Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder whose etiology is thought to have environmental (toxin) and genetic contributions. The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine (MPTP) induces pathological features of PD including loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and striatal dopamine (DA) depletion. We previously described the striatal transcriptional response following acute MPTP administration in MPTP-sensitive C57BL/6J mice. We identified three distinct phases: early (5h), intermediate (24 h) and late (72 h) and reported that the intermediate and late responses were absent in MPTP-resistant Swiss-Webster (SWR) mice. Here we show that C57BL/6J mice pre-treated with a single 40 mg/kg dose of MPTP and treated 9 days later with 4 × 20 mg/kg MPTP, display a striatal transcriptional response similar to that of MPTP-resistant SWR mice i.e. a robust acute response but no intermediate or late response. Transcriptional refractoriness is dependent upon the dose of the priming challenge with as little as 10 mg/kg MPTP being effective and can persist for more than 28 days. Priming of SWR mice has no effect on their response to subsequent challenge with MPTP. We also report that paraquat, another free radical producer, also elicits striatal transcriptional alterations but these are largely distinct from those triggered by MPTP. Paraquat-induced changes are also refractory to priming with paraquat. However neither paraquat nor MPTP elicit cross-attenuation. Thus exposure to specific toxins triggers distinct transcriptional responses in striatum that are influenced by prior exposure to the same toxin. The prolonged refractory period described here for MPTP could explain at the molecular level the reported discrepancies between different MPTP administration regimens and may have implications for our understanding of the relationship between environmental toxin exposure and PD.
Keywords: Parkinson’s disease, MPTP, C57BL/6J, Swiss Webster, dopamine, paraquat
1. Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc). The etiology of sporadic PD, the most frequent form of the disorder, remains uncertain, although it likely involves interplay between environmental toxins (Landrigan et al., 2005, Brown et al., 2006) and predisposing genetic risk factors (Huang et al., 2004, Benmoyal-Segal and Soreq, 2006, Farrer, 2006, Wood-Kaczmar et al., 2006). Several reports indicate that exposure to environmental agents and chronic use of certain substances alters the incidence of PD (Tanner et al., 2011). Cigarette smoking (Tanaka et al., 2010), chronic intake of ibuprofen (Gao and Hong, 2011), or caffeine (Costa et al., 2010), have been associated with a lower incidence of PD. On the other hand several studies indicate that viral infections (Jang et al., 2009, Henry et al., 2010), prenatal exposure to lipopolysacharide (Barlow et al., 2007), drinking well water (Koller et al., 1990), and life-long exposure to heavy metals (Weisskopf et al., 2010), pesticides and herbicides (Gatto et al., 2009), can increase the risk of developing PD.
The introduction of several toxin- and genetic-based models for PD has advanced our knowledge of the disease, although none of the models fully recapitulate the characteristics of sporadic PD (Bove et al., 2005, Meredith et al., 2008). The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine (MPTP) model represents the most widely used and better-characterized toxin-based animal model that is currently available (Smeyne and Jackson-Lewis, 2005). This neurotoxin was first identified as the cause of PD-like symptoms in humans (Ballard et al., 1985) and was subsequently used to simulate PD in various animals including mice (Dauer and Przedborski, 2003, Smeyne and Jackson-Lewis, 2005). MPTP induces the characteristic loss of DAergic neurons in SNpc and a concomitant loss of striatal dopamine seen in PD (Dauer and Przedborski, 2003, Smeyne and Jackson-Lewis, 2005). Furthermore, sensitivity to MPTP is genetically influenced: some strains of mice, such as Swiss Webster (SWR), display a limited loss of DAergic neurons after MPTP administration, whereas other strains of mice such as C57BL/6J, display a larger cell loss (Sundstrom et al., 1987, German et al., 1996, Hamre et al., 1999, Vila et al., 2001). These strain differences provide an opportunity to identify predisposing genetic determinants of MPTP toxicity.
The striatum, that is innervated by the SNpc DAergic neurons, is likely to be the proximal target of neurotoxicity in the MPTP model (Boyd et al., 2007) and PD itself (Brooks, 1991). MPTP is a protoxin that rapidly crosses the blood brain barrier and it is metabolized to 1-methyl-4-phenylpiridinium (MPP+) by monoamine oxidase B (MAO-B) in glial cells (Smeyne and Jackson-Lewis, 2005). Within hours of MPTP exposure, DA is released in the striatum from synaptic stores (Bazzu et al., 2010), and dopaminergic nerve terminals show evidence of dysfunction (Nagai et al., 2007). Several days after this initial MPTP-induced dopamine depletion, microglia are activated and SNpc DAergic neurons degenerate (Boyd et al., 2007).
Using Affymetrix chip technology coupled with quantitative reverse transcribed PCR (qRT-PCR) we previously described a stereotypic change in gene expression patterns (Pattarini et al., 2008). We identified three distinct phases of MPTP-induced gene expression changes characterized by almost unique sets of genes. The earliest response was seen 5 hour after systemic administration of MPTP, and was followed by an intermediate (24 hour), and a late (72 hour) response. The changes in transcriptional response occur long before any sign of dopaminergic neuron degeneration can be identified in the SNpc (Boyd et al., 2007).
Administration of MPTP follows different dosing regimens that simulate different types of exposures. The acute paradigm uses doses of MPTP injected over a short period of time, whereas other regimens simulate prolonged exposure to lower doses of MPTP (Meredith et al., 2008). All the dosing regimens cause loss of DAergic neurons in SNpc and are often used interchangeably (Meredith et al., 2008). Nonetheless, evidence suggests that they may differ in various respects including the ability to induce proteinaceous inclusions (Alvarez-Fischer et al., 2008, Meredith et al., 2008), the progressiveness of the DAergic lesion in the SNpc, and recovery from injury of the surviving DAergic neurons (Meredith et al., 2008).
Here we have used Affymetryx gene expression analysis to compare mRNA expression profiles in various MPTP regimens. We have complemented this by comparing the expression profiles elicited by paraquat with those of MPTP and whether there is an interaction at the level of gene transcription between these two agents.
2. Experimental procedures
2.1 Animals and Experiments
8 week old female C57BL/6J mice were purchased from the Jackson Laboratories (Bar Harbor, ME, USA), and female Swiss Webster (SWR) mice were purchased from Harlan (Strain ND4, Indianapolis, IN, USA). Animals were housed in micro-isolator units with a 12 hour light-dark schedule and constant temperature with free access to food and water. When animals were 3–4 months of age, MPTP-HCl (Sigma, St. Louis, MO, USA) was administered by intraperitoneal (IP) injections at the dosage and schedule specified (see Results and Figure Legends). Further details regarding MPTP administration and biosafety have been previously reported (Przedborski et al., 2001, Smeyne et al., 2001). Animals were sacrificed at the time points after MPTP administration specified for each experiment (see Results and Figure Legends), and the striatum was rapidly dissected and immediately frozen on dry ice to preserve RNA integrity. Samples were stored at −80°C. All studies were approved by the St. Jude Children's Research Hospital Animal Care and Use Committee (ACUC) and were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996). Efforts were made to minimize the number of animals involved in each experiment and their suffering.
2.2 RNA Isolation
Total RNA was extracted with TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer instructions. Briefly, 1 ml TRIzol® was added to frozen samples and immediately homogenized. Samples were mixed with 200 µl of chloroform and centrifuged for 15 min at 12,000 × g at 4°C. The aqueous phase was transferred to a new vial, mixed with 50 µg of glycogen from Roche Applied Science (Penzberg, Germany) and 500 µl of isopropanol and centrifuged (10 min at 12,000 × g, 4°C) to precipitate total RNA. The pellet was washed with ice cold 70% ethanol and air dried for 10 min. Total RNA was resuspended in RNase-free water and it was checked for integrity by agarose gel electrophoresis. Samples that appeared degraded were discarded.
2.3 Preparation of Samples for Microarray Analysis
Technical procedures for microarray analysis, including quality control of RNA, labeling, hybridization and scanning of the arrays were performed by the Hartwell Center for Bioinformatics & Biotechnology (HC) at St. Jude Children's Research Hospital (SJCRH) according to standard operating procedures for Affymetrix protocols (GeneChip® Expression Analysis manual, Affymetrix, Santa Clara CA, USA). Prior to use, RNA sample integrity was assessed with a 2100 Bioanalyzer Lab-on-a-chip system (Agilent Technologies, Santa Clara, CA, USA). Total RNA samples that were not degraded were labeled using the Gene Chip IVT Labeling Kit (Affymetrix) according to manufacturer instructions. Further technical details are reported in Pattarini et al. (2008).
2.4 Analysis of Microarrays
The microarrays used in this study (Mouse Genome 430 2.0 Arrays, Affymetrix) contain 45,101 probe sets, representing 39,000 transcripts and variants. Scanned images were analyzed with the Gene Chip Operating Software (GCOSv1.2, Affymetrix). Assessment of probe set present/absent calls was made using the Single Array Analysis method in GCOS using statistical algorithm with default analysis parameters (http://www.affymetrix.com/support/technical/whitepapers/sadd_whitepaper.pdf). Probe set signal values were scaled by global methods to a target value of 500.
Array analysis was performed using Spotfire® DecisionSite 8.2 from TIBCO Software Inc. (Palo Alto, CA, USA) as previously reported in Pattarini et al. (2008).
Gene ontology (GO) and Gene set enrichment analysis (GSEA)
Gene ontology analysis for the probe sets differentially expressed between SM/SS (203 probe sets) and SP/SS (70 probe sets) was performed using DAVID Bioinformatics Resources (http://david.abcc.ncifcrf.gov/). GO terms with p-value less than 0.003 were selected.
To identify biological processes and molecular functions differentially represented between MPTP and paraquat treatments, we compared all the probe sets in the array of MPTP-treated mice (SM) to those of paraquat-treated mice (SP), using a permutation approach called gene set enrichment analysis (GSEA). GSEA is a computational method that determines whether an a priori defined set of genes shows statistically significant, concordant differences between two biological states. This analysis was performed using the R-version of a publicly available program at http://www.broad.mit.edu/gsea (Mootha et al., 2003, Subramanian et al., 2005). Gene sets for this analysis were downloaded from the Broad Institute Molecular Signature database (MSigDB) at http://www.broadinstitute.org/gsea/msigdb/index.jsp. Only results with a normalized enrichment score (NES) ≥ 1.7 and false discovery rate (q) ≤ 0.07 were considered. Further details can be found in Pattarini et al. (2007).
2.5 Validation of Microarray Data by Quantitative RT-PCR
Total RNA was reverse transcribed using TaqMan® reverse transcription reagents from Applied Biosystems (Foster City, CA, USA). Primers and probes for real-time PCR (qRT-PCR) were designed with Primer Express Software version 1.5 for Windows® (Applied Biosystems) and synthesized by the HC. Real time PCR was performed using TaqMan® PCR Core Reagent Kit (Applied Biosystem), and the ABI Prism 7900HT system (Applied Biosystem). Absolute quantification was performed using standard curves for each gene of interest. Primers and probes used for qRT-PCR, for both absolute and relative (ΔΔCt) quantification are listed in Table 1.
Table 1.
