Abstract
Healthy aged individuals are more likely to suffer profound memory impairments following a challenging life event such as a severe bacterial infection, surgery, or an intense psychological stressor, than are younger adults. Importantly, these peripheral challenges are capable of producing a neuroinflammatory response, (e.g., increased pro-inflammatory cytokines). In this review we will present the literature demonstrating that in the healthy aged brain this response is exaggerated and prolonged. Normal aging primes or sensitizes microglia and this appears to be the source of this amplified response. We will review the growing literature suggesting that a dysregulated neuroendocrine response in the aged organism is skewed towards higher brain CORT levels, and that this may play a critical role in priming microglia. Among the outcomes of an exaggerated neuroinflammatory response are impairments in synaptic plasticity, and reductions in key downstream mediators such as Arc and BDNF. We will show that each of these mechanisms is important for long-term memory formation, and is compromised by elevated pro-inflammatory cytokines.
Keywords: normal aging, neuroinflammation, hippocampal-dependent memory, microglial sensitization, dysregulated neuroendocrine response, cognitive deficit
Introduction
By the year 2030, roughly 20% of the population will be over 65 years of age (Vincent and Velkoff, 2010). As life expectancy continues to increase, it is important to understand the factors underlying the decline in memory and cognition that occurs with normal healthy aging, not just those that result from neurodegenerative disorders.
Although cognitive function declines with age, there is considerable variability among individuals in the extent of this decline (Laursen, 1997). An important observation is that cognitive function of otherwise normal older individuals can be severely impaired shortly after experiencing a challenging life event such as an infection, surgery, or psychological stress, an effect not readily observed in healthy young individuals (Bekker and Weeks, 2003; VonDras et al., 2005; Wofford et al., 1996). Thus, normal aging is a vulnerability factor for the cognitive effects of these challenges (Foster, 2006; Laursen, 1997; Tsolaki et al., 1994; Unverzagt et al., 2001). Recently, there have been several advances toward understanding a) the mechanisms that mediate this vulnerability, b) the resulting memory impairments associated with vulnerable individuals presented with such challenges, and c) potential treatments and interventions to buffer against the effects of these challenges. The purpose of this article is to review those advances.
How do peripheral challenges, such as an infection or a surgical manipulation, impact cognitive function? In this review, we will first establish that these challenges activate innate immune cells, thereby inducing a peripheral inflammatory response. We will then describe how pro-inflammatory cytokines, released as part of this response, signal the brain. Next, we will introduce microglia, the brain’s resident immune cells, that are activated upon peripheral inflammatory signaling to the brain. Microglia have the main job of immunosurveillance. Upon detection of a pathogen, dead cells, or other cellular debris, microglia become activated and release a variety of inflammatory mediators including pro-inflammatory cytokines that organize host defense and restore homeostasis. Under basal conditions, microglia in the healthy aged brain exhibit phenotypic signs of activation, but do not always release elevated levels of pro-inflammatory cytokines (although this is somewhat dependent on the age and strain of the animal being studied). As we shall describe, in the face of a challenge, these primed microglia exhibit a potentiated pro-inflammatory response. We will then take a short detour to discuss the neuroendocrine response in normal aging, as it will provide a useful backdrop for understanding a possible mechanism for microglial sensitization. The exaggerated pro-inflammatory microglial response can then impact neuronal functioning to ultimately impair hippocampal-dependent memory. We will discuss a handful of putative mechanisms of pro-inflammatory cytokine-induced neuronal dysfunction. Along the way, we will review pharmacological, dietary, and behavioral interventions that have ameliorated or blocked age-related microglial sensitization, pro-inflammatory elevations, or downstream responses, to protect against memory impairments in the aged organism. See Figure 1 for schematic representation of the perspective described.
