Abstract
A novel ‘white’ laccase was purified from the deuteromycete fungus, Myrothecium verrucaria NF-05, which was a high laccase-producing strain (40.2 U·ml−1 on the thirteenth day during fermentation). SDS-PAGE and native-PAGE revealed a single band with laccase activity corresponding to a molecular weight of approximately 66 kDa. The enzyme had three copper and one iron atoms per protein molecule determined by ICP-AES. Furthermore, both UV/visible and EPR spectroscopy remained silence, indicating the enzyme a novel laccase with new metal compositions of active centre and spectral properties. The N-terminal amino acid sequence of the purified protein was APQISPQYPM. Together with MALDI-TOF analysis, the protein revealed a high homology of the protein with that from reported M. verrucaria. The highest activity was detected at pH 4.0 and at 30°C. The enzyme activity was significantly enhanced by Na+, Mn2+, Cu2+ and Zn2+ while inhibited by DTT, NaN3 and halogen anions. The kinetic constant (Km) showed the enzyme was more affinitive to ABTS than other tested aromatic substrates. Twelve structurally different dyes could be effectively decolourised by the laccase within 10 min. The high production of the strain and novel properties of the laccase suggested its potential for biotechnological applications.
Introduction
Laccase (EC 1.10.3.2, benzenediol: oxygen oxidoreductase) is a multicopper oxidase that has become an important industrially relevant enzymes that is used in paper-pulp bleaching [1], synthetic dyes decolourisation [2], bioremediation [3], chemical analysis and bioelectronics [4]. Laccases are widely distributed among in plants [5], bacteria [6] and especially fungi [7]. In fungi, laccases are produced by many ascomycetes [2], [8], basidiomycetes [9] and some deuteromycetes [10], [11], [12] which are involved in plant pathogenesis, pigmentation, detoxification and lignin degradation [7]. The deuteromycete fungus Myrothecium verrucaria is widely industrially used to produce bilirubin oxidase. The only reported laccase from this species was defined as an alkaliphilic laccase without a detailed purification and characterization [13].
Normal copper-containing laccases contain three types of copper that can be distinguished using UV/visible and EPR spectra. T1 copper gives a blue color to the protein from an absorbance at about 600 nm and is EPR detectable. T2 copper confers no color, but is EPR detectable. T3 copper is a pair of copper atoms that give a weak absorbance in the near UV and have no EPR signal [14]. However, laccases with a differently structured active site are also described in literatures [15], [16]. Enzymes lacking the maximum around 600 nm in the absorption spectrum are usually classified as ‘yellow’ or ‘white’ laccases because they have the catalytic activity inherent in typical ‘blue’ laccases [17]. Different active center might confer these laccases different properties of interest.
In the previous work, we isolated the deuteromycete, M.verrucaria NF-05, from the soil of a pine forest in the Liangshui Nature Reserve (47°10′N, 128°53′E) in China in November 2009 [18]. The present study described the purification and characterization of a novel ‘white’ laccase from the strain. The metal content, UV/visible and EPR spectra characteristics, N-terminal sequence and MALDI-TOF analysis were elaborated. The effects of pH, temperature, metal ions, putative inhibitors, organic solvents and reaction with different aromatic compounds on the purified laccase were investigated. In addition, the applications of the purified laccase in the decoloursation of various dyes were discussed.
Results and Discussion
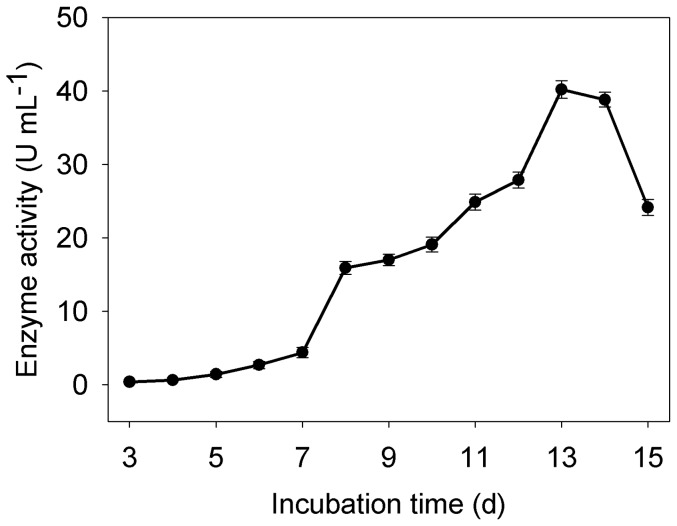
Production and purification of extracellular laccase
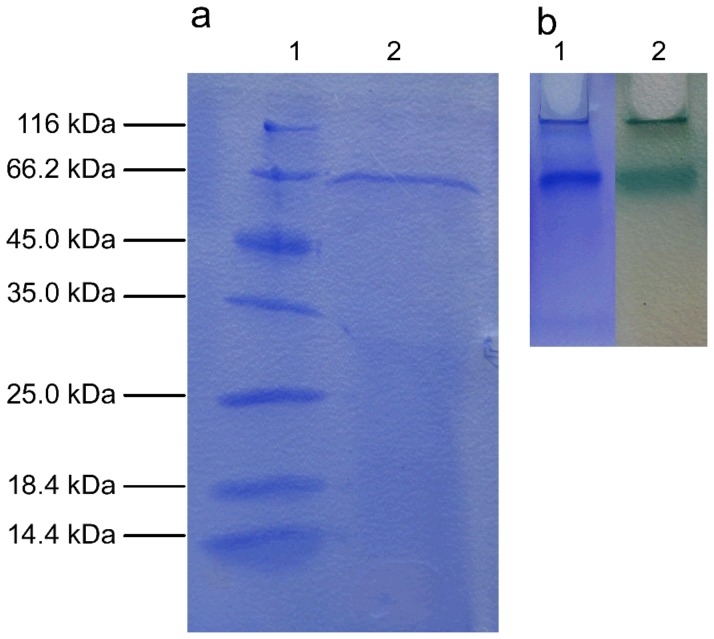
The production of laccase by M.verrucaria NF-05 was performed in shaking flask cultures at 140 rpm, 30°C that were induced with 1 mM copper for 15 days. The amount of laccase production increased rapidly after 7 days and the maximum activity was recorded on day 13 (40.2 U·ml−1) (Fig. 1). The laccase activity dropped sharply at day 15. No activity of bilirubin oxidase was detected in the fermentation liquid. The property of laccase to maintain a high production over a short time is interesting from the industrial point of view. The purification achieved a 34.7-fold increase in the activity with a yield of 15.7% (Table 1). The SDS-PAGE revealed the purity of the sample and a molecular weight of 66 kDa (Fig. 2a). The green band on native-PAGE was oxidized ABTS which indicated the laccase activity (Fig. 2b). Both SDS-PAGE and native PAGE suggested that this enzyme is a monomeric protein (Fig. 2). The only reported purified laccase from M. verrucaria 24G-4 is 62 kDa [13].
