion, cell adhesion pathways were also studied in the gene sets, but not strictly correlated with the enhanced cell growth inhibition (P for all >0.05) (Figure 3C–F).
Table 2. Gene expression profiles of TC-1 cells treated with 0.15 ug/ml Radachorin/PDT and/or 3 uM of As4O6 for 1 day.
| Symbol | As4O6 | PDT | PDT + As4O6 | Symbol | As4O6 | PDT | PDT + As4O6 |
| Odc1 | 13064.2 | −1.1 | 9131.7 | Icam1 | 1.4 | 5.0 | 1.9 |
| Cebpb | 6309.6 | 384.5 | 4319.6 | Tmepai | 5.4 | 8.6 | 1.8 |
| Igfbp4 | 4683.4 | 7315.1 | 2413.1 | Cdkn2b | 1.4 | −262.0 | 1.2 |
| Egr1 | 1317.3 | −1.1 | 1495.8 | Birc3 | −1.5 | −1.2 | 1.1 |
| Fos | 1513.1 | 5281.3 | 1043.1 | Hk2 | −2.1 | −4.3 | 1.1 |
| Jun | 977.8 | 4927.6 | 1035.9 | Bmp2 | −1.5 | −14.2 | −1.0 |
| Bmp4 | 964.3 | 1000.6 | 414.9 | Cdh1 | 4.3 | −1.1 | −1.2 |
| Cdk2 | 254.8 | −2.4 | 371.4 | Trp53 | −1.7 | −4.5 | −1.3 |
| Hspb1 | 67.8 | 81.4 | 118.3 | Bax | −2.2 | −1.5 | −1.4 |
| Ikbkb | 68.8 | 279.5 | 106.6 | Fn1 | −2.3 | 2.0 | −1.7 |
| Wisp1 | 68.8 | 150.8 | 56.0 | Ptch1 | −2.4 | 1.1 | −1.8 |
| Mdm2 | 12.1 | 71.8 | 46.1 | Hhip | 5.3 | 1.7 | −2.0 |
| Hoxa1 | 17.4 | 45.2 | 32.6 | Tfrc | 725.8 | −1.1 | −2.2 |
| Brca1 | 8.0 | −4.2 | 31.3 | Mmp7 | 4.8 | −1.1 | −2.2 |
| Ccl2 | 108.6 | −1.1 | 27.8 | Cxcl1 | −3.9 | −1.1 | −2.2 |
| Aip 1 | 3.3 | −1.1 | 17.0 | Birc5 | −1.4 | −229.7 | −2.4 |
| Bcl2 | 8.5 | 32.8 | 13.3 | Lef1 | −2.2 | −1.7 | −2.5 |
| p21 | 3.8 | 12.0 | 13.1 | Tank | 40.3 | −1.7 | −3.3 |
| Mmp10 | 2.5 | 6.0 | 12.7 | Fgf4 | 2.1 | −2.4 | −4.9 |
| Vcam1 | 6.5 | 12.4 | 10.6 | Cdkn2a | −1.7 | −1.3 | −5.3 |
| Nrip1 | 3.9 | 35.9 | 10.3 | Sele | −2.3 | −2.6 | −5.3 |
| Ptgs2 | 2.6 | 9.8 | 9.5 | Irf1 | −4.7 | −2.2 | −6.3 |
| Igfbp3 | 6.8 | 44.8 | 8.0 | Il1a | 1.2 | −1.6 | −7.5 |
| Cdkn1b | 4.0 | 43.0 | 7.0 | Nab2 | −4.2 | −2.1 | −7.9 |
| Vegfa | 3.7 | −76.3 | 5.6 | Nos2 | −2.4 | −6.3 | −12.7 |
| Bcl2l1 | 10.7 | −170.5 | 5.6 | Il2 | 1.0 | −2.6 | −17.2 |
| En1 | 2.0 | 3.0 | 4.9 | Rbp1 | −4.9 | −5.7 | −19.3 |
| Gadd45a | 2.5 | 5.2 | 4.7 | Tert | −2.7 | −17.2 | −34.6 |
| Selp | 5.1 | −1.5 | 4.4 | Nfkbia | −2.1 | −1055.2 | −2125.1 |
| Myc | 4.6 | −174.0 | 3.9 | ||||
| Atf2 | −1.1 | −55.5 | 3.3 | ||||
| Fas | 1.9 | 10.1 | 2.5 | ||||
| Ly-12(Cd5) | 1.9 | 3.3 | 2.3 | ||||
| Fasn | 1.5 | 4.2 | 2.1 |
Figure 3. Hierarchical cluster analysis.
(A) All the data were median centred and clustered using a hierarchical clustering. A cluster image representing 63 of the genes is shown in matrix format, where rows represent individual genes and columns represent each assay. Each cell in the matrix represents the expression level of a gene in an individual assay. Red and green cells reflect high and low expression levels, respectively. (B) Ten genes (p53 and NEAT pathways) showing statistically significant differences in three groups are shown as a cluster image. (C) MAPK pathway as a cluster image. (D) Cytokine-cytokine receptor interaction pathway. (E) Focal adhesion pathway. (F) Cell adhesion pathway.
Figure 4. In vitro gene expression profiles using RT2ProfilerTM PCR array of TC-1 cells treated with 0.15 ug/ml Radachlorin/PDT or/and 3 uM of As4O6 for 1 day.
The results are presented as transcript levels relative to the level in untreated control cells by using the CT method, with average mRNA level of five internal control genes, including b-actin, used as the normalization control. (A) Cell survival pathway. (B) NEAT pathway. (C) p53 pathway.
