Abstract
Determination of amyloid β (Aβ) isoforms and in particular the proportion of the Aβ 1-42 isoform in cerebrospinal fluid (CSF) of patients suspected of Alzheimer’s disease might help in early diagnosis and treatment of that illness. Due to the low concentration of Aβ peptides in biological fluids, a preconcentration step prior to the detection step is often necessary. This study utilized on-chip immunoprecipitation, known as micro-immunoprecipitation (μIP). The technique uses an immunosorbent (IS) consisting of magnetic beads coated with specific anti-Aβ antibodies organized into an affinity microcolumn by a magnetic field. Our goal was to thoroughly describe the critical steps in developing the IS, such as selecting the proper beads and anti-Aβ antibodies, as well as optimizing the immobilization technique and μIP protocol. The latter includes selecting optimal elution conditions. Furthermore, we demonstrate the efficiency of anti-Aβ IS for μIP and specific capture of 5 Aβ peptides under optimized conditions using various subsequent analytical methods, including matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS), capillary electrophoresis, microchip electrophoresis, and immunoblotting. Synthetic Aβ peptides samples prepared in buffer and spiked in human CSF were analyzed. Finally, on-chip immunoprecipitation of Aβ peptides in human CSF sample was performed.
INTRODUCTION
Alzheimer’s disease (AD) is a progressive, fatal neurodegenerative disorder characterized by deterioration in cognition and memory, progressive impairment in the ability to carry out activities of daily living, and a number of neuropsychiatric and behavioral symptoms.1 AD is giving rise to serious socioeconomic problems, as the number of AD patients requiring long-term care increases each year.2, 3 An important step toward addressing these issues in relation to AD would be to develop diagnostic tools for early detection of AD biomarkers in preclinical phase.4
At present, clinically relevant biomarkers used for AD diagnosis in cerebrospinal fluid (CSF) are amyloid β (Aβ) peptides 1-42,5, 6 total tau, and hyperphosphorylated tau protein.2, 7, 8, 9 Our work focused on Aβ peptide biomarkers. Various isoforms of the Aβ peptide, usually between 37 and 43 amino acids (AA) in length, occur naturally in our body liquids and are derived by proteolysis of a larger protein known as the amyloid precursor protein (APP).10 The Aβ 1-42 isoform (4 kDa) is the major species found in the senile plaques. It is regarded as a key molecule in AD pathology and more prone to aggregation than are the shorter Aβ isoforms.11
The concentration of Aβ 1-42 analyzed by Aβ-SDS-PAGE/immunoblot was reported by Wiltfang et al. to be in the range of 0.91–1.57 ng/ml in the CSF of AD patients but 1.56–2.88 ng/ml in the CSF of controls without dementia disease.12 The decrease of Aβ 1-42 level compared to other isoforms in the CSF of AD patients is presumably due to its lower clearance from the brain into the CSF. This might be explained by the fact that Aβ 1-42 creates senile plaques in brain tissue, and it could also be considered as a first sign of conversion from mild cognitive impairment to AD.13 Therefore, it is important not only to determine total concentration of Aβ peptide in CSF of dementia-affected patients but also the proportions for each of the Aβ isoforms.12
Differential quantification of the various Aβ peptides at such low concentration in a complex biological matrix is challenging. Various methods for preconcentration of target analyte can be applied. Impressive results were reported in Refs. 14, 15, 16 using isotachophoresis (ITP) where fluorescently labeled or unlabeled samples were enriched up to 10 000 fold from whole volume of simple mixture of standard proteins or amino acids. It is very efficient technique applicable mainly for pharmaceutical, food, and/or lipoprotein analysis.17 However, according to Lion et al.18 ITP suffers from one drawback: the sample conductivity must be carefully controlled, which is not always that easy when dealing with “real” biological samples. Therefore, we have decided in this work to use immunoprecipitation (IP) since as reported by numerous other authors,12, 19, 20, 21 IP using specific antibodies is one of the most efficient methods for isolation and preconcentration of target biomarkers from complex biological samples enabling its subsequent determinantion.22, 23 Although this method accomplishes in our hands lower preconcentration level compare to ITP, we can gain especially high selectivity which is substantially desired in such cases and, moreover, the analytes are after IP presented directly in reagent compatible with subsequent detection method. IP is very powerful tool especially when antibody providing appropriate affinity and specificity to target antigen is at hand. Recent advances in microfluidics have enabled development of on-chip immunoprecipitation (µIP), which offers such advantages as lower sample and reagent consumption, easier sample handling, and reduced protein loss due to surface adsorption.24
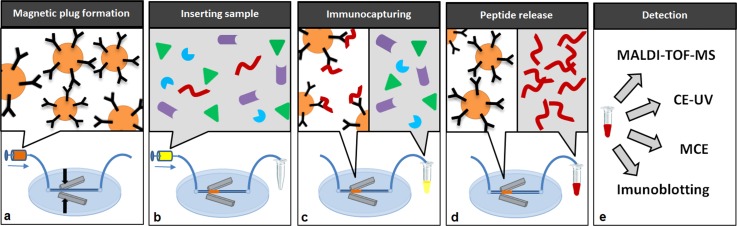
Magnetic or nonmagnetic beads can be used for creating an IP microcolumn inside a microfluidic device. Nonmagnetic beads can be fixed mechanically by a frit or weir in the microchannel, but this generally requires high pressures and furthermore reproducible packing is difficult to achieve. Thus, capturing magnetic beads in a microchannel by the action of two strong magnets has proven an efficient and convenient alternative.25 The principle of experiment setup based on specific capture of target peptides on microaffinity self-assembled column formed by magnetic anti-Aβ immunosorbent (IS) which is followed by release of captured peptides from the column is described here (see Fig. 1). By this method, we are able to enrich from CSF sample the 5 Aβ peptides using N-terminated monoclonal antibody immobilized on magnetic particles.
Figure 1.