Figure 1. Production of extracellular laccase by M.verrucaria NF-05.
Results represents means of three experiments, and error bars indicates ± standard error.
Table 1. Steps in protein purifying to homogeneity of M.verrucaria NF-05 cultures.
| Purification step | Volume (ml) | Activity (U ml−1) | Total activity (U) | Total protein (mg) | Specific activity (U mg−1) | Yield (%) | Fold purification |
| Crude culture | 200.0 | 38.6 | 7720.0 | 170.8 | 45.2 | 100.0 | 1.0 |
| (NH4)2SO4 precipitation | 20.0 | 232.6 | 4651.3 | 34.3 | 135.6 | 60.3 | 3.0 |
| DEAE-Cellulose column | 10.0 | 320.9 | 3208.6 | 3.9 | 821.0 | 41.6 | 18.2 |
| Sephadex G-75 column | 10.0 | 121.1 | 1211.1 | 0.8 | 1568.8 | 15.7 | 34.7 |
Figure 2. SDS–PAGE (a) and native PAGE (b) of purified laccase from M.verrucaria NF-05.
(a) Lane 1: denatured protein marker, Lane 2: purified laccase; (b) Lane 1: purified laccase with Coomassie Brilliant Blue R-250staining, Lane 2: purified laccase with ABTS staining.
Spectral properties of the purified protein
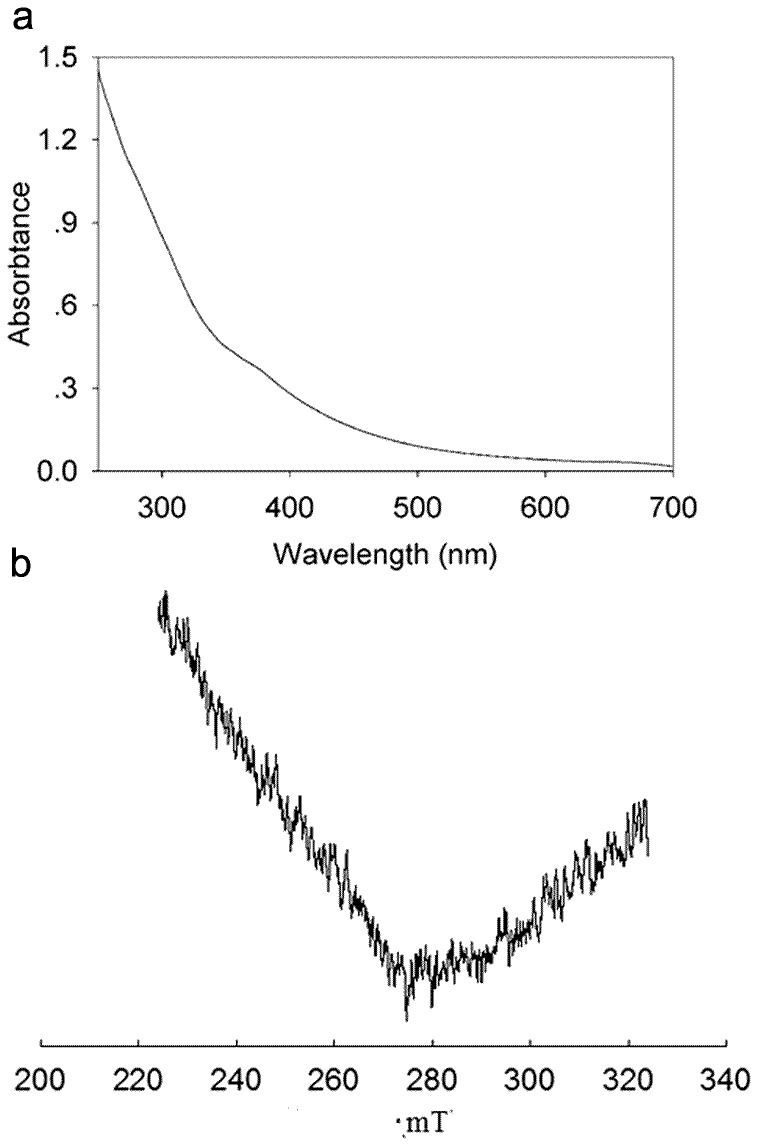
The inductively coupled plasma atom emission spectrometry (ICP-AES) showed that the enzyme contained copper and irons ions with a ratio of 3∶1. The quantitative analysis resulted in 3.08±0.3 copper atoms and 0.95±0.2 iron atoms per protein molecule. The UV-visible spectrum of purified enzyme gave an atypical spectrum (Fig. 3a). There is no peak around 600 nm corresponding to a T1 blue copper which was consonant with the colorless solution of the purified enzyme. Simultaneously, there is no shoulder at 330 nm corresponding to a T3 binuclear copper. The EPR spectra also remained silence thus no T1 and T2 Cu signal were detectable (Fig. 3b). As a rule, the Cu2+ renders the blue color of a liquid. The Cu2+ whose electron configuration is d9 engenders a d-d transition and absorbs visible light under the act of ligands so as to appear color. Excluding the contamination during purification, the colorlessness of the protein in this research could result from the change of valence state of Cu2+. In addition, no EPR signal was detected owing to Fe2+ whose electron configuration presented a low-spin state.