List of primers and probes for qRT-PCR
| Gene Name | mRNA ID # | Forward Primer | Probe | Reverse Primer |
|---|---|---|---|---|
| Cdkn1a | NM_007669 | 5’-TTCCGCACAGGAGCAAACT-3’ | 5’-Fam-CCGTTGTCTCTTCGGTCCCGTGG-BHQ-3’ | 5’-CGGCGCAACTGCTCACT-3’ |
| Edg3 | NM_010101 | 5’-AACCAGCCCAGATGCGC-3’ | 5’-Fam-TTGCAGAACGAGAGCCTATTTTCAACACTCTTC-BHQ-3’ | 5’-GCGTGCAGGCCCGAC-3’ |
| Fosb | NM_008036 | 5’-CCAGAGCCAGGCCTAGAAGAC-3’ | 5’-Fam-TCGCAGAGAGCGGAACAAGC-BHQ-3’ | 5’-CCGACGGTTCCTGCACTTA-3’ |
| Gadd45b | NM_008655 | 5’-AGGCGGCCAAACTGATGA-3’ | 5’-Fam-TGTGGACCCCGACAGCGTGG-BHQ-3’ | 5’-CATCCTCCTCTTCTTCGTCTATGG-3’ |
| Gadph | NM_001001303 | 5’-TGGATCTGACGTGCCGC-3’ | 5’-Fam-TGGAGAAACCTGCCAAGTATGATGACATCA-BHQ-3’ | 5’-TGCCTGCTTCACCACCTTC-3’ |
| Hbegf | NM_010415 | 5’-TGCTGCCGTCGGTGATG-3’ | 5’-Fam-TGAAGCTCTTTCTGGCCGCAGTGTTG-BHQ-3’ | 5’-ACCGGTCACCAACGCG-3’ |
| Hmox1 | NM_010442 | 5’-GTGATGGAGCGTCCACAGC-3’ | 5’-Fam-CGACAGCATGCCCCAGGATTTGTC-BHQ-3’ | 5’-TGGTGGCCTCCTTCAAGG-3’ |
| Mcp-1/Ccl2 | NM_011333 | 5’-GAGCATCCACGTGTTGGCT-3’ | 5’-Fam-AGCCAGATGCAGTTAACGCCCCACT-BHQ-3’ | 5’-TGGTGAATGAGTAGCAGCAGGT-3’; |
| NFkBia | NM_010907 | 5’-CAGCTCACGGAGGACGGA-3’ | 5’-Fam-ACTCGTTCCTGCACTTGGCAATCATCC-BHQ-3’ | 5’-ATGGTCAGCGGCTTCTCTTC-3’ |
| Nr4a1 | NM_010444 | 5’-GGCCACAGGGAGTGGGA-3’ | 5’-Fam-CCGGCTGGAGATGCCCTGTATTCAAG-BHQ-3’ | 5’-CGTTGCTGGTGTTCCATATTGA-3’ |
| Pdlim4 | NM_019417 | 5’-AGTGCACGCGCTGCG-3’ | 5’-Fam-ACGGGATCGTGGGAACCATTGTCA-BHQ-3’ | 5’-ATGGTAGAGCTTGTCTCTCGCC-3’ |
| Tnfrsf12a | NM_013749 | 5’-GCCGCCGGAGAGAAAAG-3’ | 5’-Fam-TTACTACCCCCATAGAGGAGACTGGTGGAGAG-BHQ-3’ | 5’-GCCACACCTGGGCAGC-3’ |
| Tnf | NM_013693 | 5’-TCTATGGCCCAGACCCTCAC-3’ | 5’-Fam-CTCAGATCATCTTCTCAAAATTCGAGTGACAAGC-BHQ-3’ | 5’-TTGCTACGACGTGGGCTACA-3’ |
Standards for absolute quantification were prepared by cloning the coding sequence (CDS) of each gene into a pcDNA3 plasmid (Invitrogen) as previously described (Pattarini et al., 2007, Pattarini et al., 2008). The primers used to prepare the standards, including the restriction site used, are listed in Table 2.
Table 2.
List of primers used to prepare the standards for qRT-PCR
| Gene name | Enzyme | Forward primer | Enzyme | Reverse primer |
|---|---|---|---|---|
| Cdkn1a | HindIII | 5’-CCCAAGCTTGGGATGTCCAATCCTGGTGATGT-3’ | EcoRI | 5’-CCGGAATTCCGGCTTCAGGGTTTTCTCTTGCA-3’ |
| Edg3 | EcoRI | 5’-CCGGAATTCCGGATGGCAACCACGCATGCGCA-3’ | XhoI | 5’-CCGCTCGAGCGGTCACTTGCAGAGGACCCCGT-3’ |
| Fosb | BamHI | 5’-CGCGGATCCGCGGTGAAACCGACAGAGCCTGG-3’ | EcoRI | 5’-CCGGAATTCCGGGTTCCTTGCGGGTTTGTTTG-3’ |
| Gadd45b | BamHI | 5’-CGCGGATCCGCGATGACCCTGGAAGAGCTGGT-3’ | EcoRI | 5’-CCGGAATTCCGGTGGGTCTCAGCGTTCCTCTA-3’ |
| Gapdh | BamHI | 5’-CGCGGATCCGCGATGGTGAAGGTCGGTGTGAA-3’ | EcoRI | 5’-CCGGAATTCCGGTTCTTACTCCTTGGAGGCCA-3’ |
| Hbegf | HindIII | 5’-CCCAAGCTTGGGATGAAGCTGCTGCCGTCGGT-3’ | EcoRI | 5’-CCGGAATTCCGGATAGCTCAGGTCCTCCTCAGTGGG-3’ |
| Hmox1 | BamHI | 5’-CGCGGATCCGCGTAGCCCAGTCCGGTGATGGA-3’ | EcoRI | 5’-CCGGAATTCCGGTGGGGGCCAGTATTGCATTT-3’ |
| Mcp-1/Ccl2 | BamHI | 5’-CGCGGATCCGCGTCTCTTCCTCCACCACCATG-3’ | EcoRI | 5’-CCGGAATTCCGGGCATCACAGTCCGAGTCACA-3’ |
| NFkBia | HindIII | 5’-CCCAAGCTTGGGAGCCATGTTTCAGCCAGCTG-3’ | XhoI | 5’-CCGCTCGAGCGGTTATAATGTCAGACGCTGGC-3’ |
| Nr4a1 | BamHI | 5’-CGCGGATCCGCGTGCTAGAAGGACTGCGGAGC-3’ | EcoRI | 5’-CCGGAATTCCGGGGCTTAAAGGCACATGGGTG-3’ |
| Pdlim4 | HindIII | 5’-CCCAAGCTTGGGATGACCCACTCGGTGACCCTG-3’ | EcoRI | 5’-CCGGAATTCCGGCAGCTCAGACAAGTTCCACCT-3’ |
| Tnf-α | BamHI | 5’-CGCGGATCCGCGATGAGCACAGAAAGCATGAT-3’ | EcoRI | 5’-CCGGAATTCCGGCTTCACAGAGCAATGACTCC-3’ |
| Tnfrsf12a | BamHI | 5’-CGCGGATCCGCGGCAATCATGGCTCCGGGTTG-3’ | EcoRI | 5’-CCGGAATTCCGGTGAATCACCACCTCGCCCCA-3’ |
2.6 Statistical Analysis
Statistical methods used to analyze microarray results are explained in the microarray analysis section. Statistical analysis for qRT-PCR results was performed with GraphPad Prism® version 5.0 for Macintosh® (GraphPad Software Inc., San Diego, CA). Results are expressed as the ratio of number of copies of a specific gene over the number of copies of glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Each time point is the average of at least three animals. The temporal profile of each gene was analyzed by one-way ANOVA followed by Bonferroni’s multiple comparisons test to assess statistical significance versus respective control.
2.7 HPLC quantitation of dopamine and its metabolites
Striata were rapidly removed at specified time points after a single dose of MPTP and placed on an ice-cooled plate for dissection of the striatum. Tissue was weighed and homogenized in 10 volumes of ice-cooled 0.1 M perchloric acid and centrifuged for 12 minutes at 10,000 × g at 4°C. The supernatant was passed through a 0.2 µm filter and stored at −70°C. Dopamine (DA), and its metabolites 3–4-dihydroxphenylacetic acid (DOPAC) and homovanillic acid (HVA) were analyzed using reverse-phase ion pairing HPLC combined with electro coupling (EC) detection under isocratic conditions (Acworth and Cunningham, 1999). The guard cell was set at +350 mV, with a screening electrode set at −150 mV, and working electrode set at +220 mV. The mobile phase consisted of 75 mM sodium dihydrogen phosphate monohydrate, 1.7 mM 1-octane sulfonic acid sodium salt, 0.73 mM triethylamine, 25 $M EDTA, 10% acetonitrile/90% water. The pH was adjusted to 3.0 with phosphoric acid. The mobile phase was delivered at a flow rate of 0.6 ml/min onto the MD-150 (3×150 mm, 3 µm) reverse phase column (ESA, Chelmsford, MA, USA). Twenty µl aliquots were injected by an autoinjector with cooling module set at 4°C. The levels of DA, DOPAC, and HVA in unknown samples were determined using standard curves created by injection of known concentrations of each substance.
3 Results
3.1 Differences between the acute and subchronic MPTP regimens
Two widely characterized MPTP dosing regimens, acute and subchronic, were used in this study (Petroske et al., 2001). The acute model consists of four injections of MPTP (20 mg/kg) spaced two hours apart, whereas the subchronic regimen consists of single daily injections of MPTP (40 mg/kg) for 4 days (Figure 1A).
Figure 1. Comparison of mRNA changes of Hmox1 in the striatum C57BL/6J mice treated with the acute or subchronic MPTP regimens.
Panel A: Schematic representation of the different MPTP injection schedules used in this study. Animals treated according to the acute regimen received 4 injections of 20 mg/kg MPTP-HCl (MPTP) spaced 2 hours apart during a single day. The subchronic model consisted of daily injections of 40 mg/kg MPTP for 4 consecutive days. Panel B: quantitative assessment of Hmox1 mRNA levels after a single variable dose of MPTP in the striatum of MPTP-sensitive C57BL/6J mice. Animals were injected at time zero with a single dose of MPTP (10, 20 or 40 mg/kg) or saline as control, and sacrificed at 24 h. Levels of mRNA for Hmox1 was determined by qRT-PCR using the ΔΔCt relative expression method as described in the Materials and Methods section. Data are presented as mean ± S.E.M. of 4 animals for each condition. Differences versus control (saline, white bar) were analyzed with one-way ANOVA and Bonferroni post-hoc tests (*** P<0.001, ** P<0.01). MPTP causes a dose-dependent increase of Hmox1 mRNA levels starting at the dose of 20 mg/kg. Panel C: quantitative assessment of Hmox1 mRNA levels after MPTP intoxication following the acute regimen in the striatum of MPTP-sensitive C57BL/6J mice. Animals were injected at time zero with 20 mg/kg MPTP according to the schematic reported on top of the bar graph. Control animals were injected with saline (white bar). Animals were sacrificed at 2, 4, 8, 12, 18, 24, 36 and 48 h after the first injection. Animals sacrificed at 2 and 4h received only 1 and 2 injections, respectively. Levels of mRNA for Hmox1 were determined by qRT-PCR using the absolute quantization methods. Data are presented as mean ± S.E.M. of 3 animals for each condition. Differences versus control (saline, white bar) were analyzed with one-way ANOVA and Bonferroni post-hoc tests (*** P<0.001). Following acute administration of MPTP, Hmox1 mRNA levels increase starting at 18 h and return to baseline at 36 h. Panel D: quantitative assessment of Hmox1 mRNA levels after MPTP intoxication following the subchronic regimen in the striatum of MPTP-sensitive C57BL/6J mice. Animals were injected with 40 mg/kg MPTP according to the schematic reported on top of the bar graph. Control animals were injected with saline (white bar). Animals were sacrificed 24 h after each injection. Mice were sacrificed on day 1, 2, 3, 4 and 8 and received 1, 2, 3, 4 and 5 injections, respectively. Levels of mRNA for Hmox1 were determined by qRT-PCR using the ΔΔCt relative expression method. Data are presented as mean ± S.E.M. of 15 (time point 0), 12 (time points 1, 2 and 3), 7 (time point 4) and 5 (time point 8) animals. Differences versus control (white bar) were analyzed with one-way ANOVA and Bonferroni post-hoc tests (*** P<0.001).
We have previously reported that 24 h after systemic injection of MPTP, mRNA levels for the inducible form of heme oxygenase-1 (Hmox1) are elevated in striatal astrocytes (Fernandez-Gonzales et al., 2000). Therefore, we used mRNA expression of this gene as a benchmark to determine the minimal dose of MPTP necessary to elicit a transcriptional response (Figure 1B). C57BL/6J mice were injected with a single dose of MPTP at concentrations of 10, 20 and 40 mg/kg, sacrificed 24 h later and striatal Hmox1 mRNA levels determined by qRT-PCR. A statistically significant increase in relative Hmox1 mRNA was detected starting with the 20 mg/kg dose (Figure 1B). Thus, single doses of MPTP used in both the acute (20 mg/kg) and subchronic (40 mg/kg) regimens are able to modulate Hmox1 expression.
We next compared striatal Hmox1 expression profiles over time in the two MPTP models by qRT-PCR. For the acute model, we intraperitoneally injected C57BL/6J mice with MPTP (20 mg/kg) or saline over an eight hour period (0, 2, 4 and 6 h) and collected striata at 2, 4, 8, 12, 18, 24, 36 and 48 h after the first injection. Levels of Hmox1 mRNA increased between 12 and 24 h and returned to basal levels by 36 h (Figure 1C), confirming results from our previous study (Fernandez-Gonzales et al., 2000). Using the subchronic regimen (Figure 1A), animals were intraperitoneally injected with MPTP (40 mg/kg) on day 0, 1, 2, and 3 and their striata were harvested 24 h after each injection (Figure 1D). Striatal Hmox1 mRNA levels were elevated 24 h after the first injection (Figure 1D), however, further injections of MPTP (40 mg/kg) failed to induce Hmox1 gene expression (Figure 1D). Even a fifth dose of MPTP (for a total of 200 mg/kg) a week after the first MPTP injection failed to elevate Hmox1 mRNA levels (Figure 1D). Thus exposure to a single dose of MPTP elicits a refractory period in which Hmox1 cannot be reinduced.
