Abstract
BACKGROUND
Lung transplant recipients (LTR) have an increased risk of cutaneous squamous cell carcinoma (SCC) due to immunosuppressive therapy. Voriconazole, which is associated with phototoxic side effects in some patients, may be an additional risk factor for SCC in this population.
METHODS
To test whether voriconazole is a risk factor for developing SCC in LTR, we evaluated cumulative exposure to voriconazole in 327 adults who underwent lung transplantation at a single center between 1991 and 2010. Voriconazole exposure was assessed as a time-varying covariate. We analyzed risk of developing SCC over time using survival analysis methods.
RESULTS
Exposure to voriconazole was associated with a 2.6-fold increased risk for SCC. This phenomenon was dose-dependent; the risk for SCC increased by 5.6% with each 60-day exposure at a standard dose of 200mg twice daily. At five years posttransplant, voriconazole conferred an absolute risk increase for SCC of 28%.
CONCLUSIONS
These results suggest that caution should be taken when using voriconazole in LTR, as this drug increases the already high risk for SCC in this population.
Keywords: Voriconazole, Squamous Cell Carcinoma, Lung Transplantation, Skin Cancer, Risk Factors
Introduction
Skin cancer is the most common malignancy in organ transplant recipients (OTRs); cutaneous squamous cell carcinoma (SCC) is the most frequently diagnosed1. Further, OTRs are at increased risk for recurrence, metastasis, and multiple primary tumors. Lung transplant recipients (LTR) have an increased risk of developing SCC compared to abdominal transplant recipients1, likely due to older age at transplant and more intense immunosuppression used to prevent allograft rejection.
In addition to malignancies, OTRs are at high risk for invasive fungal infections. In 2002, the U.S. Food and Drug Administration (FDA) approved voriconazole for the treatment of serious fungal infections. It is a second-generation triazole broad-spectrum antifungal that inhibits P450-dependent ergosterol synthesis, disrupting cell membrane lipid formation2. While its efficacy against many molds and ease of administration have led to widespread use in many, but not all, transplant centers, its use is off-label. Voriconazole is also associated with significant side effects including vision changes, hallucinations, and hepatic enzyme abnormalities3–5. It can also cause photosensitivity, which can range from mild sunburn-like erythema to blistering pseudoporphyria6. Photosensitivity may be reversible after drug discontinuation or can progress to freckling and epithelial dysplasia7–14.
The association between voriconazole phototoxicity and SCC has been reported in conditions including chronic granulomatous disease, bone marrow transplantation, graft versus host disease, and HIV15–21. It has also been recognized in LTR, which is of particular importance given its common use22, 23. Recently, a case-control study reported that voriconazole and geographic location were independent risk factors for SCC in LTR24.
Given these findings, we sought to investigate whether voriconazole is associated with an increased risk of developing SCC in LTR. To do so, we performed a 20-year retrospective single center cohort study of LTR.
Methods
To investigate the effect of voriconazole exposure on post-transplant SCC, we performed a retrospective cohort study of all patients who underwent single-, double-, or heart-lung transplantation at the University of California at San Francisco (UCSF) from 1/1/1991 to 12/31/2010. Demographic data including date of death was acquired from the Organ Procurement and Transplantation Network (OPTN) registry (STAR File #020910–16). Medical records were reviewed to determine the details of skin cancer diagnoses and to obtain the dates and doses of voriconazole administration. This study was approved by the UCSF Committee on Human Research and performed in compliance with the Declaration of Helsinki.
We collapsed pre-transplant listing diagnoses into the four groupings used in calculating the Lung Allocation Score (LAS)25. The LAS is an urgency based allocation system used in the United States to prioritize lung transplant candidates on the waiting list. Medication records are maintained on a specific flowchart for each LTR. This allows for the straightforward identification of dates of administration and doses for each medication. For the purposes of this study, we standardized post-operative day 3 after lung transplantation as our index (start) date for voriconazole dosing. Dates and doses were abstracted until the time of SCC diagnosis, patient death, or last follow-up as of 3/1/2011. If the last follow-up date was within one month of death, censoring was defined as the date of death. Three patients transitioned their clinical care to other institutions prior to developing SCC. Therefore, their SCCs were reported to OPTN after their last follow-up at UCSF. In these three patients, we were unable to determine voriconazole administration dates and doses after they left our center. We therefore right-censored their data at the date of last follow-up at UCSF. One additional patient had SCC preceding lung transplantation and was excluded.