Scheme of experiment setup in macro- and micro-scales. (a) Prepared magnetic anti-Aβ immunosorbent is inserted in PDMS microchip using syringe pump where they are self-assembled into affinity microcolumn. (b) Sample containing Aβ peptides is applied into the chip and (c) the specific immunocapturing is performed. After proper washing (d) the specifically captured peptides are eluted using compatible eluting reagent according to subsequent method (e).
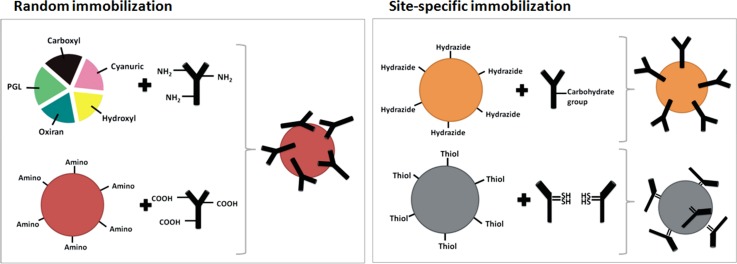
In general, different immobilization techniques, either non-covalent or covalent, may be applied for attaching antibodies onto the magnetic beads and thus for magnetic immunosorbent preparation. Nevertheless, covalent binding provides the strongest linkages compared with such other non-covalent immobilization methods as biospecific, ionic and/or affinity adsorption, and leakage of ligand from the beads is thus often minimized.26 In our work, various site-groups on magnetic beads enabling random or site-specific covalent binding of Ab were compared for their immobilization efficiency.
Two types of specific anti-Aβ antibodies were attached to beads with highest immobilization efficiency: (i) rabbit polyclonal IgG to amyloid β (1-14 AA), and/or (ii) mouse monoclonal IgG1 to amyloid β (1-16 AA). Both antibodies were directed against an N-terminus of Aβ peptide which is common for all 5 isoforms (1-37, 1-38, 1-39, 1-40, and 1-42), and hence all tested isoforms are presumed to be immunocaptured. Proportional immunocapture of both ISs, anti-Aβ IS (pAb) or anti-Aβ IS (mAb) for all 5 isoforms was investigated. When successful preconcentration of synthetic Aβ peptide mixtures on prepared ISs was confirmed, IP with spiked or native human CSF samples of non-AD patients was performed.
Preliminary testing of the prepared magnetic IS and its use under micro-immunoprecipitation (μIP) conditions for 3 synthetic Aβ peptides with urea/tricine/tris SDS-PAGE detection had been described in our previous work.27 Mohamadi et al. also had introduced application of such immunocapture in combination with microchip electrophoresis in detecting 5 Aβ isoforms.28 It was concluded that a preconcentration step was essential for sensitive detection of such low-abundance analytes. In the present paper, therefore, results from an extensive study on the development of a magnetic anti-Aβ IS and its characterization and validation for Aβ peptides using various analytical tools ranging from conventional western blotting to newly developed microchip electrophoresis are presented.
EXPERIMENTAL SECTION
Materials and reagents
SiMAG-active, maghemite-core, superparamagnetic, nonporous silica beads, 1 µm in diameter and with various active groups (SiMAG-PGL, SiMAG-Amino, SiMAG-Carboxyl, SiMAG-Hydrazide, SiMAG-Thiol, SiMAG-Cyanuric, SiMAG-Oxiran and SiMAG-Hydroxyl), were acquired from Chemicell (Berlin, Germany). Nonspecific human IgG isolated from serum and goat anti-mouse antibody marked with horseradish peroxidase were from Sigma-Aldrich (St. Louis, MO). Two anti-Aβ antibodies were used: rabbit polyclonal IgG to amyloid β (1-14 AA) from Abcam (Cambridge, UK) and mouse monoclonal IgG1 to amyloid β (clone 6E10, 1-16 AA) from Covance (Princeton, NJ). Synthetic Aβ peptides 1-37, 1-38, 1-39, 1-40, and 1-42 were purchased from Sigma-Aldrich (St. Louis, MO), Anaspec (Fremont, CA), and/or Apronex (Vestec, Czech Republic). Other chemicals, such as D-glyceraldehyde, sodium periodate, acrylamide, bis-acrylamide, EDAC (N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride), 2 -(N-morpholino)ethanesulfonic acid sodium (MES), Tween 20, sulfosuccinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate (SMCC) and acetonitrile, were from Sigma-Aldrich (St. Louis, MO). α-cyano-4-hydroxycinnamic acid (CHCA) was from LaserBio Labs (Sophia-Antipolis, France). MicrospinTM G-25 columns were from GE Healthcare (Buckinghamshire, UK). BCA Protein Assay Kit was from Pierce (Rockford, IL). ECL Plus western blot detection reagents were from Amersham Biosciences (now GE Healthcare, Buckinghamshire, UK). Trifluoroacetic acid (TFA) was from Fluka (St. Louis, MO), NH4HCO3 and urea from Lachema (Brno, Czech Republic), and formic acid and NH4OH from Merck & Co (Whitehouse Station, NJ). Laemmli and tricine sample buffer were from Bio-Rad (Hercules, CA). Sodium dodecyl sulfate (SDS) was purchased from Penta (Prague, Czech Republic) and FluoProbe 488 NHS ester from Interchim (Montlucon, France). CSF samples of non-AD patients were provided by Professor Marcus Otto from the Department of Neurology at the University of Ulm (Ulm, Germany). Collection and analysis of the CSF samples were approved by the Ethics Committee at the University of Ulm.