3.2 A single dose of MPTP elicits a long-term refractory state for Hmox-1 induction
To confirm and extend our finding of an MPTP-induced refractory period, we investigated the dose-dependency and duration of this phenomenon. C57BL/6J mice were injected on day 0 with a priming dose of MPTP ranging from 1 to 40 mg/kg (Figure 2A). Some mice were sacrificed the next day to assess the effect of the priming dose on Hmox1 mRNA levels (blue bars in figure 2A). The remaining animals received a second dose of 40 mg/kg MPTP on day 7 and were sacrificed 24 h later (brown bars in figure 2A). The dose-response at 24 h after the priming dose was similar to that reported in Figure 1B: only doses at or higher than 20 mg/kg increased Hmox1 mRNA levels (compare Figure 1B with blue bars in Figure 2A). Pre-exposure to either 20 or 40 mg/kg abolished Hmox1 gene expression after the second injection of MPTP (40 mg/kg) (Figure 2A). Interestingly, although 10 mg/kg MPTP did not alter Hmox1 mRNA levels after 24 hours (blue bar in Figure 2A), it was sufficient to lower the mRNA levels of Hmox1 after the second injection of MPTP when compared to the 1 mg/kg priming dose (brown bar in Figure 2A).
Figure 2. Dose dependency and time course of the latency period caused by a single injection of MPTP.
Panel A: MPTP-sensitive C57BL/6J mice were injected according to the schematic on the top of the bar graph. Animals received a single injection of MPTP (1, 5, 10, 20 or 40 mg/kg) on day 0. Some animals were sacrificed 24 h later (blue bars) while remaining animals were injected a second time with 40 mg/kg MPTP after 7 days and sacrificed after 24 hours (brown bars). Control animals were injected with saline using the same schedules. Levels of mRNA for Hmox1 were determined by qRT-PCR using the ΔΔCt relative expression method. Data are presented as mean ± S.E.M. of 6 to 9 animals for each condition. Differences versus control (Saline) were analyzed by one-way ANOVA followed by Bonferroni post-hoc tests and are indicated with asterisks (*** P<0.001). Differences among treatments were analyzed with one-way ANOVA and Bonferroni post-hoc tests and indicated with letters (A P<0.001). Exposure to 10 mg/kg MPTP does not significantly elevate Hmox1 mRNA levels (blue bar) but can reduce the response to acute administration of MPTP (brown bar). Panel B: MPTP-sensitive C57BL/6J mice were injected according to the schematic on top of the bar graph. Animals received a single injection of 40 mg/kg MPTP or saline as control on day 0. Some animals were sacrificed 24 h later (green and white bars) whereas remaining animals received a second injection of 40 mg/kg MPTP or saline as control either 7, 14, 21 or 28 days later and were sacrificed 24 h after the second injection. Levels of mRNA for Hmox1 were determined by qRT-PCR using the ΔΔCt relative expression method. Data are presented as mean ± S.E.M. of 7 to 27 animals for each condition. Differences versus control (Saline) were analyzed by one-way ANOVA followed by Bonferroni post-hoc tests and are indicated with asterisks (*** P<0.001). Differences versus MPTP (green bar) and between MPTP-Saline and MPTP-MPTP (blue and brown bars, respectively) were analyzed with one-way ANOVA and Bonferroni post-hoc tests and indicated with letters (A P<0.001).
To investigate the duration of refractoriness, we injected C57BL/6J mice on day 0 with a single dose of MPTP (40 mg/kg) or saline. Some animals were sacrificed after 24 h to assess the effect of the first MPTP injection (green bar in Figure 2B). The remaining animals were injected a second time with MPTP (40 mg/kg) (brown bars in Figure 2B) or saline (blue bars in Figure 2B) on either day 7, 14, 21 or 28 and were sacrificed 24 h after the second injection. Striatal Hmox1 mRNA levels were elevated 24 h after the priming dose of MPTP (40 mg/kg) (green bar in Figure 2B). When compared to mice that received MPTP followed by saline, mice that received two injections of MPTP did not have statistically significantly elevated levels of Hmox1 at any time point (compare blue and brown bars in Figure 2B). Although there was a trend towards recovery of a transcriptional response to MPTP starting at day 22, it did not reach statistical significance, indicating that the refractory period elicited by MPTP lasts at least 3–4 weeks.
3.3 mRNA levels for Cdkn1a are elevated after multiple injections of MPTP
Shortly (5 h) after administration of MPTP, the cyclin-dependent kinase inhibitor 1a (Cdkn1a a.k.a. p21Waf1/Cip1) mRNA level is elevated in striatum. This is only one of the 88 genes that are elevated starting at early time points after MPTP injections in both MPTP-sensitive (C57BL/6J) and MPTP-resistant (SWR) mice (Pattarini et al., 2008). Therefore we assessed Cdkn1a mRNA levels in striatum by qRT-PCR. C57BL/6J mice were injected with MPTP (40 mg/kg) or saline as control at day 0, 1, 2 and 3 (0, 24, 48 and 72 h, respectively; Figure 3) and sacrificed 5 and 24 h after each injection. Cdkn1a mRNA levels were elevated 5 hours after each injection, and returned to baseline 24 h later (Figure 3). Thus in contrast to Hmox1, MPTP does not induce a refractory period for Cdkn1a.
Figure 3. Quantitative assessment of Cdkn1a mRNA changes in the striatum of C57BL/6J mice treated with the subchronic MPTP regimen.
MPTP-sensitive C57BL/6J mice were injected according to the schematic shown on the top of the bar graph. Animals were injected daily with a single dose of 40 mg/kg MPTP and sacrificed 5 or 24 hr after each injection. Control animals were injected with saline (white bar). Levels of mRNA for Cdkn1a were determined by qRT-PCR using the ΔΔCt relative expression method. Data are presented as mean ± S.E.M. of 10 animals for each condition. Differences versus control (white bar) were analyzed by one-way ANOVA followed by Bonferroni post-hoc tests and are indicated with asterisks (*** P<0.001, * P<0.05). Differences among time points were analyzed with one-way ANOVA and Bonferroni post-hoc tests and indicated with letters (A P<0.001, B P<0.01).
3.4 Genome-wide transcriptional responses after pre-exposure to MPTP
We used a genome-wide approach to determine whether genes other than Hmox1 were refractory to repeated administration of MPTP. C57BL/6J mice were “primed” with a single dose of either MPTP (40 mg/kg) (M) or saline (S) on day 0. After 9 days, saline or MPTP-primed mice were injected with either saline or MPTP following the acute regimen (4×20 mg/kg every 2 hours). Mice were sacrificed 5, 24 and 72 h after the first injection. Control animals (S-S) were injected with saline on both days. Animals in the M-S group received a single dose of MPTP (40 mg/kg) on day 0 and saline on day 9 to identify genes that were still elevated 9 days after the priming dose. Animals of the S-M group were treated with saline on day 0 and MPTP on day 9 to assess the transcriptional response after acute administration of MPTP. Mice in the M-M group were injected with MPTP (40 mg/kg) on day 0 and again with the acute MPTP regimen on day 9 to assess the effect of pre-exposure to MPTP.
Following isolation of total RNA from striatum, Affymetrix analysis was performed (Figure 4). Using hierarchical cluster analysis, a direct comparison of animals in categories M-S and S-S indicated that with few exceptions (Table 3), the transcriptional response elicited by the MPTP injection on day 0 was no longer evident by day 9 (Figure 4). The S-M group revealed relatively unique gene sets in the early (5 h), intermediate (24 h) and late (72 h) phases, that were qualitatively similar to those previously reported (Pattarini et al., 2008). Animals primed with MPTP (40 mg/kg) before receiving an acute MPTP treatment (M-M group) did not differ in the early (5 h) response (compare 5 hour S-M and M-M groups in Figure 4). In contrast, mice of the M-M group lacked almost completely the intermediate and late transcriptional phases (compare 24 and 72 h S-M and M-M groups in Figure 4). Indeed only 10 genes in the intermediate phase were not affected by pre-exposure to MPTP (Table 4).
Figure 4. Hierarchical cluster analysis reveals differences between the early (5 hr) and intermediate (24 hr) MPTP-induced responses in striatum of C57BL/6J mice following a priming dose of MPTP.
Animals were injected with either 40 mg/kg MPTP or saline as control on day 0, and injected again after 9 days using the acute MPTP regimen. Mice were sacrificed and striatum harvested at 5 (3 injections), 24 or 72 hours. In this hierarchical cluster analysis each vertical column represents a single mouse treated as indicated on top of the figure and each horizontal row is an individual probe set. Probe sets that are upregulated in treated compared to control mice appear in red, those that are downregulated appear in green. The relative log2 (ratio) is reflected by the intensity of the color. Whereas the early phase occurs independent of previous exposure to MPTP, the intermediate and late phases are absent in mice pretreated with MPTP.
Table 3.
Genes with elevated mRNA levels 9 days after MPTP priming.
| Probe Set | Gene Title | Gene Symbol | SS | MS | MS/SS |
|---|---|---|---|---|---|
| 1418021_at | Complement component 4B (Childo blood group) | C4b | 802 | 1193 | 1.5 |
| 1451978_at | Lysyl oxidase-like 1 | Loxl1 | 161 | 271 | 1.7 |
| 1415897_a_at | Microsomal glutathione S-transferase 1 | Mgst1 | 531 | 800 | 1.5 |
| 1418674_at | Oncostatin M receptor | Osmr | 187 | 327 | 1.8 |
| 1417346_at | PYD and CARD domain containing | Pycard | 149 | 276 | 1.9 |
| 1428114_at | Solute carrier family 14 (urea transporter), member 1 | Slc14a1 | 137 | 290 | 2.1 |
| 1423549_at | Solute carrier family 1 (glutamate/neutral amino acid transporter), member 4 | Slc1a4 | 151 | 278 | 1.8 |
| 1449682_s_at | Tubulin, beta 2b / tubulin, beta 2a, pseudogene 2 | Tubb2b/Tubb2a-ps2 | 784 | 1177 | 1.5 |
Table 4.
Genes that are not refractory 24 h after MPTP priming.
| Probe Set Name | Gene Title | Gene Symbol | SS | MS | SM | MM | MM/SM |
|---|---|---|---|---|---|---|---|
| 1448318_at | Adipose differentiation related protein | Adfp | 88 | 95 | 222 | 184 | 0.8 |
| 1434449_at | Aquaporin 4 | Aqp4 | 1134 | 1286 | 1772 | 1445 | 0.8 |
| 1417381_at | Complement component 1, q subcomponent, alpha polypeptide | C1qa | 1651 | 1553 | 3041 | 2450 | 0.8 |
| 1449401_at | Complement component 1, q subcomponent, C chain | C1qc | 1241 | 1328 | 2036 | 1788 | 0.9 |
| 1449164_at | CD68 antigen | Cd68 | 591 | 601 | 1059 | 883 | 0.8 |
| 1436838_x_at | Coactosin-like 1 (Dictyostelium) | Cotl1 | 375 | 516 | 717 | 646 | 0.9 |
| 1456567_x_at | Granulin | Grn | 1202 | 1203 | 1921 | 1721 | 0.9 |
| 1419573_a_at | Lectin, galactose binding, soluble 1 | Lgals1 | 355 | 436 | 602 | 511 | 0.9 |
| 1418004_a_at | Transmembrane protein 176B | Tmem176b | 1015 | 967 | 1530 | 1391 | 0.9 |
| 1450792_at | TYRO protein tyrosine kinase binding protein | Tyrobp | 719 | 904 | 1243 | 1063 | 0.9 |
To confirm the gene chip array results we performed qRT-PCR for representative genes of the early (5 h) and intermediate (24 h) phases (Figures 5 and 6, respectively). Animals for this experiment were treated exactly the same as those used in the microarray experiments. Levels of mRNA for all genes in M-S treated animals were indistinguishable from those that received only saline (S-S) (compare white and brown bars in Figures 5 and 6). Some genes, such as Cdkn1a and growth arrest and DNA-damage-inducible, beta (Gadd45b), did not differ between S-M and M-M groups whereas other genes, such as nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (Nfkbia) and FBJ murine osteosarcoma viral oncogene homolog B (Fosb), displayed a blunted although still significant response after pre-exposure to MPTP (Figure 5). Nuclear receptor subfamily 4, group A, member 1 (Nr4a1) was the only early response gene that showed significant refractoriness (Figure 5). For the intermediate response, we assessed the mRNA level profiles of Hmox1, tumor necrosis factor receptor superfamily, member 12A (Tnfrsf12a), endothelial differentiation, sphingolipid G-protein-coupled receptor 3 (Edg3), PDZ and LIM domain 4 (Pdlim4), heparin-binding EGF-like growth factor (Hbegf) tumor necrosis factor alpha (Tnfα) and monocyte chemoattractant protein-1/chemokine (C-C motif) ligand 2 (Mcp1/Ccl2). Hmox1, Tnfrsf12a, Edg3 and Tnfα were profoundly refractory (Figure 6). Elevation of Pdlim4 mRNA was attenuated but not abolished. Mcp1/Ccl2 is an interesting gene as it is elevated during both the early and intermediate phases (Pattarini et al, 2007 and Figure 6), and whereas the intermediate response is refractory, the early response is only reduced following pre-exposure to MPTP.