As4O6 plus Radachlorin/PDT suppresses tumor growth in TC-1 animal model
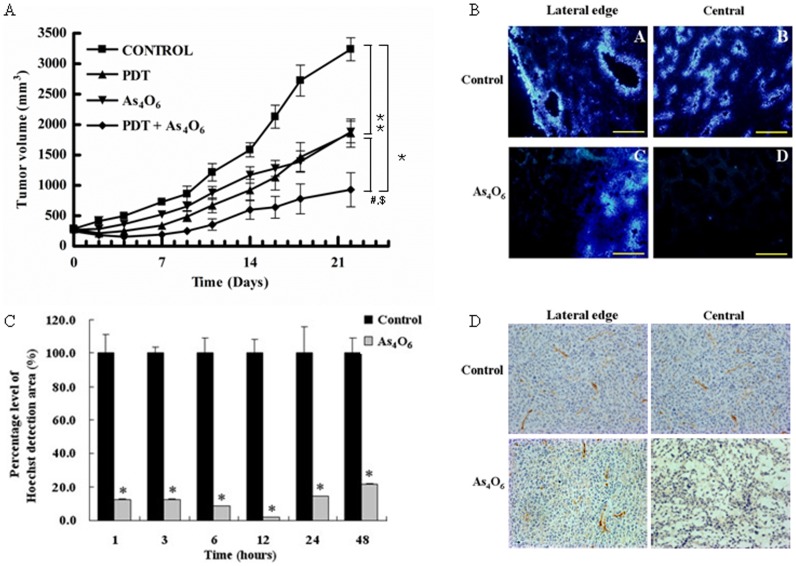
To determine the synergistic antitumor effects of As4O6 plus Radachlorin/PDT in vivo, C57BL/6 mice were challenged s.c. with TC-1 cells and then treated with As4O6 or/and Radachlorin/PDT. Treatment was initiated on day 0 when tumors were ∼230 mm3 in size. As shown in Figure 5A, As4O6 or Radachlorin/PDT treated animal groups showed significant suppression of tumor growth, compared to the untreated control group. However, animals treated with combination of As4O6 plus Radachlorin/PDT showed the most significant tumor suppression, compared to the other groups. This suggested that As4O6 may play an important role in inhibiting the growth by Radachlorin/PDT.
Figure 5. In vivo effect of the combination of As4O6 and Radachlorin/PDT on tumor growth inhibition in TC-1 cell-challenged C57BL/6 mice.
(A) Tumor growth curves for TC-1 of mice treated with Radachlorin/PDT and/or As4O6 (n = 7 for each group). Tumor bearing mice were given intravenously injected Radachlorin (10 mg/kg b.w.) and/or peritoneal injections of 7.5 mg/kg of As4O6. Three hours later, cells were exposed to laser, tumor size was monitored thereafter, as described in Materials and Methods, and its mean ellipsoid volume was plotted over time. Significant inhibition of tumor growth was detected by ANOVA. *: P<0.01, compared with the control group, #: P<0.01, compared with the Radachlorin/PDT alone group and $: P<0.01, compared with the As4O6 alone group. (B) Perfusion and morphological changes in TC-1 tumors in C57BL/6 mice treated with As4O6 (10 mg/kg b.w., i.p.). Tumor tissues were harvested after 48 h after As4O6 treatment. Tissue were viewed at a wavelength of 365 nm and photographed at x200 magnification. (C) Percent area of perfusion area of TC-1 tumors in C57BL/6 mice treated with As4O6 compared with non treated tumors. The tumor tissues were harvested at indicated times after As4O6 treatment. Sequential changes of perfused areas at 1, 3, 6, 12, 24, and 48 hr after As4O6 treatment. *P<0.05, compared to untreated controls. (D) CD31-immunostained tumor sections (original magnification, x400) and As4O6-treated sections are displayed. Twenty-four hours after As4O6 treatment, TC-1 tumors showed extensive loss of CD31 staining indicative of significant As4O6-induced vascular damage.
Effect of arsenic treatment on regional blood perfusion in tumor tissue
Hoechst 33342 was used to visualize perfused vessels as described in material and methods. In control tissue, vascular endothelial cells were clearly stained by Hoechst 33342 at both their outer edge and central region (Figure 5B). Treated tissue was centrally destained and the periphery florescence signal was almost undetectable. This phenomenon continued to the 24 h point. However, fluorescence at the periphery of cells was clearly evident at 48 h post-treatment, while central staining still remained absent. In order to observe changes of vessel function in whole tumor, tumor section were scanned field by field. The sequential perfusion area changes after As4O6 treatment are shown in Figure 5C. At each time point, the percentage of every section to tumor field was comparable to control tissue at each time point. The perfusion area was reduced immediately after As4O6 treatment. At 6 and 12 hr, the perfusion area was the minimum, at 24 h the perfusion area was 14.4% compared with tissue from PBS treated control tissue. Tumor microvessel density dimensions were measured after the administration of As4O6 to mice bearing TC-1 tumor xenografts. As shown in Figure 5D, there was a significant difference in tumor vessel density as detected by CD31 staining between the As4O6-treated group and the time-matched PBS treated control group after 24 hr of As4O6 treatment. Mice treated with As4O6 had a reduction in tumor vessel dimensions compared with the control animals, with CD31 stained microvessels still being apparent at the outer edge of the tumor.