Immobilization of antibodies onto SiMAG-active beads with various reactive groups
Selection of a suitable reactive group for antibody immobilization on the beads’ surface was performed first on a model ligand using human nonspecific serum IgG (hu IgG). We used 1 mg of beads and 100 µg of hu IgG in a 1 ml reaction volume. The immobilization was carried out according to the manufacturer’s instructions. SiMAG-PGL and/or SiMAG-Cyanuric form a covalent bond through their terminal autoreactive polyglutaraldehyde/cyanuric groups. For immobilization to SiMAG-Amino and SiMAG-Carboxyl, a two-step carbodiimide method was used. SiMAG-Hydroxyl beads were activated by CNBr, and the ligand was bound through its amino group. SiMAG-Thiol beads form a covalent bond with IgG through a sulfhydryl reactive group using a maleimide method, and SiMAG-Oxiran beads use the epoxide groups for IgG immobilization. SiMAG-Hydrazide beads use site-directed covalent coupling of IgG through their carbohydrates after oxidation by sodium periodate. In this case, the supplier’s recommended protocol was slightly modified. Antibodies were oxidized in 0.02 M sodium periodate, and the reaction was stopped by addition of 0.4 µl of ethylene glycol. The unreacted reagent was removed using G-25 micro columns. The coupling process ran overnight at RT under stirring, then the IS was repeatedly washed and stored in phosphate buffered saline (PBS; pH 7.4) with 0.05% sodium azide at 4–8 °C. Different covalent immobilization techniques were compared according to the amount of immobilized IgG molecules on the beads’ surface using SDS-PAGE and BCA assay. The difference between IgG concentration in the samples before and after immobilization was measured. Binding capacity was expressed as the percentage of hu IgG inputted to the reaction. When the most suitable beads were selected, the specific anti-Aβ antibodies were immobilized.
Batchwise immunoprecipitation
An Aβ sample (1 ml) was added to the specific anti-Aβ IS (1 mg) previously equilibrated with PBS (pH 7.4). After 1.5 h incubation at RT under stirring, the unbound proteins/peptides were removed by three washing steps. Each step consisted of 5 × 1.5 ml changing 0.1 M phosphate buffer (pH 7.0) with three different NaCl molarities (0.2 M—1 M—0 M). Subsequently, immunospecifically captured Aβ peptides were released by adding 3 × 200 µl of elution reagent followed by 15 min incubation at RT under stirring. The elution fractions were concentrated in a speed vac and analyzed off-line.
On-chip immunoprecipitation (μIP)
The single channel microchip device with one inlet and one outlet was fabricated by rapid prototyping and PDMS (polydimethylsiloxane) technology at Institut Curie (Paris), as previously described by Slovakova et al.29 The microchannel dimensions were 1 mm × 0.250 mm × 20 mm. The anti-Aβ immunosorbent suspension (1 mg) was injected into the channel using a syringe pump from KD Scientific (Holliston, MA) under flow rate 2 ml/h. The suspension was passed by two strong magnets placed along the channel in 20° angle position where the magnetic beads were reversibly trapped in the magnetic field and formed a compact dense plug. Once a microcolumn was created, equilibration with PBS at pH 7.4 (100 µl, 500 µl/h) followed as described in our previous work.27 The flow rate conditions were slightly modified. A sample containing Aβ (100 µl) was pumped into the chip under flow rate 50 µl/h at RT. The unbound peptides were removed by washing with 50 µl of 0.1 M phosphate buffer (pH 7.0) with three different NaCl molarities (0.2 M – 1 M – 0 M) at flow rate 200 µl/h at RT. Immunospecifically captured Aβ peptides were eluted by 100 µl of elution reagent under flow rate 200 µl/h. The collected elution fractions were concentrated in a speed vac and analyzed off-line.
Dot blot monitoring of μIP
The Aβ concentration level during each particular step of the μIP was studied by dot blot. Aβ 1-42 was selected to simplify the experiment’s interpretation. Each 10 µl of liquid which passed through the chip during the binding, washing, and elution phases was collected and applied onto nitrocellulose membrane to determine the Aβ concentration in the dots. Then, the membrane was blocked by 1% bovine serum albumin in PBS-T (PBS, pH 7.4, with 0.05% Tween 20) for 1 h and after a quick wash in PBS-T it was incubated overnight in a solution containing specific monoclonal mouse anti-Aβ primary Ab (1:1000). After washing in PBS-T, it was incubated with the secondary antibody goat anti-mouse marked with horseradish peroxidase (1:5000). The signal was developed using an enhanced chemiluminescence detection kit (ECL, Roche, France) and the image was captured using an LAS-3000 mini Fuji imager (Fujifilm Life Science, Dusseldorf, Germany). The image was processed using ImageJ and the concentration of Aβ 1-42 in each dot of the samples and the calibration curve in concentration range 1–50 µg/ml were determined.
Urea/tricine/tris SDS-PAGE of immunocaptured Aβ peptides
We used a 16.5%T/3%C tricine/tris SDS-PAGE gel modified with 8 M urea for batchwise analysis of IP Aβ peptides according to Klafki et al.30 The gels of 0.75 mm thickness were run in a Mini-PROTEAN Tetra electrophoresis cell from Bio-Rad (Hercules, CA) in tricine/tris/SDS (0.1 M tris, 0.1 M tricine, 0.1% [w/v] SDS) buffer. Samples were mixed with tricine sample buffer (1:1) and boiled for 2 min at 100 °C.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) of immunocaptured Aβ peptides
The preconcentrated Aβ samples were detected using a 4800 MALDI TOF/TOF™ Analyzer mass spectrometer from Applied Biosystems (Foster City, CA) in reflectron positive mode with laser intensity 3000 and range m/z 800–5000. A 1 μl volume of each sample was spotted onto the target MALDI-plate, air-dried, then covered with 1 μl of matrix solution (α-cyano-4-hydroxycinnamic acid, 50 mg/ml in 33% acetonitrile, 0.3% TFA). The mass spectra were evaluated using the program data explorer (Applied Biosystems, Carlsbad, CA).
Capillary electrophoresis (CE)
The conditions for analyzing preconcentrated Aβ samples were described in detail by Verpillot et al.31 The Beckman Coulter PA 800 ProteomLab employed for off-line analysis of Aβ samples was equipped with UV and photodiode array detection (DAD). The uncoated silica capillary had 50 µm inside diameter and 57 cm total length and was thermostated at 25 °C. The samples were introduced into the capillary by hydrodynamic injection under 3.4 kPa. The peptides were detected by UV detection (214 nm) or DAD (190 nm). The separations were carried out at 30 kV with positive polarity at the inlet and using 40 mM borate buffer (pH 9) containing 3 mM of diaminobutane as the electrolyte. The data acquisition and instrument control were directed using karat 7.0 software. Detection limits ranged from 300 to 500 nM depending on the Aβ peptide isoform.