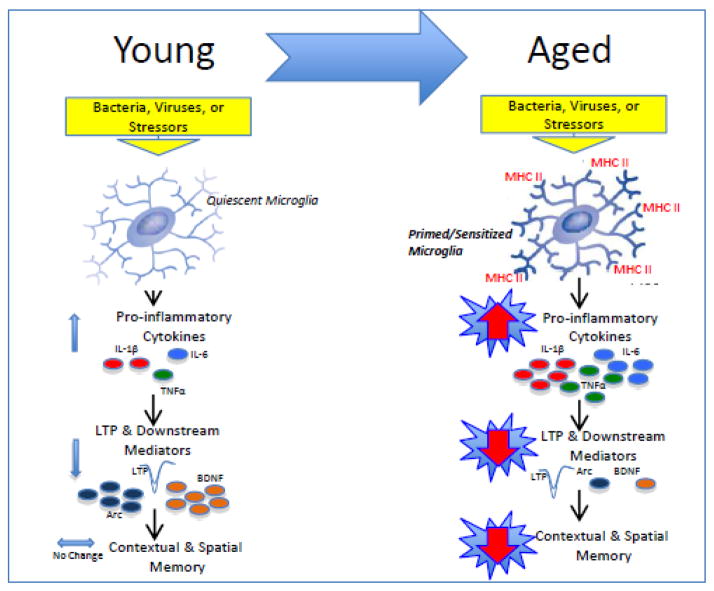
Figure 1.
Schematic depicting our working hypothesis. Upon a peripheral inflammatory, or stressor challenge in the young adult animal, once quiescent microglia are rapidly and transiently activated. Pro-inflammatory cytokines are released resulting in only moderate elevations above basal levels, and lasting no longer than 24 hours. Synaptic facilitation (LTP) and downstream mediators such as BDNF and Arc are moderately decreased resulting in mild to negligible memory impairments. In contrast, unchallenged aged microglia exhibit phenotypic markers of activation (i.e., MHCII), rendering them primed for a subsequent challenge. Upon a peripheral challenge, these microglia produce a sensitized neuroinflammatory response. Pro-inflammatory cytokine release is exaggerated and prolonged, lasting at least 8 days. Synaptic facilitation (LTP) and downstream mediators are profoundly reduced, and long-term contextual, and spatial memory is significantly impaired.
Communication between peripheral pro-inflammatory cytokines and the brain
Interleukin-1 beta (IL-1β) is a pro-inflammatory protein, synthesized and released by a variety of cell types distributed throughout the body, in response to tissue damage or on contact with a pathogen. Upon release, IL-1β exhibits diverse and overlapping activities as an intercellular mediator (a cytokine) in the generation of an inflammatory immune response. Infection or injury initiates a peripheral acute phase response that involves of a cascade of local and systemic events. As part of its role in this response, IL-1β enhances T and B lymphocyte proliferation and stimulates natural killer cell cytocidal activity to eliminate the injured cells or invading pathogen. IL-1β also induces the production of other cytokines from many cell types, for example tumor necrosis factor-alpha (TNFα), and interleukin-6 (IL-6), which in turn have secondary effects on other cells.
An important recent advance in understanding the biological basis of cognitive behavior is the recognition that there is extensive communication between the immune system and the central nervous system. As a result of this communication, neural activity is altered quite dramatically during and following peripheral infection (Maier and Watkins, 1998). Both blood-borne and neural pathways of communication have been well defined over the last two decades (Bachstetter et al., 2009; Banks et al., 1999; Dinarello et al., 1988; Ericsson et al., 1994; Gaykema et al., 1998; Goehler et al., 1997). Importantly, this communication leads to de novo cytokine production within the brain parenchyma, primarily by microglial cells (Laye et al., 1996; Nguyen et al., 1998; Turrin et al., 2001; Van Dam et al., 1995). That is, part of the neural cascade that follows peripheral inflammation includes the activation of the once resting microglia and a shift of these cells to an inflammatory phenotype.