Our study period spanned 20 years. During this period, temporal trends in the care of LTR, including immunosuppression regimens, may have impacted the risk of SCC development separate from the introduction of voriconazole. Given our modest sample size, to investigate the potential for an effect of temporal trends in LTR care, we created an era-effect variable dichotomizing subjects transplanted before or after 1/1/2004.
Voriconazole doses could not be confirmed for 52 subjects due to incomplete or missing medical records and were excluded from the analysis. They did not differ from those included with respect to the predictor variables, follow-up time, or frequency of SCC.
Statistical Analysis
Variables were analyzed with two-sided Fisher’s exact test or two-sample Wilcoxon rank-sum test. We assessed correlations between predictors including male sex, age (at transplant), white versus nonwhite race, transplant type (single, bilateral, or heart-lung), LAS diagnostic category, body mass index (BMI), and ever/never voriconazole exposure. Correlation coefficients were <±0.3 in all cases (−0.24 to 0.29) except for ever/never voriconazole exposure to voriconazole and transplant type, which had a correlation coefficient of 0.48. We identified that there was a preferential performance of bilateral lung transplantation after 2003. In addition, “ever use” of voriconazole was more frequent in patients transplanted after 2003. These findings suggested that the correlation was due to temporal factors. Stratified by time, the correlation for these two variables was 0.28 before 2003 and 0.18 after 2003.
We employed Cox proportional hazard models to assess the impact of voriconazole exposure on the risk of developing SCC. The mechanism by which voriconazole may impact the risk of developing SCC is unknown. We hypothesized that voriconazole could be related to the subsequent development of SCC in two ways: (1) any exposure to voriconazole could confer an increased risk and/or (2) the risk could be dose dependent. We therefore developed two analytic approaches to assess these potential risks. First, to assess the impact of “any” voriconazole exposure on SCC development, we created a dichotomous time-dependent variable: “ever exposed”/“never exposed”. To be considered “ever exposed”, subjects had to have received voriconazole prior to SCC development. Second, to assess how the risk of SCC development varied with increasing exposure to voriconazole, we considered cumulative dose of voriconazole as a continuous time-dependent covariate. Cumulative dose of voriconazole was calculated from the index date until subjects developed SCC, died, or the study period ended. We treated cumulative dose of voriconazole as a time-dependent covariate to align the timing of exposure and outcome, thereby eliminating the potential for immortal time bias26.
Gender and age were included in the Cox models a priori based on known associations with skin cancer after organ transplant1. We confirmed model robustness using likelihood ratio testing. We next performed binary tests of interaction between all predictors, which revealed interactions between race and gender as well as between race and age. Further, we identified that 94% of SCC developed in white subjects. Because of these interactions and the rarity of SCC development in non-white subjects, models were stratified by race (white/non-white).
The proportionality of hazards assumption was tested and confirmed with the Schoenfeld test. The goodness of fit of the models was confirmed by comparing a plot of the Cox-Snell residuals to the Nelson-Aalon cumulative hazard function.
Kaplan-Meier methods for survival curves do not to translate to the setting of competing risks27. Instead, we chose to estimate the proportion of patients in four possible states following transplant: they could develop SCC and remain alive, develop SCC and then die, die before developing SCC, or remain alive without developing SCC.
To project these cumulative incidence probabilities28, we represented the cumulative incidence function in terms of the cause-specific hazards for SCC and death and employed estimates from the Cox models for the cause specific hazards of death and SCC. We compare two scenarios: continuous voriconazole through the development of SCC or no voriconazole.
To investigate whether our findings were sensitive to the effect of transplant era, we repeated our analyses including the era-effect variable in the final multivariate models. These models were compared to the models without the era-effect variable by likelihood ratio testing.
Results
Of 327 LTR included in the analysis, 50 subjects (15%) had at least one SCC (cases), and the remaining 277 (85%) did not (controls) (Table 1). Comparing cases and controls, there were no differences in age (mean 53.2±10.4 years versus 51.2±12.9 years, p=0.37), male gender (60% versus 53%, p=0.44), transplant type (p=0.65) or listing diagnosis category (p=1.0). Race did differ, however, between cases and controls: 94% of cases were white, compared to 76% of controls (p=0.002).
Table 1.
