Abstract
Although the neuropathology of Korsakoff’s syndrome (KS) was first described well over a century ago and the characteristic brain pathology does not pose a diagnostic challenge to pathologists, there is still controversy over the neuroanatomical substrate of the distinctive memory impairment in these patients. Cohort studies of KS suggest a central role for the mammillary bodies and mediodorsal thalamus, and quantitative studies suggest additional damage to the anterior thalamus is required. Rare cases of KS caused by pathologies other than those of nutritional origin provide support for the role of the anterior thalamus and mammillary bodies. Taken together the evidence to date shows that damage to the thalamus and hypothalamus is required, in particular the anterior thalamic nucleus and the medial mammillary nucleus of the hypothalamus. As these nuclei form part of wider memory circuits, damage to the inter-connecting white matter tracts can also result in a similar deficit as direct damage to the nuclei. Although these nuclei and their connections appear to be the primary site of damage, input from other brain regions within the circuits, such as the frontal cortex and hippocampus, or more distant regions, including the cerebellum and amygdala, may have a modulatory role on memory function. Further studies to confirm the precise site(s) and extend of brain damage necessary for the memory impairment of KS are required.
Keywords: Wernicke encephalopathy, thiamin deficiency, alcoholism, diencephalon, memory
The key neuropsychological features of Korsakoff’s syndrome (KS), that are discussed in detail elsewhere in this issue, are profound deficits in memory for new material (anterograde) and temporally-graded deficits in remote memory (retrograde). The neuroanatomical regions that subserve these functions are a complex circuit of cortical and subcortical nuclei connected by white matter tracts. Although we have learned a great deal from the study of lesions in any one of these nuclei or tracts, focussing on a single site does not adequately reflect the degree of interconnectedness of the circuit. Neuropathological examinations of well characterized patients with KS have yielded valuable information on both the type and topography of pathology in KS, however the site or sites of pathology that are both necessary and sufficient to cause KS have not been fully elucidated. Furthermore, the mechanism(s) of cell damage and the explanation for the regional vulnerability are not known. There is also controversy over the relative contribution of alcohol abuse and whether a number of different lesions affecting different nuclei can result in a similar clinical syndrome.
Neuropathological studies have the advantage of being able to detect small or subtle lesions below the resolution of imaging modalities, to allow the investigation of co-existing or contributory pathologies and to study multiple nuclei in the same individual; however, they are inherently retrospective and the interval between the onset of the disorder and autopsy is often prolonged. Adaptive changes, such as secondary degeneration of nuclei or fiber tracts following injury to interconnected brain regions, can alter the pathological picture. Such changes can obscure primary pathologies and may result in erroneous conclusions being drawn. A further drawback is the marked decline over the past decades in the number of autopsies being performed. This means that individuals with chronic disorders are often not autopsied, so the potential for more refined studies using improved histological and molecular techniques is reduced. Newly developed imaging techniques have enhanced the in vivo diagnosis of the Wernicke-Korsakoff syndrome (WKS) and, for the first time, allowed us to study in real time brain connectivity as well as anatomy. These significant advances would be made even more valuable if coupled with detailed histopathological assessments.
Neuropathology of WKS
Macroscopic examination of the brain in patients with KS reveals little overt pathology. Shrinkage and brown discolouration of the mammillary bodies and dilatation of the ventricular system are seen to varying degrees in the majority of patients. Rarely, grey discolouration indicative of necrosis is seen in the regions surrounding the third and fourth ventricles. The most striking microscopic feature is gliosis that results, in part, from an increase in packing density of cells due to atrophy of these regions. In most instances there is apparent sparing of neurons, although quantitative studies reveal fewer neurons in most regions compared with controls (see section below). Evidence of prior haemorrhage, indicated by the presence of hemosiderin-laden macrophages, and endothelial hypertrophy indicative of Wernicke encephalopathy (Harris et al., 2008) are seen in some patients.
Much of what we have learned of the distribution of pathology in the Wernicke-Korsakoff syndrome (WKS), and the correlates with clinical deficits, comes from the large autopsy series of Malamud and Skillicorn (N=70; (Malamud and Skillicorn, 1956)) and Victor and colleagues (N=245; 82 with neuropathological examination;(Victor et al., 1989)). These series were collected in California between 1946 and 1956, and Massachusetts between 1950 and 1961, and are noteworthy for the extent of the neuropathological examination and the rich clinical data used for correlations. Table 1 outlines the characteristics of the groups studied. In both series the male to female ratio is approximately 2:1 and the mean age at onset is in the 50s, although the range from 20s to 70s. Malamud and Skillicorn (Malamud and Skillicorn, 1956) studied inpatients from psychiatric facilities who were institutionalized due to “mental deterioration”. Sixty-two (90%) had a history of alcoholism. In Victor and colleagues series (Victor et al., 1989), which was derived from general hospital admissions, only two patients were confirmed as not alcoholic. Of the remaining alcoholism was confirmed in 175.
Table 1.