In vivo gene expression levels of tumor tissues treated with As4O6 plus Radachlorin/PDT
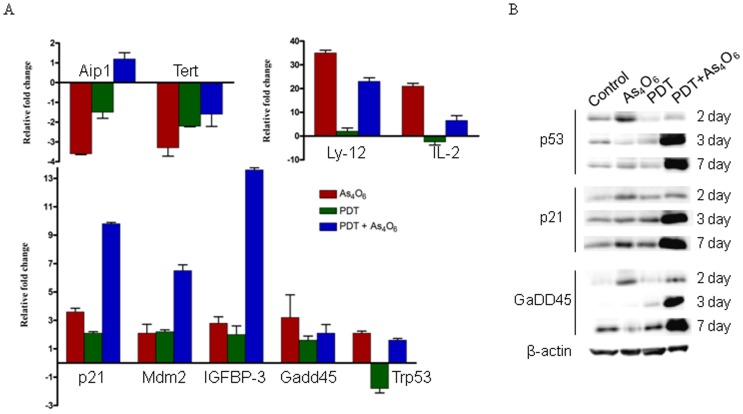
TC-1 cells were treated with As4O6 and Radachlorin/PDT for 1 day, as indicated in the “Materials and Methods” section. The expression of genes mRNA in tumor tissues was examined by qRT-PCR (Figure 6A). The results are presented as transcript levels relative to the level in untreated control cells by using the CT method, and b-actin mRNA levels were used as the normalization control. Using CD5 antigen in the combination therapy group, Ly-12 and IL-2 gene expression increased 26 and 17 fold, respectively, compared to the control group, showing that p53 was involved in regulating the NFAT pathway of p21, Gadd45, IGFBP-3, Mdm-2 and other genes. Gene expression was found increased in three consecutive experiments. To determine the roles of p53 signaling pathways in the synergistic antitumor effects of As4O6 plus Radachlorin/PDT in vivo, we further investigated the p53, p21, and Gadd45 protein levels in vivo (Figure 6B). As4O6 or Radachlorin/PDT treated animal groups showed no significant expression changes, compared to the untreated control group. In contrast, p53, p21, and Gadd45 protein were significantly increased in the combination treatment with As4O6 plus Radachlorin/PDT.
Figure 6. In vivo gene expression profiles in tumor tissues treated with 0.15 ug/ml Radachlorin/PDT or/and 3 uM of As4O6 for 1 day.
(A) The expression of genes mRNA in tumor tissues was examined by Q-PCR. The results are presented as transcript levels relative to the level in untreated control cells by using the CT method, with b-actin mRNA levels used as the normalization control. (B) Western blot analysis of in vivo tumor tissues treated with 0.15 ug/ml Radachlorin/PDT or/and 3 uM of As4O6 for several days.
Discussion
In this study, we comparatively analyzed results of single treatment of As4O6 or photodynamic therapy groups with those obtained by the co-treatment using E6/E7 expressing TC-1 cell line and C57BL/6 mouse model transplanted with this cell. We finally report the enhanced cell growth inhibition and antitumor effects and changes in gene expression levels of genes related with cell death, i.e. p53 and NFAT pathway, induced by this As4O6 and PDT co-treatment method.
These effects were produced by the photodynamic therapy depending on the concentration of the photosensitizer Radachlorin, and by the As4O6 treatment in a concentration dependent manner. In the PDT-untreated cells, the cell growth was consistently maintained until the end of the observation period. PDT-treated cells were regressed only for 1 day post-PDT, but the cell growth was re-increased after 2 days post-PDT. The lower the As4O6 level was, the slower the cell growth inhibiting effect was shown. Under 3 uM of As4O6, cell growth inhibition seemed to begin on the 1st day of treatment and was consistently maintained under 60∼80% level of cell growth during the first 4 days of treatment. In contrast, concentrations of As4O6 higher than 3 uM seemed to be cytotoxic. In the co-treatment group of As4O6 and PDT, more significant effects on cell growth inhibition and more morphological changes were found, compared to each single-treatment group. It has been known that antitumor agents generate reactive oxygen and reactive nitrogen species to induce apoptosis and necrosis of the cell [17]–[19]. As one of the antitumor agents, the antitumor mechanism of arsenic compound to generate reactive oxygen and nitrogen species in the colorectal cancer cell line has also been reported [20]. The supply of sufficient oxygen and the generation of reactive oxygen species (ROS) are known to be significant factors determining the effect of photodynamic therapy [4], [21]. Therefore, induction of ROS mediated by antitumor agents might have contributed to enhancing the efficiency of PDT.
Next, we comparatively analyzed the change of intracellular signal pathway of each single-/co-treatment group, using the RT2ProfilerTM PCR array method, and those results were confirmed by the real-time PCR examination using 13 different primers. Using the real-time PCR method, we analyzed the change in expression levels of 84 genes involved in 15 signaling pathways. We found significant changes in the expression levels of genes known to be involved in cell survival pathway (e.g., Naip1, Tert, Aip1, etc.), p53 pathway (e.g., Bax, p21, Fas, Gadd45, IGFBP-3, Mdm-2, etc.), and NFAT (nuclear factor of activate T-cell) pathway (e.g., Ly-12, CD178, IL-2, etc.) involved in modulation of the immune response. Especially, expression levels of Naip1, which is known as the apoptosis inhibitory gene, and Tert, the telomerase reverse transcriptase, were decreased in every cell survival pathway, whereas the expression level of the apoptosis inducing gene Aip1 was enhanced. Moreover, the PCR array results showed decreased levels of Tert gene expression of 2.7, 17.1, and 34.6 fold in As4O6 alone, PDT alone, and co-treatment groups, respectively, each compared to the control group. Tert gene is known to be related with activation of telomerase, and in case of cervical cancer, the c-myc gene seems to induce telomerase activation by Tert expression [22]. Therefore, we suggest that down-regulation of the Tert gene induced by this co-treatment of As4O6 and PDT might have been involved in cell death. The inhibition of Tert gene was also confirmed by the real-time PCR examination performed after RT2 ProfilerTM PCR array analysis, and this reduction of Tert gene expression level was consistently shown in the co-treatment group, both in vitro and in vivo.
We also observed changes in the expression levels of p21, Fas, Gadd45, IGFBP-3, and Mdm-2 genes, known to be involved in the p53 pathway, and the co-treatment group showed more significant increase compared to each single-treatment group. However, any significant change of Bax gene expression (known to be activated by p53) was not observed. Moreover, from the absence of significant changes of p53 level, those changes of p21, Fas, Gadd45, IGFBP-3, and Mdm-2 gene expression levels seem to be mediated by p53-independent pathways.