Microchip electrophoresis (MCE)
A chip-based method for Aβ profiling recently described by Mohamadi et al. was performed.28 The electrophoresis chip has channel cross section dimensions of 100 μm × 50 μm × 35 mm. The channel surface was coated by introducing a 0.2% solution of a homemade copolymer PDMA-AGE in ddH2O. For separation, methylcellulose at various concentrations (at least 0.2%) with 0.01% Tween 20 was added to the electrophoresis buffers. The fluorescently labeled samples containing synthetic Aβ 1-37 and Aβ 1-42 peptides (1:1) preconcentrated on anti-Aβ IS were injected into the electrophoresis channel by pinched injection method. An Olympus IX71 inverted fluorescence microscope equipped with a 10 × 0.3 NA objective lens (Olympus, Tokyo, Japan) was used to detect fluorescent intensity of labeled Aβ peptides in real time within the detection zone using a Nikon digital DS-Qi1 camera. Illumination was by 100 W mercury arc lamp.
Immunoblotting analysis
CSF aliquots of 0.5 ml from non-AD patients were received from the University of Ulm on dry ice and then stored at −20 °C until analysis. Before analysis, the CSF sample was thawed and diluted 1:1 (v/v) with working buffer and the batchwise IP or μIP was immediately performed on anti-Aβ ISs. The Aβ samples for analysis were dried on a speed vac and sent at 4 °C to the University of Duisburg-Essen for analysis. The Aβ-SDS-PAGE/immunoblot according to Wiltfang et al. was used for Aβ isoforms quantification.12
RESULTS AND DISCUSSION
Magnetic microbeads for μIP
Nonporous silica superparamagnetic microbeads 1 μm in diameter were selected for IS preparation, as they were supplied with a wide range of terminal reactive groups on their surfaces (hydrazide, polyglutaraldehyde, cyanuric, hydroxyl, amino, carboxyl, oxiran, and thiol). In order to obtain high density of Ab on the bead surface, the amount of IgG immobilized on each type of beads was determined by SDS-PAGE or BCA test. The protocols for ligand immobilization differed in their process requirements, the reagents used, and the orientation of immobilized ligand (Fig. 2). Therefore, the final decision as to which site-group would be used for μIP was based upon how simple was the protocol providing random or site-specific binding of IgG, whether the reagents were or were not harmful, and what was the IgG capacity on 1 mg of the beads. Our results from SDS-PAGE (Fig. 3) and the bicine-choninic acid (BCA) test (Fig. S-1)32 show high binding capacity of immobilized IgG was achieved using hydrazide, cyanuric, or thiol beads. In the end, microbeads with the hydrazide site-group were determined to be most suitable for further experiments with specific anti-Aβ IgG because they afforded a site-specific, covalent type of IgG binding in combination with high density and reasonable binding protocol.
Figure 2.
Scheme of immobilization techniques employed for IgG on magnetic beads with various site-groups
Figure 3.
Binding efficiency (%) of SiMAG-active Chemicell microbeads (1 mg) with different site-groups for human serum IgG (100 µg) immobilization as determined by SDS-PAGE (3 replicates)
Optimization of elution step in IP of Aβ peptides
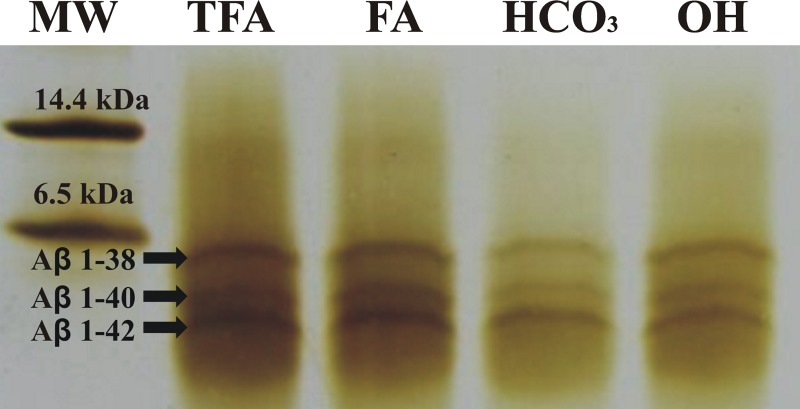
Immunocaptured analytes are supposed to be released from the anti-Aβ IS by using an elution reagent which ideally is compatible with the subsequent detection method. Toward this end, various elution conditions using 0.05% TFA (pH 2.3), 0.25% formic acid (pH 2.35), 0.16% ammonium hydroxide (pH 11.0), 100 mM ammonium bicarbonate (pH 8.0), 6 M urea (pH 8.2) and 2% SDS (pH 8.2) were tested. The determining criteria for evaluating elution efficiency were the concentration of batchwise immunoprecipitated Aβ peptides (1-38, 1-40, 1-42) in elution fractions as analyzed by urea/tricine/tris SDS-PAGE (Fig. 4). Chaotropic reagents such as urea and SDS in such concentrations were not compatible with subsequent analytical methods, but efficient elution of all tested Aβ isoforms was achieved using the other tested reagents. Therefore, elution reagents employed for releasing of captured peptides were adapted according to the analytical method that followed: For MALDI-TOF-MS analysis, 0.05% TFA was used, while for CE, chip electrophoresis and/or immunoblotting 0.16% NH4OH was preferred. For CE, moreover, 100 mM NH4HCO3 or 0.25% formic acid also was suitable.
Figure 4.
16.5% urea/tricine/tris SDS-PAGE of Aβ peptides (1-38, 1-40, 1-42) eluted by the various elution reagents: 0.05% trifluoroacetic acid (TFA; pH 2.3), 0.25% formic acid (FA; pH 2.35), 0.16% ammonium hydroxide (OH; pH 11.0), and 100 mM ammonium bicarbonate (NH4HCO3; pH 8.0); molecular weight standard 1.4–26.6 kDa (MW, BioRad).