Characteristics and neuropathological findings in alcoholic WKS
| Malamud & Skillicorn | Victor et al. | |
|---|---|---|
| N | 70 | 245 |
| Gender (M:F) | 52:18 | 154:91 |
| Mean age at onset (y) | 58 | 51 (est.) |
| History of alcoholism (N, %) | 63 (90%) | 243 (99%) |
| Neuropathology (%) | ||
| Mammillary bodies | 96 | 100 |
| Dorsomedial thalamus | 53 | 88 |
| Pulvinar | 4 | 85 |
| Brainstem nuclei | 23 | 55 |
| Cerebellum | 34 | 56 |
Neuropathological examination revealed lesions in the mammillary bodies of virtually all patients, however the frequency of lesions in other periventricular regions varied markedly between the two studies (table 1). These differences in prevalence of lesions may reflect methodological differences in the neuropathological examination. For example, in many cases Victor and colleagues examined serial sections of periventricular regions increasing their probability of detecting abnormalities. Although both studies are extensive in the breadth of neuropathological examination neither comment on the severity of pathology or the involvement of various nuclei within a single patient. For these reasons it is difficult to develop a complete picture of the anatomical loci that are key to the clinical deficits in KS. Victor and colleagues conclude that it is involvement of the mammillary bodies, mediodorsal nucleus of the thalamus and medial pulvinar that correlates with the presence of amnesia in their series (Victor et al., 1989). The absence of pathology in the mediodorsal nucleus of the thalamus in five patients with Wernicke encephalopathy but no evidence of amnesia led to the final conclusion that this nucleus plays a key role in development of memory dysfunction.
Not all authors agree that damage to the mediodorsal nucleus of the thalamus is crucial for KS. Mair and colleagues (Mair et al., 1979) described two patients with well documented KS in that there was damage to the mammillary bodies and gliosis adjacent to the third ventricle but no apparent neuronal loss from the mediodorsal nucleus, or from elsewhere in the thalamus. The authors concluded, following an extensive review of the literature of the time, that the mammillary bodies are the key site of pathology in KS. A similarly extensive clinicopathological study of two cases by Mayes and colleagues confirmed the finding of gliosis adjacent to the third ventricle and sparing of the mediodorsal nucleus of the thalamus (Mayes et al., 1988).
Involvement of memory circuits in KS
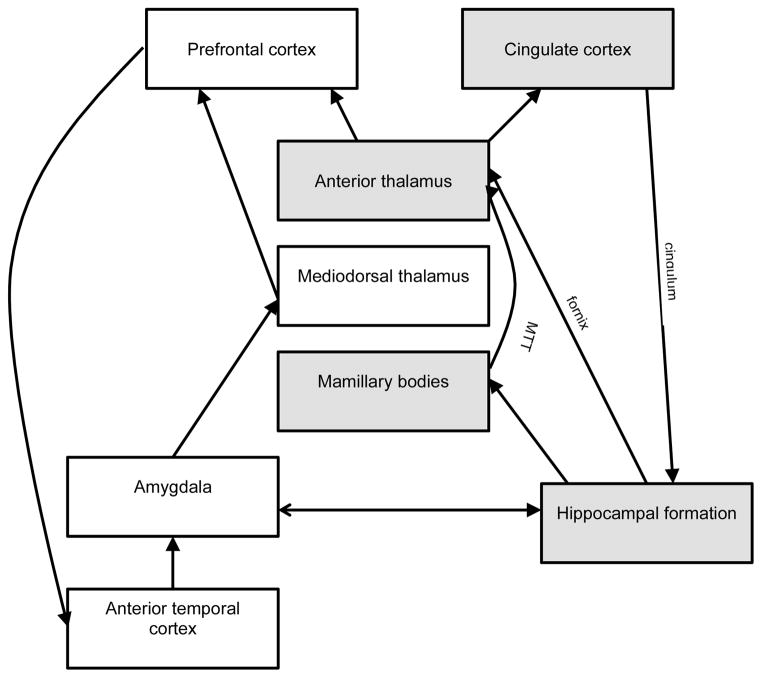
Our current understanding of the nuclei and pathways involved in the various aspects of memory function has been derived from a combination of investigations using patients with brain lesions, behavioural studies, functional imaging and electrophysiology. These data have been enhanced with studies of animal models. Some of the principal brain areas involved in episodic memory are the hippocampal formation, mammillary bodies, anterior thalamic nucleus and cingulate cortex (Aggleton and Brown, 1999). These regions are connected by the fornix, mamillothalamic tract and cingulum (figure 1).
Figure 1.
A schematic diagram of the brain regions and connections involved in memory. The major hippocampus-anterior thalamus axis (shaded) involves the hippocampus, anterior thalamus, mammillary bodies and cingulate cortex and is connected by the fornix, mamillothalamic tract (MTT) and cingulum. The extended connections involving the amygdala, mediodorsal thalamus, and temporal and frontal cortices have a modulatory role (for description see (Aggleton and Brown, 1999)).