In addition, considering the expression levels of genes like Ly-12, CD178, and IL-2, known to be involved in the NFAT pathway, Ly-12 expression increased, whereas CD178 and IL-2 expressions rather decreased in the co-treatment group, compared to each single-treatment group. As the intracellular pathway of NFAT has been known to be associated with increased immune responses, we expected that it might be up-regulated by PDT; however, the gene expression levels in this pathway, including IL-2, decreased after PDT treatment and further decline was found in the co-treatment group. However, when we tried to confirm the results of RT2ProfilerTM PCR array using the real-time PCR method, we found opposite results compared to those of RT2ProfilerTM PCR array, i.e. IL-2 expression decreased in the PDT alone treatment group and increased in the co-treatment group, both showing similar results under in vitro and in vivo conditions. It was reported that PDT might stimulate tumor growth-promoting immune signals and could be improved by controlling unwanted growth-stimulatory pathways [23], [24]. Therefore, our results for IL-2 seem to show that PDT decreased the expression of this gene, whereas it was increased by the arsenic compound treatment, indicating that the combination therapy combine two advantages by inducing immune mediators and antitumor effects. In addition, this concurrent treatment increased the in vitro and in vivo expression levels of the CD5 antigen gene and Ly-12 up to 23 and 26 fold, respectively. However, expression levels of Ly-12 or IL-2 did not show significant changes in the PDT alone treatment group; therefore, the increased gene expression seemed to be induced by the arsenic compound. So far, however, no other studies have reported the relationship between the combined PDT-As4O6 treatment and the NFAT pathway, and more intensive studies should be carried out.
Similarly, our TC-1 cell line-transplanted mouse model showed significantly enhanced antitumor effect in the PDT and As4O6 co-treatment group, compared to each single-treatment group. To identify the mechanism of this effect, we performed real-time PCR experiment using 10 different primers and finally observed significant changes in the expression levels of genes known to be involved in the cell survival pathway, p53 pathway, and NFAT pathway, similar with those obtained from in vitro experiments. Our western blot data demonstrates that co-treatment increased expression of p53, p21, Gadd45 at 7 day compared to single-agent treatment and control. Activation of p53 protein plays a crucial role in the control of tumor cell response to chemotherapeutic agents and DNA-damaging agents. p21 and GaDD45, downstream effectors of p53, were activated in combination treatment producing apoptosis in TC-1 tumor cells. These results suggest that apoptosis may be mediated through the p53 signaling pathway via up-regulation of proapoptotic proteins.
During PDT, intracellular depletion of oxygen and collapsed blood vessels might induce intracellular hypoxia and this could up-regulate the level of VEGF expression to catalyze angiogenesis [25]. Many studies on PDT have been trying to increase the efficiency of this method by supporting the generation of intracellular ROS, or by performing this method in combination with several inhibitors of angiogenesis, such as COX-2, MMP, or VEGF [9], [26]–[31]. Arsenic compounds are currently used as therapeutic agents for acute leukemia and various solid tumors, and they also have been reported to generate ROS and inhibit the VEGF expression [17], [19]. In this study, the vascular disruption effect of As4O6 was determined by Hoechst33342 perfusion and CD31 immunohistochemistry staining. Blood perfusion area decreased rapidly after As4O6 treatment. From the 3 h time point, the central part of tumors disappeared with respect to perfusion activity and, finally, almost no signal could be detected in the whole tumor area, even the outer edge. Interestingly, this reduction of the perfusion area was recovered from the 24 h time point, and the florescence appeared from the outer edge clearly at 48 and 72 h. Even though the blood perfusion appeared to be recovered from the outer edge 72 h after treatment, no signal was detected from inside of the tumors. These data showed that much more intensive immunoreactive microvessels were observed in tumor tissues from mice treated with PBS, but little CD31 staining was present in the central region of tumor tissues from mice treated with As4O6. The results echo those of studies involving As2O3 and Conbretastatin A-4 [32], [33]. As2O3 decreased blood perfusion relative to controls up to 6 h after As2O3 treatment with some recovery at 24 h [32], and also CA-4-P, 24 h after treatment on P22 tumors vascular functions displayed partial recover [33]. Therefore, in this study, we used one of the arsenic compounds, i.e. As4O6, in combination with the PDT method to investigate the increasing antitumor effect and also its intracellular signaling pathways. These data showed that the effect of the combinatory therapy on VEGF and associated pathways were strengthened with the assessments of tumor-associated vasculature in the animal model and of angiogenesis markers in the tumor lesions. We finally found that changes in the expression levels were important, particularly of genes known to be involved in the cell survival pathway, p53 pathway, and NFAT pathway involved in modulation of the immune response. This co-treatment of As4O6 and PDT induced significantly enhanced antitumor effects, both in vitro and in vivo, compared to those of each treatment alone. This seems to indicate that the presence of As4O6 might increase the antitumor effect of PDT by inducing changes in the expression of genes involved in cell survival, p53, and NFAT pathways. In the future, this co-treatment method of PDT and As4O6 may lead to improved results of antitumor therapy.
Materials and Methods
Ethics Statement
All procedure of animal research were provided in accordance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals and the Guidelines and Policies for Rodent experiment provided by the IACUC (Institutional Animal Care and Use Committee) in school of medicine, The Catholic University of Korea [permit no: CUMC-2008-0061-02].
