Summary
Every moment of every day, our skin and its embedded sensory neurons are bombarded with mechanical cues that we experience as pleasant or painful. Knowing the difference between innocuous and noxious mechanical stimuli is critical for survival and relies on the function of mechanoreceptor neurons that vary in their size, shape, and sensitivity. Their function is poorly understood at the molecular level. This review emphasizes the importance of integrating analysis at the molecular and cellular levels and focuses on the discovery of ion channel proteins co-expressed in the mechanoreceptors of worms, flies, and mice.
Introduction
All sensory neurons are alike. Each detects a physical stimulus and produces an electrical signal that gives rise to behavioral responses, conscious perceptions, or both. Many operate near the physical limits of detection over a dynamic range of several orders of magnitude (Bialek, 1987; Block, 1992). These properties suggest that they are endowed with a detector, an amplifier, and mechanisms for gain-control. One of the most striking and well-understood examples is the ability of photoreceptors to detect single photons while retaining sensitivity to light intensities that vary by nine orders of magnitude (Rieke and Rudd, 2009).
Each somatosensory neuron is distinct. The somatosensory system is a collection of neurons innervating the skin, muscle, joints, tendons and internal organs to establish and maintains sensitivity across a range of stimulus intensities and frequencies. This collection includes nociceptors that require stimulation above a high threshold for activation. Nociceptors are responsible for informing the brain about damage to peripheral tissues, an essential function that enables the nervous system to execute and coordinate appropriate protective behaviors. They are often polymodal neurons responding to multiple types of stimulation including extreme temperatures, intense force, acid, and noxious chemicals. Other somatosensory neurons respond to less intense stimulation and detect either temperature changes or mechanical stimulation, but not both. These cells provide information about warmth, cooling or the shape and texture of objects.
Theater of sensation
The skin is our largest sensory surface, extending nearly two square meters in an average human. Mechanoreceptor neurons are principal actors in this theater. They are responsible not only for detecting mechanical cues, but also for encoding and transmitting all relevant information to the central nervous system. Their performance is shaped by ion channels that include, but are not limited to, sensory transduction channels. Agents that activate or inhibit mechanoreceptor neurons can exert their influence by acting on channels other than transduction channels. For example, naked mole rats are insensitive to the persistent skin acidification that is a feature of their environment. These animals have acid-gated ion channels (ASICs) with a similar sensitivity to protons (H+) as those found in mice (Smith et al., 2011). However the voltage-gated Na+ channels expressed in their C-fiber nociceptors are hypersensitive to inhibition by protons and this inhibition counterbalances the excitation due by ASIC activation, rendering animals insensitive to acidification. Thus, the difference in nociceptor sensitivity stems from variation in voltage-gated Na+ sodium channels that are essential for action potential generation rather than any variation in sensory transduction.
Though mechanoreceptor neurons were first studied more than 75 years ago (Adrian, 1926; Adrian and Zotterman, 1926a, b), the events that link sensory stimulation to neuronal activation are only beginning to be understood. Today, the protein partners responsible for detecting mechanical stimuli have been identified only for a few mechanoreceptor neurons in the nematode Caenorhabditis elegans. Genetic screens for animals defective in touch sensation have revealed critical roles for genes encoding TRP channels and DEG/ENaCs in behavioral responses to mechanical inputs. The key insights derived from genetic approaches have been reviewed elsewhere (Arnadottir and Chalfie, 2010; Ernstrom and Chalfie, 2002). We review data demonstrating that TRP channels and DEG/ENaC channels are widely distributed in the sensory neurons of vertebrates and invertebrates and examine the idea that these channels have conserved, but distinct functions. We rely on investigations of somatosensory mechanoreceptors in nematodes, flies, and mice, but recognize that on-going investigations in humans and other animals have the potential to deepen and expand understanding of how mechanoreceptors function.
Sensing with ion channels
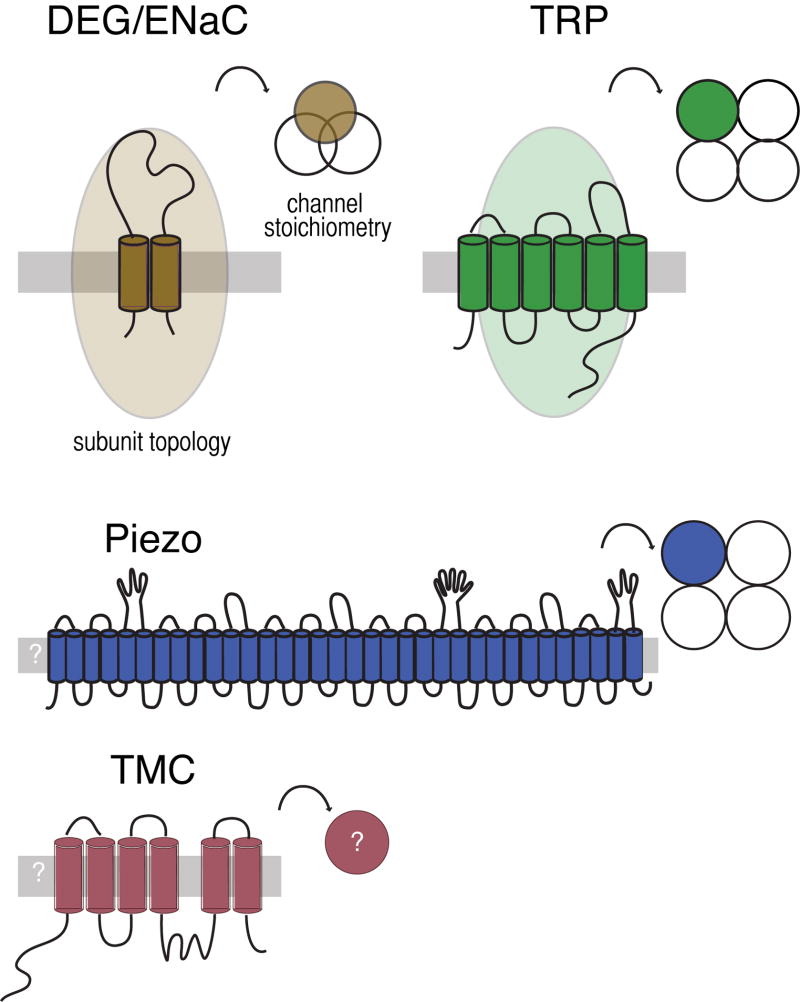
For several decades, attention has focused on the idea that mechano-electrical transduction (MeT) channels are formed by proteins enriched in mechanoreceptor neurons and required for their function. Figure 1 summarizes current knowledge about proteins proposed to form MeT channels in animals. The mec-4 DEG/ENaC and osm-9 TRP channel genes were the first candidates identified from classical genetic screens. The DEG/ENaC genes are conserved in animals, but absent from plants, bacteria, and fungi (Goodman and Schwarz, 2003; Hunter et al., 2012) and encode proteins with two transmembrane domains and a large extracellular domain. As revealed in high-resolution crystal structures (Gonzales et al., 2009; Jasti et al., 2007), three DEG/ENaC proteins assemble to form an ion channel. Both homomeric and heteromeric channels has been observed (Akopian et al., 2000; Deval et al., 2004; Donier et al., 2008; Gründer et al., 2000; Hesselager et al., 2004; Lingueglia et al., 1997). The TRP channel genes comprise a large superfamily conserved in eukaryotes and encode proteins predicted to have six transmembrane domains. Four TRP channel proteins assemble into homomeric or heteromeric ion channels (Venkatachalam and Montell, 2007). Recently, two additional classes of membrane proteins (Piezo and TMC) have been linked to mechanotransduction (Coste et al., 2010; Coste et al., 2012; Kawashima et al., 2011; Kim et al., 2012).
Figure 1. Topology and stoichiometry of proteins proposed to form MeT channels in animals.