In Aggleton and Brown’s description of the circuitry of episodic memory they designate as central the hippocampus-anterior thalamus axis and then as secondary, a more diffuse and extended network of connections that involves the temporal and frontal cortices (Aggleton and Brown, 1999). The anterior thalamus receives direct input from the hippocampus via the fornix and indirect input from the hippocampus via the mammillary bodies and the mamillothalamic tract (figure 1). Disruption to these connections is at the heart of anterograde amnesia, although there is likely involvement of other midline thalamic nuclei. Connections between the anterior thalamus and frontal lobe (prefrontal and cingulate cortices), reciprocal connections between the frontal lobe and medial temporal cortices and between the mediodorsal thalamus and medial temporal cortices provide modulatory influences on memory function. Lesions in these cortical regions and the cingulum have a less marked effect on memory function (Aggleton and Brown, 1999). Furthermore, some authors have highlighted the modulatory role of the amygdala (Mair et al., 1979; Richardson et al., 2004).
Quantitative, postmortem studies in KS are rare and the sample sizes small. Using unbiased stereological techniques we compared the brains of KS patients (N=8) with those from WKS patients with pathological evidence of thiamin deficiency but without amnesia (N=5), alcoholics without WKS or amnesia (N=5), and normal controls (N=6) (Harding et al., 2000). When compared with controls, non-amnestic alcoholics showed no significant reduction in either the volume or neuron number in the mammillary body, anterior principal or mediodorsal nuclei of the thalamus (table 2). WKS patients without amnesia showed significantly fewer neurons in both the mammillary bodies (53% of control mean) and mediodorsal thalamus (52% of control mean), but not the anterior principal nucleus. Compared with controls, KS patients showed both smaller volume and fewer neurons in all three nuclei (table 2; figure 2; (Harding et al., 2000)).
Table 2.
Quantitative neuropathological assessment of brain regions involved in memory function in KS in comparison with alcoholics without KS and those with thiamin deficiency but no amnesia (WE). Values expressed as percentage of control mean.
| Region | Volume | Neuron number | Ref | ||||
|---|---|---|---|---|---|---|---|
| Alcoholic | WE | KS | Alcoholic | WE | KS | ||
| Mediodorsal thalamus | 100 | 88 | 56# | 100 | 52# | 36# | 1 |
| Anterior principal thalamus | 93 | 87 | 63# | 100 | 86 | 47# | 1 |
| Mammillary bodies | 89 | 78 | 33# | 98 | 53# | 32# | 1 |
| Dorsolateral prefrontal ctx (BA8) | 77# | 80# | 84 | 2 | |||
| Primary motor ctx (BA4) | 93 | 81 | 85 | 2 | |||
| Hippocampal CA1 | 100 | - | 100 | 3 | |||
| Cerebellar vermis | 84 | 100* | - | 79 | 75* | - | 4 |
| Basal nucleus of Meynert | - | - | - | 100 | 76# | 79# | 5 |
significantly reduced compared with control values. * 3 of 6 WE cases had memory impairment.
References: 1=Harding et al. 2000; 2=Kril et al. 1997; 3 = Harding et al. 1997; 4 = Baker et al. 1999; 5 =Cullen et al. 1997
Figure 2.
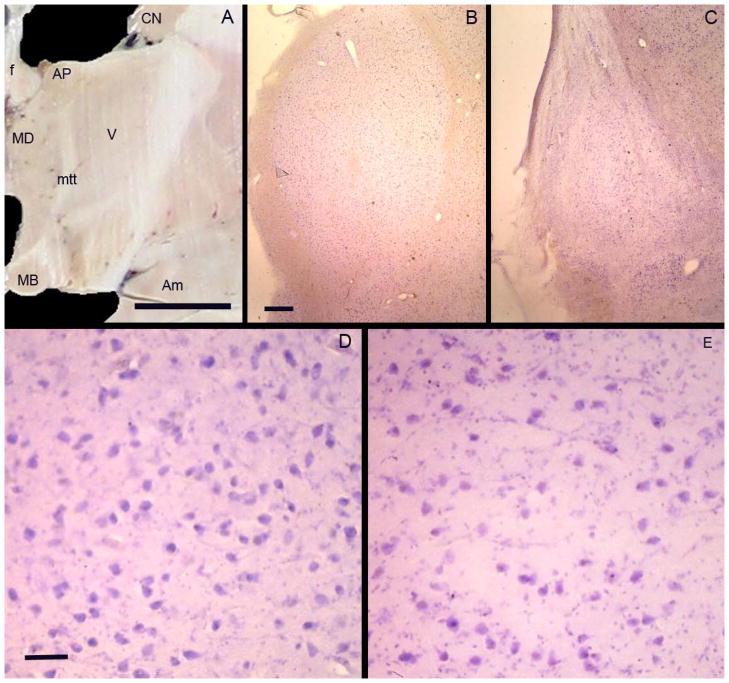
(A) A photograph of a coronal section through the diencephalon of a normal brain showing the nuclei involved in memory circuits. Nuclei labelled are AP= anterior principal nucleus of the thalamus; MD = mediodorsal nucleus of the thalamus; MB = mammillary body (medial mammillary nucleus of the hypothalamus). The tracts connecting these nuclei are the mamillothalamic tract (mtt) and fornix (f). The caudate nucleus (CN) and amygdala (Am) are also labelled. Photomicrographs of the anterior principal nucleus of the thalamus in a control subject (B, D) and KS patient (C, E). The volume of the nucleus is smaller in KS (C) compared with the control (B). A decreased density of neurons and increased density of glia can be seen in KS (E). Bar in A = 1cm. Bar in B = 1mm (equivalent for C). Bar in D = 50μm (equivalent for E).