Figure 5. Quantitative assessment of striatal mRNA changes of genes of the early phase in the striatum of C57BL/6J mice previously exposed to 40mg/kg MPTP.
MPTP sensitive C57BL/6J mice were injected at time zero with a single priming dose of 40 mg/kg MPTP or saline. Mice received a second exposure to either saline or the acute MPTP regimen starting 9 days after the priming injection, and animals were sacrificed and striatum harvested 5 (3 injections), 24 or 72 hours later. Levels of mRNA for candidate genes of the early (Cdkn1a, NFkBia, FosB, Gadd45b and Nr4a1) response phase were evaluated by qRT-PCR using the absolute quantitative method. Results are expressed as the number of copies of a specific mRNA normalized versus the number of copies of Gapdh mRNA. Data are presented as mean ± S.E.M. of 4 (saline-treated) and 3 (MPTP-treated) animals. Differences versus control (Saline-Saline) were analyzed by one-way ANOVA followed by Bonferroni post-hoc tests and are indicated with asterisks (*** P<0.001, ** P<0.01). Differences among treatments were analyzed with one-way ANOVA and Bonferroni post-hoc tests and indicated with letters (B P<0.01, C P<0.05).
Figure 6. Quantitative assessment of striatal mRNA changes of genes of the intermediate phase in the striatum of C57BL/6J mice previously exposed to 40 mg/kg MPTP.
MPTP-sensitive C57BL/6J mice were injected at time zero with a single priming dose of 40 mg/kg MPTP or saline. Mice received a second exposure to either saline or the acute MPTP regimen starting 9 days after the priming injection, and animals were sacrificed and striatum harvested 5 (3 injections), 24 or 72 hours later. Levels of mRNA for candidate genes of the intermediate (Hmox1, Tnfrsf12a, Edg3, Pdlim4, Hbegf, Tnf-α and Mcp1/Ccl2) response phase were evaluated by qRT-PCR using the absolute quantitative method. Results are expressed as the number of copies of a specific mRNA normalized versus the number of copies of Gapdh mRNA. Data are presented as mean ± S.E.M. of 4 (control) and 3 (MPTP-treated) animals. Differences versus control (Saline-Saline) were analyzed by one-way ANOVA followed by Bonferroni post-hoc tests and are indicated with asterisks (*** P<0.001, * P<0.05). Differences among treatments were analyzed with one-way ANOVA and Bonferroni post-hoc tests and indicated with letters (A P<0.001, C P<0.05).
3.5 Effect of MPTP pre-exposure in MPTP-sensitive (C57BL/6J) and MPTP-resistant (SWR) strains of mice
We previously described that the striatal transcriptional response in the MPTP-sensitive C57BL/6J strain of mice differs from that of the MPTP-resistant, SWR strain (Pattarini et al., 2007, Pattarini et al., 2008). Here we assessed if pre-exposure to MPTP modified the transcriptional response in MPTP-resistant SWR mice. We injected both SWR and C57BL/6J mice with MPTP (30 mg/kg) or saline, on day 0 and day 9, and sacrificed animals 5, 24 and 72 hours after the second injection. The dosage used in this experiments was chosen to minimize death of SWR mice due to peripheral toxicity and has been shown previously to elicit transcriptional responses in both strains (Pattarini et al., 2007, Pattarini et al., 2008). qRT-PCR was performed for genes of the early phase (Cdkn1a, Fosb, Gadd45b and Nfkbia; Figure 7) and intermediate phase (Hmox1, Edg3, Tnfrsf12a, and Pdlim4; Figure 8). mRNA levels in animals treated with MPTP on day 0 followed by saline (M-S) did not differ from mice injected with saline on both days (S-S, Figures 7 and 8). Animals in the saline-MPTP group, displayed a qualitatively similar response to that previously reported (Pattarini et al., 2008). In particular, the early response occurred in both strains of mice with some genes induced to similar levels (Cdkn1a and Nfkbia) and others at even higher levels in SWR compared to C57BL/6J mice (Fosb and Gadd45b) (Figure 7). In contrast, the intermediate response was much reduced (Tnfrsf12a and Pdlim4) or absent (Hmox1 and Edg3) in the S-M group of the MPTP-resistant, SWR strain (white bars in Figure 8). Pre-exposure to MPTP (M-M group) did not alter gene expression in the intermediate phase in SWR mice (Figure 8); i.e. priming doses of MPTP do not convert the transcriptional profile of SWR mice to one resembling an MPTP sensitive strain. As anticipated in C57BL/6J mice of the M-M group, the early phase was largely unaffected (Figure 7) whereas the intermediate response was absent or attenuated (Figure 8).
Figure 7. Comparison of the MPTP-induced refractory state between MPTP-sensitive C57BL/6J and MPTP-resistant SWR strains of mice.
C57BL/6J and SWR mice were injected with either 30 mg/kg MPTP or saline on day zero. After 9 days they were injected again with either a single injection of 30 mg/kg MPTP or saline and sacrificed 5, 24 and 72 hour later and striata collected. Levels of mRNA for candidate genes of the early (Cdkn1a, Fosb, Gadd45b and NFkBia) response phase were evaluated by qRT-PCR. Results are expressed as the number of copies of a specific mRNA normalized versus the number of copies of Gapdh mRNA. Data are presented as mean ± S.E.M. of 5 to 6 animals per condition. Differences versus control (S-S) were analyzed by one-way ANOVA followed by Bonferroni post-hoc tests and are indicated with asterisks (*** P<0.001). Differences among treatments were analyzed with one-way ANOVA and Bonferroni post-hoc tests and indicated with letters (A P<0.001, B P<0.01). Results indicate that mRNA levels for genes of the early response phase were elevated after MPTP injection independent of previous exposures in both strains of mice.
Figure 8. Comparison of the MPTP-induced refractory state between MPTP-sensitive C57BL/6J and MPTP-resistant SWR strains of mice.
C57BL/6J and SWR mice were injected with either 30 mg/kg MPTP or saline (control) on day zero. After 9 days they were injected again with either a single injection of 30 mg/kg MPTP or saline and sacrificed 5, 24 and 72 hour later and striata collected. Levels of mRNA for candidate genes of the intermediate (Hmox1, Edg3, Tnfrsf12a and Pdlim4) response phase were evaluated by qRT-PCR. Results are expressed as the number of copies of a specific mRNA normalized versus the number of copies of Gapdh mRNA. Data are presented as mean ± S.E.M. of 5 to 6 animals per condition. Differences versus control (S-S) were analyzed by one-way ANOVA followed by Bonferroni post-hoc tests and are indicated with asterisks (*** P<0.001). Differences among treatments were analyzed with one-way ANOVA and Bonferroni post-hoc tests and indicated with letters (A P<0.001). Results indicate that mRNA levels for the genes of the intermediate phase were not elevated following MPTP pre-exposure, and SWR mice did not respond differently to MPTP compared to C57BL/6J after priming.
3.6 Paraquat and MPTP trigger distinct and refractory transcriptional responses but do not elicit cross-attenuation
Several environmental agents have been associated with an elevated risk of PD (Moretto and Colosio, 2011). Therefore it is conceivable that exposure to one toxin may alter the response to future encounters with other toxins. In addition, distinct agents may trigger different transcriptional responses. Paraquat (PQ) is structurally similar to MPP+ (Brooks et al., 1999) and can elicit loss of DAergic neurons of the SNpc in mice (McCormack et al., 2005). Therefore, we assessed whether exposure to PQ triggers a transcriptional response similar to MPTP and whether pre-exposure to PQ modifies the response to PQ or MPTP and vice versa. C57BL/6J mice were treated on day 0 with one dose of saline, MPTP (40 mg/kg) or paraquat (40 mg/kg). Four days later mice received injections of saline, MPTP or PQ and were sacrificed 24 hour later. Total striatal RNA was isolated and used for Affymetrix® array analysis as described.
In animals pretreated with saline, PQ induced a transcriptional response that was limited to relatively few genes (66 probe sets corresponding to 56 genes listed in Table 5) that by and large did not overlap with those induced by MPTP (compare columns S-M and S-P in Figure 9 and see Table 6 for a list of 5 genes modulated by both toxicants). This was in striking contrast to the extensive changes elicited by MPTP in the S-M group (column S-M in Figure 4 and column S-M in Figure 9). Pre-exposure to priming doses of MPTP again eliminated or attenuated the intermediate response (M-M group, Figure 9). Interestingly, although PQ induced a small group of genes (Table 5), this response was blunted or absent in PQ pre-treated mice (P-P group in Figure 9). In contrast, mice primed with one toxin, and then challenged with the other one, did not show any sign of refractoriness (compare columns P-M and M-P versus S-M and S-P columns in Figure 9). Therefore, PQ and MPTP elicit distinct striatal transcriptional responses and pre-exposure to one toxin can only alter the response to a subsequent challenge with the same toxin but they do not elicit cross-attenuation.
Table 5.
List of genes modulated in the striatum 24 h after paraquat treatment.
| Probe Set Name | Gene Title | Gene Symbol | SS | SP | SS/SP | |
|---|---|---|---|---|---|---|
| 1418687_at | Activity regulated cytoskeletal-associated protein | Arc | 2281 | 319 | 0.1 | ↓ |
| 1452975_at | Alanine-glyoxylate aminotransferase 2-like 1 | Agxt2l1 | 595 | 2101 | 3.5 | ↑ |
| 1417765_a_at | Amylase 1, salivary | Amy1 | 309 | 682 | 2.2 | ↑ |
| 1444564_at | Apolipoprotein D | Apod | 334 | 640 | 1.9 | ↑ |
| 1428352_at | Arrestin domain containing 2 | Arrdc2 | 230 | 409 | 1.8 | ↑ |
| 1419758_at | ATP-binding cassette, sub-family B (MDR/TAP), member 1A | Abcb1a | 220 | 366 | 1.7 | ↑ |
| 1416250_at | B-cell translocation gene 2, anti-proliferative | Btg2 | 566 | 332 | 0.6 | ↓ |
| 1459372_at | Bhlh-PAS type transcription factor NXF | Nxf | 389 | 119 | 0.3 | ↓ |
| 1428485_at | Carbonic anyhydrase 12 | Car12 | 1106 | 679 | 0.6 | ↓ |
| 1456314_x_at | CDK2 (cyclin-dependent kinase 2)-associated protein 1 | Cdk2ap1 | 1839 | 1038 | 0.6 | ↓ |