The TRP channel genes are conserved in eukaryotes and encode proteins predicted to have six transmembrane domains and assemble into tetrameric ion channels. Many TRPs have ankyrin repeats in their intracellular amino terminal; some have more than ten such repeats (Venkatachalam and Montell, 2007). The DEG/ENaC genes are absent from plants, yeast and other microbes, but conserved in animals (Goodman and Schwarz, 2003). They encode proteins with two transmembrane domains and a large extracellular domain. Three DEG/ENaC proteins assemble to form an ion channel. Both TRP channels and DEG/ENaC proteins can form homomeric and heteromeric channels, increasing the potential for channel diversity. Recently, two additional classes of membrane proteins (Piezo and TMC) have been linked to mechanotransduction in mammals (Coste et al, 2010; Kawashima et al, 2011) and Drosophila fruit flies (Kim et al., 2012). Piezo is sufficient to produce stretch activated channels in heterologous cells (Coste et al, 2010; Coste et al., 2012; Bae et al., 2011) and purified Piezo forms a channel in lipid bilayers (Coste et al., 2012). TMC1 and TMC2 are required for mechanotransduction by sound- and vibration-sensing hair cells in mice (Kawashima et al, 2011). Both Piezo and TMC have homologs in invertebrates; the C. elegans TMC homolog is expressed in the multidendritic PVD nociceptors. The predicted topology and stoichiometry of Piezo and TMC await further experimental confirmation.
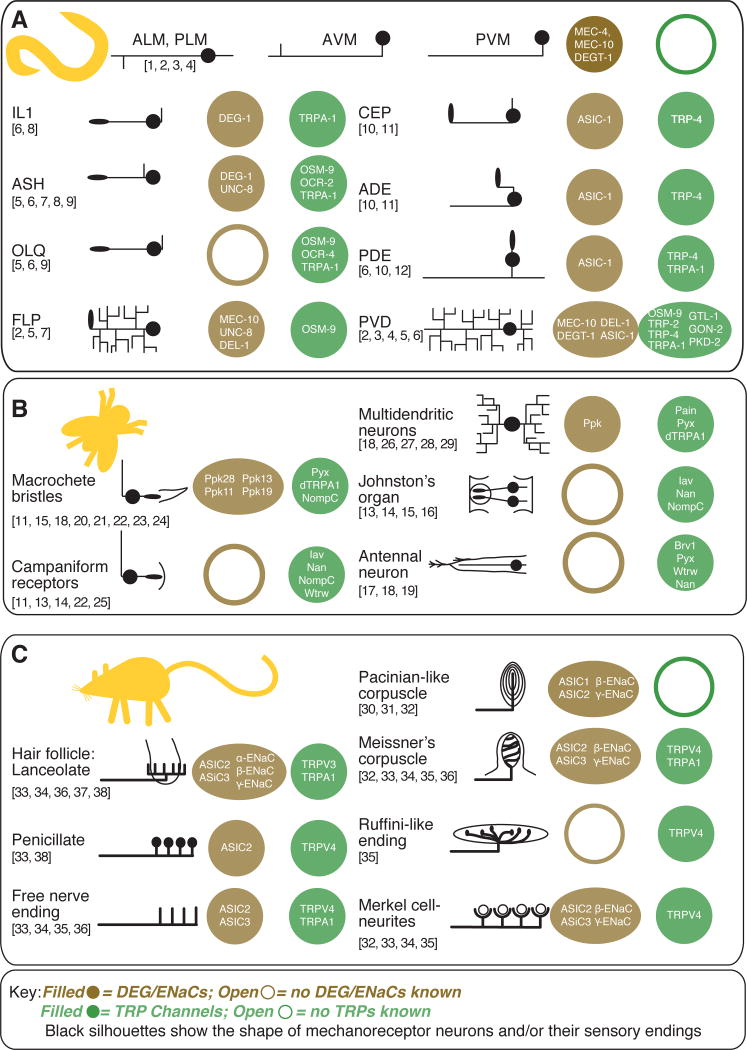
Both TRPs and DEG/ENaCs are broadly expressed in somatosensory neurons. Several mechanoreceptor neurons are known to co-express multiple TRPs and multiple DEG/ENaC channels. Figure 2 aggregates evidence that these channels are co-expressed in mechanoreceptor neurons from the growing, but essentially independent literatures on TRP and DEG/ENaC channels expression and function. Excluding reviews, PubMed lists 1687 entries for DEG/ENaCs, 2341 for TRP channels, and only 15 entries for both ion channel families.1 Here we focus on two invertebrates, Caenorhabditis elegans nematodes and Drosophila melanogaster fruitflies, and one mammal, the laboratory mouse. Despite the fact that members of these gene families are coexpressed, the cellular and behavioral function of individual TRP and DEG/ENaC channels is often explored subunit by subunit. But, genetic redundancy within each ion channel family and the potential for functional redundancy between the two families limits insight derived from this approach. Additional complications include alteration of channel function by their association with heteromeric channel complexes and through alternative splicing of ion channel genes.
Figure 2. DEG/ENaC and TRP channel proteins co-expressed in mechanoreceptor neurons in C. elegans nematodes, Drosophila melanogaster fruitflies, and Mus musculus mice.
This graphical table illustrates the gross morphology of entire mechanoreceptor neurons (C. elegans) or peripheral sensory endings (Drosophila, mice) and lists ion channel subunits that are expressed in each class of mechanoreceptor cell. Sources for C. elegans mechanoreceptor expression are listed by number above: 1) Driscoll and Chalfie, 1991, 2) Huang and Chalfie, 1994, 3) Chatzigeorgiou et al., 2010, 4) Smith et al., 2010, 5) Colbert et al., 1997, 6) Kindt et al., 2007, 7) Tavernarakis et al., 1997, 8) Hall et al., 1997, 9) Tobin et al., 2002, 10) Voglis and Tavernarakis, 2008, 11) Walker et al., 2000, 12) Li et al., 2006. Drosophila melanogaster sourcesare: 13) Gong et al., 2004, 14) Kim et al., 2003, 15) Lee et al., 2010, 16) Liang et al., 2011, 17) Gallio et al., 2011, 18) Lee et al., 2005, 19) Liu et al., 2007, 20) Hamada et al., 2008, 21) Kim et al., 2010, 22) Cheng et al., 2010, 23) Chen et al., 2010, 24) Liu et al., 2003, 25) Bechstedt et al., 2010, 26) Tracey et al., 2003, 27) Zhong et al., 2012, 28) Adams et al., 1998, 29) Zhong et al., 2010). These sources establishing expression in peripheral endings in mice and humans were consulted: 30) Calavia et al., 2010, 31) Montaño et al., 2009, 32)Drummond et al., 2000, 33) García-Añoveros et al., 2001, 34) Price et al., 2001, 35) Suzuki et al., 2003b, 36) Kwan et al., 2009, 37) Price et al., 2000, 38) Fricke et al., 2000, 39) Xu et al., 2002).
Stagecraft for sensory biology
A fundamental block to progress in understanding how mechanoreceptor neurons function is that studying stimulus-initiated behavior, action potential generation or intracellular calcium dynamics does not allow researchers to separate the initial step of mechanotransduction from amplification, gain control and transmission. Behavior, spikes, and calcium dynamics depend on the action of all of the ion channels expressed by sensory neurons. These channels are interconnected in feedback loops regulated by a common control variable—membrane potential. The voltage-clamp uncouples such feedback loops by holding membrane potential constant and allows researchers to examine transduction independently of amplification, gain control, and spike generation. Within this heuristic, deleting molecules needed for the formation or function of MeT channels should eliminate mechanoreceptor currents, but leave other ionic currents and mechanisms for amplification, gain control and spike generation intact. Conversely, deleting molecules essential for post-transduction signal should leave mechanoreceptor currents intact, but produce defects in other ionic currents or in amplification, gain control, and spike generation. Marrying in vivo voltage clamp with genetic dissection of identified mechanoreceptor neurons in C. elegans has revealed that the pore-forming subunits of MeT channels are DEG/ENaCs in two classes of mechanoreceptors (Geffeney et al., 2011; O’Hagan et al., 2005) and a TRP channel in a third class of mechanoreceptors (Kang et al., 2010).