Microglial phenotype in normal aging: a shift towards an immunologically primed state
Microglia, as part of the myelomonocytic lineage, constitute the predominant innate immune cell in the brain parenchyma and serve many functions including immunosurveillance of the brain microenvironment for pathogen invasion, cellular debris, apoptotic cells, and alterations in neuronal phenotype (Kreutzberg, 1996). Our focus here is on evidence showing that microglia undergo profound immunophenotypic and functional changes with normal brain aging. An important issue that merits attention here is the distinction between “normal” brain aging and “pathological” brain aging. Our work, as well as the preponderance of studies reviewed here, has focused on studying normal aging in which obvious neurodegeneration and senescence is not a prominent feature. Here, older animals exhibit primed neuroinflammatory and behavioral responses that require a challenge for overt action to occur. Outside the scope of the present review, a considerable literature has studied senescent animals, which exhibit basal behavioral and brain cytokine profiles dramatically different from younger animals, and whose brains are generally classified under the heading of “neurodegeneration” (Cacabelos et al., 1994; Luterman et al., 2000; Remarque et al., 2001).
In normal brain aging, the immunophenotype of microglia is characterized by up-regulation of glial activation markers including major histocompatibility complex II (MHC II) and complement receptor 3 (CD11b), a finding that has been reported in several species including human post-mortem tissue, rodent, canine, and non-human primates (Perry et al., 1993; Rogers et al., 1988; Rozovsky et al., 1998; Sheffield and Berman, 1998; Tafti et al., 1996). This up-regulation of MHCII occurs also at the mRNA level (Frank et al., 2006a). Importantly, MHCII is expressed at very low levels on microglia of younger animals under basal conditions (Perry, 1998), providing a clear baseline to detect aging-related changes in microglia immunophenotype. A key question is how do changes in microglia immunophenotype (up-regulated MHCII) relate to changes in microglia immune function with normal brain aging. Increased MHCII could result from aging-induced increases in microglia number, or from increases in antigen presentation. Although there are not a large number of studies, they favor the idea that there is microglial sensitization. A stereological assessment of microglia numbers in hippocampal sub-regions indicated that microglia numbers appear to remain stable across the life span (Long et al., 1998). Moreover, flow cytometry on microglia isolated from young and aged mice conclusively showed that microglia MHCII expression increases on a per cell basis in aged animals (Henry et al., 2009). Further, we have rapidly isolated microglia from hippocampus in young and aged rats, and MHCII, CD11b, and Iba-1 gene expression were all strongly up-regulated in aged animals, while controlling for microglia cell number (Frank et al., 2006a). A critical point to bear in mind with regard to the use of isolated microglia is the effect the isolation procedure may have on antigen expression. To address this concern, we have shown that the microglia isolation procedure preserves the in vivo immunophenotype of microglia as measured by flow cytometry and real time PCR (Frank et al., 2007; Frank et al., 2006B). Several cell surface proteins (MHCII, CD163, and CD11b) were undetectable using flow cytometry on isolated hippocampal microglia from young rats, suggesting the methodology per se does not impact antigen expression (Frank et al., 2006b). Whether an age-related vulnerability exists to elicit an up-regulation of activation antigens in these cells following this procedure is unknown. Nevertheless, our findings using this method are consistent with the preponderance of evidence from researchers using other methods suggesting that aging results in the progressive up-regulation of microglia “activation” antigens such as MHCII. This phenotype represents a progressive shift in the state of microglia from quiescent to primed. In the primed state, microglia are skewed towards exaggerated pro-inflammatory immune responses to subsequent challenges.