Demographics. Data presented as n (%) or mean ± SD.
| Characteristic | Cases | Controls | p value | |
|---|---|---|---|---|
| N=50 | N=277 | |||
| Age at Transplant (years) | 53.2 ± 10.4 | 51.2 ±12.9 | 0.37 | |
| Age < 60 | 35 (70) | 199 (71.8) | 0.86 | |
| Age ≥ 60 | 15 (30) | 78 (28.2 | ||
| Sex (male) | 30 (60) | 146 (53.09) | 0.44 | |
| Ethnicity | ||||
| White, non-Hispanic | 47 (94.0) | 210 (75.81) | 0.002 | |
| Hispanic | 2 (4.0) | 30 (10.83) | (white vs. | |
| Black/African-American | 0 | 19 (6.86) | nonwhite) | |
| Asian | 0 | 12 (4.33) | ||
| Other/Missing | 1 (2.0) | 6 (2.17) | ||
| Body Mass Index | 24.8 ± 4.8 | 24.7 ± 5.0 | 0.98 | |
| Type of Transplant | ||||
| Bilateral Lung | 37 (68.52) | 235 (72.76) | 0.65 | |
| Single Lung | 15 (27.78) | 80 (24.77) | ||
| Heart-Lung | 2 (3.70) | 8 (2.48) | ||
| Indication for Transplant by Diagnostic Category* | ||||
| Group A | 17 (34.0) | 88 (32.12) | 1.0 | |
| Group B | 5 (10.00) | 28 (10.22) | ||
| Group C | 5 (10.0) | 24 (8.76) | ||
| Group D | 23 (46.0) | 134 (48.91) | ||
| Voriconazole, ever exposed** | 40 (80.0) | 202 (72.92) | ||
Diagnostic grouping used for calculation of the Lung Allocation Score27.
Does not account for timing of exposure relative to SCC development.
Overall, 242 subjects (74%) were “ever exposed” to voriconazole. Subjects who were “ever exposed” manifested a 2.6-fold increased risk of subsequent SCC development compared to those who were “never exposed” (Hazard Ratio [HR] 2.62, 95% Confidence interval [CI]: 1.21–5.65; p=0.014; Table 2). Importantly, this risk was dose-dependent. For each additional one-gram of voriconazole exposure, subjects experienced a 0.2% increased risk of developing SCC (HR 1.002, 95%CI: 1.001–1.004; p=0.006). Clinically, most invasive fungal infections are treated for 6–8 weeks. For each 60-day exposure to voriconazole at standard dosing of 200mg BID (approximately 8 weeks of treatment,) subjects manifested a 6% increased risk of developing SCC (HR 1.06, 95%CI: 1.02–1.10; p=0.006). Both male sex and age ≥60 demonstrated trends towards increased risk for developing SCC (Table 2).
Table 2.
Relative risk of developing cutaneous squamous cell carcinoma (hazard ratio) in lung transplant recipients exposed to voriconazole.
| Hazard Ratio* | 95% CI | p value | |||
|---|---|---|---|---|---|
| Cumulative Voriconazole Dose** | 1.002 | 1.001 | - | 1.004 | 0.006 |
| Male Gender | 1.57 | 0.87 | - | 2.83 | 0.138 |
| Age ≥ 60 | 1.57 | 0.84 | - | 2.94 | 0.16 |
| Hazard Ratio | 95% CI | p value | |||
| Any Exposure to Voriconazole† | 2.62 | 1.21 | - | 5.65 | 0.014 |
| Male Gender | 1.65 | 0.91 | - | 2.98 | 0.097 |
| Age ≥ 60 | 1.43 | 0.76 | - | 2.69 | 0.27 |
A hazard ratio of >1.0 represents greater risk for developing squamous cell carcinoma for subjects who were exposed to voriconazole after transplant compared to subjects who were not. Models were stratified by race (white versus not-white).
Cumulative voriconazole dose is the HR per 1 gram exposure to voriconazole.