On-chip Aβ preconcentration
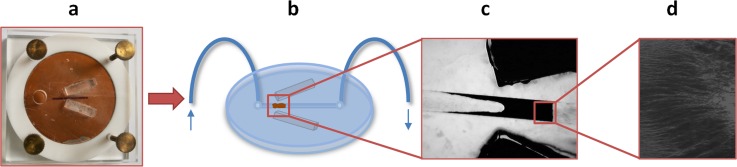
On-chip IP (μIP) runs on anti-Aβ IS self-organized in the channel when using two permanent magnets to generate an affinity microcolumn (Fig. 5). The volume of the plug formed in the channel was calculated from its height (0.25 mm) and width (1 mm) and length (2.33 mm). The length may vary, thence 3 different images underwent the image analysis using software NIS Elements AR (Nikon, Tokyo, J), where the length was measured in 5 different places of plug and the average of the 15 measurements were calculated (2.33 ± 0.44 mm). Final volume of plug was determinated as 0.58 mm3.
Figure 5.
(a) Master for PDMS molding, (b) scheme of PDMS microfluidic device, and (c),(d) detail of anti-Aβ plug/affinity microcolumn in microfluidic channel (1 mm × 0.25 mm × 20 mm) formed by magnetic microbeads self-organized using 2permanent magnets.
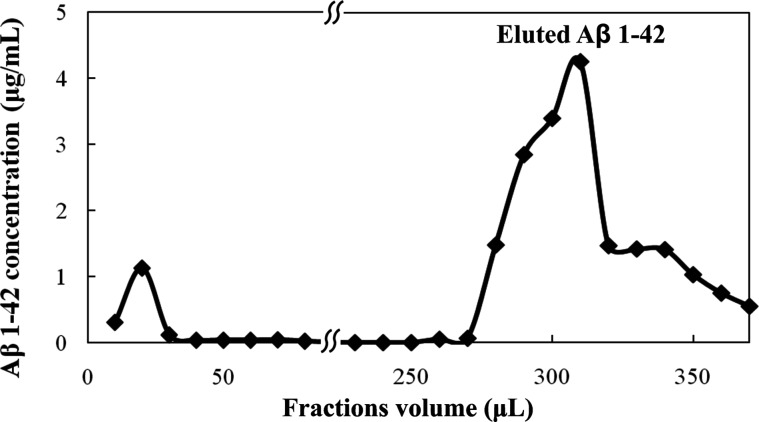
Although batchwise IP and μIP include a number of the same steps, such as binding, washing, and elution, there also are some basic differences. What is considered as reaction or incubation time in batchwise IP experiments can be transposed into flow rate in on-chip immunocapture (µIP). Hence, the μIP process was monitored by dot blot to confirm that the μIP produced the same results as did conventional batchwise IP. This technique was selected because it is a sensitive and quantitative method enabling easy monitoring of Aβ 1-42 concentration at each particular step of μIP. The record of Aβ 1-42 concentrations in collected fractions (10 µl) from the chip device under optimized flow rates is presented in Fig. 6. This result clearly shows that mostly all of the Aβ 1-42 applied into the chip was biospecifically captured during the binding step and efficiently released during the elution step.
Figure 6.
Monitoring of on-chip Aβ 1-42 immunocapture on anti-Aβ IS (pAb) by dot blot on nitrocellulose membrane. The process consists of Aβ 1-42 capture (0–100 μl; flow rate 50 μl/h), washing (110–260 μl; flow rate 200 μl/h), and elution of captured Aβ 1-42 (270–370 μl; flow rate 200 μl/h) by 0.05% TFA.
Concentration factor of μIP setup may vary and depends on different parameters, such as primer concentration of sample, sample volume, amount of the beads, flow rate, and volume of the elution reagent. In this case, we were able to preconcentrate the Aβ peptide on the IS surface from the volume 100 μl to volume 0.58 μl which means at least 170 fold and obtain peptide in high purity presented in reagent suitable for subsequent separation and/or detection method.
Detection of Aβ peptides preconcentrated by IP or μIP
Several methods were utilized for comparing and confirming immunocapture efficiency of the two prepared ISs (mAb and pAb) for the 5 Aβ peptide isoforms: First MALDI-TOF-MS for qualitative analysis, next CE or MCE for Aβ profiling, and then western blotting for the final quantitative and sensitive analysis of Aβ peptides preconcentrated from human CSF.
MALDI-TOF-MS
MALDI-TOF-MS is a qualitative method suitable for preliminary IS evaluation and allowed us to check whether the desired peptides were present in samples preconcentrated by IP. Its results did not give us information, however, about the proportions of the different Aβ peptides in samples, and, at least in our hands, the method was not sufficiently sensitive to detect Aβ peptides when their concentrations were close to the common levels in CSF samples. We therefore tried CSF samples spiked with Aβ synthetic peptides (6 µg/ml of 1-37, 1-38, 1-39, 1-40, and 1-42 Aβ isoforms). The Aβ isoforms 1-37 and 1-39 were not detectable in the samples preconcentrated by IP (Fig. S-2).32 Thus, to evaluate Aβ immunocapture in biologic samples, other methods were utilized.