Figure 3. UV–visible absorption (a) and electron paramagnetic resonance spectra (b) of laccase from M.verrucaria NF-05 in 10 mM citric acid buffer (pH 4.0).
Though it was not clear the arrangement of these metal atoms and which copper was replaced by iron, it could be deduced that the lack of absorption in visible light range probably resulted from the existence of incomplete oxidation state of copper (Cu+), which had a fully occupied electron configuration of d10 and no d-d transition could take place. The easiness of electron transfer in active center of the laccase which was conferred by the incomplete oxidation of metal ions might render the protein extra high activity. The partial silence of spectrum and EPR detection have been found in the ‘white’ laccase POXA1 from Pleurotus ostreatus which contained only one copper atom, together with two zinc and one iron atoms per molecule [15], ‘white’ laccase from Phellinus ribis which contained one copper, one manganese and two zinc atoms [19]. To sum up, the metal content in active center and total silence on UV/visible and EPR spectra indicated the purified ‘white’ laccase was different from all reported laccases.
The N-terminal amino acid sequence of the purified protein was determined up to 10 amino acids as APQISPQYPM, exhibited high homology with that of alkaliphilic laccase from M.verrucaria 24G-4, APQISPQYPM [19] and bilirubin oxidase from M. verrucaria MT-1, VAQISPQYPM [20]. Peptides identified by MALDI-TOF of the protein revealed only 49% of homology with bilirubin oxidase from M. verrucaria (gi 2833236). These results showed the homospecificity of the protein with those from M. verrucaria, but not common laccases.
Effects of temperature and pH value
The purified laccase was more active in the temperature range of 20–60°C (Fig. 4a), which corresponded to the optimal temperature range for fungal laccase activity (30–60°C) indicated in the literature. The enzyme retained approximately 60% activity after incubation at 20–30°C for 1 h. However, the enzyme activity sharply decreased when the temperature was increased to 40°C and almost no activity was detected at 80°C. The laccase could still oxidise ABTS when incubated at 90°C for 3 min. However, the incubation of the enzyme without the substrate under the same condition demonstrated that the enzyme did not display any activity. The results revealed that the presence of the substrate protects the enzyme from being inactivated at high temperatures. Similar observations have been obtained in laccase from Fusarium solani [21], in cellulose [22] and in xylanase [23]. The pH profile for the laccase activity with the substrate ABTS showed a peak of maximum activity at pH 4.0 (Fig. 4b). The enzyme remained active at pH values from 2.0 to 7.0, and its activity at 2.0 and 7.0 were 57.0% and 42.8% of that at pH 4.0, respectively. The enzyme was stable over a pH range from 3.0 to 7.0 for 1 h and maintained for more than 40% activity. The preference for the acidic to neutral pH range is similar to most fungal laccases [2], [21] but is different form the alkaliphilic laccase that is produced by M.verrucaria 24G-4 [13].
Figure 4. Optima and stability of (a) temperature and (b) pH value for purified laccase from M.verrucaria NF-05 reacting with ABTS.
Optima curve (•); stability curve (○). The highest value of activity for each analytical curve was considered as 100%, and error bars shown are standard deviations of triplicate samples.
Effects of different organic solvent
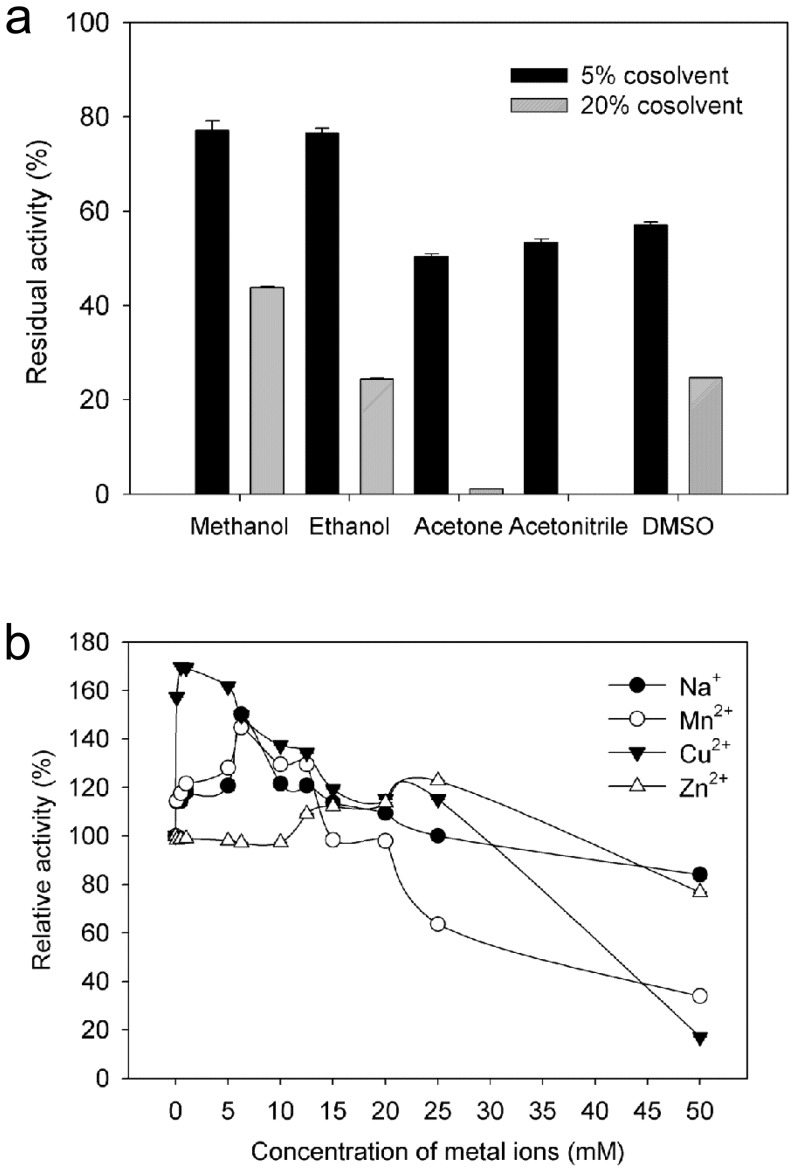
The addition of water miscible organic solvents caused a decrease in the enzymatic activity by altering the pH of the aqueous solution. The purified laccase retained approximately 80% of its initial activity in the presence of 5% methanol and ethanol (Fig. 5a). These results indicated that the enzyme might be suitable for use in reactions that require a similar concentration of these solvents. However, the inhibitory effect increased with the increasing concentration of solvents. The activity was almost completely inhibited in the presence of 20% acetone and acetonitrile. The activity was completely inhibited when the concentration of all of the tested solvents increased to 50% (data not shown).