Any exposure to voriconazole is defined as any exposure before the development of squamous cell carcinoma
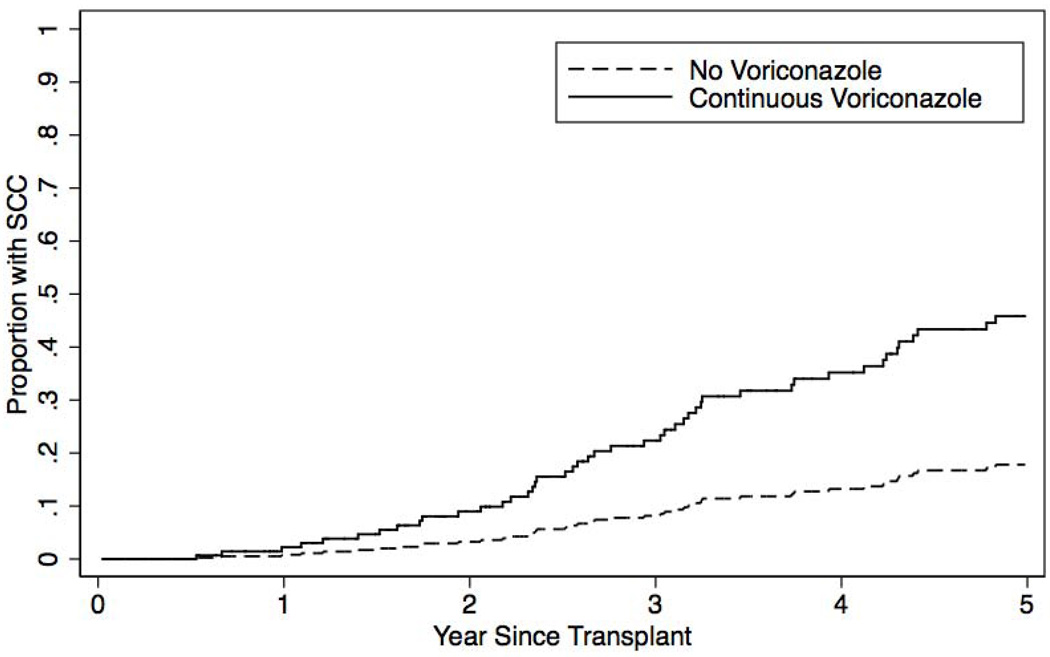
The 3-year, 5-year, and 10-year cumulative incidence of SCC in the overall cohort was 11.9%, 29.8%, and 45.5%, respectively. Figure 1 illustrates the extrapolated incidence of SCC in subjects “ever exposed” to voriconazole accounting for death as a competing risk. In this model, SCC development was predicted at 5 years after transplant. At 5 years, 46% of subjects ever exposed to voriconazole developed SCC compared to 18% of subjects never exposure corresponding to an absolute risk increase of developing SCC of 28%.
Figure 1.
Cumulative incidence model of squamous cell carcinoma in lung transplant recipients exposed to voriconazole. Model based on continuous exposure to voriconazole.
In a sensitivity analysis, the era of lung transplantation (before or after 1/1/2004) did not impact the effect sizes or statistical significance of our findings (likelihood ratio p-value=0.42 for any exposure and 0.87 for cumulative dose).
Discussion
We found that voriconazole is associated with the development of cutaneous squamous cell carcinoma (SCC) after lung transplantation. We identified that any exposure to voriconazole confers a 2.6-fold increased risk of SCC development and that, importantly, this risk is dose-dependent. Indeed, each 8-week exposure to voriconazole at 200mg BID dosing (a common duration of therapy for invasive fungal infections) increases the risk of developing SCC by 6%. Lastly, we found that 5-years after lung transplantation, 46% of subjects ever exposed to voriconazole developed SCC compared to 18% of those never exposed; an absolute risk increase of 28%.
Overall, our cohort suffered from a high incidence of SCC with a cumulative incidence of 30% at 5 years and 46% at 10 years. The median time to development of SCC was 3.6 years.
The cumulative incidence reported here is markedly higher than that reported in renal transplant recipients at 10 years, which ranges from 5 to 25%29–32. The difference in incidence of SCC in LTR underscores the importance of identifying risk factors for the development of SCC in this population. While LTR are typically exposed to more intensive immunosuppression regimens compared to renal transplant recipients, it is unlikely that these differences can entirely be ascribed to levels of immunosuppression or sun exposure. Other important potential explanations may include voriconazole exposure, older age at transplant and other, yet to be determined factors.
Our results build on a recent nested case-control study of LTR24. In 17 LTR with SCC and 51 controls, Vadnerkar et al. demonstrated that duration of voriconazole exposure was associated with an increased risk of SCC24. Subjects in that study had a shorter median time to SCC than is reported here (1.6 versus 3.6 years). It is possible that induction regimen may be a factor in explaining this difference. The authors postulated that the short time to the development of SCC might have been due to induction with alemtuzumab24. Our center’s typically uses basiliximab, a less immunosuppressive agent. In part, due to this more intense induction Varderkar reports using six-months of voriconazole prophylaxis while our center uses three-months. Based on our findings, the higher cumulative exposure to voriconazole could be an alternative explanation for our differences in time to SCC development. Another potential explanation is that the median age at transplant in their cases was greater than cases in our cohort (63 versus 53 years).