| 1435509_x_at | CDK2 (cyclin-dependent kinase 2)-associated protein 1 | Cdk2ap1 | 2090 | 1194 | 0.6 | ↓ |
| 1417574_at | Chemokine (C-X-C motif) ligand 12 | Cxcl12 | 989 | 586 | 0.6 | ↓ |
| 1416332_at | Cold inducible RNA binding protein | Cirbp | 815 | 2105 | 2.6 | ↑ |
| 1434369_a_at | Crystallin, alpha B | Cryab | 1963 | 963 | 0.5 | ↓ |
| 1452956_a_at | DNA segment, Chr 12, ERATO Doi 647, expressed | D12Ertd647e | 992 | 591 | 0.6 | ↓ |
| 1437204_a_at | DNA segment, Chr 8, ERATO Doi 325, expressed | D8Ertd325e | 255 | 443 | 1.7 | ↑ |
| 1446190_at | Double cortin and calcium/calmodulin-dependent protein kinase-like 1 | Dcamkl1 | 1402 | 2399 | 1.7 | ↑ |
| 1448830_at | Dual specificity phosphatase 1 | Dusp1 | 1513 | 812 | 0.5 | ↓ |
| 1415834_at | Dual specificity phosphatase 6 | Dusp6 | 1187 | 682 | 0.6 | ↓ |
| 1417065_at | Early growth response 1 | Egr1 | 14097 | 7195 | 0.5 | ↓ |
| 1436329_at | Early growth response 3 | Egr3 | 2839 | 1642 | 0.6 | ↓ |
| 1428142_at | Ets variant gene 5 | Etv5 | 1139 | 691 | 0.6 | ↓ |
| 1433551_at | Expressed sequence AI427515 | AI427515 | 2067 | 1196 | 0.6 | ↓ |
| 1442025_a_at | Expressed sequence AI467657 | AI467657 | 218 | 392 | 1.8 | ↑ |
| 1442026_at | Expressed sequence AI467657 | AI467657 | 474 | 941 | 2.0 | ↑ |
| 1435940_at | Expressed sequence AI836758 | AI836758 | 1606 | 2790 | 1.7 | ↑ |
| 1450779_at | Fatty acid binding protein 7, brain | Fabp7 | 685 | 357 | 0.5 | ↓ |
| 1423100_at | FBJ osteosarcoma oncogene | Fos | 964 | 200 | 0.2 | ↓ |
| 1437247_at | Fos-like antigen 2 | Fosl2 | 346 | 88 | 0.3 | ↓ |
| 1428642_at | Frc, fringe-like 1 (Drosophila) | Frcl1 | 845 | 517 | 0.6 | ↓ |
| 1455444_at | Gamma-aminobutyric acid (GABA-A) receptor, subunit alpha 2 | Gabra2 | 856 | 506 | 0.6 | ↓ |
| 1448397_at | Gap junction membrane channel protein beta 6 | Gjb6 | 661 | 1176 | 1.8 | ↑ |
| 1417859_at | Growth arrest specific 7 | Gas7 | 387 | 237 | 0.6 | ↓ |
| 1435194_at | Heat shock protein 4 | Hspa4 | 1262 | 699 | 0.6 | ↓ |
| 1431182_at | Heat shock protein 8 | Hspa8 | 438 | 253 | 0.6 | ↓ |
| 1455789_x_at | Heat shock protein 8 | Hspa8 | 21704 | 13291 | 0.6 | ↓ |
| 1415899_at | Jun-B oncogene | Junb | 799 | 458 | 0.6 | ↓ |
| 1449490_at | Methyl-cpg binding domain protein 4 | Mbd4 | 132 | 217 | 1.6 | ↑ |
| 1422860_at | Neurotensin | Nts | 389 | 220 | 0.6 | ↓ |
| 1416505_at | Nuclear receptor subfamily 4, group A, member 1 | Nr4a1 | 3523 | 1074 | 0.3 | ↓ |
| 1449374_at | Pipecolic acid oxidase | Pipox | 284 | 165 | 0.6 | ↓ |
| 1418595_at | Plasma membrane associated protein, S3-12 | S3-12 | 268 | 945 | 3.5 | ↑ |
| 1448684_at | Protein phosphatase 1, regulatory (inhibitor) subunit 2 | Ppp1r2 | 4593 | 7927 | 1.7 | ↑ |
| 1419247_at | Regulator of G-protein signaling 2 | Rgs2 | 3148 | 1891 | 0.6 | ↓ |
| 1419248_at | Regulator of G-protein signaling 2 | Rgs2 | 3284 | 1849 | 0.6 | ↓ |
| 1447830_s_at | Regulator of G-protein signaling 2 | Rgs2 | 5817 | 3077 | 0.5 | ↓ |
| 1456339_at | RIKEN cDNA 2810410D24 gene | 2810410D24Rik | 485 | 293 | 0.6 | ↓ |
| 1428375_at | RIKEN cDNA 4932415G12 gene | 4932415G12Rik | 467 | 784 | 1.7 | ↑ |
| 1436387_at | RIKEN cDNA C330006P03 gene | C330006P03Rik | 1776 | 412 | 0.2 | ↓ |
| 1456280_at | RIKEN cDNA E130314M08 gene | E130314M08Rik | 814 | 480 | 0.6 | ↓ |
| 1422660_at | RNA binding motif protein 3 | Rbm3 | 1435 | 3304 | 2.3 | ↑ |
| 1429169_at | RNA binding motif protein 3 | Rbm3 | 248 | 120 | 0.5 | ↓ |
| 1434347_s_at | RUN and FYVE domain-containing 2 | Rufy2 | 127 | 211 | 1.7 | ↑ |
| 1416041_at | Serum/glucocorticoid regulated kinase | Sgk | 1649 | 4070 | 2.5 | ↑ |
| 1457373_at | Similar to cadherin 19, type 2 preproprotein (LOC227485), mRNA | --- | 201 | 334 | 1.7 | ↑ |
| 1424481_s_at | Similar to hypothetical protein FLJ12969 (LOC385421), mRNA | --- | 203 | 355 | 1.7 | ↑ |
| 1427345_a_at | Sulfotransferase family 1A, phenol-preferring, member 1 | Sult1a1 | 292 | 863 | 3.0 | ↑ |
| 1424330_at | SUMO1/sentrin specific protease 1 | Senp1 | 280 | 166 | 0.6 | ↓ |
| 1416029_at | TGFB inducible early growth response 1 | Tieg1 | 373 | 184 | 0.5 | ↓ |
| 1436094_at | Transcribed sequence with weak similarity to protein pir:S05381 (R.norvegicus) S05381 VGF8a protein precursor - rat | --- | 6183 | 2368 | 0.4 | ↓ |
| 1439163_at | Transcribed sequence with weak similarity to protein ref:NP_079268.1 (H.sapiens) hypothetical protein FLJ12547 [Homo sapiens] | --- | 533 | 882 | 1.7 | ↑ |
| 1430153_at | Tropomodulin 2 | Tmod2 | 403 | 664 | 1.6 | ↑ |
| 1417374_at | Tubulin, alpha 4 | Tuba4 | 6174 | 3755 | 0.6 | ↓ |
| 1423778_at | Ubiquitin specific protease 20 | Usp20 | 201 | 434 | 2.2 | ↑ |
| 1419063_at | UDP-glucuronosyltransferase 8 | Ugt8 | 2003 | 1020 | 0.5 | ↓ |
| 1420909_at | Vascular endothelial growth factor A | Vegfa | 663 | 326 | 0.5 | ↓ |
Figure 9. Hierarchical cluster analysis of basal gene expression differences in striata of MPTP-sensitive C57BL/6J mice following pretreatment with either MPTP or paraquat.
C57BL/6J mice were injected on day 0 with a single injection of saline, 40 mg/kg MPTP or 40 mg/kg paraquat. Mice were treated again after 4 days with saline, 40 mg/kg MPTP or 40 mg/kg paraquat and sacrificed 24 h later. All possible treatment combinations were tested. Striatal RNA was extracted and hybridized onto the Affymetrix Mouse Genome 430 2.0 array to identify genes with differential expression between treatments. Each vertical column represents a single mouse treated as indicated on top of the figure (a total of 52 animals were used: 9 each were treated with saline only and PQ-MPTP; 7 each with saline-MPTP, MPTP-MPTP, saline-PQ and PQ-PQ; and 6 with MPTP-PQ), and each horizontal row is an individual probe set. Probe sets that are upregulated in treated compared to control mice appear in red, those that are downregulated appear in green. The relative log2 (ratio) is reflected by the intensity of the color. Pre-exposure to MPTP and PQ altered subsequent striatal transcriptional response to the same substance but not to the other one.
Table 6.
List of genes modulated in the striatum 24 h after injection of MPTP or paraquat.
| Probe Set Name | Gene Title | Gene Symbol | SS | SM | SP | SP/SS | SM/SS | ||
|---|---|---|---|---|---|---|---|---|---|
| 1444564_at | Apolipoprotein D | Apod | 334 | 878 | 640 | 1.9 | ↑ | 2.6 | ↑ |
| 1418687_at | Activity regulated cytoskeletal-associated protein | Arc | 2281 | 804 | 319 | 0.1 | ↓ | 0.4 | ↓ |
| 1416250_at | B-cell translocation gene 2, anti-proliferative | Btg2 | 566 | 337 | 332 | 0.6 | ↓ | 0.6 | ↓ |
| 1459372_at | Bhlh-PAS type transcription factor NXF | Nxf | 389 | 161 | 119 | 0.3 | ↓ | 0.4 | ↓ |
| 1434369_a_at | Crystallin, alpha B | Cryab | 1963 | 1174 | 963 | 0.5 | ↓ | 0.6 | ↓ |
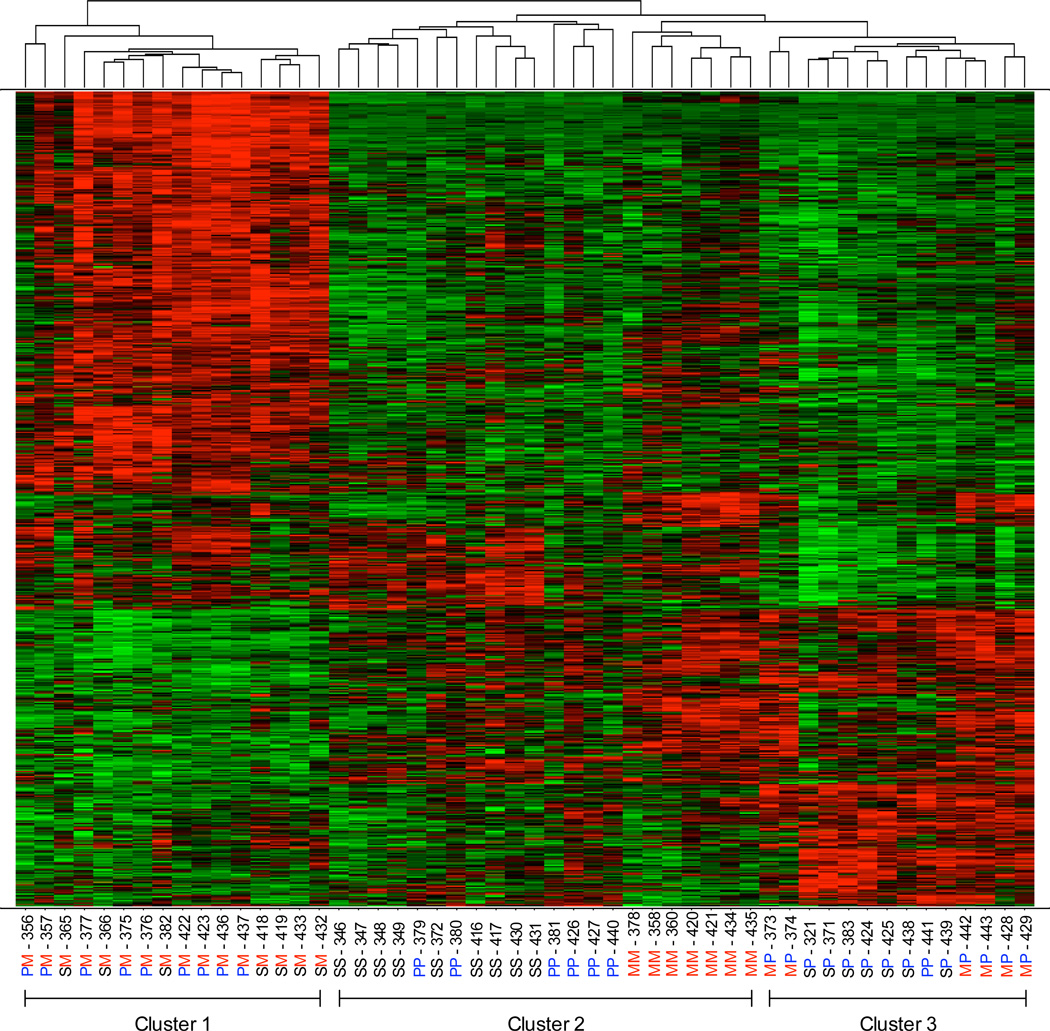
In Figure 9, arrays from animals receiving the same treatment (e.g. S-M) were grouped together, and these groups were compared versus the S-S group using t-tests. This method identified 245 probe sets that differed between the S-S group and the other groups. To identify potential similarities among treatments not revealed with the previous method, we analyzed data using a different approach. First we compared all treatment groups to each other using ANOVA instead of t-tests to identify probe sets differentially expressed among treatments. This method identified 1171 probe sets that differed significantly. The 1171 probe sets on all the arrays were then compared to each other based on their similarity to one another, rather than the experimental condition. This hierarchical clustering approach revealed three major subgroups (Figure 10): cluster 1 includes mice pre-exposed to either PQ or saline, and then treated with MPTP on day 9; cluster 2 includes animals treated with the same toxins both on day 0 and day 9 (MM and PP), and mice injected only with saline (SS); cluster 3 includes arrays from mice pre-exposed to either MPTP or saline, and then treated with PQ on day 9. This served to reinforce the notion that MPTP and PQ induce distinct gene expression profile changes and that mice treated twice with the same toxin were indistinguishable from saline-treated animals.
Figure 10. Unsupervised hierarchical cluster analysis of gene expression profiles in striata of C57BL/6J mice following treatment with MPTP and/or paraquat.
C57BL/6J mice were treated on day 0 with a single injection of saline, 40 mg/kg MPTP or 40 mg/kg paraquat. Mice were treated again after 4 days with saline, 40 mg/kg MPTP or 40 mg/kg paraquat and sacrificed 24 h later. All possible treatment combinations were tested. Striatal RNA was extracted and hybridized onto the Affymetrix Mouse Genome 430 2.0 array and probe sets with differential expression between any two treatments identified by ANOVA. These data were then compared to each other, in an unsupervised cluster analysis based on their similarity to one another regardless of the experimental condition. Probe sets that are upregulated appear in red, those that are downregulated appear in green. The relative log2 (ratio) is reflected by the intensity of the color. Each vertical column represents a single mouse treated as indicated at the bottom of the column (a total of 52 animals were used: 9 each were treated with saline only and PQ-MPTP; 7 each with saline-MPTP, MPTP-MPTP, saline-PQ and PQ-PQ; and 6 with MPTP-PQ). Each horizontal row represents a probe set identified by ANOVA. Three major clusters were identified: cluster 1 includes mice pre-exposed to either PQ or saline, and then treated with MPTP on day 9; cluster 2 includes animals treated with the same toxins both on day 0 and day 9 (MM and PP), and mice injected only with saline (SS); cluster 3 includes arrays from mice pre-exposed to either MPTP or saline, and then treated with PQ on day 9. This hierarchical cluster analysis highlights the fact that mice treated twice with the same toxin (MM or PP) are most similar to those treated only with saline (SS).