Aging–related sensitization of the neuroinflammatory response to challenge
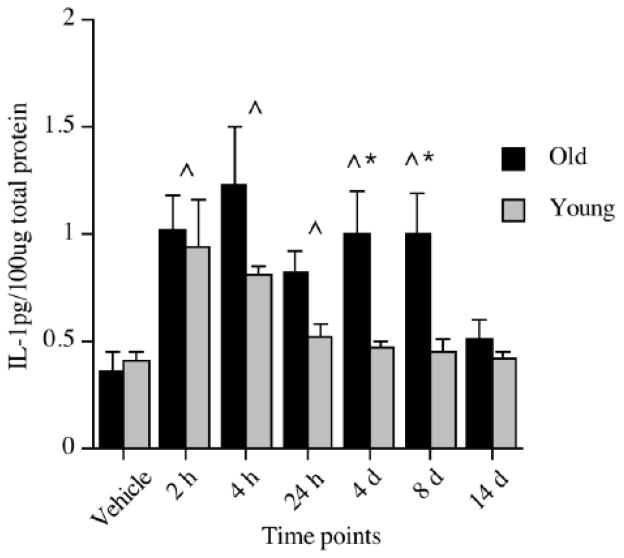
Significantly elevated levels of pro-inflammatory cytokines, such as IL-1β, in key brain regions responsible for mediating memory, such as the hippocampus, have been shown to impair memory (Akana et al., 1999; Barrientos et al., 2002; Barrientos et al., 2003; Barrientos et al., 2004; Gibertini et al., 1995; Hauss-Wegrzyniak et al., 1998; Hauss-Wegrzyniak et al., 2002; Hauss-Wegrzyniak et al., 1999; Hein et al., 2010; Pugh et al., 1998; Pugh et al., 1999). Thus, an obvious question is whether the neuroinflammatory response to challenge is sensitized in normal aging. Though earlier we established that healthy aged animals exhibit a marked shift in microglia activation state, it cannot be assumed that this translates to a sensitized neuroinflammatory response to challenge. To directly answer this question, our laboratory, along with several others, began by examining the neuroinflammatory response of aged and young animals following a challenge with a bacteria or virus (Abraham et al., 2008; Barrientos et al., 2009; Barrientos et al., 2006; Godbout et al., 2005; Godbout et al., 2008; Sparkman et al., 2005), a stressor (Buchanan et al., 2008; Sparkman and Johnson, 2008), or a surgical intervention (Rosczyk et al., 2008). Regardless of the type of challenge, aged animals exhibited a clear and exaggerated neuroinflammatory response compared to young adult animals; that is, a sensitized response. Our laboratory, for example, showed that adult and aged F344xBN rats had dramatically different cytokine responses to a live, replicating Escherichia coli (E. coli) infection. IL-1β protein was measured 2 hours, 4 hours, 24 hours, 4 days, 8 days, and 14 days following the infection. Both adult and aged rats had elevated levels of IL-1β in the hippocampus at 2 hours post-E. coli, with IL-1β in adult rats returning to vehicle control levels at 24 hours. However, aged rats showed sustained elevations through day 8 post-infection, with a return to vehicle control levels at day 14 (See Fig. 2)(Barrientos et al., 2009). A study by Godbout and colleagues found similar results in the BALB/c mouse. A peripheral injection of lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, resulted in both exaggerated and prolonged elevations in IL-1β and IL-6 (both protein and mRNA) in aged mice (Godbout et al., 2005). Importantly, in both of these studies, the exaggerated cytokine response was restricted to the brain and did not occur in the periphery (Barrientos et al., 2009; Godbout et al., 2005). In fact, our laboratory found this amplified response to be particular to the hippocampus. We did not observe exaggerated or protracted responses in hypothalamus, parietal cortex, pre-frontal cortex, spleen or serum (Barrientos et al., 2009). Similarly, Buchanan and colleagues reported that 30 min of restraint stress repeated over four days resulted in IL-1β mRNA expression that was exaggerated in the hippocampus of aged mice compared to non-stressed age-matched controls (Buchanan et al., 2008). This augmented response was not observed in hypothalamus or in peripheral plasma samples, implicating a hippocampus-specific response. Along the same lines, Rosczyk and colleagues found IL-1β mRNA expression in the hippocampus of aged mice to be amplified 24 hours after abdominal surgery compared to age-matched sham controls (Rosczyk et al., 2008). Yet, young adult mice that underwent the same surgical procedure showed no differences in IL-1β mRNA compared to their age-matched sham controls. Taken together, these studies provide an abundance of evidence indicating that a challenging event indeed results in a potentiated neuroinflammatory response in the aged organism, and in many cases, this response is specific to the hippocampal formation, a structure important for memory function.