Neuron by neuron
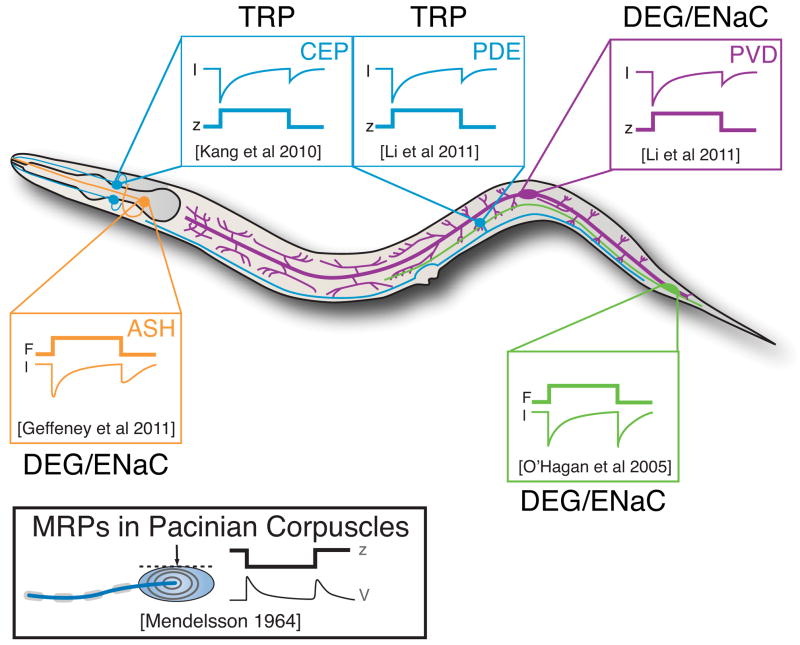
C. elegans nematodes are microscopic animals with a compact nervous system consisting of only 302 neurons, about 30 of which are classified as mechanoreceptor neurons (Goodman, 2006). Because the mechanoreceptor neurons can be identified in living animals, and because of their small size, it is possible to record mechanoreceptor currents (MRCs) and mechanoreceptor potentials (MRPs) in vivo. MRCs have been recorded from the body touch receptor neurons known collectively as the TRNs, the cephalic CEP neurons and two classes of nociceptors, the ciliated ASH neurons and the multi-dendritic PVD neurons. In all four of these mechanoreceptors, stimulation activates inward currents (Figure 3) and evokes transient increases in intracellular calcium. Strikingly, MRCs are activated in response to both the application and withdrawal of stimulation. Such response dynamics were first described 50 years ago in recordings from Pacinian corpuscles in mammals (Alvarez-Buylla and Ramirez de Arellano, 1953; Gray and Sato, 1953) and are emerging as a conserved property of somatosensory mechanoreceptor neurons.
Figure 3. Mechanoreceptor currents in C. elegans mechanoreceptor neurons activate in response to the application and removal of mechanical stimulation.
Mechanoreceptor currents have been recorded using in vivo whole-cell patch clamp recording and the predominant ion channel type identified by genetic dissection. Traces adapted from the following: PLM: O’Hagan et al. (2005); CEP: Kang et al. (2010); PDE, PVD: Li et al. (2011b); ASH: Geffeney et al. (2011). Displacement stimuli were applied to activate CEP, PDE, and PVD, while mechanical stimuli delivering known forces were applied to activate PLM and ASH. Receptor potentials in PLM and ASH mirror the receptor currents (Geffeney et al., 2011; O’Hagan et al., 2005) and are reminiscent of the response dynamics of Pacinian corpuscles in cats (inset, lower left).
The TRNs (ALM, PLM, AVM, and PVM) express several DEG/ENaC channel proteins, but no TRP channel subunits have been reported (Figure 2A). External mechanical loads open sodium-dependent, amiloride-sensitive mechanotransduction (MeT) channels. MEC-4 is essential, while MEC-10 is dispensable for the generation of MeT currents (Arnadottir et al., 2011; O’Hagan et al., 2005). Both proteins are pore-forming subunits of the native MeT channel since missense mutations of a conserved glycine in the second transmembrane domain alter the permeability of the MeT current (O’Hagan et al., 2005). These protein partners were the first to be linked to native MeT currents in any animal.
CEP expresses at least one DEG/ENaC and one TRP channel protein, TRP-4 (Figure 2A). Two lines of evidence support the idea that the TRP-4 protein is an essential pore-forming subunit of MeT channels in CEP: 1) loss of TRP-4 eliminates MRCs in CEP and 2) mutations in the putative pore domain of the channel alter the reversal potential of MRCs (Kang et al., 2010). These latter data are strong indicators that TRP-4 is a pore-forming subunit of the MeT channel in CEP.
The ASH neurons function as nociceptors in the animal because they require more intense forces for activation than PLM and larger displacements for activation than CEP (Geffeney et al., 2011). These cells express multiple DEG/ENaC and TRP channel proteins (Figure 2A), but the major mechanoreceptor current is carried by a MeT channel formed by the DEG/ENaC channel protein, DEG-1. A minor current remains in deg-1 null mutants and is carried by a biophysically-distinct channel (Geffeney et al., 2011). Though it is possible that DEG-1 and the channel responsible for the minor current function in series with DEG-1 amplifying the minor current, the data support a model where the channels function in parallel because loss of DEG-1 does not alter the rise rate of MRCs in ASH. The TRPV proteins OSM-9 and OCR-2 are essential for ASH-mediated behaviors (Colbert et al., 1997; Tobin et al., 2002), but loss of these channel subunits has no effect on either the major or minor current in ASH (Geffeney et al., 2011). In ASH, TRPV channels likely regulate cell activity downstream of mechanotransduction, as suggested by their importance for calcium signaling in ASH following mechanical stimulation (Hilliard et al., 2005).
From analysis of ASH, we learn that DEG/ENaC channels can act in parallel with a second MeT channel and that TRPV channels are important for post-transduction signaling. This complex pathway for mechanoreceptor neuron signaling may be shared with other nociceptors responsible for detecting noxious and potentially damaging sensory stimuli. An additional, conserved function of nociceptors is their sensitization in response to injury and their regulation by biogenic amines (Walters and Moroz, 2009). Such sensitization is also apparent in C. elegans and reflected in the finding that ASH-dependent behaviors are regulated by various biogenic amines, including serotonin (Chao et al., 2004). Collectively, these observations raise the possibility that biogenic amines might regulate the sensitivity of nociceptors to mechanical cues and that such regulation may affect MeT channels, post-transduction signaling or both.
The multidendritic PVD neuron is a polymodal neuron activated by mechanical and thermal stimuli and is proposed to function as a nociceptor. Like ASH, PVD expresses multiple TRP and DEG/ENaC channel subunits (Figure 2A). As in ASH and the touch receptor neurons, mechanoreceptor currents in PVD are amiloride-sensitive and sodium-dependent (Li et al., 2011b). These biophysical properties suggest that MRCs are carried primarily by a DEG/ENaC channel. Consistent with this idea, Chatzigeorgiou et al. (2010) demonstrated that both DEGT-1 and MEC-10 are required for mechanically-evoked calcium transients in PVD. But, as reported for the touch receptor neurons (Arnadottir et al., 2011), loss of mec-10 had no effect on mechanoreceptor currents (Li et al., 2011b). Thus, mec-10 may function redundantly with the three other DEG/ENaC channels expressed in PVD: degt-1, del-1, asic-1. Additional studies are needed to clarify this issue and to determine the function of all of these ion channel proteins in PVD. One approach developed recently exploits optogenetics to identify PVD-expressed genes needed for post-transduction signaling (Husson et al., 2012). Using this strategy, Husson et al. (2012) show that PVD-selective knockdown of asic-1 alters light-evoked behavioral responses. (Light responses were unaffected in animals by mec-10, del-1, and degt-1 knockdown.)