Cell cultures
TC-1 cells prepared by transformation of C57BL/6 primary mouse lung cells with HPV16 E6/E7 oncogene and activated H-RAS as have been kindly provided from the cell line bank at Johns Hopkins University (a kind gift from Dr. Wu, Johns Hopkins University, MD, USA). The cells were routinely propagated in monolayer cultures in RPMI-1640 medium, supplemented with 5% heat-inactivated fetal bovine serum, 0.22% sodium bicarbonate, and penicillin/streptomycin. The cells were cultured in a 5% CO2 incubator at 37°C.
Photosensitizer and laser
The PDT was carried out using a diode laser generator apparatus (Won-PDT D662, Won Technology, Daejon, Korea) equipped with a halogen lamp, a band-pass filter and a fiber optics bundle. The wavelength was set at 662± 2 nm. Under PDT treatment, duration of the light irradiation was calculated taking into account the effective dose of light energy in J/cm2. Radachlorin (RADA-PHARMA Co, Ltd., Moscow, Russia) was used as a photosensitizer.
Arsenic compound
As4O6 was provided by Chonjisan Co. (Seoul, Korea). These chemicals were diluted in phosphate-buffered saline (PBS) and kept at 4°C.
MTT assay
To assess cell viability by PDT plus As4O6, TC-1 cells (3×103) were treated with 3 uM of As4O6 and/or 0.15 ug/ml, and 2.0 ug/ml of Radachlorin (RADA-Parma). After incubation for 12 hrs, the cells were washed with fresh medium and exposed to PDT (6.25 J/cm2). The cells were then further incubated at 37°C for 1, 2, 3, or 4 days, in a humidified incubator. After incubation with PDT plus As4O6 co-treatment, 100 ul of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (2 mg/ml) was added to each well and cultured for 4 h. One hundred microliters of dimethyl sulfoxide (DMSO) was added to each well for 10 min, and the absorbance was measured with an automated spectrophotometric microtiter plate reader (SpectraMax 340; Molecular Devices, Sunnyvale, CA), using a 570-nm filter. The morphological changes of the cells were determined by optical microscopy.
Evaluation of in vitro combination effects by the Chou-Talalay method
The combined effects of Radachlorin/PDT and As4O6 on cell survival were analyzed using Chou-Talalay method, which applies the median-effect equation of Chou and the CI equation of Chou and Talalay [34]. TC-1 cells were plated in 96-well microplates as above were exposed in triplicate to each agent or both in combination using the constant ratio combination design for 96 hours, followed by the MTT assay for cell viability determination. Calculated CIs were used to ascertain the presence of strong synergism (CI<0.3), moderate synergism (0.3< CI<0.9), additive effect (CI = 1), antagonism (CI>1) and strong antagonism (CI>3.3) between Radachlorin/PDT and As4O6.
FACS analysis
TC-1 cells were washed twice with PBS and then resuspended in 1X binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Then, 1×105 cells per tube were added with 5 μl of Annexin V-FITC and 10 μl of propidium iodide (BD, San Jose, CA), followed by incubation at 22°C for 15 min. Each tube was added with 100 μ of 1X binding buffer and then the cells were analyzed by a flow cytometer (BD). The samples were read using flow cytometer (BD). Cell debris and fixation artifacts were gated out using the CellQuest program.
Real-time PCR microarray analysis with TC-1 cell
TC-1 cells were treated with 3 uM of As4O6 and/or 0.15 ug/ml of Radachlorin (RADA-Parma). After incubation for 12 hrs, the cells were washed with fresh medium and exposed to light. The following four groups were used in this study: control, 3 uM of As4O6, 0.15 ug/ml of Radachlorin/irradiation, and 3 uM of As4O6 plus 0.15 ug/ml of Radachlorin/irradiation. For all groups, cells were harvested 24 h after irradiation. Total RNA was isolated from cells according to the manufacturer's recommendations using TRIzol and the Absolutely RNA kit from Stratagene. RNA (2 μg) was reverse transcribed in a total volume of 20 μl, using 200 U of Superscript II (Invitrogen) reverse transcriptase, 100 pmol oligo-dT, 0.5 mM dNTP, and 40 U RNasin (Promega). The resultant cDNA was diluted 1∶100 with nuclease-free water. Five microliters of diluted cDNA was used in subsequent PCR reactions. Samples for each group were analyzed according to the manufacturer's recommendations, using the “Mouse Signal Transduction PathwayFinder (89 genes including 5 housekeeping genes)” array in conjunction with the RT2 Profiler PCR Array System from SuperArray Bioscience (Frederick, MD). The results are presented as transcript levels relative to the levels in untreated control cells with average mRNA levels of five internal control genes, including beta-actin, used as the normalization control. To verify changes in gene expression, real-time PCR was carried out on 12 selected genes. All primers were designed based on nucleotide sequences retrieved from Genbank using the Primer Express software (PE Applied Biosystems) (Supplementary Table S1).
Analyses
Genes that showed differences in their expression levels were, of at least 2.0 fold, selected for the different analyses (hierarchical cluster analysis, functional cluster analysis, biological pathway analysis). Hierarchical clustering (GENE CLUSTER v3.0) and display programs (TREE VIEW) were also used for analysis (http://rana.stanford.edu/software). We performed unsupervised hierarchical clustering based on the most variably expressed genes using the Euclidean distance as the similarity metric and the average linkage method as the between-cluster distance metric. A t-test was also performed to find genes that have changed between PDT alone and As4O6 alone, and PDT plus As4O6 treatment. Supervised clustering of experimental samples was performed by reducing the number of genes by statistical analysis. To classify the gene expression profiles, functional analyses and KEGG (Kyoto Encyclopedia for Genes and Genomes) pathway analyses (http://www.genome.jp/kegg/pathway.html) were carried out as previously described [35], [36]. To perform a KEGG analysis, differentially expressed genes of each treatment group were used for the calculation of their attribution to pre-defined KEGG signaling pathways and analyzed by pair-wise comparisons. The different number of genes were seen in a given pathway. The Ingenuity Pathway Analysis software (IPA, Ingenuity Systems, Mountain View, CA) was utilized to identify networks of interacting genes and other functional groups. Semantically consistent pathway relationships were modeled based on a continual, formal extraction from the public domain literature (www.ingenuity.com/products/pathways_knowledge.html).