By contrast, compared with controls the volume of the hippocampus is smaller in alcoholics, including those with KS, but there is no difference in neuron number in any of the subregions including the CA1 (Harding et al., 1997). Interestingly, the smaller volume is a result of less white matter rather than a grey matter deficit and relates to whether or not the alcoholic patient was drinking at the time of death or had remained abstinent, but was independent of amnestic state (Harding et al., 1997). In the cortex, the volume of the cingulate cortex in KS is similar to controls (Kril et al., 1997), and there is no difference in neuron density in alcoholics with WKS (Kril and Harper, 1989), although the latter were not selected to include only KS patients. The only cortical region where atrophy is identified at postmortem is the dorsolateral prefrontal cortex (Kril et al., 1997). Alcoholics show approximately 20% fewer neurons in this region but this is equivalent in all alcoholic groups regardless of the presence or absence of KS (Kril et al., 1997). Atrophy and neuron loss from the amygdala is equivalent in WKS patients with and without amnesia (Kril & Halliday, unpublished data). In response to the report by Arendt and colleagues (Arendt et al., 1983) that neuron loss from the cholinergic basal forebrain is important for the development of KS, we undertook a 3-dimensional study of this region in WKS patients with and without amnesia (Cullen et al., 1997). We showed that compared with controls neuron number is reduced, but that it did not differ between groups (Cullen et al., 1997) (table 2).
Taken together these studies suggest that damage to the anterior principal nucleus of the thalamus is required for the clinical manifestation of KS; however, as the mammillary bodies, and mediodorsal nucleus of the thalamus are also damaged in these patients it is not possible, from these studies, to determine whether it is the anterior nucleus alone or a combination of two or all three nuclei that is required for KS.
KS in malnourished patients without alcoholism
Although it is well established that thiamin deficiency results in Wernicke encephalopathy in both alcoholic and non-alcoholic populations, and the underlying neuropathology is the same in both groups, KS is rarely seen in those without a history of alcoholism (Kopelman, 1995; Homewood and Bond, 1999)
This may be a reflection of the relative rarity in recent decades of thiamin deficiency outside the alcoholic population or suggest an etiological role for alcohol-related brain damage in KS. Alternatively, it could reflect differences in the natural history of thiamin deficiency in alcoholic and non-alcoholic subjects brought about, in part, by the exacerbation of thiamin deficiency by alcohol consumption (Butterworth, 1989; Kopelman, 1995; Thomson and Marshall, 2006). Alcoholic patients who develop Wernicke encephalopathy require larger doses of thiamin for successful treatment implying more severe thiamin deficiency (Cook et al., 1998). It has been also hypothesized that thiamin deficiency in non-alcoholic subjects may be more readily identified at an earlier stage of disease as symptoms are more clearly recognized in those without obvious signs of alcohol misuse (Kopelman, 1995; Thomson and Marshall, 2006). This results in an earlier and more active treatment response. Furthermore, WKS in alcoholics may result from multiple sub-clinical episodes of thiamin deficiency (Harper and Kril, 1994) whereas the insult in non-alcoholic individuals is usually a single episode.
Although they are rare, reports of KS in non-alcoholic subjects provide useful insights into the anatomic substrate(s) of the disease. Unfortunately, however, the majority of reports do not include pathological confirmation of disease or investigation of the extent and severity of neuropathology (Parkin et al., 1991). Nevertheless, the improved diagnosis of WKS with neuroimaging has allowed the topography of lesions in these patients to be explored.
One of the clearest descriptions of thiamin deficiency in non-alcoholic subjects comes from de Wardener and Lennox’s description of 52 prisoners-of-war (De Wardener and Lennox, 1947). Of the 31 patients who survived the initial episode of Wernicke encephalopathy, 29 recovered following treatment with thiamin or thiamin-rich foods and one recovered without treatment. Only a single patient showed no improvement in mental symptoms, despite physical recovery, suggesting a persistent amnesia.
Recently, Karaiskos and colleagues (Karaiskos et al., 2008) described a 44 year old man with poor dietary food intake who developed dysphagia followed by confusion, disorientation and ultimately a reduced level of consciousness. His symptoms resolved quickly following intravenous thiamin administration; however, he showed the marked anterograde and retrograde amnesia characteristic of KS. T2 and FLAIR MRI sequences, performed at the onset of mental signs, showed symmetric signal hyperintensities in the medial thalami, periaqueductal grey matter and floor of the fourth ventricle. A repeat MRI following treatment, and performed prior to discharge from hospital two months after onset of symptoms, showed no hyperintensities. Caulo and colleagues (Caulo et al., 2005) describe a 34 year old man with malnutrition due to oesophageal stenosis following surgery for cancer. The patient developed Wernicke encephalopathy and then, five days later, KS that persisted at re-assessment five months later. Initial FLAIR sequence MRI, performed 7 days after onset of Wernicke encephalopathy, showed signal hyperintensities in the medial thalamus, mammillary bodies and fornix. These hyperintensities were no longer visible on a repeat MRI performed five months after onset of symptoms.