Our study has notable strengths. Our detailed medication records and analytic approach allowed us to consider voriconazole exposure that occurred only before SCC development. In doing so, we accounted for both competing risks and the risk of immortal time bias. Our methods and findings both confirm the work of Vadnerkar et al. as well as provide clinically relevant evidence that the risk of SCC from voriconazole exposure is dose dependent. Indeed, there does not appear to be a threshold below which voriconazole is without risk. Additionally, our findings are derived from a 20-year retrospective cohort study of 327 lung transplant recipients. This represents the largest analysis of voriconazole as a risk factor for development of SCC in LTR to date. Lastly, we selected biologically known risk factors for SCC and employed statistically rigorous methods for selecting covariates for inclusions in multivariate models.
Our study also faces limitations. First, we were unable to retrospectively ascertain the Fitzpatrick skin type or prior sun exposure history, or determine whether photosensitivity preceded dysplasia in those subjects who developed SCC. Prospective studies employing a combination of survey based sun exposure and physical assessment methods could address these limitations. Second, although our study represents the largest cohort study of SCC in LTR, it is a single-center study with a relatively modest sample size. Thus, it may have been underpowered to identify associations between SCC and male sex or age. Third, this study did not account for the types and intensity of immunosuppression. While it likely plays a role in SCC development, a single effective and accepted measure of overall immunosuppression intensity is lacking. Given that immunosuppressive agents are generally weaned over time (with occasional increases in the setting of acute allograft rejection), a comprehensive consideration of immunosuppression intensity would have treated each agent as its own time varying covariate. Despite this limitation, we suspect voriconazole does confer an increased risk for SCC development. In this study, cases and controls were generally treated similarly according to established treatment protocols. Analytically, a sensitivity analysis of transplant era did not have a substantive or significant effect on our findings. Nevertheless, accounting for immunosuppression intensity in future prospective studies of this question will be important.
Although single-center studies in lung transplantation are often limited by modest sample sizes, in general, investigators benefit from access to more detailed patient-level data. This allows for more accurate cancer assessment than registry-based studies. This is particularly important for studies of non-melanoma skin cancer, which are not captured in standard cancer registries and share a single ICD-9 code with several other cancer diagnoses including SCC, basal cell carcinoma, adnexal carcinomas among others. Notably, in our study, 18 of 50 SCC cases were identified by internal medical record review that had not been reported to the OPTN. This suggests that future studies on this subject should not rely solely on UNOS or other registry data for ascertainment of non-melanoma skin cancer.
Since the FDA approved voriconazole, our center’s approach has been to use this drug as standard antifungal prophylaxis for the first three-months after transplant. Voriconazole is discontinued thereafter if voriconazole-sensitive fungus is not identified on surveillance broncheoalveolar lavage and/or CT scans of the chest do not reveal findings consistent with an invasive fungal infection. Voriconazole is reinstituted for the treatment of invasive fungal infections or if increased immunosuppression is required for acute allograft rejection. Since other transplant centers may employ different protocols, tracking cumulative dose exposure may allow physicians to identify patients at increased risk for SCC.
Our center does not routinely check serum voriconazole trough levels. Clinically, levels are checked if there are concerns about drug absorption in the setting of gastroparesis or if patients are not improving radiographically on standard therapy. Given our findings, it could be hypothesized that higher serum levels are a biomarker for SCC risk. This assay, however, cannot be recommended for SCC risk assessment until studies investigate its clinical utility.
In summary, voriconazole is an independent risk factor for the development of cutaneous squamous cell carcinoma in lung transplant recipients. Its efficacy and ease of administration makes voriconazole an extremely attractive and important therapeutic agent to combat invasive fungal infections. It is important, however, to be aware of the increased risk of SCC associated with this agent. The risks and benefits of using voriconazole as prophylaxis and treatment compared to alternative antifungal medications should be weighed carefully. When voriconazole is used, we recommend heighted attention to risk factors for photosensitivity and/or SCC as well as skin cancer screening. This is especially important in patients with other known non-modifiable risk factors for SCC such as fair skin, male sex, and older age.