PVD appears to express seven TRP channels, including TRPA-1 and OSM-9 (Figure 2A). Neither TRPA-1 nor OSM-9 are required for calcium transients induced by noxious mechanical stimuli. However, TRPA-1 is needed for responses to noxious cold (Chatzigeorgiou et al., 2010). Less is known about the function of the other TRP channels, but an optogenetics-based approach reveals that GTL-1 is required for normal light-evoked behaviors and strongly suggests that this TRPM channel plays an essential role in post-transduction signal amplication (Husson et al., 2012). Collectively, these studies paint a picture of PVD function in which a DEG/ENaC channel acts as a force sensor, TRPA-1 detects thermal stimuli, and ASIC-1 and GTL-1 contribute to post-transduction signaling.
The FLP neurons are multidendritic neurons and are activated by noxious mechanical and thermal stimuli (Chatzigeorgiou and Schafer, 2011; Chatzigeorgiou et al., 2010). The FLP neurons innervate the body surface anterior to PVD and co-express osm-9 and three DEG/ENaC genes: mec-10, unc-8, and del-1 (Figure 2A). Apart from the observation that mechanical stimuli activate calcium transients in FLP in a MEC-10-dependent manner (Chatzigeorgiou and Schafer, 2011), little is known about the function of MEC-10, UNC-8, and DEL-1 in FLP. The contribution of TRP channel genes to FLP function is complex, as FLP mechanosensitivity depends on OSM-9 and TRPA-1-dependent signaling in the OLQ mechanoreceptors.
In a cell that expresses multiple TRP channel subunits and no DEG/ENaC subunits, each TRP protein appears to have a distinct cellular function. The OLQ neuron expresses three TRP channel subunits and two of these have been examined for their role in initiating behavioral responses and calcium transients in response to mechanical stimulation. Loss of TRPA-1 decreases two behaviors influenced by OLQ, the cessation of foraging for food and reversal of forward movement induced by mechanical stimulation (Kindt et al., 2007). Surprisingly, loss of TRPA1 had a very subtle effect on calcium transients in OLQ. The first response to mechanical stimulus was not affected, but the magnitude of the second response was reduced (Kindt et al., 2007). A role for the TRPV channel subunit OSM-9 is evident from the finding that osm-9 mutant OLQ neurons lack mechanically-evoked calcium transients (Chatzigeorgiou et al., 2010). Because MRCs have yet to be measured in this mechanoreceptor neuron, it is not known whether loss of TRPA-1 or OSM-9 affect MRCs or the events that follow their activation.
These examples in C. elegans nematodes establish the rule that mechanoreceptor neurons commonly express multiple DEG/ENaC and TRP channel proteins and that these channels operate together to enable proper sensory function. The ability to directly measure MRCs in vivo has revealed that both DEG/ENaC and TRP channels can form MeT channels. Evidence from the ASH and PVD nociceptors suggest that some TRP channels are essential for post-transduction events essential for sensory signaling. These case studies provide evidence for the idea that TRP channels can be crucial elements in both sensory transduction and in post-transduction signaling. They also illustrate the powerful insights available when detailed physiological analysis of identified mechanoreceptor neurons is merged with genetic dissection.
It is rare for deletion of a single DEG/ENaC gene to induce strong behavioral defects in C. elegans. Indeed, there is only one such DEG/ENaC gene known so far: mec-4. By contrast, deleting the DEG/ENaC genes mec-10, deg-1, unc-8, and unc-105 fails to produce clear behavioral phenotypes, although gain-of-function alleles significantly disrupt several behaviors. Though only a subset of the DEG/ENaC genes have been studied in this way, these findings suggest there is considerable redundancy in C. elegans mechanosensation. The case of mec-4 and mec-10 illustrate this idea clearly: both genes are co-expressed in the TRNs and encode pore-forming subunits of the MeT channel required for gentle touch sensation (O’Hagan et al., 2005). Whereas deleting mec-4 eliminates mechanoreceptor currents and behavioral responses to touch, deleting mec-10 produces a mild defect in touch sensation and has little effect on mechanoreceptor currents (Arnadottir et al., 2011).
Case studies on the fly
The peripheral nervous system of Drosophila larvae has three main types of neurons (Bodmer et al., 1987; Bodmer and JAN, 1987; Ghysen et al., 1986). External sensory and chordotonal neurons have a single sensory dendrite and innervate specific mechanosensory organs. In contrast, multidendritic neurons have a variable number of fine dendritic processes that lie beneath the epidermis and do not innervate a specific structure. Different subclasses of these neurons provide information about touch and body position as well as function as nociceptors (Hughes and Thomas, 2007; Song et al., 2007; Zhong et al., 2010). In the adult, external sensory and chordotonal neurons innervate more elaborate structures formed by the cuticle including bristles and antennae. These cells continue to be responsible for proprioception and touch sensation. Multidendritic neurons persist into adulthood after extensive arbor rearrangements (Shimono et al., 2009).
Polymodal nociceptor neurons in Drosophila larvae called Class IV multidendritic or md neurons innervate the body surface and express both TRP and DEG/ENaC channel subunits (Figure 2B). These neurons initiate aversive, nocifensive responses to heat, mechanical loads and UV light (Hwang et al., 2012; Tracey et al., 2003; Xiang et al., 2010; Zhong et al., 2012; Zhong et al., 2010). The md neurons express three TRPA genes: painless, pyrexia and dTRPA1 (Figure 2B). None are expressed exclusively in md neurons, suggesting that these genes have additional functions. Painless is present in the larval cardiac tube (Sénatore et al., 2010) and in adult sensilla, including gustatory bristles in the proboscis, the leg and the wing margin (Al-Anzi et al., 2006); Pyrexia is expressed in neurons that innervate sensory bristles and antennae (Lee et al., 2005); and dTRPA1 is expressed both in chemoreceptor neurons and in central neurons required for temperature-sensing in adult flies (Hamada et al., 2008; Kim et al., 2010).
The contribution of Pyrexia to the mechanosensitivity of md neurons has not been studied, but genetic deletion of Painless and dTRPA1 increase the threshold for aversive responses to heat and force (Tracey et al., 2003; Zhong et al., 2012). In contrast, loss of either a DEG/ENaC channel subunit, Pickpocket, or DmPiezo reduces the response to intense mechanical stimuli, but has no effect on the response to noxious heat (Kim et al., 2012; Zhong et al., 2010). Decreasing the expression of both Pickpocket and DmPiezo renders larvae insensitive to noxious mechanical stimuli, but has little effect on responses to noxious heat. Additionally, cultured md neurons from DmPiezo knockout mutants lack mechanically activated currents that are present in cell isolated from wild-type animals (Kim et al., 2012). These findings suggest that Pickpocket and DmPiezo could function in parallel as MeT channels in md neurons.
Recent studies reveal that the painless and dTRPA1 genes encode multiple isoforms (Hwang et al., 2012; Zhong et al., 2012). The longest isoform of Painless, Painlessp103, has eight ankyrin repeats in the amino-terminal domain and the shortest, Painlessp60, has none. Both isoforms are expressed in md neurons, but only the shortest isoform rescues mechanonociception (Hwang et al., 2012). In contrast, dTRPA1 isoforms differ in regions of the protein that flank the ankyrin repeats (Zhong et al., 2012) and two isoforms of the gene are expressed in md neurons. One isoform, dTrpA1-C, restores normal thermal nociception but not mechanonociception. It is not clear whether dTRPA1-D or an as-yet-unidentified isoform is important for mechanonociception in md neurons. Thus, an unexpectedly complex model for md neuron-mediated mechanonociception is emerging—Pickpocket and DmPiezo detect mechanical loads in parallel, while Painlessp60 and perhaps a dTRPA1 isoform are required for post-transduction signaling, including amplification.