Other pathologies resulting in KS
In addition to KS in patients with established nutritional deficiency, there are other examples of neuropathological lesions that give rise to a severe amnesic syndrome, disproportionate to any other cognitive impairment, similar to KS. These cases are not alcohol dependent nor do they have thiamin deficiency and include lesions of traumatic, neoplastic and vascular origin. It has been argued that the term KS is best reserved for those cases with persistent memory impairment and the characteristic pathological changes of thiamin deficiency, while the term “amnestic syndrome” be used for other etiologies (Kopelman, 2002). The similarities and differences in amnestic disorders of different etiologies have been reviewed by Kopelman (Kopelman, 2002); however, the similarity in memory deficits suggests that much can be learned from these cases. A significant limitation of many of these studies is that they do not have neuropathological assessments and, when present, these assessments are qualitative rather than quantitative. Furthermore, the complexity of the anatomical regions damaged often makes interpretation of such studies difficult. Simple descriptions of the anatomical disruption caused by the pathological processes are inadequate as it has been shown that routine assessment of some regions, such as the anterior principal nucleus of the thalamus and cerebral cortex, can overlook critical volume and neuronal density changes found in quantitative studies (Kril et al., 1997; Harding et al., 2000). Although the literature contains numerous case reports of KS-like features following a variety of neurological insults, only those that shed light on the neuroanatomical substrates of KS are reviewed here.
Two historical case reports of penetrating injuries to the brain, one with a miniature fencing foil (patient NA; (Teuber et al., 1968)) and another with a snooker cue (patient BJ; (Dusoir et al., 1990)), show marked anterograde amnesia with relative preservation of other cognitive abilities, similar to KS. In each case the implement entered through the nostril and penetrated the basal regions of the brain. In BJ a structural MRI revealed bilateral damage to the hypothalamus, including the mammillary bodies, but no damage to the thalamus (Dusoir et al., 1990). CT scanning of NA performed 17 years after the injury (Squire and Moore, 1979) showed damage to the left mediodorsal thalamus; however, subsequent MRIs performed 26 and 27 years after the injury showed more extensive damage that included many of the intralaminar nuclei of the thalamus, the ventral lateral and ventral anterior nuclei, ventral parts of the mediodorsal nucleus, the posterior hypothalamus and bilateral mammillary bodies (Squire et al., 1989). In addition, there was damage to parts of the anterior temporal lobe and amygdala. Importantly, the fiber tracts of the internal medullary lamina, mamillothalamic tract and postcommissural fornix were also disrupted. The extent of the damage, together with the secondary degeneration over time, damage to white matter tracts and the temporal lobe, make it difficult to assess the relative contribution of diencephalic nuclei to the memory impairment seen in this patient. Although there are other reports in the literature of amnesia similar to KS following traumatic brain injury (Jarho, 1973; Brion et al., 2001) imaging and neuropathology are either unavailable or uninformative in these cases.
Cases with clinical syndromes similar to KS have also been described following a range of neurovascular insults. KS following dominant hemisphere thalamic infarction was described in five patients by Cole and colleagues (Cole et al., 1992). In one patient the lesion involved the mediodorsal and anterior principal nuclei of the thalamus with uncertain involvement of the mamillothalamic tract. More extensive involvement of the thalamus was seen in three of the remaining cases, including bilateral involvement on one case, and the lesion was not able to be precisely located in the fourth. Bilateral infarcts involving the mamillothalamic tracts were described in a 56 year old man with acute onset of anterograde and retrograde amnesia (Yoneoka et al., 2004). Interestingly, the infarcts were of different ages with the right being remote and the left acute, suggesting a pivotal role for the left hemisphere. The patient was followed for only six months but showed no improvement in his memory function. Renou and colleagues (Renou et al., 2008) describe a 66 year old man with MRI evidence of infarction of the genu of the corpus callosum and the anterior columns of the fornix, bilaterally. The patient presented with acute onset of anterograde and retrograde amnesia and neuropsychological assessment confirmed KS. Diffusion tensor tractography performed 11 days after presentation showed marked degeneration of commissural fibres and disruption of frontal connections. At one year follow-up the severe amnesia remained.
Similarly some tumours, because of their anatomical position, are more likely to cause symptoms similar to KS. There are case reports of KS-like syndromes in patients with craniopharyngioma (Yarde et al., 1995), following removal of colloid cysts of the third ventricle (Konovalov et al., 1998; Tsivilis et al., 2008), and other periventricular tumours such as lymphomas (Toth et al., 2002) that impinge on hypothalamic and thalamic structures. One such example is the case report by McEntee and colleagues (McEntee et al., 1976) where amnesia was observed in a patient with a bilateral thalamic tumour that involved the mediodorsal nuclei, but spared both the mammillary bodies and anterior thalamic nuclei. However, the complexity of the anatomical regions involved in such cases, the often diffuse spread of the tumour and the brain’s reaction to the tumour makes interpretation difficult and mean these cases do not contribute greatly to our understanding of the anatomical substrate of KS.