CE detection
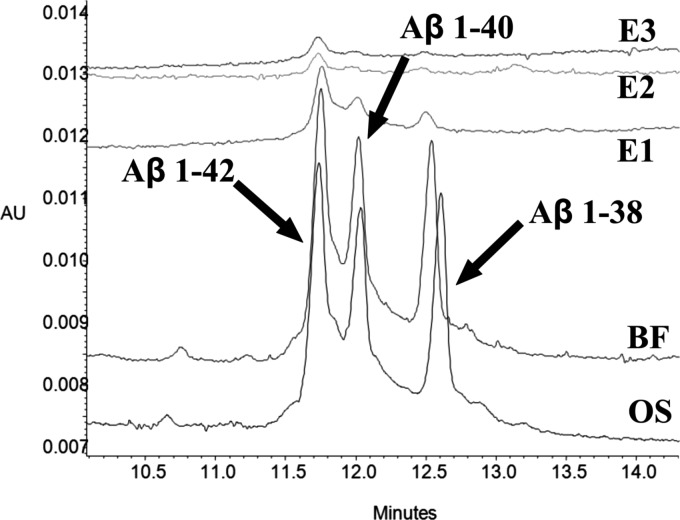
In their work, Verpillot et al. had presented Aβ peptide separation and detection by ultraviolet visible CE (CE-UV). Compatibility with 0.16% NH4OH and 100 mM NH4HCO3 elution reagents allowed analysis of the preconcentrated Aβ peptides. Original samples (OS), binding fraction (BF; supernatant with non-IP Aβ peptides), and three successive elution fractions (E1–3) from batch IP on anti-Aβ IS (pAb) were analyzed by CE-UV (Fig. 7). Despite there being the same initial concentration of each Aβ isoform, we observed higher peak intensity for eluted Aβ 1-42 (66%) than for the shorter Aβ isoforms 1-40 (23%) and 1-38 (9%). These results were confirmed also by urea/tricine/tris SDS-PAGE, where the proportions of eluted Aβ peptides were 74% for Aβ 1-42, 21% for Aβ 1-40, and 5% for Aβ 1-38 (Table S-I).32 We thus concluded that the anti-Aβ IS (pAb) showed preferential capture of Aβ 1-42. To confirm this tendency, other methods for Aβ detection were also employed.
Figure 7.
CE-UV of Aβ mixture of three synthetic peptides (1-38, 1-40, 1-42) from IP performed on anti-Aβ IS (pAb): original sample (OS; sample before IP), binding fraction (BF; sample with non-IP Aβ peptides), and three successive elution fractions (E1–3; fractions with IP Aβ peptides eluted by 0.16% NH4OH). E1–3 show preferential capture of Aβ 1-42. Analytical conditions were according to Verpillot et al. (2008).
MCE for comparing magnetic anti-Aβ IS (mAb and pAb)
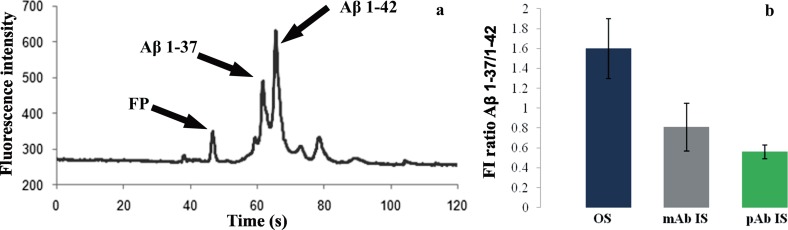
In our previous work, we presented an MCE method with fluorescent detection for profiling of 5 Aβ isoforms.28 Here, the MCE method was used for monitoring the μIP process by comparing the ratio of Aβ isoforms in the sample before the μIP and in the elution fraction using two types of immunosorbents: one coated with mAb and one with pAb anti-Aβ IgG (Fig. 8a). The results presented in Figure 8b (average of 3 repetitions) show that when sample consisting of Aβ 1-37 and Aβ 1-42 (Aβ 1-37/Aβ 1-42 = 1.6) was immunoprecipitated, the Aβ peptides were eluted with different ratios according to the Ab-coated immunosorbents: pAb (0.6) and mAb (0.8). In other words, the results show that both anti-Aβ ISs (pAb and mAb) demonstrated preferential capture of Aβ 1-42 over Aβ 1-37. Such tendency might be caused by their structural difference and higher hydrophobicity of Aβ 1-42 in comparison to the other 4 isoforms.33, 34 This feature might possibly change accessibility of the epitope for Ab, which is known as epitope masking. These observations were discussed by Wiltfang et al. in their study that compared their results from Aβ-SDS-PAGE/immunoblot with those of other authors determining Aβ concentrations in CSF.12 They reported finding that differences in Aβ isoforms concentration were due to the different types of antibodies that had been used. Thus, the origins of different antibodies, and despite their having the same specificity, could create different reactivity with immunoprecipitated peptides.
Figure 8.
(a) Electropherogram from MCE of fluorescently labeled Aβ 1-37 and 1-42 from μIP on anti-Aβ IS (mAb); FP = FluoProbe. (b) Comparison of fluorescence intensity (FI) ratio of Aβ 1-37/1-42 peptides in samples obtained from μIPs on anti-Aβ IS (pAb) and anti-Aβ IS (mAb) (averages of 3 repetitions). OS = original sample (before IP).
Immunoblotting of Aβ captured by µIP from human CSF
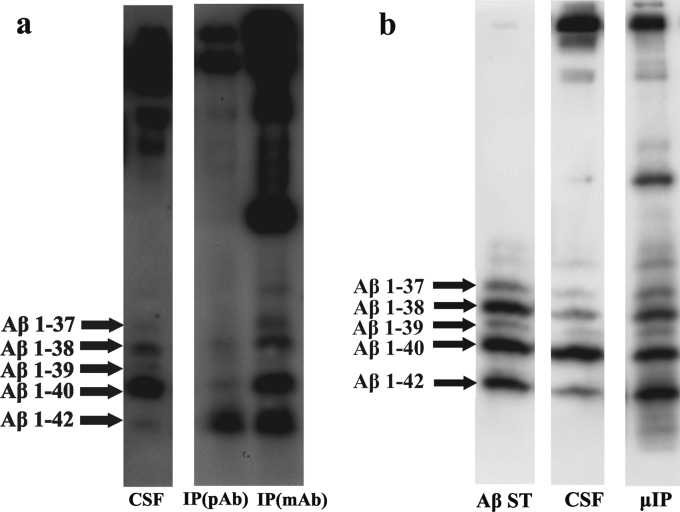
Previous testing of both anti-Aβ ISs was done with samples containing synthetic Aβ peptides or spiked CSF samples in concentration range higher than is usually found in CSF samples. The ability of both anti-Aβ ISs (pAb and mAb) to capture Aβ peptides from real human CSF samples of non-AD patients was thus tested by immunoblotting since this method affords sensitivity compatible with the concentration level of these peptides in biological samples. The two ISs were compared to show proportional immunocapture of Aβ isoforms in human CSF samples (Fig. 9a). Both antibodies used were presumed to immunocapture all 5 Aβ isoforms. Significant differences between the two ISs were observed, however, as shown in the previous results, with anti-Aβ IS (pAb) having higher affinity for the Aβ 1-42 isoform than for the other Aβ isoforms. The anti-Aβ IS (mAb) provided a more uniform and efficient capture of all 5 Aβ isoforms from CSF.