Evaluation of in vivo antitumor effect
TC-1 cells (2×105) were injected subcutaneously into the right flank of C57BL/6 mice. Tumor-bearing mice were injected ip with 7.5 mg/kg of As4O6 and/or intravenously injected with Radachlorin (10 mg/kg). Three hours after injection, the animals were anaesthetized and the tumors were irradiated with 300 J/cm2 via a light fiber inserted into the tumor mass. Each group included 7 animals. Tumor volume was determined in vivo by external caliper using the pi-based ellipsoid volume formula (length x depth x width x 0.5233) [37], [38]. The average value and standard deviation are based on calculated tumor volumes from the eight mice in each group. To assess tumor response, tumor growth was recorded every 2–3 days for 25 days. Tumor growth was measured 2–3 times a week using calipers.
Western blot analysis
TC-1 cells were treated with 3 uM of As4O6 and/or 0.15 ug/ml of Radachlorin. The cell lysates (approximately 30 μg of protein) were separated in 12% polyacrylamide SDS-gels and transferred to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). This was then immersed in blocking buffer (5% skim milk and 0.1% Tween 20 in PBS, pH 7.4) for 1 h at room temperature and incubated with primary antibodies: (SantaCruz Biotechnology, Inc., California, USA), p53 (1∶200), GaDD45 (1∶200), p21 (1∶200), and actin (1∶5000) in blocking buffer overnight at 4°C. After incubation, the membrane was probed with horseradish peroxidase-labeled anti-mouse IgG antibody (1∶5000) in PBS (containing of 0.05% Tween 20 and 5% skim milk powder) for 30 min at room temperature. The proteins in the membrane were detected by an enhanced chemiluminescence detection system (Amersham, Buckinghamshire, UK) and the bands were visualized by autoradiography using X-ray film (Amersham).
Blood perfusion analysis after As4O6 treatment
Hoechst 33342 (Sigma-Aldrich) was dissolved in a sterile phosphate-buffered saline (PBS) at a concentration of 3 mg/ml and injected intravenously (i.v.) 0.10 ml/20 g mouse. TC-1 tumor-bearing mice (n = 3) were divided into 1, 3, 6, 12, 24, 48, 72, 168 h post-As4O6 treatment groups. As the control, three mice (i.p. injected PBS) used in each time point. One minute before killing, mice were perfused with 15 mg/kg Hoechst 33342. Tumors were excised and immediately frozen in liquid nitrogen. Frozen tissues were embedded into OCT cryofixative (Sakura Finetek, Janpan) and tumor cryo-sections were cut using a CM1850UV cryostat microtome (Leica, Germany) and air-dried. All the processes were performed quickly and samples were protected from direct expose to light. Hoechst 33342 distribution was assessed using a AX70, TR-62A02 fluorescent microscope (Olympus, Tokyo, Japan). A scan consisted of a field-by field movement of the scanning stage based on a selectable meander pattern, depending on the tumor size. In each field, the microscopic image was recorded and processed; during the image processing using the Image Pro Plus digital image analysis system (Media Cybernetics, USA). Each picture was converted into a gray scale and the positive range value was fixed, and the percentage of the positive area to the whole tumor area was determined.
Immunohistochemistry
Immunohistochemistry was used to analyze the expression of CD31. Tumor tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 4 μm thickness. Tumor sections were deparaffinized, rehydrated, and quenched with 3% hydrogen peroxide for 10 min at room temperature. The sections were incubated in protein blocking solution for 20 min before the addition of the primary antibody. The sections were incubated for 2 h at 37°C with a 1∶50 dilution of rabbit anti-mouse CD31 (Abcam, UK). After incubation, the sections were washed in PBS for 5 min and anti-rabbit secondary biotinylated antibody was applied. After washings, the avidin-biotin complex was then applied to the sections, followed by extensive washing steps. A diaminobenzidine chromogen kit (Abcam) was used to develop sections.
Histopathology
Animals were treated with 10 mg/kg of As4O6. At selected time points, tumors were excised and fixed in 4% neutral formalin and embedded in paraffin. Paraffin-embedded tissues were sectioned for routine staining with hematoxylin and eosin.
Real-time PCR microarray analysis with TC-1 tumor tissue
TC-1 cells (2×105) were injected subcutaneously into the right flank of C57BL/6 mice. Tumor-bearing mice were injected ip with 7.5 mg/kg As4O6 and/or intravenously injected with Radachlorin (10 mg/kg). Three hours after injection, the animals were anaesthetized and the tumors were irradiated with 300 J/cm2 via a light fiber inserted into the tumor mass. One day after treatment, animals were sacrificed and tumor tissue was harvested. Total RNA was isolated from tumor tissue according to the manufacturer's recommendations, using TRIzol and the Absolutely RNA kit from Stratagene. Real-time PCR was carried out on 13 selected genes, as described previously.
Treatments interaction analysis
To determine the nature of the interaction between Radachlorin/PDT and As4O6 treatment, the data from the MTT assay were analyzed as reported previously [34] using CalcuSyn V2.0 software, (Biosoft, Cambridge, UK) [39]. The interaction of treatments was quantified determining a combination index (CI). CI < or >1 indicated synergy or antagonism respectively, whereas a CI value of 1 indicates additivity [40].
Statistical analysis
Statistical analysis included ANOVA and the Student's t-test. The values for the different groups were compared. P values of less than 0.05 were considered statistically significant.