3.7 Gene ontology (GO) and gene set enrichment analysis (GSEA)
Although PQ and MPTP largely affected expression of distinct sets of genes, it is possible that they still influence similar biological processes. Therefore, to compare the responses to MPTP and PQ, gene ontology (GO) and gene set enrichment analyses (GSEA) were performed.
GO analysis revealed several biological and molecular functions (12 GO terms) regulated after MPTP treatment (Table 7), including response to wounding (GO:0009611), protein dimerization activity (GO:0046983), and inflammatory-related responses such as inflammatory response (GO:0006954), acute inflammatory response (GO:0002526), and activation of immune response (GO:0002253). These results are consistent with both the available literature on MPTP (Dauer and Przedborski, 2003, Bove et al., 2005, Smeyne and Jackson-Lewis, 2005, Wood-Kaczmar et al., 2006) and our previous results (Pattarini et al., 2007, Pattarini et al., 2008). GO analysis revealed only protein dimerization activity (GO:0046983), to be regulated after PQ administration (Table 7). This molecular process is also affected by MPTP, although genes regulated by PQ (Fos, Fosl2, Junb, Npas4, Nr4a1, Vegfa, Zbtb16) differ from those modulated by MPTP (C1qb, Cav2, Cebpb, Cebpd, Jun, Npas4, Pycard, S100a16, Sim1, Stat3, Vwf, Wwtr1,).
Table 7.
GO Analysis of biological processes and molecular functions enriched after MPTP or PQ treatment. GO terms enriched 24 hours after MPTP treatment (*).
| Biological Processes | Size | GiG | % | p Value | Genes |
|---|---|---|---|---|---|
| Response to wounding (GO:0009611) | 327 | 17 | 9.88 | 0.000001 | C1qa, C1qb, C1qc, C4b, Cd44, Gja1, Hbegf, Lcp1, Ly86, Nupr1, Pycard, Pros1, S1pr3, Stat3, Tnfrsf1a, Trf, Vwf |
| Defense response (GO:0006952) | 376 | 17 | 9.88 | 0.000004 | C1qa, C1qb, C1qc, C4b, Cd44, Clic1, Fcer1g, H2-K1, Irgm1, Ly86, Nupr1, Pycard, Ptprc, S1pr3, Stat3, Tnfrsf1a, Trf |
| Inflammatory response (GO:0006954) | 208 | 12 | 6.98 | 0.000021 | C1qa, C1qb, C1qc, C4b, Cd44, Ly86, Nupr1, Pycard, S1pr3, Stat3, Tnfrsf1a, Trf, |
| Acute inflammatory response (GO:0002526) | 73 | 7 | 4.07 | 0.000159 | C1qa, C1qb, C1qc, C4b, Nupr1, Stat3, Trf |
| Positive regulation of response to stimulus (GO:0048584) | 169 | 9 | 5.23 | 0.000602 | C1qa, C1qb, C1qc, C4b, Fcer1g, H2-K1, Ptprc, Tgm2, Tnfrsf1a, |
| Activation of immune response (GO:0002253) | 75 | 6 | 3.49 | 0.001482 | C1qa, C1qb, C1qc, C4b, Fcer1g, Ptprc |
| Immune effector process (GO:0002252) | 114 | 7 | 4.07 | 0.001717 | C1qa, C1qb, C1qc, C4b, Fcer1g, Ptprc, Ptx3 |
| Response to metal ion (GO:0010038) | 50 | 5 | 2.91 | 0.002295 | Ccnd1, Mgp, Mt1, Mt2, S100a16 |
| Complement activation, classical pathway (GO:0006958) | 24 | 4 | 2.33 | 0.002318 | C1qa, C1qb, C1qc, C4b |
| Positive regulation of immune response (GO:0050778) | 121 | 7 | 4.07 | 0.002323 | C1qa, C1qb, C1qc, C4b, Fcer1g, H2-K1, Ptprc |
| Molecular functions | Size | GiG | GiG% | p Value | Genes |
| Protein dimerization activity (GO:0046983) | 326 | 12 | 7.0 | 0.001 | C1qb, Cav2, Cebpb, Cebpd, Jun, Npas4, Pycard, S100a16, Sim1, Stat3, Vwf, Wwtr1 |
| Identical protein binding (GO:0042802) | 279 | 10 | 5.8 | 0.003 | C1qb, Cav2, Cebpb, Cebpd, Cldn10, Lcp1, Pycard, S100a16, Vwf, Wwtr1 |
| GO terms enriched 24 hours after PQ treatment (*). | |||||
| Molecular Function | Size | GiG | % | p Value | Genes |
| Protein dimerization activity (GO:0046983) | 340 | 7 | 11.86 | 0.001 | Fos, Fosl2, Junb, Npas4, Nr4a1, Vegfa, Zbtb16 |
Only GO terms with p-value less than 0.003 are reported.
GSEA confirmed the GO results in that MPTP influenced many biological processes (Table 8), whereas we were unable to identify any over-expressed function in PQ treated mice. Table 8 shows the most prominent biological processes and molecular functions that are significantly higher in MPTP-treated mice. These results are comparable to those reported by several authors (Dauer and Przedborski, 2003, Bove et al., 2005, Smeyne and Jackson-Lewis, 2005, Wood-Kaczmar et al., 2006, Pattarini et al., 2007, Pattarini et al., 2008). Similar to GO analysis, we were unable to identify any biological process or molecular function that is more represented in PQ-treated mice.
Table 8.
GSEA analysis of biological processes and molecular functions enriched in MPTP-versus PQ-treated mice.
| Biological processes | Size | NES | FDR q-val | Gene List |
|---|---|---|---|---|
| Negative regulation of apoptosis (GO:0043066) | 136 | 2.08 | 0.029 | Anxa5, Arhgdia, Aven, Bax, Bcl10, Bcl2, Bcl3, Becn1, Bnip1, Ccl2, Cd74, Clcf1, Cryab, Dad1, Ddah2, Gpx1, Hsp90b1, Hspa1b, Hspb1, Ier3, Il2, Krt18, Mcl1, Myo18a, Nme2, Notch2, Npm1, Rtn4, Scg2, Sema4d, Sfrp1, Socs3, Sphk1, Tnfaip8 |
| Jak stat cascade (GO:0007259) | 29 | 2.08 | 0.011 | Ccl2, Clcf1, Hcls1, Lyn, Nmi, Socs3, Stat3 |
| Positive regulation of signal transduction (GO:0009967) | 108 | 2.07 | 0.009 | Bcl10, Bst2, Casp1, Cdkn1c, Clcf1, Cxxc5, Eef1d, Eef1e1, Fadd, Flna, Golt1b, Hcls1, Hmox1, Il12a, Lgals1, Lgals9, Litaf, Ltbr, Lyn, Myd88, Nek6, Notch2, Ptprc, Rasgrp4, Rela, Rhoc, Rpl17, Slc44a2, Tfg, Tmem101, Tnfrsf1a, Zdhhc13 |
| Regulation of IKK NFκB cascade (GO:0043122) | 76 | 2.05 | 0.008 | Bcl10, Bst2, Casp1, Cxxc5, Eef1d, Fadd, Flna, Golt1b, Hmox1, Lgals1, Lgals9, Litaf, Ltbr, Myd88, Nek6, Rela, Rhoc, Rpl17, Slc44a2, Tfg, Tmem101, Tnfrsf1a, Zdhhc13 |
| Positive regulation of phosphorylation (GO:0042327) | 25 | 2.02 | 0.007 | Ccnd1, Ccnd2, Clcf1, Hcls1, Il5, Lyn |
| Regulation of signal transduction (GO:0009966) | 197 | 2.001 | 0.008 | Ambp, Arf6, Bcl10, Bst2, Casp1, Cdkn1c, Clcf1, Cxxc5, Eef1d, Eef1e1, Eid2, Fadd, Flna, Git1, Git2, Golt1b, Grk5, Hcls1, Hmox1, Il12a, Lgals1, Lgals9, Litaf, Ltbr, Lyn, Mdfic, Myd88, Nek6, Notch2, Otx2, Plce1, Ptprc, Rasgrp4, Rela, Rgs2, Rgs20, Rhoc, Rpl17, Slc44a2, Smad2, Tax1bp3, Tfg, Tgfb1, Tmem101, Tnfrsf1a, Zdhhc13 |
| Molecular functions | Size | NES | FDR q-val | Gene List |
|---|---|---|---|---|
| Structural constituent of cytoskeleton (GO:0005200) | 55 | 2.09 | 0.002 | Arpc1b, Gfap, Msn, Vim, |
| Specific RNA POL-II transcription factor activity (GO:0003704) | 31 | 2.01 | 0.007 | Atf4, Fos, Glis3, Notch2, Nr2f1, Sox9, Usf1, Znf202 |
| Protease inhibitor activity (GO:0030414) | 32 | 1.92 | 0.030 | Ambp, Cst7, Pros1, Renbp, Serpinb12, Serping1, Serpinh1, Spink5, Spint2, Timp1 |
| Unfolded protein binding (GO:0051082) | 39 | 1.92 | 0.023 | Cct3, Cct4, Cct6a, Cct7, Cdc37, Chaf1b, Grpel1, Hspa1b, Hspa9, Hspd1, Mkks, Nap1l4, Npm1, Ppib, Ppic, Ptges3, Ruvbl2, Spg7, Tapbp, Tcp1 |
| Enzyme inhibitor activity (GO:0004857) | 107 | 1.90 | 0.026 | Abce1, Ambp, Anxa5, Cdc42se1, Cdkn1c, Csn2, Cst7, Dnajc3, Gps1, Gpx1, Pkig, Pros1, Prpsap1, Renbp, Rnh1, Serpinb12, Serping1, Serpinh1, Socs3, Spink5, Spint2, Tesc, Timp1, Tnfaip8 |
| Oxidoreductase activity (GO:0016616) | 50 | 1.86 | 0.041 | Akr1b1, Akr1b10, Akr7a2, Gpd1, Hpgd, Hsd3b2, Idh1, Idh3b, Impdh2, Ldha, Me1, Rdh5, Rdh10, Rdh16, Spr, Ugdh |
| Oxidoreductase activity acting on CHOH groups of donors (GO:0016614) | 56 | 1.82 | 0.051 | Akr1b1, Akr1b10, Akr1c4, Akr7a2, Gpd1, Gpd2, H6pd, Hadha, Hpgd, Hsd17b4, Hsd3b2, Idh1, Idh3b, Impdh2, Ldha, Me1, Me2, Rdh5, Rdh10, Rdh16, Spr, Ugdh |
| Electron carrier activity (GO:0009055) | 71 | 1.80 | 0.054 | Aifm1, Akr1b1, Akr1c4, Akr7a2, Cox11, Cox5a, Cyb561, Cyb5r3, Cyb5r4, Cyba, Cycs, Dhrs3, Dmgdh, Etfa, Etfb, Etfdh, Fdx1, Gpx2, Gsr, Haao, Idh3b, Kmo, Me1, Me2, Mtrr, Ndufa13, Ndufa9, Ndufs1, Ndufs3, Ndufs7, Ndufs8, Nqo1, P4ha2, Por, Rdh16, Sardh, Txndc2, Ugdh |
| Transferase activity transferring acyl groups (GO:0016746) | 52 | 1.76 | 0.072 | Aanat, Agpat2, Edf1, Hadha, Hat1, Lpcat1, Sat1, Smarce1, Soat1, Taf5, Tgm1, Tgm2, Tgm3, Tgm5 |
3.8 Temporal correlation between DA depletion and refractoriness
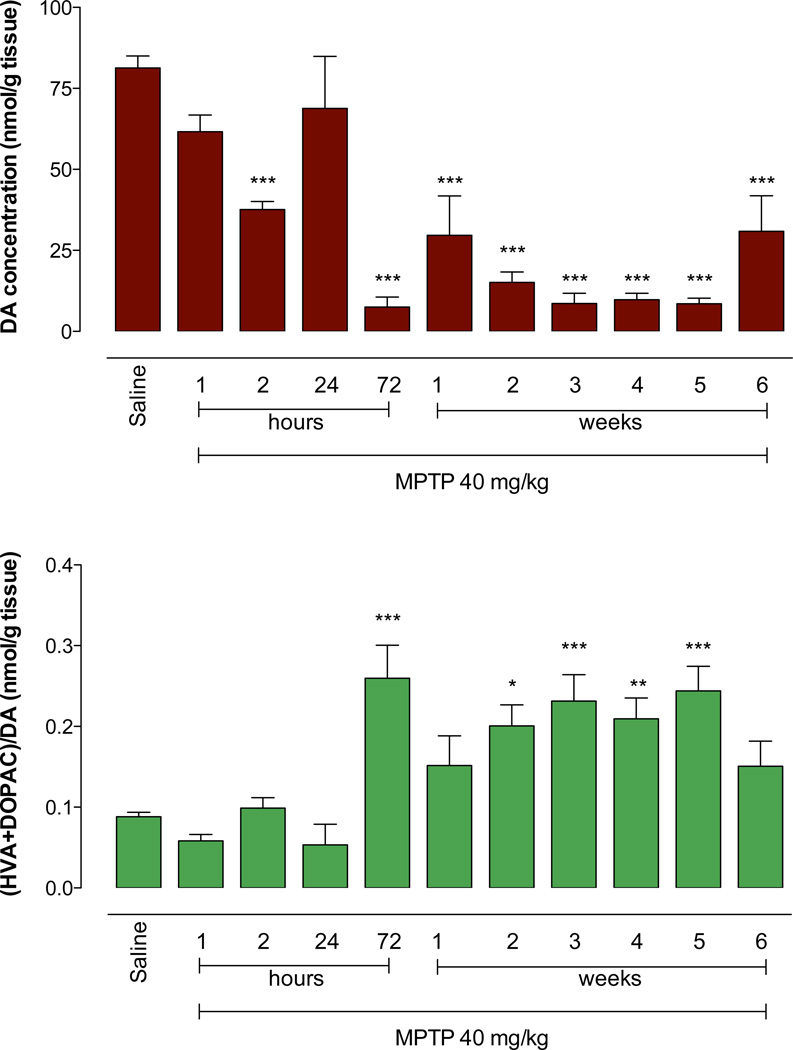
DA, but not other catecholamines, induces transcription of Hmox1 in glial cells (Schmidt et al., 1999). Since DA is known to be rapidly released from SNpc nerve endings as an acute response to MPTP intoxication (Chang and Ramirez, 1986), this catecholamine or some unidentified substance emanating from DAergic nerve endings may trigger part of the transcriptional response. If true, the refractory period might be coincident with the period of DA depletion in striatum. Therefore, we assessed the effect of MPTP on striatal DA levels. Animals were administered a single injection of MPTP (40 mg/kg) and the striatal concentrations of DA and its two main metabolites, 3–4-dihydroxphenylacetic acid (DOPAC) and homovanillic acid (HVA) assessed at 1, 2, 24 and 72 hours and 1, 2, 3, 4, 5 and 6 weeks following treatment. The concentration of DA in the striatum was substantially reduced 2 h after MPTP administration, and transiently recovered to basal levels at 24 h before decreasing again at day 3, and remaining low for up to 6 weeks (Figure 11). The ratio between DA concentration and that of its two metabolites HVA and DOPAC, confirmed that the turnover of DA in the striatum is significantly altered for a prolonged period of time. At the longest time point there was a suggestion that DA levels were beginning to recover. Nevertheless, the long-term refractory period occurs concomitantly with a reduction of striatal DA.