Cerebellar involvement in KS
In recent years it has been shown that the cerebellum, in addition to its established role in motor control, is involved in cognition. Correlations between motor dysfunction and cerebellar pathology have been well documented using radiological (Sullivan et al., 2000; Sullivan et al., 2006) and neurological (Allsop and Turner, 1966) studies. It has also been postulated that cerebello-cerebral disconnection could have a role in the memory and executive dysfunction of KS (Wijnia and Goossensen, 2010; Zahr et al., 2010). Fewer cerebellar Purkinje cells compared with controls have been demonstrated in alcoholics (Phillips et al., 1987) including those with WKS (Phillips et al., 1990; Baker et al., 1999). Although in this study cases of KS were not analyzed separately, the marked loss of Purkinje cells in thiamin deficient alcoholics with mental state signs (Baker et al., 1999) adds support to the hypothesis that disruption of cerebello–cerebral pathways might contribute to neurocognitive dysfunction in KS. Nevertheless, the relative contributions of thiamin deficiency and alcoholism remain to be fully elucidated.
Animal models of KS
A number of animal models of alcohol-related disorders have been developed (reviewed by (Vetreno et al., 2011)). Pyrithiamine induced thiamin deficiency (PTD) in the rat is a well validated experimental model to study the pathophysiology of human Wernicke encephalopathy where the histological lesions generally mirror those seen in the human disorder. If rats are allowed to progress through a severe bout of thiamin deficiency, they develop lesions in the anterior and midline thalamus as well as the mammillary bodies (Zhang et al., 1995; Pfefferbaum et al., 2007). Furthermore, these animals show impaired memory function (Langlais and Savage, 1995) and older animals demonstrate greater susceptibility to brain damage (Pitkin and Savage, 2001). The PTD model has assisted researchers to address the relative contribution of different anatomical regions to the memory deficits in KS.
In addition, animal models have also been used to establish the relative contribution of alcohol to KS. Experiments in chicks exposed to both thiamin deficiency and ethanol have shown that they develop irreversible memory impairment, even after thiamin replacement. By contrast, the memory of chicks exposed to thiamin deficiency alone returns to normal following correction of thiamin deficiency. Thus, there is some experimental evidence that alcohol permanently damages the thiamin-depleted brain to a greater degree than thiamin depletion alone (Ciccia and Langlais, 2000; Crowe and El-Hadj, 2002). White matter also appears to be more susceptible to the combined effects of thiamin deficiency and ethanol exposure. Rats exposed to PTD plus ethanol had greater thinning of myelin in the corpus callosum than did those that received only PTD treatment (He et al., 2007).
Conclusions
Despite considerable evidence for the involvement of the diencephalon in the memory impairment of KS, some uncertainly still exists as to the precise location of the minimum lesion required. The absence of pure, focal lesions in humans has meant that reports from a variety of diseases, involving various combinations of nuclei and their connections, have been used to infer the neuroanatomical basis of KS. Taken together, the studies reviewed above would suggest that damage to the thalamus and hypothalamus are required, in particular the anterior thalamic nucleus and the medial mammillary nucleus of the hypothalamus. This damage can be achieved either by direct involvement of the nuclei or damage to the major connections, namely the fornix and mamillothalamic tract. Although damage to other parts of the circuit, such as cortical regions of the frontal and medial temporal lobes, or other modulatory loci such as the cerebellum and amygdala, may have a contributory effect it would appear that in isolation lesions of these regions are insufficient to result in KS. A combination of rigorous neuropsychological assessment, longitudinal in vivo neuroimaging and quantitative neuropathology is required to provide the definitive answer to the question of the neuroanatomical substrates for the various cognitive and function impairments seen in KS.
Acknowledgments
The research described in this article was facilitated by the NSW Tissue Resource Centre at the University of Sydney which is supported by the National Health and Medical Research Council of Australia, Schizophrenia Research Institute and the National Institute of Alcohol Abuse and Alcoholism (NIH (NIAAA) R24AA012725).