Figure 5. Effect of different organic solvents (a)and enhancement effects of metal ions (b) on the activity of purified laccase from M.verrucaria NF-05.
All the experiments are performed with the same purified laccase, and activity without the addition of organic solvents was considered as 100%. Error bars shown are standard errors of triplicate samples.
Effects of metal ions and enzyme inhibitors
Metal ions especially heavy metal ions are common environmental pollutants and can affect the production and stability of the extracellular enzymes 1. Table 2 showed that the laccase activity was reduced or completely inhibited by Li+, K+, Ag+, Hg+, Mg2+, Ca2+, Cd2+, Fe2+, Co2+, Ba2+, Al3+, and Fe3+ at the tested concentrations of metal ions. The laccase activity was significantly increased by the presence of Na+ (6.25 mM, 150.2%), Zn2+, (25 mM, 122.75%) and Mn2+ (6.25 mM, 144.7%) (Fig. 5b). The laccase activity (0.5 mM, 169.7%) was enhanced by Cu2+ at a concentration of 0.1–25 mM. However, the laccase activity (17.2%) decreased when the Cu2+ concentration was raised to 50 mM (Fig. 5b). The large extent activation of activity by Cu2+ might be caused by the filling of type-2 copper binding sites with Cu2+ [13]. Many metal ions at specific concentrations have been reported to be activators for laccases such as Na+, Fe2+, Mn2+, Ba2+ [2], Mg2+, Zn2+, Fe3+ [24], Cu2+ [24], [25], Hg2+ [21] and Mo6+ [26].
Table 2. Effects of metal ions on laccase activity.
| Metal ions | Residual activity (%) | |||
| 6.25 mM | 12.5 mM | 25 mM | 50 mM | |
| Li+ | 41.9±0.2 | 19.8±0.2 | 17.8±0.2 | 6.4±0.1 |
| K+ | 52.2±0.5 | 47.0±0.3 | 33.6±0.2 | 17.3±0.1 |
| Ag+ | 8.3±0.6 | 8.1±0.1 | 8.6±0.1 | 6.9±0.1 |
| Hg+ | 13.2±0.3 | 5.2±0.1 | 4.4±0.1 | 3.4±0.1 |
| Mg2+ | 57.4±0.4 | 55.5±0.3 | 29.3±0.3 | 1.4±0.1 |
| Ca2+ | 37.1±0.4 | 36.1±0.2 | 9.9±0.2 | 0 |
| Cd2+ | 53.8±0.6 | 34.2±0.2 | 28.8±0.3 | 27.6±0.1 |
| Fe2+ | 4.8±0.1 | 4.1±0.1 | 6.7±0.1 | 4.4±0.1 |
| Co2+ | 49.1±0.2 | 28.3±0.2 | 4.4±0.1 | 0.4±0.1 |
| Ba2+ | 13.5±0.3 | 14.2±0.2 | 9.5±0.1 | 0 |
| Al3+ | 91.7±1.1 | 19.3±0.3 | 12.3±0.2 | 6.5±0.1 |
| Fe3+ | 42.6±0.3 | 7.8±0.1 | 2.0±0.1 | 0 |
The enzymatic activity was completely inhibited by sodium azide (an inhibitor of oxidase), which suggested the function of laccase as an oxidase. DTT, which is a strong reducing agent on disulphide bonds, strongly inhibited the enzyme. These results indicate the existence of a disulphide structure in the active domain. L-cysteine and SDS caused complete inactivation of the enzyme. In addition, EDTA partially inhibited the laccase, which suggested that existence of a metal-binding domain in the protein. The enzyme revealed sensitivity to halogen anions, which are typical inhibitors of laccases (Table 3).
Table 3. Effect of inhibitors on laccase activity.