Figure 9.
Aβ peptides separated by Aβ-SDS-PAGE/immunoblot on polyvinylidene fluoride membrane (according to Wiltfang et al. (2002)). (a) Batchwise IP of CSF sample on anti-Aβ IS (pAb) and anti-Aβ IS (mAb). CSF = CSF sample before IP; IP (pAb) = batchwise IP on anti-Aβ IS (pAb); IP (mAb) = batchwise IP on anti-Aβ IS (mAb). (b) μIP of CSF sample on anti-Aβ IS (mAb). Aβ ST = Aβ standard peptides mixture corresponding to levels in CSF; μIP = μIP on anti-Aβ IS (mAb).
The ability of anti-Aβ IS (mAb) to preconcentrate the Aβ isoforms from CSF on-chip was also confirmed. The μIP of CSF sample was performed and the preconcentrated Aβ peptides were analyzed by immunoblotting (Fig. 9b). An IS with monoclonal antibodies showed a representation of all Aβ isoforms in proportions nearly similar to those present in the initial CSF sample, albeit still with a slight preference for the Aβ 1-42 isoform (see Table TABLE I.).
TABLE I.
Quantification of amyloid β (Aβ) isoforms in human CSF before and after μIP.
| Aß isoforms | CSF before μIP (ng/ml) | CSF after μIP (ng/ml) | CSF before μIP (%) | CSF after μIP (%) |
|---|---|---|---|---|
| 1-37 | 2.32 | 0.15 | 6.0 | 6.1 |
| 1-38 | 4.81 | 0.34 | 12.4 | 13.7 |
| 1-39 | 2.60 | 0.14 | 6.7 | 5.7 |
| 1-40 | 24.25 | 0.88 | 62.7 | 35.6 |
| 1-42 | 4.69 | 0.96 | 12.1 | 38.8 |
CONCLUSION
Micro-immunoprecipitation is a suitable method for isolation and preconcentration of Aβ peptide biomarkers occurring in low concentrations from CSF. Variability in elution reagents allows releasing the target molecules in a medium compatible with the subsequent detection method, be that performed by MALDI-TOF-MS, CE, MCE and/or immunoblotting. CE provided the best combination of sensitivity, convenience, and resolution, with a baseline resolution of the three isoforms investigated. Microchip electrophoresis, however, revealed promising potential for on-line connection and/or integration of immunocapture and detection into a single microfluidic device. A certain rate of Aβ 1-42 preferential capture was observed for both investigated antibodies (mAb or pAb), although with anti-Aβ IS (mAb) the representation of Aβ peptides was much closer to the composition of the initial human CSF sample. The proportional capture of Aβ isoforms has great significance for the clinical diagnosis of AD and in research as to the disease’s pathogenesis. Hence, this effect will be considered in our next investigation. Our future work will also focus on developing an integrated lab-on-chip device for preconcentration, separation, and detection of 5 Aβ isoforms. In the aim to enhance the sensitivity of the analysis, an additional preconcentration component is now under testing to be integrated into the microfluidic device. Such system will be evaluated on samples from patients affected by AD or other dementias and which might contribute in the future to rapid Aβ profiling of patients suspected of having AD and to their diagnosis.
ACKNOWLEDGMENTS
This work was supported by the European Union’s NADINE project under Contract No. 246513. Special thanks are due to H.-W. Klafki for his advice and suggestions related to IS preparation.
References
- Cummings J. L., Doody R., and Clark C., Neurology 69, 1622 (2007). 10.1212/01.wnl.0000295996.54210.69 [DOI] [PubMed] [Google Scholar]
- Jalbert J. J, Daiello L. A., and Lapane K. L., Epidemiol. Rev. 30, 15 (2008). 10.1093/epirev/mxn008 [DOI] [PubMed] [Google Scholar]
- Feldman H. H., Van Baelen B., Kavanagh S. M., and Torfs K. E., Alzheimer Dis. Assoc. Discord. 19, 29 (2005). 10.1097/01.wad.0000157065.43282.bc [DOI] [PubMed] [Google Scholar]
- Brookmeyer R., Gray S., and Kawas C., Am. J. Public Health 88, 1337 (1998). 10.2105/AJPH.88.9.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B., and Selkoe D. J., Nature 359, 322 (1992). 10.1038/359322a0 [DOI] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schiossmacher M., Whaley J., Swindlehurst C., McCormack R., Wolfert R., Selkoe D., Lieberburg I., and Schenk D., Nature 359, 325 (1992). 10.1038/359325a0 [DOI] [PubMed] [Google Scholar]
- Andreasen N., Minthon L., Davidsson P., Vanmechelen E., Vanderstichele H., Winblad B., and Blennow K., Arch. Neurol. 58, 373 (2001). 10.1001/archneur.58.3.373 [DOI] [PubMed] [Google Scholar]
- Kanai M., Matsubara E., Isoe K., Urakami K., Nakashima K., Arai H., Sasaki H., Abe K., Iwatsubo T., Kosaka T., Watanabe M., Tomidokoro Y., Shizuka M., Mizushima K., Nakamura T., Igeta Y., Ikeda Y., Amari M., Kawarabayashi T., Ishiguro K., Harigaya Y., Wakabayashi K., Okamoto K., Hirai S., and Shoji M., Ann. Neurol. 44, 17 (1998). 10.1002/ana.410440108 [DOI] [PubMed] [Google Scholar]