The Johnston’s organ (JO) of adult Drosophila antennae is a near-field sound receptor and like other animal ears, the JO relies on mechanical amplification and frequency-selective tuning to optimize sound sensitivity (Göpfert et al., 2006; Göpfert et al., 2005; Robert and Göpfert, 2002; Tsujiuchi et al., 2007). Sound is not the only mechanical stimulus detected by the JO, however. This array of hundreds of mechanoreceptor neurons also responds to displacements induced by wind and gravity (Kamikouchi et al., 2009; Sun et al., 2009; Yorozu et al., 2009). Mechanoreceptor cells in the JO project their axons into the antennal nerve and express five TRP channels (Figure 2B): NOMPC, Nan, Iav, Painless, and Pyrexia. Genetic dissection of hearing and gravitaxis reveals that some channels (Painless, Pyrexia) are needed to sense gravity, others for hearing (NOMPC), and that the TRPV proteins Nan and Iav are expressed broadly and needed for both hearing and gravity sensing.
In sound-sensitive chordotonal neurons, the exact function of each TRP channel is matter of continuing investigation. One model (Göpfert et al., 2006) is that NOMPC is essential for detecting sound-induced mechanical stimuli and Nan and Iav work together to both refine mechanical amplification and ensure the proper transmission of stimulus-evoked action potentials in the antennal nerve. In this schema, NOMPC functions like its C. elegans homolog, TRP-4, and forms the pore of a sensory MeT channel. Techniques for measuring mechanoreceptor currents in the JO are needed to directly test this model, but functional specialization of NOMPC and Nan/Iav is supported by the fact that they occupy distinct compartments in the sensory cilium of JO mechanoreceptors (Lee et al., 2010; Liang et al., 2011).
Several TRP proteins may be co-expressed in the chordotonal organs of the adult leg that provide information about joint position (Gong et al., 2004; Kim et al., 2003; Liang et al., 2011). These include, NOMPC, Iav and Waterwitch (Wtrw), which appear to be co-expressed in the campaniform sensilla that detect cuticle deformation in the wings and halteres (Gong et al., 2004; Kim et al., 2003; Liang et al., 2011). The coexpression of these proteins in other mechanoreceptor neurons suggests that an understanding of how these cells enable mechanosensitivity may depend on cellular context and the entire ensemble of ion channels expressed in each mechanoreceptor. Finally, NOMPC is famous for its expression in the mechanoreceptors that innervate large bristle sensilla on the fly’s body (Walker et al., 2000). NompC mutants lack transient, but retain sustained trans-epithelial mechanoreceptor currents (Walker et al., 2000). Thus, in bristle mechanoreceptors, there appears to be another mechanotransduction channel. Bristle receptors express many other TRP channel subunits as well as DEG/ENaC channel subunits (Figure 2B). These data raise the possibility that another channel may function in parallel to the NOMPC channel in the bristle receptor neuron.
The company of flies and nematodes
There is evidence that DEG/ENaC and TRP channels function as MeT channel subunits in distinct mechanoreceptor neurons in both C. elegans and Drosophila. One evolving paradigm is that mechanonociceptors (md neurons in Drosophila and ASH neurons in C. elegans)rely on DEG/ENaC proteins to detect noxious mechanical stimuli and on TRP channels for essential post-mechanotransduction signaling. Another is the notion that TRP channels can play multiple roles within a single mechanoreceptor neuron, exemplified by the finding that a trio of TRP channels is critical for mechanotransduction and post-transduction signal essential for hearing in Drosophila. A third paradigm is the presence of multiple MeT channels as found in Drosophila bristles, md neurons and C. elegans ASH nociceptors, suggesting that functional redundancy may be a shared feature of mechanoreceptors.
The mouse and its skin
As in nematodes and flies, mechanoreceptor neurons in mice co-express DEG/ENaC and TRP channel proteins and are among the principal actors that give rise to somatic sensations. Except for nociceptors associated with painful perceptions, the performance of mechanoreceptors in mammals depends on affiliation with specialized sensory organs in the skin presumed to be the locus of mechanotransduction. Figure 2C summarizes current research showing that DEG/ENaC and TRP channel proteins localize to the skin in mice, a property that suggests these proteins could form MeT channels in mammals. Most investigations of mechantransduction in mammals have relied on behavioral studies, analysis of dorsal root ganglia (DRG) or trigeminal (TG) neurons in culture, and extracellular, single-unit recordings in vivo and ex vivo. The recent demonstration of in vivo, whole-cell patch-clamp recordings from DRG neurons (Ma et al., 2010) is an exciting new tool that is just beginning to be applied.
The nerve endings emanating form TG and DRG neurons are diverse and are classified according to the expression of signaling peptides and receptors, their functional properties or their morphology and anatomy (Delmas et al., 2011; Lewin and Moshourab, 2004). For most somatosensory neurons, however, anatomical and functional properties are only loosely connected (Boulais and Misery, 2008). For instance, fibers that share multiple electrophysiological features such as Aβ-like conduction velocities as well as rapidly-adapting, low-threshold responses to mechanical stimuli innervate multiple peripheral end organs (Brown and Iggo, 1967; Vallbo et al., 1995). An example of the converse situation in which fibers with distinct electrophysical properties innervate a common peripheral end organ has recently come to light. In particular, Li et al. (2011a) show that Aβ, Aδ, and C fibers all form lanceolate endings that surround hair follicles. This discovery relied on developing a suite of genetic markers that were exploited in two ways. First, they were used to determine the peripheral endings associated with marked sensory sub-types. Second, they were used to link electrophysiological properties derived from in vivo intracellular recordings to the marker suite and hence to mechanoreceptor subtype. Thus, knowledge of conduction velocity and force sensitivity is not sufficient to infer the identity of the peripheral organ being stimulated. Nevertheless, many investigations of the contribution of ion channel proteins to somatosensation rely on functional classifications (see Figure 4).
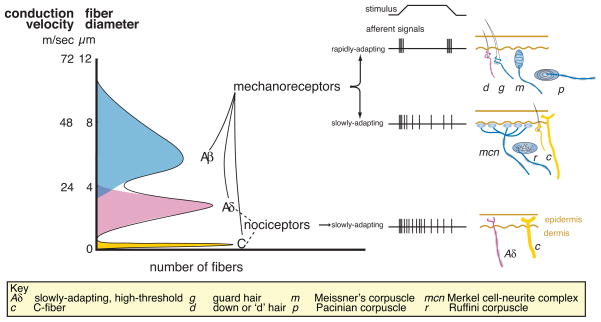
Figure 4. Classification schemes for skin mechanoreceptors in mammals.
Mammalian mechanoreceptor nerve fibers are classified by according to three physiological properties: 1) the speed of action potential propagation (which depends on fiber diameter and myelination state); 2) the threshold for activation; and 3) the rate of adaptation to mechanical stimuli. The broad categories of Aβ, Aδ and C-fibers are defined by their propagation speeds where Aβ-fibers have the most rapid propagation speeds and the slender, unmyelinated C-fibers have the slowest. Most fibers in these categories share other properties. For example, C fibers have slow rates of adaptation to mechanical stimuli and many have high mechanical thresholds. In contrast, most Aβ-fibers have low mechanical thresholds and these fibers are thought to innervate light-touch receptors in the skin. The challenge is to link fiber properties to the diverse endings in the skin.
Genetic deletion of single DEG/ENaC or TRP channel proteins in mice alters sensitivity to mechanical stimulation, but leaves both functions largely intact. While these studies cast doubt on the idea that DEG/ENaC or TRP channel proteins are essential for mechanotransduction in mammals, they also suggest that the mammalian somatosensory system is robust to genetic deletion. Such robustness could reflect molecular redundancy within or between ion channel gene families. Additionally, robustness could be conferred by functional degeneracy among mechanoreceptor neurons. The potential for degeneracy arises from the fact that each and every skin dermatome contains a mixture of peripheral sensory structures and is innervated by multiple classes of somatosensory neurons that fasciculate into a common nerve. For example, low-threshold, rapidly adapting Aβ fibers are thought to innervate both Pacinian and Meissner corpuscles in the skin (Brown and Iggo, 1967; Burgess et al., 1968; Vallbo et al., 1995). In addition to having distinct morphologies, each of these endings also expresses different DEG/ENaC and TRP channel proteins (Calavia et al., 2010; García-Añoveros et al., 2001; Kwan et al., 2009; Price et al., 2001; Suzuki et al., 2003B). In this scenario, loss of a single ion channel protein is expected to have only a minor effect on the entire class of such fibers.