| Inhibitor (mM) | Inhibition (%) | Inhibitor (mM) | Inhibition (%) |
| EDTA (0.1) | 17.2±0.3 | Cl−1 (20) | 80.1±1.1 |
| EDTA (1) | 19.0±0.5 | Cl−1 (200) | 96.7±1.2 |
| EDTA (5) | 19.9±0.2 | Cl−1 (800) | 98.5±0.5 |
| L-Cysteine (0.1) | 83.1±0.7 | Br−1 (20) | 82.3±1.2 |
| L-Cysteine (1) | 100 | Br−1 (200) | 91.2±0.9 |
| SDS (0.1) | 56.1±0.3 | Br−1 (800) | 98.8±0.6 |
| SDS (1) | 77.8±0.3 | I−1 (20) | 88.4±0.8 |
| SDS (5) | 100 | I−1 (200) | 98.6±1.1 |
| DTT (0.1) | 96.8±0.3 | I−1 (800) | 99.1±1.2 |
| DTT (1) | 100 | ||
| NaN3 (0.1) | 100 |
Substrate and kinetic analysis
Table 4 showed the Michaelis-Menten constants for laccase in the presence of different substrates. The enzyme exhibited the highest activity with ABTS with the Km value of 85.9 µM, which was determined using the Lineweaver-Burk plot. The apparent Km values that were determined for hydroquinone, catechol and guaiacol were 2091.7, 329.3 and 312.2 µM, respectively. The k cat/Km value for ABTS was 3.1×106 (s−1 ·M−1), which was higher than that for hydroquinone, catechol and guaiacol. These results indicated that ABTS had a more effective catalysis process. The laccase oxidised syringaldazine. However, the reaction rate was very low under the conditions that were provided. The affinity for ABTS [21], [24], [26] and the inaction to syringaldazine [21] have been observed in other fungal laccases.
Table 4. Substrate specificity of the purified laccase.
| Substrate | K m (µM) | V max (µM min−1 mg−1) | k cat (s−1) | k cat/K m (s−1 M−1) |
| ABTS | 85.9 | 555.6 | 267.1 | 3.1×106 |
| Hydorquinone | 2091.7 | 3333.3 | 1587.3 | 7.6×105 |
| Catechol | 329.3 | 1666.7 | 973.7 | 3.0×106 |
| Guciacol | 312.2 | 416.7 | 198.4 | 6.4×105 |
Degradation and colourisation of synthetic dyes
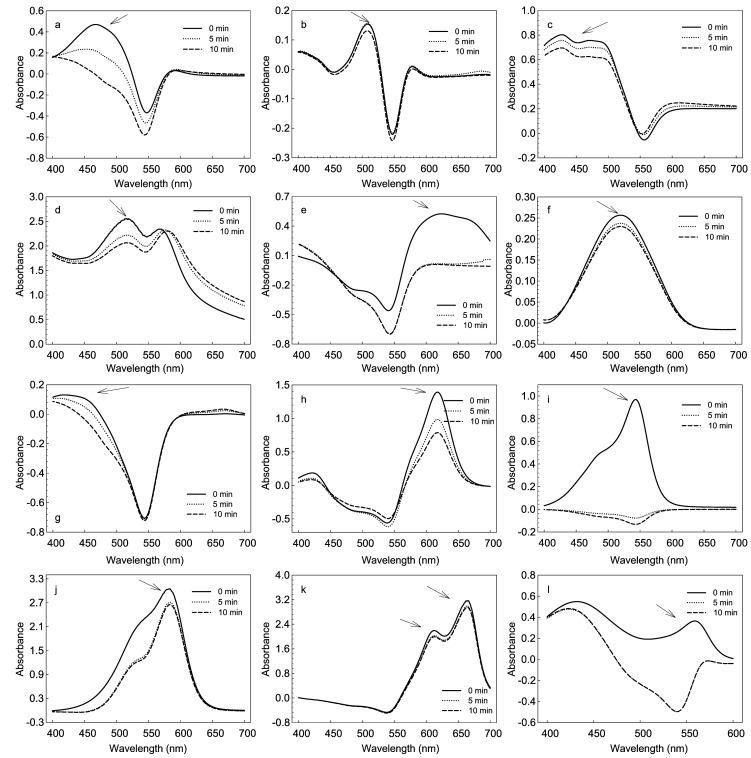
Figure 6 showed the degradation of twelve structurally different dyes including azo, anthraquinone, arylmethyl and other structure type dyes by the purified laccase. Four out of twelve tested dyes, orange I, eriochrome black T, fuchsin basic and phenol red showed a total decolourisation by NF-05 laccase within 10 min. The remaining eight dyes were degraded to different extend within 24 h as revealed in table 5. There was no significant relation between structure and decolourisation efficiency of dyes. Though lots of papers reported the degradation and decolourisation of synthetic dyes by laccases [4], [10], the broad substrates specificity of NF-05 laccase rendered its great potentials in industrial applications, such as degradation of dyes from acidic textile effluents.
Figure 6. Degradation of azo dyes, a: orange I, b: amaranth, c: sudan II, d: sudan III, e: eriochrome black T; anthraquinone dyes, f: alizarin red, g: alizarin; arylmethane dyes, h: malachite green, i: fuchsin basic, j: crystal violet; and other dyes, k: methylen blue, l: phenol red by purified laccase from M.verrucaria NF-05.
Table 5. Decolourisation of dyes by M.verrucaria NF-05 laccase.
| Dyes | Decolourisation (%) | ||
| 5 min | 10 min | 24 h | |
| orange I | 53.02% | 100% | 100% |
| Amaranth | 0 | 14.72% | 26.96% |
| sudan II | 5.64% | 13.36% | 37.45% |
| sudan III | 13.27% | 19.29% | 45.66% |
| eriochrome black T | 96.78% | 98.32% | 99.45% |
| alizarin red | 7.49% | 10.51% | 58.82% |
| Alizarin | 19.84% | 51.00% | 80.26% |
| malachite green | 29.32% | 43.46% | 56.33% |
| fuchsin basic | 100% | 100% | 100% |
| crystal violet | 11.02% | 12.97% | 36.25% |
| methylen blue | 6.12%/7.46% | 7.24%/9.25% | 34.22%/45.36% |
| phenol red | 100% | 100% | 100% |
In conclusions, the purified ‘white’ laccase from the deuteromycete fungus M.verrucaria NF-05 was a monomeric protein displaying the typical properties as an oxidoreductase. The metal content and spectral properties indicated its novelty. The dye degradation ability added to the advantages that the organism and its enzyme possessed for bioremediation and biotransformation. Further studies should be focused on the analysis on the laccase-encoded gene, the mechanism of electron transfer in the active center of the laccase and also investigation on whether the laccase could be involved in the direct oxidization of different polycyclic aromatic hydrocarbons in vitro.