- Blennow K. and Hampel H., Lancet Neurol. 2, 605 (2003). 10.1016/S1474-4422(03)00530-1 [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K., Multhaup G., Beyreuther K., and Muller-Hill B., Nature 325, 733 (1987). 10.1038/325733a0 [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T. and Shoji M., Curr. Opin. Psychiatr. 21, 3 (2008). 10.1097/YCO.0b013e3282fc989f [DOI] [PubMed] [Google Scholar]
- Wiltfang J., Esselmann H., Bibl M., Smirnov A., Otto M., Paul S., Schmidt B., Klafki H.-W., Maler M., Dyrks T., Bienert M., Beyermann M., Rüther E., and Kornhuber J., J. Neurochem. 81, 481 (2002). 10.1046/j.1471-4159.2002.00818.x [DOI] [PubMed] [Google Scholar]
- De Leon M. J., Mosconi L., Blennow K., DeSanti S., Zinkowski R., Mehta P. D., Pratico D., Tsui W., Saint L. A., Sobanska L., Brys M., Li Y., Rich K., Rinne J., and Rusinek H., Ann. N. Y. Acad. Sci. 1097, 114 (2007). 10.1196/annals.1379.012 [DOI] [PubMed] [Google Scholar]
- Bottenus D., Jubery T. Z., Ouyang Y., Dong W.-J., Dutta P., and Ivory C. F., Lab Chip 11, 890 (2011). 10.1039/c0lc00490a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana T. K. and Santiago J. G., Lab Chip 9, 1377 (2009). 10.1039/b815460k [DOI] [PubMed] [Google Scholar]
- Mohamadi M. R., Kaji N., Tokeshi M., and Baba Y., Anal.Chem. 79, 3667 (2007). 10.1021/ac0623890 [DOI] [PubMed] [Google Scholar]
- Gebauer P. and Bocek P., Electrophoresis 23, 3858 (2002). 10.1002/elps.200290006 [DOI] [PubMed] [Google Scholar]
- Lion N., Rohner T. C., Dayon L., Arnaud I. L., Damoc E., Youhnovski N., Wu Z.-Y., Roussel CH., Josserand J., Jensen H., Rossier J., Przybylski M., and Girault H. H., Electrophoresis 24, 3533 (2003). 10.1002/elps.200305629 [DOI] [PubMed] [Google Scholar]
- Clarke N. J., Tomlinson A. J., Ohyagi Y., Younkin S., and Naylor S., FEBS Lett. 430, 419 (1998). 10.1016/S0014-5793(98)00706-6 [DOI] [PubMed] [Google Scholar]
- Gelfanova V., Higgs R. E., Dean R. A., Holtzman D. M., Farlow M. R., Siemers E. R., Boodhoo A., Qian Y., He X., Jin Z., Fisher D. L., Cox K. L., and Hale J. E., Brief. Funct. Genomic. Proteomic. 6, 149 (2007). 10.1093/bfgp/elm010 [DOI] [PubMed] [Google Scholar]
- Portelius E., Tran A. J., Andreasson U., Persson R., Brinkmalm G., Zetterberg H., Blennow K., and Westman-Brinkmalm A., J. Proteome Res. 6, 4433 (2007). 10.1021/pr0703627 [DOI] [PubMed] [Google Scholar]
- Chen T., Lei J., and Tong A., Luminescence 20, 256 (2005). 10.1002/bio.858 [DOI] [PubMed] [Google Scholar]
- Kaboord B. and Perr M., in Sample Preparation and Fractionation: Volume 1, edited by Posch A. (Humana, New York, 2008), p. 349. [Google Scholar]
- Kim J., Johnson M., Hill P., and Gale B. K., Integr. Biol. 1, 574 (2009). 10.1039/b905844c [DOI] [PubMed] [Google Scholar]
- Le Nel A., Minc N., Smadja C., Slovakova M., Bilkova Z., Peyrin J., Viovy J., and Taverna M., Lab Chip 8, 294 (2008). 10.1039/b715238h [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Science 184, 56 (1974). 10.1126/science.184.4132.56 [DOI] [Google Scholar]
- Jankovicova B., Zverinova Z., Mohamadi M. R., Slovakova M., Hernychova L., Faigle W., Viovy J., and Bilkova Z., in New Trends in Alzheimer and Parkinson Related Disorders, edited by Fisher A. and Hanin E. (Medimond S.r.l., Bologna, Italy, 2009), pp. 239–244. [Google Scholar]
- Mohamadi M. R., Svobodova Z., Verpillot R., Esselmann H., Wiltfang J., Otto M., Taverna M., Bilkova Z., and Viovy J., Anal. Chem. 82, 7611 (2010). 10.1021/ac101337n [DOI] [PubMed] [Google Scholar]
- Slovakova M., Minc N., Bilkova Z., Smadja C., Faigle W., Futterer C., Taverna M., and Viovy J., Lab Chip 5, 935 (2005). 10.1039/b504861c [DOI] [PubMed] [Google Scholar]
- Klafki H.-W., Wiltfang J., and Staufenbiel M, Anal. Biochem. 237, 24 (1996). 10.1006/abio.1996.0195 [DOI] [PubMed] [Google Scholar]
- Verpillot R., Otto M., Klafki H.-W., and Taverna M., J. Chromatogr. A 1214, 157 (2008). 10.1016/j.chroma.2008.10.051 [DOI] [PubMed] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.4722588 for Fig. S-1, Fig. S-2, and Table S-I.
- Jarrett J. T., Berger E. P., and P. T.Lansbury, Jr., Biochemistry 32, 4693 (1993). 10.1021/bi00069a001 [DOI] [PubMed] [Google Scholar]
- Soto C., Branes M. C., Alvarez J., and Inestrosa N. C., J. Neurochem. 63, 1191 (1994). 10.1046/j.1471-4159.1994.63041191.x [DOI] [PubMed] [Google Scholar]