The acid-sensing ion channels or ASICs are a vertebrate sub-division of the conserved DEG/ENaC superfamily. Most, if not all of the ASIC proteins are expressed in cell bodies in the trigeminal and dorsal root ganglia (reviewed in Deval et al., 2010) and localize to the peripheral endings in the skin (Figure 2C). For instance, ASIC1 is expressed in nerves innervating Pacinian corpuscles in human skin (Calavia et al., 2010; Montaño et al., 2009). However, genetic deletion of ASIC1 has no effect on the threshold or firing frequency of fibers innervating mouse skin (Page et al., 2004), but alters visceral sensory function (Page et al., 2004; Page et al., 2005). ASIC2 and ASIC3 are expressed in the majority of mechanoreceptor endings in mouse skin (Figure 2C; García-Añoveros et al., 2001; Price et al., 2000; Price et al., 2001), but deleting ASIC2 or ASIC3 has only subtle effects on the activity of mechanoreceptor fibers (Price et al., 2000; Price et al., 2001). Moreover, neither ASIC2 nor ASIC3 is essential for MeT currents studied in cultured DRG neurons (Drew et al., 2004). Collectively, these investigations indicate that no single ASIC subunit is essential for the function of mechanoreceptor neurons in mice and suggest that the function of such neurons is robust to genetic deletion of channel proteins.
In principle, genetic deletion of a shared auxiliary subunit could reveal more severe deficits in behavioral and cellular responses to touch because such proteins might affect the function of multiple channel-forming subunits. The impact of genetic deletion of SLP3 illustrates the power of this idea (Wetzel et al., 2007). SLP3 is a stomatin-like protein that binds to both ASIC2 and ASIC3 and alters the activity of ASIC channels in heterologous cells. It is orthologous to the C. elegans protein MEC-2, which is required for MeT currents in vivo and enhances MEC-4-dependent currents in heterologous cells (Goodman et al., 2002; Huang and Chalfie, 1994; O’Hagan et al., 2005). Genetic deletion of SLP3 decreases the proportion of mechanically sensitive Ab and Ad fibers that innervate the skin and the proportion of dissociated DRG neurons with mechanosensitive currents (Wetzel et al., 2007). Additionally, loss of SLP3 disrupts texture sensing. These data suggest that many, but not all mechanoreceptors depend on SLP3 and its DEG/ENaC binding partners to detect mechanical stimuli.
Mirroring the effects of single ASIC gene deletions are those of TRP channel gene deletions: loss of a single channel gene has only subtle effects on somatosensory nerve fiber function and no single TRP channel gene deletion leads to a loss of mechanosensitivity in an individual fiber class (Kwan et al., 2006; Kwan et al., 2009; Liedtke and Friedman, 2003; Suzuki et al., 2003a). But, loss of TRP channel proteins has clear effects on the response of nociceptors to inflammation. Here we provide three examples. First, noxious chemical agents such as mustard oil potentiate behavioral responses to mechanical stimuli, an effect which is muted in TRPA1 knockout mice (Bautista et al., 2006). Loss of TRPA1 also disrupts sensitization produced by injection of bradykinin, a peptide released by damaged tissue (Kwan et al., 2009). Second, genetic deletion of TRPV4 has subtle effects on behavioral and neural responses to mechanical cues (Chen et al., 2007; Suzuki et al., 2003a), but produces significant deficits in inflammation-induced sensitization. Compounds induced by inflammation (prostaglandin E2 and serotonin) decrease the threshold for mechanical activation of C fiber nociceptors in wild type, but not in TRPV4−/− (Alessandri-Haber et al., 2005; Chen et al., 2007). In addition, the spontaneous activity of C fibers is increased following inflammation, but there is no change in the rate of spontaneous activity in C fibers isolated from TRPV4−/− mice. Finally, TRPC1-deficient mice have reduced firing rates in Aβ-fibers and reduced probability of withdrawal from light touch (Garrison et al., 2012). A reduction of TRPC1 has a much more dramatic effect on the ability of the animals to respond to the inflammation mediators, prostaglandin E2 and serotonin (Alessandri-Haber et al., 2009). Thus, loss of TRP channels has subtle effects on baseline responses and significant effects on the ability of animals to respond to inflammation.
Conclusions and Perspectives
Over the past decades, a great deal of attention has focused on discovering the protein partners that form MeT channels in somatic mechanoreceptors. Two classes of ion channel proteins are leading candidates: DEG/ENaC and TRP channel proteins. Two others have recently joined their ranks: Piezo and TMC. Here, we surveyed the literature to establish that most, if not all mechanoreceptor neurons in worms, flies, and mice express multiple DEG/ENaC and TRP channel proteins. Piezo is expressed in a subset of somatosensory neurons in mice, but its representation relative to other channels is not known. Little is known about expression of TMC proteins in mechanoreceptor neurons. But, the landscape of ion channel co-expression in mechanoreceptor neurons is only beginning to be mapped. Future work aimed at refining such maps for mammalian mechanoreceptor neurons will be critical for deeper understanding. Also, each of these potential MeT channel subunits operates within a large company of other ion channel actors that increase the complexity, flexibility, and robustness of somatosensory neuron function.
Both DEG/ENaC and TRP channel proteins can function as essential, pore-forming subunits of MeT channels in three classes of mechanoreceptor neurons in worms: the touch receptor neurons, the CEP texture sensors, and the ASH nociceptors. Unexpectedly, some mechanoreceptor neurons rely on a single class of channels to detect mechanical cues (TRNs and CEP), while others (ASH) use at least two, genetically- and biophysically-distinct channels. Although such functional redundancy has been established only in invertebrates so far, it could explain some of the notable failures of genetic deletion of putative MeT channel subunits to disrupt touch and pain sensation in mice. From worms we also learn that some, but not all pore-forming MeT channel subunits are essential for mechanosensation. This situation is likely to exist in other mechanoreceptor neurons, including those responsible for touch and pain sensation in mammals. These findings strongly recommend adopting a cautionary stance in interpreting the modest effects of genetic deletion of a single MeT channel subunit.
Epilogue
Somatic sensation of gentle and noxious mechanical cues gives rise to our sense of touch and acute pain and also provides crucial information that regulates body movements and essential functions like blood pressure. The robustness that makes full loss of somatosensory function extremely rare complicates traditional genetic dissection of somatic sensation in mammals. Progress towards identifying the composition of MeT channels in mammalian mechanoreceptor neurons would be enhanced by refining current methods for categorizing somatosensory neurons and their fibers to better reflect their true functional organization. Perhaps, following the example of Li, et al. (2011a) and mapping the channel proteins co-expressed in peripheral endings in the skin will provide a reliable method for linking morphological subtypes to specific neuronal functions. When robust categorization of DRG and TG neurons can be combined with sub-type selective gene markers, the curtain could rise on a new scene in which selected classes of sensory neurons can be identified and targeted for in vivo whole-cell patch recording in transgenic mice, as they are in worms.
Acknowledgments
We thank the Goodman laboratory for reviewing many drafts and for lively discussion and dialog; two anonymous reviewers for their critiques; Rebecca Agin for artwork in Figures 1 and 2; we are grateful to wormbase and flybase for enabling investigations of what is known. Research supported by NIH grants RO1NS047715 and RO1EB006745 (MBG) and a Helen Hay Whitney Fellowship (SLG).