Figure 2.
IL-1β protein levels in hippocampus of young and old rats following a peripheral vehicle or E. coli injection at 2 hours, 4 hours, 24 hours, 4 days, 8 days, and 14 days. Note that vehicle samples were collected at 3 hours and 24 hours and because they did not differ, were pooled to form just one vehicle control group for each age group. ^Significant difference between E. coli-treated and vehicle-treated groups. *Significant difference between age groups. Error bars indicate ± SEM. Modified with permission from Barrientos, et al., 2009.
From the studies above, the cellular source of this age-related potentiated neuroinflammatory response was unknown. An early study using mixed glial cultures demonstrated that these cells taken from aged mice exhibited greater spontaneous IL-6 mRNA expression compared to those taken from younger animals (Ye and Johnson, 1999). Flow cytometric analysis strongly suggested that microglia were the primary cellular source of this age-related response. In 2005, Cunningham and colleagues first demonstrated that a compromised brain with a phenotype of chronic microglial activation and minimal proinflammatory cytokine expression (a phenotype shared by normal aged animals) was primed to produce exaggerated inflammatory cytokines in response to subsequent challenges (Cunningham et al., 2005). They showed that an intracerebral administration of LPS into prion diseased mice indeed resulted in a potentiated microglial IL-1β response compared to the response induced by the same challenge in healthy mice. These data confirmed that microglia produced the potentiated inflammatory response. Whether the potentiated neuroinflammatory response was mediated by sensitized microglia per se was later addressed by Godbout and colleagues who demonstrated that neuroinflammatory mediators, measured from enriched microglia isolated following an in vivo, peripheral LPS challenge, were potentiated in microglia taken from aged mice compared to those taken from adult mice (Henry et al., 2009). These findings strongly pointed to microglia as the sensitized cell producing the exaggerated response. It could be argued however, that the immune challenge, administered in vivo, acted at some other cell type, whether in a central or peripheral compartment, to deliver an exaggerated signal to the microglia in the aging subjects. Therefore, to further address whether a potentiated neuroinflammatory response in aged animals is mediated specifically by sensitized microglia, we isolated microglia from unchallenged adult and aged animals and stimulated them with LPS ex vivo (Frank et al., 2010b). Our findings supported what others had concluded. That is, we found that enriched hippocampal microglia isolated from normal aged (but not adult) animals were sensitized, as they produced a potentiated pro-inflammatory cytokine response (i.e. IL-1β, TNFα) when subsequently challenged with LPS (Frank et al., 2010b).
Aging and loss of CNS immunoregulatory control of microglia
The evidence presented thus far suggests that aging is accompanied by a progressive shift in the activation state of microglia, resulting in these cells being chronically sensitized. They could be described as hyper-vigilant to disruptions in central homeostasis. It is as if with aging, microglia are in a refractory state of immunologic activation and thus cannot revert to a ground state of quiescent central housekeeping function. In other words, the functional plasticity of microglia may be compromised with aging.