Acknowledgements
This work was supported by an American Society for Dermatology Surgery Cutting Edge Research Grant to A.B. J.P.S. is supported by an NIH/NHLBI F32 HL107003-01. S.T.A. is supported by an NIH/NCRR/OD UCSF-CTSI grant number KL2 RR024130. Work was also supported by the Health Resources and Services Administration contract 231-00-0115. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services. The funding agencies played no role in the collection, analysis, interpretation, or publication of this data. The authors wish to thank the UCSF Lung Transplant team and the Nina Ireland Lung Disease Center for their support in this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors have no relevant financial interests to disclose.
References
- 1.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: Advances in therapy and management: Part 1. Epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011 Aug;65:253–261. doi: 10.1016/j.jaad.2010.11.062. 2011. [DOI] [PubMed] [Google Scholar]
- 2.Georgopapadakou NH. Update on antifungals targeted to the cell wall: focus on beta-1,3-glucan synthase inhibitors. Expert Opin Investig Drugs. 2001 Feb;10(2):269–280. doi: 10.1517/13543784.10.2.269. [DOI] [PubMed] [Google Scholar]
- 3.Hilliard T, Edwards S, Buchdahl R, et al. Voriconazole therapy in children with cystic fibrosis. J Cyst Fibros. 2005 Dec;4(4):215–220. doi: 10.1016/j.jcf.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Johnson LB, Kauffman CA. Voriconazole: a new triazole antifungal agent. Clin Infect Dis. 2003 Mar 1;36(5):630–637. doi: 10.1086/367933. [DOI] [PubMed] [Google Scholar]
- 5.Walsh TJ, Lutsar I, Driscoll T, et al. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr Infect Dis J. 2002 Mar;21(3):240–248. doi: 10.1097/00006454-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Voriconazole [package insert] New York: Pfizer; 2009. [Google Scholar]
- 7.Denning DW, Griffiths CE. Muco-cutaneous retinoid-effects and facial erythema related to the novel triazole antifungal agent voriconazole. Clin Exp Dermatol. 2001 Nov;26(8):648–653. doi: 10.1046/j.1365-2230.2001.00909.x. [DOI] [PubMed] [Google Scholar]
- 8.Dolan CK, Hall MA, Blazes DL, Norwood CW. Pseudoporphyria as a result of voriconazole use: a case report. Int J Dermatol. 2004 Oct;43(10):768–771. doi: 10.1111/j.1365-4632.2004.02177.x. [DOI] [PubMed] [Google Scholar]
- 9.Frick MA, Soler-Palacin P, Martin Nalda A, Guarner ME, Nadal CF. Photosensitivity in immunocompromised patients receiving long-term therapy with oral voriconazole. Pediatr Infect Dis J. 2010 May;29(5):480–481. doi: 10.1097/INF.0b013e3181d60a82. [DOI] [PubMed] [Google Scholar]
- 10.Kwong WT, Hsu S. Pseudoporphyria associated with voriconazole. J Drugs Dermatol. 2007 Oct;6(10):1042–1044. [PubMed] [Google Scholar]
- 11.Malani AN, Aronoff DM. Voriconazole-induced photosensitivity. Clin Med Res. 2008 Sep;6(2):83–85. doi: 10.3121/cmr.2008.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Racette AJ, Roenigk HH, Jr, Hansen R, Mendelson D, Park A. Photoaging and phototoxicity from long-term voriconazole treatment in a 15-year-old girl. J Am Acad Dermatol. 2005 May;52(5 Suppl 1):S81–S85. doi: 10.1016/j.jaad.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Rubenstein M, Levy ML, Metry D. Voriconazole-induced retinoid-like photosensitivity in children. Pediatr Dermatol. 2004 Nov-Dec;21(6):675–678. doi: 10.1111/j.0736-8046.2004.21614.x. [DOI] [PubMed] [Google Scholar]
- 14.Tolland JP, McKeown PP, Corbett JR. Voriconazole-induced pseudoporphyria. Photodermatol Photoimmunol Photomed. 2007 Feb;23(1):29–31. doi: 10.1111/j.1600-0781.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- 15.Brunel AS, Fraisse T, Lechiche C, Pinzani V, Mauboussin JM, Sotto A. Multifocal squamous cell carcinomas in an HIV-infected patient with a long-term voriconazole therapy. AIDS. 2008 Apr 23;22(7):905–906. doi: 10.1097/QAD.0b013e3282f706a9. [DOI] [PubMed] [Google Scholar]