Footnotes
Search conducted on 15 April 2012 using search terms: “TRP channels”, “ENaC OR ASIC OR degenerin”, and the union of both terms.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CM, Anderson MG, Motto DG, Price MP, Johnson WA, Welsh MJ. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J Cell Biol. 1998;140:143–152. doi: 10.1083/jcb.140.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED. The impulses produced by sensory nerve-endings: Part 4. Impulses from Pain Receptors. J Physiol (Lond) 1926;62:33–51. doi: 10.1113/jphysiol.1926.sp002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED, Zotterman Y. The impulses produced by sensory nerve endings: Part 3. Impulses set up by Touch and Pressure. J Physiol (Lond) 1926a;61:465–483. doi: 10.1113/jphysiol.1926.sp002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED, Zotterman Y. The impulses produced by sensory nerve-endings: Part II. The response of a Single End-Organ. J Physiol (Lond) 1926b;61:151–171. doi: 10.1113/jphysiol.1926.sp002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–2222. doi: 10.1097/00001756-200007140-00031. [DOI] [PubMed] [Google Scholar]
- Al-Anzi B, Tracey WD, Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci. 2009;29:6217–6228. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Alavarez-Buylla R, Ramirez de Arellano J. Local responses in Pacinian corpuscles. Am J Physiol. 1953;172:237–244. doi: 10.1152/ajplegacy.1952.172.1.237. [DOI] [PubMed] [Google Scholar]
- Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- Arnadottir J, O’Hagan R, Chen Y, Goodman MB, Chalfie M. The DEG/ENaC protein MEC-10 regulates the transduction channel complex in Caenorhabditis elegans touch receptor neurons. J Neurosci. 2011;31:12695–12704. doi: 10.1523/JNEUROSCI.4580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50:6295–6300. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bechstedt S, Albert JT, Kreil DP, Müller-Reichert T, Göpfert MC, Howard J. A doublecortin containing microtubule-associated protein is implicated in mechanotransduction in Drosophila sensory cilia. Nat Commun. 2010;1:11. doi: 10.1038/ncomms1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek W. Physical limits to sensation and perception. Annu Rev Biophys Biophys Chem. 1987;16:455–478. doi: 10.1146/annurev.bb.16.060187.002323. [DOI] [PubMed] [Google Scholar]
- Block SM. Biophysical principles of sensory transduction. Soc Gen Physiol Ser. 1992;47:1–17. [PubMed] [Google Scholar]
- Bodmer R, Barbel S, Sheperd S, Jack JW, Jan LY, Jan YN. Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell. 1987;51:293–307. doi: 10.1016/0092-8674(87)90156-5. [DOI] [PubMed] [Google Scholar]
- Bodmer R, JAN YN. Morphological-differentiation of the embryonic peripheral neurons in Drosophila. Roux Arch Dev Biol. 1987;196:69–77. doi: 10.1007/BF00402027. [DOI] [PubMed] [Google Scholar]
- Boulais N, Misery L. The epidermis: a sensory tissue. Eur J Dermatol:EJD. 2008;18:119–127. doi: 10.1684/ejd.2008.0348. [DOI] [PubMed] [Google Scholar]
- Brown AG, Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol (Lond) 1967;193:707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PR, Petit D, Warren RM. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968;31:833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- Calavia MG, Montaño JA, García-Suárez O, Feito J, Guervós MA, Germanà A, Del Valle M, Pérez-Piñera P, Cobo J, Vega JA. Differential localization of Acid-sensing ion channels 1 and 2 in human cutaneus pacinian corpuscles. Cell Mol Neurobiol. 2010;30:841–848. doi: 10.1007/s10571-010-9511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci USA. 2004;101:15512–15517. doi: 10.1073/pnas.0403369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou M, Schafer WR. Lateral Facilitation between primary mechanosensory neurons controls nose touch perception in C. elegans. Neuron. 2011;70:299–309. doi: 10.1016/j.neuron.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, Kindt KS, Hwang SW, Miller DM, Treinin M, Driscoll M, Schafer WR. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci. 2010;13:861–868. doi: 10.1038/nn.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Alessandri-Haber N, Levine JD. Marked attenuation of inflammatory mediator-induced C-fiber sensitization for mechanical and hypotonic stimuli in TRPV4−/− mice. Molecular pain. 2007;3:31. doi: 10.1186/1744-8069-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang Q, Wang Z. The amiloride-sensitive epithelial Na+ channel PPK28 is essential for drosophila gustatory water reception. J Neurosci. 2010;30:6247–6252. doi: 10.1523/JNEUROSCI.0627-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010;67:373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci. 2011;12:139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- Deval E, Gasull X, Noël J, Salinas M, Baron A, Diochot S, Lingueglia E. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. 2010;128:549–558. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Deval E, Salinas M, Baron A, Lingueglia E, Lazdunski M. ASIC2b-dependent regulation of ASIC3, an essential acid-sensing ion channel subunit in sensory neurons via the partner protein PICK-1. J Biol Chem. 2004;279:19531–19539. doi: 10.1074/jbc.M313078200. [DOI] [PubMed] [Google Scholar]
- Donier E, Rugiero F, Jacob C, Wood JN. Regulation of ASIC activity by ASIC4--new insights into ASIC channel function revealed by a yeast two-hybrid assay. Eur J Neurosci. 2008;28:74–86. doi: 10.1111/j.1460-9568.2008.06282.x. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol (Lond) 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 1991;349:588–593. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- Drummond HA, Abboud FM, Welsh MJ. Localization of beta and gamma subunits of ENaC in sensory nerve endings in the rat foot pad. Brain Res. 2000;884:1–12. doi: 10.1016/s0006-8993(00)02831-6. [DOI] [PubMed] [Google Scholar]
- Ernstrom GG, Chalfie M. Genetics of sensory mechanotransduction. Annu Rev Genet. 2002;36:411–453. doi: 10.1146/annurev.genet.36.061802.101708. [DOI] [PubMed] [Google Scholar]
- Fricke B, Lints R, Stewart G, Drummond H, Dodt G, Driscoll M, von Düring M. Epithelial Na+ channels and stomatin are expressed in rat trigeminal mechanosensory neurons. Cell Tissue Res. 2000;299:327–334. doi: 10.1007/s004419900153. [DOI] [PubMed] [Google Scholar]
- Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell. 2011;144:614–624. doi: 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Añoveros J, Samad TA, Zuvela-Jelaska L, Woolf CJ, Corey DP. Transport and localization of the DEG/ENaC ion channel BNaC1alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. J Neurosci. 2001;21:2678–2686. doi: 10.1523/JNEUROSCI.21-08-02678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison SR, Dietrich A, Stucky CL. TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J Neurophysiol. 2012;107:913–922. doi: 10.1152/jn.00658.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffeney SL, Cueva JG, Glauser DA, Doll JC, Lee THC, Montoya M, Karania S, Garakani AM, Pruitt BL, Goodman MB. DEG/ENaC but not TRP Channels are the major mechanoelectrical transduction channels in a C. elegans nociceptor. Neuron. 2011;71:845–857. doi: 10.1016/j.neuron.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A, Damblychaudiere C, Aceves E, Jan LY, Jan YN. Sensory neurons and peripheral pathways in Drosophila embryos. Roux Arch Dev Biol. 1986;195:281–289. doi: 10.1007/BF00376060. [DOI] [PubMed] [Google Scholar]
- Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB. Mechanosensation. WormBook : the online review of C elegans biology. 2006:1–14. doi: 10.1895/wormbook.1.62.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, Ernstrom GG, Chelur DS, O’Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- Goodman MB, Schwarz EM. Transducing touch in Caenorhabditis elegans. Annu Rev Physiol. 2003;65:429–452. doi: 10.1146/annurev.physiol.65.092101.142659. [DOI] [PubMed] [Google Scholar]
- Göpfert MC, Albert JT, Nadrowski B, Kamikouchi A. Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci. 2006;9:999–1000. doi: 10.1038/nn1735. [DOI] [PubMed] [Google Scholar]
- Göpfert MC, Humphris ADL, Albert JT, Robert D, Hendrich O. Power gain exhibited by motile mechanosensory neurons in Drosophila ears. Proc Natl Acad Sci USA. 2005;102:325–330. doi: 10.1073/pnas.0405741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Sato M. Properties of the receptor potential in Pacinian corpuscles. J Physiol (Lond) 1953;122:610–636. doi: 10.1113/jphysiol.1953.sp005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründer S, Geissler HS, Bässler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport. 2000;11:1607–1611. doi: 10.1097/00001756-200006050-00003. [DOI] [PubMed] [Google Scholar]