Materials and Methods
Microorganism and cultivation
Strain NF-05 was isolated from soil in Liangshui Native Nature Reserve, China and identified as M.verrucaria according to the internal transcribed spacer nucleotide sequence as well as the microscopic morphology based on the method of [18]. No specific permits were required for the described field studies. The fungus was monthly transferred to fresh PDA slants which contained (g l−1): potato, 200; glucose, 20; MgSO4·7H2O, 1.5; KH2PO4, 3; agar, 20 and stored at 30°C. For purification of the laccase, strain NF-05 was inoculated into the liquid PDA which contained (g l−1): potato, 200; glucose, 40; peptone, 35; MgSO4·7H2O, 1.5; KH2PO4, 3; pH 6.0. The culture medium was supplemented with 1 mM CuSO4·5H2O at the fourth day to induce the laccase production. Each flasks was then incubated with two fungal discs (1-cm diameter), which had been incubated 7–9 days earlier on PDA plates at 30°C. Each flasks (250 ml) contained 100 ml medium. Inoculated flasks were cultivated in a time course of 15 days.
Protein and enzyme assays
The protein concentration was determined based on the Lowry procedure using bovine serum albumin (BSA) as the standard [27]. The activity of laccase was spectrophotometrically determined by applying ABTS (2,2′-azinobis-(3- ethylbenzothiazoline-6-sulphonic acid)) which was used as the substrate at 30°C in 0.2 M citric acid (pH 4.0). The reaction solution consisted of 2.95 ml of citric acid buffer (0.2 M, pH 4.0), 1 ml of ABTS (1 mM) and 50 µl of enzyme solution. The change in the absorbance due to the oxidation was monitored at 420 nm and recorded after 3 min. One unit (U) of activity was defined as the production of 1 µmol of product per minute under the condition of 30°C and pH 4.0. The activity of bilirubin oxidase was measured as follows: 2.0 ml of 30 µM bilirubin dissolved in 0.2 M Tris-H2SO4 buffer (pH 8.4) was added to 0.2 ml of the enzyme solution, followed by incubation at 37°C. Measurement of the absorbance decrease of bilirubin was carried out at 440 nm. One unit was defined as the amount of enzyme which oxidized 1 µM bilirubin min−1 [28]. The activity against hydroquinone, catechol, guaiacol and 4-hydroxy-3,5-dimethoxybenzaldehyde azine (syringaldazine) were also determined under the same reaction conditions with different initial concentrations. The absorbance coefficients were as follows: ε420 nm=36,000 M−1 ·cm−1 for ABTS, ε248 nm=10,400 M−1 ·cm−1 for hydroquinone, ε410 nm=2211 M−1 ·cm−1 for catechol, ε470 nm=6740 M−1 ·cm−1 for guaiacol, and ε525 nm=65,000 M−1 cm−1 for syringaldazine [16], [29]. One unit (U) of activity was defined as the production of 1 µmol product per minute under the condition of 30°C and pH 4.0.
Enzyme purification
The laccase was purified from a cell-free culture medium by addition of ammonium sulfate to 70% saturation and centrifugation of salted-out proteins at 4°C, 10,000 g for 30 min. The supernatant was decanted and the precipitate was dissolved in 0.2 M citric acid buffer (pH 4.0) and dialyzed (membrane molecular weight cut off 14,000 Da) at 4°C overnight against the same buffer. The dialysate was loaded onto a DEAE-Cellulose column pre-equilibrated with the same buffer. Proteins were eluted with a linear gradient of sodium phosphate (0–0.8 M) in 10 mM citric acid buffer (pH 4.0) at a flow rate of 1 ml min−1. Fractions of 5 ml were collected, and those with laccase activity were pooled and concentrated using polyethylene glycol. The concentrate was then loaded onto a Sephadex G-75 column pre-equilibrated with the same buffer. Proteins were eluted with the same buffer at a flow rate of 0.5 ml min−1. Fractions with laccase activity were also pooled and the purified enzyme was stored at −20°C. The purification was carried out in the room temperature.
Determination of molecular mass and metal content of purified laccase
The purity and molecular mass of the purified laccase was determined by SDS-PAGE using a 12% resolving gel and a 4% stacking gel. Proteins were visualised by staining with coomassie brilliant blue R-250. The unstained protein molecular weight marker was purchased from Fermentas (#SM0431) (Ontario, Canada). The zymogram process was performed by a native PAGE using a 12% resolving gel. The band of interest was visualised by incubating the gel in 0.2 M citric acid buffer containing 5 mM ABTS (pH 4.0) at 30°C. The metal content was determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) using an Optima 7300DV, PerkinElmer.
The spectrometric studies
The spectroscopic characterization of the purified enzyme was carried out in 10 mM citric acid buffer (pH 4.0) from 200 to 700 nm using a lambda750, PerkinElmer.
Electron paramagnetic resonance (EPR)
An electron paramagnetic resonance (JEOL, Japan) spectrum was recorded with a JES-FA200 EPR spectrometer at 9.5 GHz, a modulation frequency of 100 kHz, a modulation amplitude of 2 G (200 µT), a sweep time/scan of 120 s and a microwave power of 5.0 mW. The enzyme sample was prepared in 10 mM citric acid buffer, pH 4.0. Probe temperature was regulated with a liquid nitrogen cryostat equipped with a temperature control unit and maintained at 100 K.
N-terminal amino acid sequencing
The purified laccase was electrophoresed on 12% (w/v) SDS–PAGE, transferred onto a polyvinylidene fluoride membrane (Millipore Corp., Bedford, Mass.) by electroblotting and then stained. The polyvinylidene fluoride membrane slice containing the laccase was excised and sequenced on an ABI PROCISETM494cLC sequencer that employs Edman degradation to sequentially cleave and identify amino acids starting at the amino terminus (N-terminus) of the protein.