Supporting Information
In vitro cell growth inhibitory effects of As4O6 and/or Radachlorin/PDT on HaCaT, HeLa, and SiHa cells. Each cell was cultured with 3 uM of As4O6 and/or 0.15 ug/ml of Radachlorin/PDT, respectively. Cell viability was determined based on the MTT assay. Each bar represents a mean [± SD (vertical line)] of three replicates (n = 3).
(TIF)
Cell apoptosis analysis using Annexin V/PI staining. TC-1 cells were cultured with 3 uM of As4O6 and/or 0.15 ug/ml of Radachlorin/PDT for 1 day. Cell pellet was resuspended in 500 μl annexin V HEPES solution (10 mM HEPES-NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) and incubated on ice for 30 min in the dark. Cells were then washed once in ice-cold HEPES buffer and PI was added just before FACS analysis. The results were analyzed with a FACS.
(TIF)
Primer sequences used for PCR assays.
(DOC)
Acknowledgments
We thank Ph.D student Pankaj Kumar Chaturvedi for analysis and technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was supported by The Catholic Harvard Wellman Photomedicine Core Technique Development Center, Seoul, Republic of Korea (Grant #5-2012-A0154-00001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Al-Waili NS, Butler GJ, Beale J, Hamilton RW, Lee BY, et al. Hyperbaric oxygen and malignancies: a potential role in radiotherapy, chemotherapy, tumor surgery and phototherapy. Med Sci Monit. 2005;11:RA279–289. [PubMed] [Google Scholar]
- 2.Moserova I, Kralova J. Role of ER Stress Response in Photodynamic Therapy: ROS Generated in Different Subcellular Compartments Trigger Diverse Cell Death Pathways. PLoS One. 2012;7:e32972. doi: 10.1371/journal.pone.0032972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corti L, Mazzarotto R, Belfontali S, De Luca C, Baiocchi C, et al. Photodynamic therapy in gynaecological neoplastic diseases. J Photochem Photobiol B. 1996;36:193–197. doi: 10.1016/s1011-1344(96)07371-x. [DOI] [PubMed] [Google Scholar]
- 4.Gomer CJ, Rucker N, Ferrario A, Wong S. Properties and applications of photodynamic therapy. Radiat Res. 1989;120:1–18. [PubMed] [Google Scholar]
- 5.Wei Y, Kong B, Song K, Qu X, Jin Q, et al. Involvement of mitochondria-caspase pathway in Hemoporfin-mediated cell death. Photochem Photobiol. 2007;83:1319–1324. doi: 10.1111/j.1751-1097.2007.00160.x. [DOI] [PubMed] [Google Scholar]
- 6.Separovic D, Bielawski J, Pierce JS, Merchant S, Tarca AL, et al. Increased tumour dihydroceramide production after Photofrin-PDT alone and improved tumour response after the combination with the ceramide analogue LCL29. Evidence from mouse squamous cell carcinomas. Br J Cancer. 2009;100:626–632. doi: 10.1038/sj.bjc.6604896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung WM, Chu PW, Kwong YL. Effects of arsenic trioxide on the cellular proliferation, apoptosis and differentiation of human neuroblastoma cells. Cancer Lett. 2007;246:122–128. doi: 10.1016/j.canlet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Woo SH, Park MJ, An S, Lee HC, Jin HO, et al. Diarsenic and tetraarsenic oxide inhibit cell cycle progression and bFGF- and VEGF-induced proliferation of human endothelial cells. J Cell Biochem. 2005;95:120–130. doi: 10.1002/jcb.20329. [DOI] [PubMed] [Google Scholar]
- 9.Yoo MH, Kim JT, Rhee CH, Park MJ, Bae IJ, et al. Reverse effects of tetraarsenic oxide on the angiogenesis induced by nerve growth factor in the rat cornea. J Vet Med Sci. 2004;66:1091–1095. doi: 10.1292/jvms.66.1091. [DOI] [PubMed] [Google Scholar]
- 10.Ahn WS, Bae SM, Lee KH, Kim YW, Lee JM, et al. Comparison of effects of As2O3 and As4O6 on cell growth inhibition and gene expression profiles by cDNA microarray analysis in SiHa cells. Oncol Rep. 2004;12:573–580. [PubMed] [Google Scholar]
- 11.Liu LZ, Jiang Y, Carpenter RL, Jing Y, Peiper SC, et al. Role and mechanism of arsenic in regulating angiogenesis. PLoS One. 2011;6:e20858. doi: 10.1371/journal.pone.0020858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Germolec DR, Yoshida T, Gaido K, Wilmer JL, Simeonova PP, et al. Arsenic induces overexpression of growth factors in human keratinocytes. Toxicol Appl Pharmacol. 1996;141:308–318. doi: 10.1006/taap.1996.0288. [DOI] [PubMed] [Google Scholar]
- 13.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 14.Shao W, Fanelli M, Ferrara FF, Riccioni R, Rosenauer A, et al. Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR alpha protein in acute promyelocytic leukemia cells. J Natl Cancer Inst. 1998;90:124–133. doi: 10.1093/jnci/90.2.124. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee N, Banerjee M, Ganguly S, Bandyopadhyay S, Das JK, et al. Arsenic-induced mitochondrial instability leading to programmed cell death in the exposed individuals. Toxicology. 2008;246:101–111. doi: 10.1016/j.tox.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Laparra JM, Velez D, Barbera R, Farre R, Montoro R. As2O3-induced oxidative stress and cycle progression in a human intestinal epithelial cell line (Caco-2). Toxicol In Vitro. 2008;22:444–449. doi: 10.1016/j.tiv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Rigas B, Sun Y. Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. Br J Cancer. 2008;98:1157–1160. doi: 10.1038/sj.bjc.6604225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rigas B. The use of nitric oxide-donating nonsteroidal anti-inflammatory drugs in the chemoprevention of colorectal neoplasia. Curr Opin Gastroenterol. 2007;23:55–59. doi: 10.1097/MOG.0b013e32801145b0. [DOI] [PubMed] [Google Scholar]