- 16.Cowen EW, Nguyen JC, Miller DD, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol. 2010 Jan;62(1):31–37. doi: 10.1016/j.jaad.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy KL, Playford EG, Looke DF, Whitby M. Severe photosensitivity causing multifocal squamous cell carcinomas secondary to prolonged voriconazole therapy. Clin Infect Dis. 2007 Mar 1;44(5):e55–e56. doi: 10.1086/511685. [DOI] [PubMed] [Google Scholar]
- 18.Vanacker A, Fabre G, Van Dorpe J, Peetermans WE, Maes B. Aggressive cutaneous squamous cell carcinoma associated with prolonged voriconazole therapy in a renal transplant patient. Am J Transplant. 2008 Apr;8(4):877–880. doi: 10.1111/j.1600-6143.2007.02140.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller DD, Cowen EW, Nguyen JC, McCalmont TH, Fox LP. Melanoma associated with long-term voriconazole therapy: a new manifestation of chronic photosensitivity. Arch Dermatol. 2010 Mar;146(3):300–304. doi: 10.1001/archdermatol.2009.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisch S, Askari SK, Beaty SR, Burkemper CN. X-linked chronic granulomatous disease with voriconazole-induced photosensitivity/ photoaging reaction. J Drugs Dermatol. 2010 May;9(5):562–564. [PubMed] [Google Scholar]
- 21.Gomez-Moyano E, Vera-Casano A, Moreno-Perez D, Sanz-Trelles A, Crespo-Erchiga V. Lupus erythematosus-like lesions by voriconazole in an infant with chronic granulomatous disease. Pediatr Dermatol. 2010 Jan 1;27(1):105–106. doi: 10.1111/j.1525-1470.2009.01058.x. [DOI] [PubMed] [Google Scholar]
- 22.Epaulard O, Saint-Raymond C, Villier C, et al. Multiple aggressive squamous cell carcinomas associated with prolonged voriconazole therapy in four immunocompromised patients. Clin Microbiol Infect. 2009 Nov 20; doi: 10.1111/j.1469-0691.2009.03124.x. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim SF, Singer JP, Arron ST. Catastrophic Squamous Cell Carcinoma in Lung Transplant Patients Treated with Voriconazole. Dermatol Surg. 2010 Jun 1; doi: 10.1111/j.1524-4725.2010.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vadnerkar A, Nguyen MH, Mitsani D, et al. Voriconazole exposure and geographic location are independent risk factors for squamous cell carcinoma of the skin among lung transplant recipients. J Heart Lung Transplant. 2010 Nov;29(11):1240–1244. doi: 10.1016/j.healun.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 26.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008 Feb 15;167(4):492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 27.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993 Apr 30;12(8):737–751. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 28.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004 Oct 4;91(7):1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behrend M, Kolditz M, Kliem V, et al. Malignancies in patients under long-term immunosuppression after kidney transplantation. Transplant Proc. 1997 Feb-Mar;29(1–2):834–835. doi: 10.1016/s0041-1345(96)00154-6. [DOI] [PubMed] [Google Scholar]
- 30.Carroll RP, Ramsay HM, Fryer AA, Hawley CM, Nicol DL, Harden PN. Incidence and prediction of nonmelanoma skin cancer post-renal transplantation: a prospective study in Queensland, Australia. Am J Kidney Dis. 2003 Mar;41(3):676–683. doi: 10.1053/ajkd.2003.50130. [DOI] [PubMed] [Google Scholar]
- 31.Fuente MJ, Sabat M, Roca J, Lauzurica R, Fernandez-Figueras MT, Ferrandiz C. A prospective study of the incidence of skin cancer and its risk factors in a Spanish Mediterranean population of kidney transplant recipients. Br J Dermatol. 2003 Dec;149(6):1221–1226. doi: 10.1111/j.1365-2133.2003.05740.x. [DOI] [PubMed] [Google Scholar]
- 32.Naldi L, Fortina AB, Lovati S, et al. Risk of nonmelanoma skin cancer in Italian organ transplant recipients. A registry-based study. Transplantation. 2000 Nov 27;70(10):1479–1484. doi: 10.1097/00007890-200011270-00015. [DOI] [PubMed] [Google Scholar]