Matrix assisted laser desorption/ionization-time of flight (MALDI-TOF)
After SDS-PAGE, the protein band corresponding to the zone of the enzyme was excised and washed three times with dd·H2O. The sample was decolored using 30% acetonitrile and 10 mM NH4HCO3, and the sample was then dehydrated with 100% acetonitrile and subsequently digested with 15 µl trypsin at 37°C for 16 h. The peptides were then extracted with 60% acetonitrile and 0.1% trifluoroacetic acid. 1 µl solution was used for further analysis. The sample was analyzed in reflectron mode using a 4800 Plus MALDI TOF/TOF™ Analyzer (Applied Biosystems, USA). Spectra were deisotoped using a detection threshold that was manually adjusted to exclude spectral noise, and the resulting peak list was used to search the NCBInr database using the GPS Explorer™ v 3.6.
Enzyme characterization
The optimal temperature and the thermal stability were investigated using 0.2 M citric acid buffer (pH 4.0) with 1 mM ABTS as the substrate. The optimal temperature was determined from 10–90°C. The thermal stability was measured after incubating the proteins under different temperatures for 1 h before adding 1 mM ABTS-containing reaction buffer. The optimal pH value and the pH stability was determined in 0.2 M phosphate buffer (pH 2.0), 0.2 M citric acid buffer (pH 3.0–8.0), and 0.1 M glycine-NaOH buffer (pH 9.0–10.0). The stability at pH 2.0–10.0 was tested after incubating the enzyme for 1 h at 30°C. Metal ions (Li+, Na+, K+, Ag+, Hg+, Mg2+, Ca2+, Cd2+, Mn2+, Fe2+, Co2+, Zn2+, Ba2+, Cu2+, Al3+, Fe3+, each at 6.25, 12.5, 25 and 50 mM), inhibitors (EDTA, SDS, dithiothreitol (DTT), sodium azide, cysteine, 0.1, 1 and 5 mM; Cl−1, Br−1 and I−1, each at 20, 200 and 800 mM) and different organic solvents (methanol, ethanol, acetone, acetonitrile, DMSO, each at 5%, 20% and 50%) were mixed with the 1 mM ABTS-containing reaction buffer to obtain the respective final concentrations. The effect of these additives on the laccase activity was also determined by incubating the additives at 30°C for 1 h.
Decolourisation of synthetic dyes
All the tested dyes were purchased from Sigma Company, detailed information was shown in Table 6. The degradation of twelve structurally different dyes by the purified laccase was determined by full spectrum scan among 400–700 nm at 5 min and 10 min, respectively. The decolorization of test dyes was calculated at 5 min, 10 min and 24 h, respectively. The reaction mixture for the standard assay contained respective dye (0.6 mg) in 10 mM citric acid buffer at pH 4.0 and the enzyme solution (15 U) in a total volume of 3 ml. The decolorization rate of dye, expressed as dye decolorization (%), was calculated as the formula: decolorization (%)=[(Ai−At)/Ai] * 100, where Ai: initial absorbance of the dye, At: absorbance of the dye along the time. All experiments were performed in triplicate.
Table 6. Characteristics of dyes tested in this work.
| Dyes | Type | Chemical formular | λmax (nm) |
| orange I | azo | C16H11N2NaO4S | 467 |
| Amaranth | azo | C20H11N2Na3O10S3 | 508 |
| sudan II | azo | C18H16N2O | 427 |
| sudan III | azo | C22H16N4O | 516 |
| eriochrome black T | azo | C20H12N3NaO7S | 624 |
| alizarin red | anthraquinone | C14H7NaO7S·H2O | 520 |
| Alizarin | anthraquinone | C14H8O4 | 417 |
| Malachite green | arylmethane | C23H25ClN2 | 617 |
| fuchsin basic | arylmethane | C20H20ClN3 | 543 |
| crystal violet | arylmethane | C25H30ClN3·9H2O | 583 |
| methylen blue | other | C16H18ClN3S·3H2O | 666/613 |
| phenol red | other | C19H14O5S | 558 |
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the the National Nature Science Foundation of China (No. 31170553, 30671702, 30170775). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Valls C, Colom JF, Baffert C, Gimbert I, Roncero MB. Comparing the efficiency of the laccase-NHA and laccase-HBT systems in eucalyptus pulp bleaching. Biochemical Engineering Journal. 2010;49:401–407. [Google Scholar]
- 2.Halaburgi VM, Sharma S, Sinha M, Singh TP, Karegoudar TB. Purification and characterization of a thermostable laccase from the ascomycetes Cladosporium cladosporioides and its applications. Process Biochemistry. 2011;46:1146–1152. [Google Scholar]
- 3.Miele A, Giardina P, Sannia G, Faraco V. Random mutants of a Pleurotus ostreatus laccase as new biocatalysts for industrial effluents bioremediation. Journal of applied microbiology. 2010;108:998–1006. doi: 10.1111/j.1365-2672.2009.04505.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez Couto S, Toca Herrera JL. Industrial and biotechnological applications of laccases: A review. Biotechnology Advances. 2006;24:500–513. doi: 10.1016/j.biotechadv.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Lafayette P, Eriksson K, Dean J. Characterization and heterologous expression of laccase cDNAs from xylem tissues of yellow-poplar (Liriodendron tulipifera). Plant molecular biology. 1999;40:23–35. doi: 10.1023/a:1026437406859. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Goel R, Capalash N. Bacterial laccases. World Journal of Microbiology and Biotechnology. 2007;23:823–832. [Google Scholar]