Figure 11. Temporal profile of dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) concentrations after a single injection of 40 mg/kg MPTP.
C57BL/6J mice were injected on day 0 with a single dose of either saline or 40 mg/kg MPTP and sacrificed 1, 2, 24, 72 h and 1, 2, 3, 4, 5 and 6 weeks later. Top Panel: MPTP elicits a rapid depletion of dopamine in striatum within 2 hours of administration that recovers by 24 hours. However, this recovery is transient as dopamine falls to approximately 10% of control levels by 3 days post-treatment and remains significantly reduced through 6 weeks. Bottom Panel: Dopamine turnover can be measured by examining the ratio of its metabolites (DOPAC + HVA)/DA. Three days after a single injection of 40mg/kg MPTP there is a significant increase in (DOPAC + HVA)/DA that remains elevated though 6 weeks. Higher (DOPAC + HVA)/DA ratios suggest dopamine displacement (dumping) since higher cytoplasmic dopamine is converted to DOPAC and rapidly released via reverse transport to promote the formation of HVA. Differences versus control (Saline) were analyzed with one-way ANOVA and Bonferroni post-hoc tests (*** P<0.001, ** P<0.01, * P<0.05).
4 Discussion
Although the etiology of sporadic PD remains to be fully elucidated, it is thought to involve both environmental agents and predisposing genetic factors (Vance et al., 2010). The current study provides an experimental platform that can potentially lead to a better understanding of both aspects of this issue.
Many epidemiological studies have shown a link between exposure to various pesticides and herbicides and the risk of developing PD (Alavanja et al., 2004, Landrigan et al., 2005, Gatto et al., 2009). Our data show an unexpected relationship between the frequency of exposure to a neurotoxin and the molecular response it elicits, suggesting this parameter might play a role in the etiology of PD. The different regimens used here to induce experimental Parkinsonism may model different exposure profiles in man. The mode of administration of MPTP can elicit different pathologies both in terms of their scope and timing. Sonsalla and Heikkila (1986) were the first to report that dose and dose intervals influenced MPTP neurotoxicity. More recently, differences in striatal glutamate synaptic regulation were reported between the acute and subchronic MPTP regimens (Robinson et al., 2003). In addition to the scope of injury, the mechanism of SNpc DAergic neuron death induced by MPTP has also been shown to be dependent on MPTP administration schedule: apoptosis is implicated in the subchronic model (Tatton and Kish, 1997), whereas necrosis was described following the acute regimen (Jackson-Lewis et al., 1995). Finally, although controversial (Alvarez-Fischer et al., 2008), Fornai and colleagues reported the presence of Lewy body-like aggregates in animals continuously perfused with MPTP (30 mg/kg/day) for a prolonged period of time (Fornai et al., 2005). The data presented here suggest that some of these differences could be the result of distinct transcriptional responses triggered in the acute and subchronic MPTP regimens and further that prior exposure to a toxin can profoundly influence the transcriptional response to a second encounter with the same toxin.
A major question raised by this study is the relevance of the various phases of gene expression to pathological outcome. SWR mice lack the delayed transcriptional responses to MPTP and do not exhibit degeneration (Figure 8 and Pattarini et al., 2007, Pattarini et al., 2008); i.e. the presence of the delayed (but not acute) response is predictive of degeneration, but it is unclear whether this is cause or effect. As shown in Figure 4, C57BL/6J mice primed with MPTP show an almost identical gene expression profile following subsequent challenges with MPTP to the MPTP-resistant SWR mice. Therefore, if the expression of one or more genes in the delayed response directly contributes to neuronal demise, then priming with MPTP might render normally MPTP-sensitive strains resistant to subsequent challenges with the same toxin. Alternatively, genes within the intermediate and late response groups may contribute to neuroprotection, in which case priming may further sensitize the animals to subsequent challenges with MPTP. Finally, the delayed phases of gene expression may represent the response to damage rather than being the cause of the damage and therefore be a reflection of the underlying biological processes that accompany the response to an MPTP lesion. Future detailed studies of cell loss in the SNpc in these dosing scenarios will help shed light on the pathological relevance of the various transcriptional alterations triggered by MPTP.
Another key finding in the present study is that whereas MPTP and paraquat both elicit parkinsonian-like responses in mice (Dauer and Przedborski, 2003, Smeyne and Jackson-Lewis, 2005, Tanner et al., 2011) they trigger quite distinct transcriptional responses (Figure 9). Epidemiological studies have linked rural living (Rajput et al., 1987) and exposure to various pesticides and herbicides including rotenone, paraquat and manganese ethylene bisdithiocarbamate (Maneb) to risk for PD (Alavanja et al., 2004, Landrigan et al., 2005). Additionally, there are reported interactions between PD-associated toxins in animal models. For example, the extent of the lesion achieved by concomitant administration of PQ and Maneb is considerably larger than that obtained with either toxin alone (Thiruchelvam et al., 2003). Priming mice with lipopolysaccharide (LPS), to cause a persistent inflammatory response, also increased the neurodegenerative effects of PQ (Mangano and Hayley, 2009) as did pre-treatment with MPTP (Shepherd et al., 2006). The current data show that MPTP, but not PQ, triggers transcriptional changes with the hallmark of an inflammatory response and this inflammation could underlie the synergy reported between these agents. That the two mechanisms of action are distinct is further underlined by our finding that priming with MPTP or PQ makes the transcriptional response to subsequent exposure to the same toxin refractory but it does not result in cross-attenuation. The lack of overlapping biological responses and molecular functions revealed by GO and GSEA analysis further highlights the differences between the toxicity of MPTP and PQ. It will be important in the future to assess the transcriptional profiles elicited by different toxins that cause PD-like lesions in mice to establish whether they all have different repertoires of gene expression changes or whether they fall into distinct categories, suggesting common mechanisms of action. If we can define toxin mechanisms by gene expression profiling it will then be interesting to establish the gene expression patterns triggered by co-administration of agents from different categories and relate this to pathological outcomes.
Another key question is the molecular and cellular basis of the refractoriness induced by MPTP. Genes within the acute phase have an over-representation of immediate-early transcription factors such as Fosb and c-Jun (Pattarini et al., 2008). Therefore, it was postulated that these gene products contributed to the regulation of genes in the delayed response phases in a temporal transcriptional cascade (Pattarini et al., 2008). However, the present data do not support this hypothesis. MPTP triggers a robust acute transcriptional response in both sensitive and resistant strains of mice as well as in a sensitive strain of mice primed with MPTP, yet the delayed responses are greatly attenuated or absent in the latter two circumstances (Figures 4 and 6). Therefore, it is more likely that the acute response underlies a global, stereotyped cellular stress reaction to MPTP intoxication rather than MPTP priming causing an uncoupling of transcriptional regulation of delayed genes by acute phase genes.
The duration of the refractory period elicited by a single administration of MPTP (Figures 1, 2 and 4) is coincident with the period of depleted striatal dopamine content in this model (Figure 11). Dopamine, but not other catecholamines, triggers expression of Hmox1 in cultured astrocytes (Schmidt et al., 1999), and striatal astrocytes are the source of elevated Hmox1 in the MPTP model (Fernandez-Gonzales et al., 2000). Therefore, dopamine dumping from SNpc DAergic nerve endings in the striatum as a result of MPTP intoxication might play a role as the initial trigger for the delayed transcriptional responses. However, once dopamine pools are depleted, subsequent treatments with MPTP cannot trigger a second delayed response. Although further studies are required to prove this interpretation, it would also fit the observation that the delayed response is selective for the striatum, in that it receives extensive DA-ergic innervation, whereas other brain areas with lower DA content only show an acute response to MPTP (Pattarini et al., 2008). The fact that the acute response is still intact in MPTP-primed mice (Figure 4) and occurs globally in brain (Pattarini et al., 2008) indicates that this rapid transcriptional response is likely triggered by something other than dopamine.
The intermediate response has an over-representation of genes involved in inflammation (Table7 and 8) such as cytokines, chemokines and their receptors (Pattarini et al., 2007, Pattarini et al., 2008). This is consistent with these alterations being a reflection of the inflammatory response known to be elicited in the striatum of MPTP treated mice (Pattarini et al., 2007, Pattarini et al., 2008) and also observed in PD patients, as well as humans intoxicated with MPTP (Langston et al., 1999, Lee et al., 2009). Therefore, the refractoriness of the delayed responses following priming may be a result of long-term negative feedback mechanisms limiting the re-initiation of an inflammatory response. This might occur through the production of anti-inflammatory cytokines and/or a shift in the functional status of cells in the brain that mediate and mount such responses. Nevertheless, the finding that exposure to a neurotoxin, even at sub-clinical doses, can have a long lasting effect on the ability of the mouse to mount an inflammatory response to a much higher level of toxin, has significant implications for our understanding of the role of environmental toxin exposure to the genesis of PD.
Genetic predisposing factors have been linked to the etiology of sporadic PD (Gao and Hong, 2011). The MPTP model is potentially useful to identify these predisposing genes as susceptibility to MPTP has a genetic component (Giovanni et al., 1991, 1994, Miller et al., 1998, Hamre et al., 1999). The present (Figure 4) and a previous gene expression study of MPTP-resistant SWR mice (Pattarini et al., 2008) demonstrate an attenuated intermediate and late transcriptional response after administration of MPTP. Strains of mice have also been generated that harbor genetic mutations implicated in familial and sporadic PD (Meredith et al., 2008). It will now be interesting to assess both the basal and MPTP-treated transcriptional profiles of these mice to determine if specific PD-associated mutations influence particular genes, or phases of the response. This may then point to additional cellular and molecular targets for therapeutic intervention in PD.
Acknowledgements
The authors would like to thank the Hartwell Center for Bioinformatics and Biotechnology for the synthesis of qRT-PCR primers and probes, sequencing and for processing samples for Affymetrix GeneChip technology. This work was supported in part by the National Institutes of Health Cancer Center CORE Grant CA 21765, the American Lebanese Syrian Associated Charities (ALSAC) and the National Institutes of Health grants R01-NS042828 and R01-ES010772 to J.I.M and R01-NS39006 to R.J.S.