- 19.Gao J, Liu X, Rigas B. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc Natl Acad Sci U S A. 2005;102:17207–17212. doi: 10.1073/pnas.0506893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Rigas B. The thioredoxin system mediates redox-induced cell death in human colon cancer cells: implications for the mechanism of action of anticancer agents. Cancer Res. 2008;68:8269–8277. doi: 10.1158/0008-5472.CAN-08-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta. 2007;1776:86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Sagawa Y, Nishi H, Isaka K, Fujito A, Takayama M. The correlation of TERT expression with c-myc expression in cervical cancer. Cancer Lett. 2001;168:45–50. doi: 10.1016/s0304-3835(01)00501-8. [DOI] [PubMed] [Google Scholar]
- 23.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kammerer R, Buchner A, Palluch P, Pongratz T, Oboukhovskij K, et al. Induction of immune mediators in glioma and prostate cancer cells by non-lethal photodynamic therapy. PLoS One. 2011;6:e21834. doi: 10.1371/journal.pone.0021834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solban N, Selbo PK, Sinha AK, Chang SK, Hasan T. Mechanistic investigation and implications of photodynamic therapy induction of vascular endothelial growth factor in prostate cancer. Cancer Res. 2006;66:5633–5640. doi: 10.1158/0008-5472.CAN-06-0604. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa H, Matsumiya T, Sakaki H, Imaizumi T, Kubota K, et al. Expression of vascular endothelial growth factor by photodynamic therapy with mono-L-aspartyl chlorin e6 (NPe6) in oral squamous cell carcinoma. Oral Oncol. 2007;43:544–550. doi: 10.1016/j.oraloncology.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Ferrario A, von Tiehl KF, Rucker N, Schwarz MA, Gill PS, et al. Antiangiogenic treatment enhances photodynamic therapy responsiveness in a mouse mammary carcinoma. Cancer Res. 2000;60:4066–4069. [PubMed] [Google Scholar]
- 28.Ferrario A, Von Tiehl K, Wong S, Luna M, Gomer CJ. Cyclooxygenase-2 inhibitor treatment enhances photodynamic therapy-mediated tumor response. Cancer Res. 2002;62:3956–3961. [PubMed] [Google Scholar]
- 29.Ferrario A, Fisher AM, Rucker N, Gomer CJ. Celecoxib and NS-398 enhance photodynamic therapy by increasing in vitro apoptosis and decreasing in vivo inflammatory and angiogenic factors. Cancer Res. 2005;65:9473–9478. doi: 10.1158/0008-5472.CAN-05-1659. [DOI] [PubMed] [Google Scholar]
- 30.Ferrario A, Chantrain CF, von Tiehl K, Buckley S, Rucker N, et al. The matrix metalloproteinase inhibitor prinomastat enhances photodynamic therapy responsiveness in a mouse tumor model. Cancer Res. 2004;64:2328–2332. doi: 10.1158/0008-5472.can-04-0071. [DOI] [PubMed] [Google Scholar]
- 31.Gomer CJ, Ferrario A, Luna M, Rucker N, Wong S. Photodynamic therapy: combined modality approaches targeting the tumor microenvironment. Lasers Surg Med. 2006;38:516–521. doi: 10.1002/lsm.20339. [DOI] [PubMed] [Google Scholar]
- 32.Lew YS, Brown SL, Griffin RJ, Song CW, Kim JH. Arsenic trioxide causes selective necrosis in solid murine tumors by vascular shutdown. Cancer Res. 1999;59:6033–6037. [PubMed] [Google Scholar]
- 33.Tozer GM, Prise VE, Wilson J, Locke RJ, Vojnovic B, et al. Combretastatin A-4 phosphate as a tumor vascular-targeting agent: early effects in tumors and normal tissues. Cancer Res. 1999;59:1626–1634. [PubMed] [Google Scholar]
- 34.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 35.Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, et al. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 2002;12:712–723. doi: 10.1016/s0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- 36.Wang JL, Lin YW, Chen HM, Kong X, Xiong H, et al. Calcium prevents tumorigenesis in a mouse model of colorectal cancer. PLoS One. 2011;6:e22566. doi: 10.1371/journal.pone.0022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodwin SC, Bonilla SC, Sacks D, Reed RA, Spies JB, et al. Reporting standards for uterine artery embolization for the treatment of uterine leiomyomata. J Vasc Interv Radiol. 2003;14:S467–476. doi: 10.1097/01.rvi.0000094620.61428.9c. [DOI] [PubMed] [Google Scholar]
- 38.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 39.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds CP, Maurer BJ. Evaluating response to antineoplastic drug combinations in tissue culture models. Methods Mol Med. 2005;110:173–183. doi: 10.1385/1-59259-869-2:173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vitro cell growth inhibitory effects of As4O6 and/or Radachlorin/PDT on HaCaT, HeLa, and SiHa cells. Each cell was cultured with 3 uM of As4O6 and/or 0.15 ug/ml of Radachlorin/PDT, respectively. Cell viability was determined based on the MTT assay. Each bar represents a mean [± SD (vertical line)] of three replicates (n = 3).
(TIF)
Cell apoptosis analysis using Annexin V/PI staining. TC-1 cells were cultured with 3 uM of As4O6 and/or 0.15 ug/ml of Radachlorin/PDT for 1 day. Cell pellet was resuspended in 500 μl annexin V HEPES solution (10 mM HEPES-NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) and incubated on ice for 30 min in the dark. Cells were then washed once in ice-cold HEPES buffer and PI was added just before FACS analysis. The results were analyzed with a FACS.
(TIF)
Primer sequences used for PCR assays.
(DOC)