- 7.Baldrian P. Fungal laccases ‘occurrence and properties’. FEMS Microbiology Reviews. 2006;30:215–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- 8.Minussi RC, Miranda MA, Silva JA, Ferreira CV, Aoyama H, et al. Purification, characterization and application of laccase from Trametes versicolor for colour and phenolic removal of olive mill wastewater in the presence of 1-hydroxybenzotriazole. African Journal of Biotechnology. 2010;6:1248–1254. [Google Scholar]
- 9.Patrick F, Mtui G, Mshandete A, Johansson G, Kivaisi A. Purification and characterization of a laccase from the basidiomycete Funalia trogii (Berk.) isolated in Tanzania. African Journal of Biochemistry Research. 2009;3:250–258. [Google Scholar]
- 10.Hao J, Song F, Huang F, Yang C, Zhang Z, et al. Production of laccase by a newly isolated deuteromycete fungus Pestalotiopsis sp. and its decolorization of azo dye. Journal of Industrial Microbiology and Biotechnology. 2007;34:233–240. doi: 10.1007/s10295-006-0191-3. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Zhang D, Hua Z, Li J, Du G, et al. A newly isolated Paecilomyces sp. WSH-L07 for laccase production: isolation, identification, and production enhancement by complex inducement. Journal of Industrial Microbiology and Biotechnology. 2009;36:1315–1321. doi: 10.1007/s10295-009-0615-y. [DOI] [PubMed] [Google Scholar]
- 12.Liu ZY, Zhang DX, Hua ZZ, Li JH, Du GC, et al. Improvement of laccase production and its properties by low-energy ion implantation. Bioprocess and biosystems engineering. 2010;33:639–646. doi: 10.1007/s00449-009-0389-7. [DOI] [PubMed] [Google Scholar]
- 13.Sulistyaningdyah W, Ogawa J, Tanaka H, Maeda C, Shimizu S. Characterization of alkaliphilic laccase activity in the culture supernatant of Myrothecium verrucaria 24G-4 in comparison with bilirubin oxidase. FEMS Microbiology Letters. 2004;230:209–215. doi: 10.1016/S0378-1097(03)00892-9. [DOI] [PubMed] [Google Scholar]
- 14.Solomon E, Sundaram U, Machonkin T. Multicopper oxidases and oxygenases. Chem Rev. 1996;96:2563–2606. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 15.Palmieri G, Giardina P, Bianco C, Scaloni A, Capasso A, et al. A novel white laccase from Pleurotus ostreatus. Journal of biological chemistry. 1997;272:31301–31307. doi: 10.1074/jbc.272.50.31301. [DOI] [PubMed] [Google Scholar]
- 16.Pozdnyakova N, Turkovskaya O, Yudina E, Rodakiewicz-Nowak Y. Yellow laccase from the fungus Pleurotus ostreatus D1: purification and characterization. Applied biochemistry and microbiology. 2006;42:63–69. [PubMed] [Google Scholar]
- 17.Morozova OV, Shumakovich GP, Gorbacheva MA, Shleev SV, Yaropolov AI. "Blue"; laccases. Biochemistry. 2007;72:1136–1150. doi: 10.1134/s0006297907100112. [DOI] [PubMed] [Google Scholar]
- 18.Zhao M, Wang HD, Zhao D, Gu HQ, Zhang X. Isolation and decolorization of azo dye of a laccase-producing Myrothecium verrucaria NF-05. Mycosystema. 2011;30:604–611. [Google Scholar]
- 19.Min KL, Kim YH, Kim YW, Jung HS, Hah YC. Characterization of a novel laccase produced by the wood-rotting fungus Phellinus ribis. Archives of Biochemistry and Biophysics. 2001;392:279–286. doi: 10.1006/abbi.2001.2459. [DOI] [PubMed] [Google Scholar]
- 20.Koikeda S, Ando K, Kaji H, Inoue T, Murao S, et al. Molecular cloning of the gene for bilirubin oxidase from Myrothecium verrucaria and its expression in yeast. J Biol Chem. 1993;268:18801–18809. [PubMed] [Google Scholar]
- 21.Wu Y, Luo Z, Chow R, Vrijmoed L. Purification and characterization of an extracellular laccase from an anthracene-degrading fungus Fusarium solani MAS2. Bioresource Technology. 2010;102:9772–9777. doi: 10.1016/j.biortech.2010.07.091. [DOI] [PubMed] [Google Scholar]
- 22.Wang HT, Hsu JT. Usage of enzyme substrate to protect the activities of cellulase, protease and α-amylase in simulations of monogastric animal and avian sequential total tract digestion. Asian-australasian journal of animal sciences. 2006;19:1164–1173. [Google Scholar]
- 23.Bendl RF, Kandel JM, Amodeo KD, Ryder AM, Woolridge EM. Characterization of the oxidative inactivation of xylanase by laccase and a redox mediator. Enzyme and Microbial Technology. 2008;43:149–156. [Google Scholar]
- 24.Zhang GQ, Wang YF, Zhang XQ, Ng TB, Wang HX. Purification and characterization of a novel laccase from the edible mushroom Clitocybe maxima. Process Biochemistry. 2010;45:627–633. [Google Scholar]
- 25.Sadhasivam S, Savitha S, Swaminathan K, Lin F. Production, purification and characterization of mid-redox potential laccase from a newly isolated Trichoderma harzianum WL1. Process Biochemistry. 2008;43:736–742. [Google Scholar]
- 26.Chakroun H, Mechichi T, Martinez MJ, Dhouib A, Sayadi S. Purification and characterization of a novel laccase from the ascomycete Trichoderma atroviride: Application on bioremediation of phenolic compounds. Process Biochemistry. 2010;45:507–513. [Google Scholar]
- 27.Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Shimizu A, Kwon JH, Sasaki T, Satoh T, Sakurai N, et al. Myrothecium verrucaria bilirubin oxidase and its mutants for potential copper ligands. Biochem. 1999;38:3034–3042. doi: 10.1021/bi9819531. [DOI] [PubMed] [Google Scholar]
- 29.Sengupta NDS, Mukherjee M. Importance of laccase in vegetative growth of Pleurotus florida. Appl Environ Microbiol. 1997. pp. 4120–4122. [DOI] [PMC free article] [PubMed]