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. The Behavioral and brain sciences. 1999;22:425–44. discussion 44–89. [PubMed] [Google Scholar]
- Allsop J, Turner B. Cerebellar degeneration associated with chronic alcoholism. Journal of the neurological sciences. 1966;3:238–58. doi: 10.1016/0022-510x(66)90024-4. [DOI] [PubMed] [Google Scholar]
- Arendt T, Bigl V, Arendt A, Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer’s disease, paralysis agitans and Korsakoff’s psychosis. Acta Neuropathologica. 1983;61:101–8. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Harding AJ, Kril JJ, Harper CG. Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience. 1999;91:429–38. doi: 10.1016/s0306-4522(98)90664-9. [DOI] [PubMed] [Google Scholar]
- Brion S, Plas J, Mikol J, Jeanneau A, Brion F. Post-traumatic Korsakoff’s syndrome: clinical and anatomical report. Encephale. 2001;27:513–26. [PubMed] [Google Scholar]
- Butterworth RF. Effects of thiamine deficiency on brain metabolism: implications for the pathogenesis of the Wernicke-Korsakoff syndrome. Alcohol and Alcoholism. 1989;24:271–9. doi: 10.1093/oxfordjournals.alcalc.a044913. [DOI] [PubMed] [Google Scholar]
- Caulo M, Van Hecke J, Toma L, Ferretti A, Tartaro A, Colosimo C, Romani GL, Uncini A. Functional MRI study of diencephalic amnesia in Wernicke-Korsakoff syndrome. Brain. 2005;128:1584–94. doi: 10.1093/brain/awh496. [DOI] [PubMed] [Google Scholar]
- Ciccia RM, Langlais PJ. An examination of the synergistic interaction of ethanol and thiamine deficiency in the development of neurological signs and long-term cognitive and memory impairments. Alcoholism, clinical and experimental research. 2000;24:622–34. [PubMed] [Google Scholar]
- Cole M, Winkelman MD, Morris JC, Simon JE, Boyd TA. Thalamic amnesia: Korsakoff syndrome due to left thalamic infarction. Journal of the neurological sciences. 1992;110:62–7. doi: 10.1016/0022-510x(92)90010-i. [DOI] [PubMed] [Google Scholar]
- Cook CC, Hallwood PM, Thomson AD. B-vitamin deficiency and neuro-psychiatric syndromes in alcohol misuse. Alcohol and Alcoholism. 1998;33:317–36. doi: 10.1093/oxfordjournals.alcalc.a008400. [DOI] [PubMed] [Google Scholar]
- Crowe SF, El-Hadj D. Phenytoin ameliorates the memory deficit induced in the young chick by ethanol toxicity in association with thiamine deficiency. Pharmacology, biochemistry, and behavior. 2002;71:215–21. doi: 10.1016/s0091-3057(01)00650-5. [DOI] [PubMed] [Google Scholar]
- Cullen KM, Halliday GM, Caine D, Kril JJ. The nucleus basalis (Ch4) in the alcoholic Wernicke-Korsakoff syndrome: Reduced cell number in both amnesic and non-amnesic patients. Journal of Neurology, Neurosurgery and Psychiatry. 1997;63:315–20. doi: 10.1136/jnnp.63.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wardener HE, Lennox B. Cerebral beriberi. Lancet Jan. 1947;4:11–7. [PubMed] [Google Scholar]
- Dusoir H, Kapur N, Byrnes DP, McKinstry S, Hoare RD. The role of diencephalic pathology in human memory disorder. Evidence from a penetrating paranasal brain injury. Brain. 1990;113 ( Pt 6):1695–706. doi: 10.1093/brain/113.6.1695. [DOI] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123 (Pt 1):141–54. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Wong A, Svoboda M, Kril JJ, Halliday GM. Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus. 1997;7:78–87. doi: 10.1002/(SICI)1098-1063(1997)7:1<78::AID-HIPO8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J. An introduction to alcohol-induced brain damage and its causes. Alcohol and Alcoholism. Supplement. 1994;2:237–43. [PubMed] [Google Scholar]
- Harris J, Chimelli L, Kril JJ, Ray D. Nutritional deficiencies, metabolic disorders and toxins affecting the nervous system. In: Love S, Louis DN, Ellison DW, editors. Greenfield’s Neuropathology. 8. London: Edward Arnold Ltd; 2008. pp. 675–73. [Google Scholar]
- He X, Sullivan EV, Stankovic RK, Harper CG, Pfefferbaum A. Interaction of thiamine deficiency and voluntary alcohol consumption disrupts rat corpus callosum ultrastructure. Neuropsychopharmacology. 2007;32:2207–16. doi: 10.1038/sj.npp.1301332. [DOI] [PubMed] [Google Scholar]
- Homewood J, Bond NW. Thiamin deficiency and Korsakoff’s syndrome: failure to find memory impairments following nonalcoholic Wernicke’s encephalopathy. Alcohol. 1999;19:75–84. doi: 10.1016/s0741-8329(99)00027-0. [DOI] [PubMed] [Google Scholar]
- Jarho L. Korsakoff-like amnesic syndrome in penetrating brain injury. A study of Finnish war veterans. Acta Neurol Scand Suppl. 1973;54:3–156. [PubMed] [Google Scholar]
- Karaiskos I, Katsarolis I, Stefanis L. Severe dysphagia as the presenting symptom of Wernicke-Korsakoff syndrome in a non-alcoholic man. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2008;29:45–6. doi: 10.1007/s10072-008-0859-8. [DOI] [PubMed] [Google Scholar]
- Konovalov AN, Dobrokhotova TA, Voronina IA, Urakov SV. Case of Korsakoff syndrome and colloid cyst of the 3rd ventricle. Zh Nevrol Psikhiatr Im S S Korsakova. 1998;98:49–51. [PubMed] [Google Scholar]
- Kopelman MD. The Korsakoff syndrome. The British journal of psychiatry : the journal of mental science. 1995;166:154–73. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Disorders of memory. Brain. 2002;125:2152–90. doi: 10.1093/brain/awf229. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–98. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of alcoholic brains. Acta neuropathologica. 1989;79:200–4. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Savage LM. Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behavioural brain research. 1995;68:75–89. doi: 10.1016/0166-4328(94)00162-9. [DOI] [PubMed] [Google Scholar]