Abbreviations
- Cdkn1a
cyclin-dependent kinase inhibitor 1a
- DA
dopamine
- DOPAC
3–4-dihydroxphenylacetic acid
- Edg3
endothelial differentiation, sphingolipid G-protein-coupled receptor 3
- Fosb
FBJ murine osteosarcoma viral oncogene homolog B
- Gadd45b
growth arrest and DNA-damage-inducible, beta
- Hbegf
heparin-binding EGF-like growth factor
- Hmox1
heme oxygenase 1
- HVA
homovanillic acid
- Mcp1/Ccl2
monocyte chemoattractant protein-1/chemokine (C-C motif) ligand 2
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine
- Nfkbiai
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha
- Nr4a1
Nuclear receptor subfamily 4, group A, member 1
- PD
Parkinson’s disease
- Pdlim4
PDZ and LIM domain 4
- qRT-PCR
quantitative RT-PCR
- SNpc
substantia nigra pars compacta
- SWR
Swiss Webster mice
- Tnfα
tumor necrosis factor alpha
- Tnfrsf12a
tumor necrosis factor receptor superfamily, member 12A
- MAO-B
monoamine oxidase B
- PQ
paraquat
- MPP+
1-methyl-4-phenylpyridinium
- IP
intraperitoneal
- CDS
coding sequence
- LPS
lipopolysaccharide
References
- Acworth IN, Cunningham ML. The measurement of monoamine neurotransmitters in microdialysis perfusates using HPLC-ECD. In: Harry J, Tilson HA, editors. Methods in Molecular Medicine. vol. 22. New Jersey: Humana Press; 1999. pp. 219–236. [DOI] [PubMed] [Google Scholar]
- Alavanja MC, Hoppin JA, Kamel F. Health effects of chronic pesticide exposure: cancer and neurotoxicity. Annu Rev Public Health. 2004;25:155–197. doi: 10.1146/annurev.publhealth.25.101802.123020. [DOI] [PubMed] [Google Scholar]
- Alvarez-Fischer D, Guerreiro S, Hunot S, Saurini F, Marien M, Sokoloff P, Hirsch EC, Hartmann A, Michel PP. Modelling Parkinson-like neurodegeneration via osmotic minipump delivery of MPTP and probenecid. J Neurochem. 2008;107:701–711. doi: 10.1111/j.1471-4159.2008.05651.x. [DOI] [PubMed] [Google Scholar]
- Ballard PA, Tetrud JW, Langston JW. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): seven cases. Neurology. 1985;35:949–956. doi: 10.1212/wnl.35.7.949. [DOI] [PubMed] [Google Scholar]
- Barlow BK, Cory-Slechta DA, Richfield EK, Thiruchelvam M. The gestational environment and Parkinson's disease: evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod Toxicol. 2007;23:457–470. doi: 10.1016/j.reprotox.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Bazzu G, Calia G, Puggioni G, Spissu Y, Rocchitta G, Debetto P, Grigoletto J, Zusso M, Migheli R, Serra PA, Desole MS, Miele E. alpha-Synuclein- and MPTP-generated rodent models of Parkinson's disease and the study of extracellular striatal dopamine dynamics: a microdialysis approach. CNS Neurol Disord Drug Targets. 2010;9:482–490. doi: 10.2174/187152710791556177. [DOI] [PubMed] [Google Scholar]
- Benmoyal-Segal L, Soreq H. Gene-environment interactions in sporadic Parkinson's disease. J Neurochem. 2006;97:1740–1755. doi: 10.1111/j.1471-4159.2006.03937.x. [DOI] [PubMed] [Google Scholar]
- Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson's disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JD, Jang H, Shepherd KR, Faherty C, Slack S, Jiao Y, Smeyne RJ. Response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) differs in mouse strains and reveals a divergence in JNK signaling and COX-2 induction prior to loss of neurons in the substantia nigra pars compacta. Brain Res. 2007;1175:107–116. doi: 10.1016/j.brainres.2007.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999;823:1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- Brooks DJ. Detection of preclinical Parkinson's disease with PET. Geriatrics. 1991;46(Suppl 1):25–30. [PubMed] [Google Scholar]
- Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson's disease--is there a link? Environ Health Perspect. 2006;114:156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GD, Ramirez VD. The mechanism of action of MPTP and MPP+ on endogenous dopamine release from the rat corpus striatum superfused in vitro. Brain Res. 1986;368:134–140. doi: 10.1016/0006-8993(86)91050-4. [DOI] [PubMed] [Google Scholar]
- Costa J, Lunet N, Santos C, Santos J, Vaz-Carneiro A. Caffeine exposure and the risk of Parkinson's disease: a systematic review and meta-analysis of observational studies. J Alzheimers Dis. 2010;20(Suppl 1):S221–S238. doi: 10.3233/JAD-2010-091525. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Reviews. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzales A, Perez-Otano I, Morgan JI. MPTP selectively induces Heme Oxygenase-1 expression in striatal astrocytes. Eur J Neurosci. 2000;12:1573–1583. doi: 10.1046/j.1460-9568.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, Pellegrini A, Nicoletti F, Ruggieri S, Paparelli A, Sudhof TC. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci U S A. 2005;102:3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Gene-environment interactions: key to unraveling the mystery of Parkinson's disease. Prog Neurobiol. 2011;94:1–19. doi: 10.1016/j.pneurobio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B. Well-water consumption and Parkinson's disease in rural California. Environ Health Perspect. 2009;117:1912–1918. doi: 10.1289/ehp.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Nelson EL, Liang CL, Speciale SG, Sinton CM, Sonsalla PK. The neurotoxin MPTP causes degeneration of specific nucleus A8, A9 and A10 dopaminergic neurons in the mouse. Neurodegeneration. 1996;5:299–312. doi: 10.1006/neur.1996.0041. [DOI] [PubMed] [Google Scholar]
- Giovanni A, Sieber BA, Heikkila RE, Sonsalla PK. Correlation between the neostriatal content of the 1-methyl-4-phenylpyridinium species and dopaminergic neurotoxicity following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration to several strains of mice. J Pharmacol Exp Ther. 1991;257:691–697. [PubMed] [Google Scholar]
- Giovanni A, Sieber BA, Heikkila RE, Sonsalla PK. Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Part 1: Systemic administration. J Pharmacol Exp Ther. 1994;270:1000–1007. [PubMed] [Google Scholar]
- Hamre K, Tharp R, Poon K, Xiong X, Smeyne RJ. Differential strain susceptibility following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration acts in an autosomal dominant fashion: quantitative analysis in seven strains of Mus musculus. Brain Res Mol Brain Res. 1999;828:91–103. doi: 10.1016/s0006-8993(99)01273-1. [DOI] [PubMed] [Google Scholar]
- Henry J, Smeyne RJ, Jang H, Miller B, Okun MS. Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism Relat Disord. 2010 doi: 10.1016/j.parkreldis.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Cheung L, Rowe D, Halliday G. Genetic contributions to Parkinson's disease. Brain Res Mol Brain Res. 2004;46:44–70. doi: 10.1016/j.brainresrev.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jang H, Boltz D, Sturm-Ramirez K, Shepherd KR, Jiao Y, Webster R, Smeyne RJ. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A. 2009;106:14063–14068. doi: 10.1073/pnas.0900096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller W, Vetere-Overfield B, Gray C, Alexander C, Chin T, Dolezal J, Hassanein R, Tanner C. Environmental risk factors in Parkinson's disease. Neurology. 1990;40:1218–1221. doi: 10.1212/wnl.40.8.1218. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environ Health Perspect. 2005;113:1230–1233. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lee JK, Tran T, Tansey MG. Neuroinflammation in Parkinson's disease. J Neuroimmune Pharmacol. 2009;4:419–429. doi: 10.1007/s11481-009-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano EN, Hayley S. Inflammatory priming of the substantia nigra influences the impact of later paraquat exposure: Neuroimmune sensitization of neurodegeneration. Neurobiol Aging. 2009;30:1361–1378. doi: 10.1016/j.neurobiolaging.2007.11.020. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J Neurochem. 2005;93:1030–1037. doi: 10.1111/j.1471-4159.2005.03088.x. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Sonsalla PK, Chesselet MF. Animal models of Parkinson's disease progression. Acta Neuropathol. 2008;115:385–398. doi: 10.1007/s00401-008-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Ali SF, O'Callaghan JP, Laws SC. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann N Y Acad Sci. 1998;844:153–165. [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Moretto A, Colosio C. Biochemical and toxicological evidence of neurological effects of pesticides: the example of Parkinson's disease. Neurotoxicology. 2011;32:383–391. doi: 10.1016/j.neuro.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Obayashi S, Ando K, Inaji M, Maeda J, Okauchi T, Ito H, Suhara T. Progressive changes of pre- and post-synaptic dopaminergic biomarkers in conscious MPTP-treated cynomolgus monkeys measured by positron emission tomography. Synapse. 2007;61:809–819. doi: 10.1002/syn.20431. [DOI] [PubMed] [Google Scholar]
- Pattarini R, Rong Y, Qu C, Morgan JI. Distinct mechanisms of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine resistance revealed by transcriptome mapping in mouse striatum. Neuroscience. 2008;155:1174–1194. doi: 10.1016/j.neuroscience.2008.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattarini R, Smeyne RJ, Morgan JI. Temporal mRNA profiles of inflammatory mediators in the murine 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine model of Parkinson's disease. Neuroscience. 2007;145:654–668. doi: 10.1016/j.neuroscience.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: A comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Uitti RJ, Stern W, Laverty W, O'Donnell K, O'Donnell D, Yuen WK, Dua A. Geography, drinking water chemistry, pesticides and herbicides and the etiology of Parkinson's disease. Can J Neurol Sci. 1987;14:414–418. doi: 10.1017/s0317167100037823. [DOI] [PubMed] [Google Scholar]
- Robinson S, Freeman P, Moore C, Touchon JC, Krentz L, Meshul CK. Acute and subchronic MPTP administration differentially affects striatal glutamate synaptic function. Exp Neurol. 2003;180:74–87. doi: 10.1016/s0014-4886(02)00050-x. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Mertz K, Morgan JI. Regulation of heme oxygenase-1 expression by dopamine in cultured C6 glioma and primary astrocytes. Brain Res Mol Brain Res. 1999;73:50–59. doi: 10.1016/s0169-328x(99)00231-4. [DOI] [PubMed] [Google Scholar]
- Shepherd KR, Lee ES, Schmued L, Jiao Y, Ali SF, Oriaku ET, Lamango NS, Soliman KF, Charlton CG. The potentiating effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on paraquat-induced neurochemical and behavioral changes in mice. Pharmacol Biochem Behav. 2006;83:349–359. doi: 10.1016/j.pbb.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Smeyne M, Goloubeva O, Smeyne RJ. Strain–dependent susceptibility to MPTP and MPP+-induced Parkinsonism is determined by glia. Glia. 2001;74:73–80. [PubMed] [Google Scholar]
- Smeyne RJ, Jackson-Lewis V. The MPTP model of Parkinson's disease. Brain Res Mol Brain Res. 2005;134:57–66. doi: 10.1016/j.molbrainres.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Heikkila RE. The influence of dose and dosing interval on MPTP-induced dopaminergic neurotoxicity in mice. Eur J Pharmacol. 1986;129:339–345. doi: 10.1016/0014-2999(86)90444-9. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom E, Stromberg I, Tsutsumi T, Olson L, Jonsson G. Studies on the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in C57BL/6 mice. Comparison with three other strains of mice. Brain Res. 1987;405:26–38. doi: 10.1016/0006-8993(87)90986-3. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Miyake Y, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Miki T, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M. Active and passive smoking and risk of Parkinson's disease. Acta Neurol Scand. 2010;122:377–382. doi: 10.1111/j.1600-0404.2010.01327.x. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, Cory-Slechta DA. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson's disease phenotype. Eur J Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- Vance JM, Ali S, Bradley WG, Singer C, Di Monte DA. Gene-environment interactions in Parkinson's disease and other forms of parkinsonism. Neurotoxicology. 2010;31:598–602. doi: 10.1016/j.neuro.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, Korsmeyer SJ, Przedborski S. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:2837–2842. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Weuve J, Nie H, Saint-Hilaire MH, Sudarsky L, Simon DK, Hersh B, Schwartz J, Wright RO, Hu H. Association of cumulative lead exposure with Parkinson's disease. Environ Health Perspect. 2010;118:1609–1613. doi: 10.1289/ehp.1002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood-Kaczmar A, Gandhi S, Wood NW. Understanding the molecular causes of Parkinson's disease. Trends Mol Med. 2006;12:521–528. doi: 10.1016/j.molmed.2006.09.007. [DOI] [PubMed] [Google Scholar]