- Hall DH, Gu G, García-Añoveros J, Gong L, Chalfie M, Driscoll M. Neuropathology of degenerative cell death in Caenorhabditis elegans. J Neurosci. 1997;17:1033–1045. doi: 10.1523/JNEUROSCI.17-03-01033.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem. 2004;279:11006–11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Chalfie M. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature. 1994;367:467–470. doi: 10.1038/367467a0. [DOI] [PubMed] [Google Scholar]
- Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol Cell Neurosci. 2007;35:383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S, Jones P, Mitchell A, Apweiler R, Attwood TK, Bateman A, Bernard T, Binns D, Bork P, Burge S, et al. InterPro in 2011: new developments in the family and domain prediction database. Nucleic Acids Res. 2012;40:D306–312. doi: 10.1093/nar/gkr948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson SJ, Costa WS, Wabnig S, Stirman JN, Watson JD, Spencer WC, Akerboom J, Looger LL, Treinin M, Miller DM, et al. Optogenetic analysis of a nociceptor neuron and network reveals ion channels acting downstream of primary sensors. Curr Biol. 2012 doi: 10.1016/j.cub.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang RY, Stearns NA, Tracey WD. The ankyrin repeat domain of the TRPA protein Painless in important for thermal nociception but not mechanical nfociception. PLoS ONE. 2012;7:e30090. doi: 10.1371/journal.pone.0030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales E, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Göpfert MC, Ito K. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZS. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Géléoc GSG, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, Griffith AJ. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel–like genes. J Clin Invest. 2011 doi: 10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci USA. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, Schafer WR. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Moon S, Cha Y, Chung YD. Drosophila TRPN(=NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS ONE. 2010;5:e11012. doi: 10.1371/journal.pone.0011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee Y, Lee J, Bang S, Hyun S, Kang J, Hong ST, Bae E, Kaang BK, Kim J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Moshourab R. Mechanosensation and pain. J Neurobiol. 2004;61:30–44. doi: 10.1002/neu.20078. [DOI] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011a;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Feng Z, Sternberg PW, Xu XZS. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kang L, Piggott BJ, Feng Z, Xu XZS. The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nat Commun. 2011b;2:315. doi: 10.1038/ncomms1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Madrid J, Saleh HS, Howard J. NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton (Hoboken) 2011;68:1–7. doi: 10.1002/cm.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- Liu L, Leonard AS, Motto DG, Feller MA, Price MP, Johnson WA, Welsh MJ. Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron. 2003;39:133–146. doi: 10.1016/s0896-6273(03)00394-5. [DOI] [PubMed] [Google Scholar]
- Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- Ma C, Donnelly DF, LaMotte RH. in vivo visualization and functional characterization of primary somatic neurons. Journal of Neuroscience Methods. 2010;191:60–65. doi: 10.1016/j.jneumeth.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaño JA, Calavia MG, García-Suárez O, Suarez-Quintanilla JA, Gálvez A, Pérez-Piñera P, Cobo J, Vega JA. The expression of ENa(+)C and ASIC2 proteins in Pacinian corpuscles is differently regulated by TrkB and its ligands BDNF and NT-4. Neurosci Lett. 2009;463:114–118. doi: 10.1016/j.neulet.2009.07.073. [DOI] [PubMed] [Google Scholar]
- O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Martinez-Salgado C, Wemmie JA, Brennan TJ, Symonds E, Omari T, Lewin GR, Welsh MJ, Blackshaw LA. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127:1739–1747. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, et al. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Rieke F, Rudd ME. The challenges natural images pose for visual adaptation. Neuron. 2009;64:605–616. doi: 10.1016/j.neuron.2009.11.028. [DOI] [PubMed] [Google Scholar]
- Robert D, Göpfert MC. Acoustic sensitivity of fly antennae. J Insect Physiol. 2002;48:189–196. doi: 10.1016/s0022-1910(01)00163-9. [DOI] [PubMed] [Google Scholar]
- Sénatore S, Rami Reddy V, Sémériva M, Perrin L, Lalevée N. Response to mechanical stress is mediated by the TRPA channel painless in the Drosophila heart. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono K, Fujimoto A, Tsuyama T, Yamamoto-Kochi M, Sato M, Hattori Y, Sugimura K, Usui T, Kimura K-i, Uemura T. Multidendritic sensory neurons in the adult Drosophila abdomen: origins, dendritic morphology, and segment- and age-dependent programmed cell death. Neural Dev. 2009;4:37. doi: 10.1186/1749-8104-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Watson JD, Spencer WC, O’Brien T, Cha B, Albeg A, Treinin M, Miller DM. Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Dev Biol. 2010;345:18–33. doi: 10.1016/j.ydbio.2010.05.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ESJ, Omerbašić D, Lechner SG, Anirudhan G, Lapatsina L, Lewin GR. The molecular basis of acid insensitivity in the African naked mole-rat. Science. 2011;334:1557–1560. doi: 10.1126/science.1213760. [DOI] [PubMed] [Google Scholar]
- Song W, Onishi M, Jan LY, Jan YN. Peripheral multidendritic sensory neurons are necessary for rhythmic locomotion behavior in Drosophila larvae. Proc Natl Acad Sci USA. 2007;104:5199–5204. doi: 10.1073/pnas.0700895104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liu L, Ben-Shahar Y, Jacobs JS, Eberl DF, Welsh MJ. TRPA channels distinguish gravity sensing from hearing in Johnston’s organ. Proc Natl Acad Sci USA. 2009;106:13606–13611. doi: 10.1073/pnas.0906377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003a;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Watanabe Y, Oyama Y, Mizuno A, Kusano E, Hirao A, Ookawara S. Localization of mechanosensitive channel TRPV4 in mouse skin. Neurosci Lett. 2003b;353:189–192. doi: 10.1016/j.neulet.2003.09.041. [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Shreffler W, Wang S, Driscoll M. unc-8, a DEG/ENaC family member, encodes a subunit of a candidate mechanically gated channel that modulates C. elegans locomotion. Neuron. 1997;18:107–119. doi: 10.1016/s0896-6273(01)80050-7. [DOI] [PubMed] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Tsujiuchi S, Sivan-Loukianova E, Eberl DF, Kitagawa Y, Kadowaki T. Dynamic range compression in the honey bee auditory system toward waggle dance sounds. PLoS ONE. 2007;2:e234. doi: 10.1371/journal.pone.0000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J, Kakuda N. Receptive field characteristics of tactile units with myelinated afferents in hairy skin of human subjects. J Physiol (Lond) 1995;483(Pt 3):783–795. doi: 10.1113/jphysiol.1995.sp020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglis G, Tavernarakis N. A synaptic DEG/ENaC ion channel mediates learning in C. elegans by facilitating dopamine signalling. EMBO J. 2008;27:3288–3299. doi: 10.1038/emboj.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Walters ET, Moroz LL. Molluscan memory of injury: evolutionary insights into chronic pain and neurological disorders. Brain Behav Evol. 2009;74:206–218. doi: 10.1159/000258667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445:206–209. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, Kamikouchi A, Ito K, Anderson DJ. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458:201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Bellemer A, Yan H, Honjo K, Robertson J, Hwang RY, Pitt GS, Tracey WD. Thermosensory and non-thermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat sensor domains of a thermoTRP channel. Cell Rep. 2012;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Hwang RY, Tracey WD. Pickpocket Is a DEG/ENaC Protein Required for Mechanical Nociception in Drosophila Larvae. Curr Biol. 2010;20:429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]