- Mair WGP, Warrington EK, Weiskrantz L. Memory disorder in Korsakoff’s psychosis. A neuropathological and neuropsychological investigation of two cases. Brain. 1979;102:749–83. doi: 10.1093/brain/102.4.749. [DOI] [PubMed] [Google Scholar]
- Malamud N, Skillicorn SA. Relationship between the Wernicke and the Korsakoff syndrome. Archives of Neurology and Psychiatry. 1956;76:585–96. doi: 10.1001/archneurpsyc.1956.02330300015003. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Meudell PR, Mann D, Pickering A. Location of lesions in Korsakoff’s syndrome: neuropsychological and neuropathological data on two patients. Cortex; a journal devoted to the study of the nervous system and behavior. 1988;24:367–88. doi: 10.1016/s0010-9452(88)80001-7. [DOI] [PubMed] [Google Scholar]
- McEntee WJ, Biber MP, Perl DP, Benson DF. Diencephalic amnesia: a reappraisal. J Neurol Neurosurg Psychiatry. 1976;39:436–41. doi: 10.1136/jnnp.39.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin AJ, Blunden J, Rees JE, Hunkin NM. Wernicke-Korsakoff syndrome of nonalcoholic origin. Brain Cogn. 1991;15:69–82. doi: 10.1016/0278-2626(91)90016-2. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Bell RL, Sullivan EV. Development and resolution of brain lesions caused by pyrithiamine- and dietary-induced thiamine deficiency and alcohol exposure in the alcohol-preferring rat: a longitudinal magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2007;32:1159–77. doi: 10.1038/sj.npp.1301107. [DOI] [PubMed] [Google Scholar]
- Phillips SC, Harper CG, Kril J. A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain. 1987;110:301–14. doi: 10.1093/brain/110.2.301. [DOI] [PubMed] [Google Scholar]
- Phillips SC, Harper CG, Kril JJ. The contribution of Wernicke’s encephalopathy to alcohol-related cerebellar damage. Drug Alcohol Review. 1990;9:53–60. doi: 10.1080/09595239000185071. [DOI] [PubMed] [Google Scholar]
- Pitkin SR, Savage LM. Aging potentiates the acute and chronic neurological symptoms of pyrithiamine-induced thiamine deficiency in the rodent. Behavioural brain research. 2001;119:167–77. doi: 10.1016/s0166-4328(00)00350-8. [DOI] [PubMed] [Google Scholar]
- Renou P, Ducreux D, Batouche F, Denier C. Pure and acute Korsakoff syndrome due to a bilateral anterior fornix infarction: a diffusion tensor tractography study. Archives of neurology. 2008;65:1252–3. doi: 10.1001/archneur.65.9.1252. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–85. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Squire LR, Amaral DG, Zola-Morgan S, Kritchevsky M, Press G. Description of brain injury in the amnesic patient N.A. based on magnetic resonance imaging. Experimental neurology. 1989;105:23–35. doi: 10.1016/0014-4886(89)90168-4. [DOI] [PubMed] [Google Scholar]
- Squire LR, Moore RY. Dorsal thalamic lesion in a noted case of human memory dysfunction. Ann Neurol. 1979;6:503–6. doi: 10.1002/ana.410060607. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology. 2000;14:341–52. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cereb Cortex. 2006;16:1077–86. doi: 10.1093/cercor/bhj048. [DOI] [PubMed] [Google Scholar]
- Teuber HL, Milner B, Vaughan HG. Persistent anterograde amnesia after stab wound of the basal brain. Neuropsychologia. 1968;6:267–82. [Google Scholar]
- Thomson AD, Marshall EJ. The natural history and pathophysiology of Wernicke’s Encephalopathy and Korsakoff’s Psychosis. Alcohol and Alcoholism. 2006;41:151–8. doi: 10.1093/alcalc/agh249. [DOI] [PubMed] [Google Scholar]
- Toth C, Voll C, Macaulay R. Primary CNS lymphoma as a cause of Korsakoff syndrome. Surgical neurology. 2002;57:41–5. doi: 10.1016/s0090-3019(01)00650-4. [DOI] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11:834–42. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Hall JM, Savage LM. Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiology of learning and memory. 2011;96:596–608. doi: 10.1016/j.nlm.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and related Neurological Disorders of Alcoholism and Malnutrition. Philadelphia: FA Davis Co; 1989. [Google Scholar]
- Wijnia JW, Goossensen A. Cerebellar neurocognition and Korsakoff’s syndrome: an hypothesis. Medical hypotheses. 2010;75:266–8. doi: 10.1016/j.mehy.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Yarde WL, Kepes JJ, O’Boynick P. Craniopharyngioma presenting as Korsakoff psychosis. Kans Med. 1995;96:22–3. [PubMed] [Google Scholar]
- Yoneoka Y, Takeda N, Inoue A, Ibuchi Y, Kumagai T, Sugai T, Takeda K, Ueda K. Acute Korsakoff syndrome following mammillothalamic tract infarction. AJNR American journal of neuroradiology. 2004;25:964–8. [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Pitel AL, Chanraud S, Sullivan EV. Contributions of studies on alcohol use disorders to understanding cerebellar function. Neuropsychol Rev. 2010;20:280–9. doi: 10.1007/s11065-010-9141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Weilersbacher GS, Henderson SW, Corso T, Olney JW, Langlais PJ. Excitotoxic cytopathology, progression, and reversibility of thiamine deficiency-induced diencephalic lesions. Journal of neuropathology and experimental neurology. 1995;54:255–67. doi: 10.1097/00005072-199503000-00012. [DOI] [PubMed] [Google Scholar]