Understanding the mechanism(s) that sensitize microglia during normal brain aging is of considerable importance in the context of understanding and attenuating neuroinflammatory-induced cognitive impairments. There is now considerable evidence that the CNS microenvironment expresses proteins that inhibit microglia activation as well as pro-inflammatory immune responses (Biber et al., 2007). Our focus here will be on the age-related decreases in two of these proteins, CD200 and fractalkine (CX3CL1), which are expressed preferentially on neurons and function to inhibit microglia through their cognate receptor expressed predominately on myelomonocytic cell types. We found that aging reduced CD200 gene expression (Frank et al., 2006a). Lyons and colleagues found similar results with CD200 protein (Lyons et al., 2007). Interestingly, CD200 knockout mice constitutively displayed a chronically activated microglia phenotype characterized by a less ramified morphology and increased expression of CD11b (Hoek et al., 2000), a phenotype remarkably similar to that observed in aged animals. Likewise, young mice deficient in the microglial fractalkine receptor (CX3CR1−/−) demonstrated long-lasting microglial activation (Corona et al., 2010) and neurotoxicity (Cardona et al., 2006) in response to a peripheral LPS challenge. Normal aged animals demonstrated a failure to up-regulate CX3CR1 24 hours after an LPS challenge, which coincided with a potentiated neuroinflammatory response and a reduction in social behavior, which is known to be modulated by pro-inflammatory cytokine changes (Wynne et al., 2010). The inability of aged microglia to up-regulate CX3CR1 in the face of an immune challenge again suggests a loss of microglia functional plasticity. The ligand, CX3CL1, also showed an age-related decrease in the CNS (Bachstetter et al., 2009; Wynne et al., 2010). Importantly, treatment with exogenous CX3CL1 reversed the age-related decrease in hippocampal neurogenesis and reduced the number of MHCII+ cells in the hippocampus (Bachstetter et al., 2009). Of note, infusion of a CX3CR1 blocking antibody resulted in a considerable increase in the number of MHCII+ cells in the hippocampus, as well as levels of IL-1β protein (Bachstetter et al., 2009). Moreover, co-treatment with the IL-1 receptor antagonist (IL-1RA) abrogated the effects of CX3CR1 blockade. These studies provide compelling evidence that neuronal inhibitory control over central pro-inflammatory processes may be compromised with normal aging, thereby predisposing the aged brain to exaggerated pro-inflammatory response in the face of immune challenges.
Neuroendocrine response in normal aging and interactions with hippocampal microglia
To further explore plausible explanations for how microglia become chronically sensitized by normal aging, we now turn to the neuroendocrine system and glucocorticoid (GC) hormones (cortisol in primates, corticosterone in rodents), as there is a large literature demonstrating that these hormones are chronically elevated in the aged organism, and a growing literature suggesting that these hormones may alter microglial function.
Central to the neuroendocrine system is the hypothalamic-pituitary-adrenal (HPA) axis in which the hypothalamus controls the secretion of adrenocorticotropic hormone from the anterior pituitary, which in turn stimulates the secretion of corticosterone (CORT) from the adrenal glands. CORT is one of the principal glucocorticoids released into the bloodstream in a circadian and pulsatile fashion, and participates in the control of whole body homeostasis (Jasper and Engeland, 1991) through the regulation of energy and metabolism, immune reactions, and stress responses (Clark et al., 1992; Munck et al., 1984). CORT in the bloodstream readily crosses the blood-brain-barrier, and so this hormone acts at both peripheral and central sites to produce many adaptive responses. It should be noted however, that approximately 90% of CORT in the bloodstream is bound to corticosteroid binding globulin (CBG), rendering it biologically inactive, and bound CORT is unable to cross the blood-brain barrier (reviewed in(Henley and Lightman, 2011). That is, under normal circumstances only a small portion of the CORT that is released by the adrenal glands is free to cross into the brain parenchyma. Unbound CORT can diffuse across the cell membrane and once in the cytoplasm binds to two different receptors, the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR). MRs have a ten-fold higher affinity for GCs than do GRs. The majority of MRs are occupied under basal conditions while GRs show low affinity for GCs and become occupied when CORT is elevated. On binding CORT, they translocate to the nucleus where they regulate gene transcription (reviewed in (Reul et al., 1990). Also of importance here, CORT secretion is under negative feedback control, with CORT exerting inhibitory control at the level of the pituitary, the hypothalamus, and the hippocampus (reviewed in (Reul et al., 1990).
The link between extensive CORT exposure and aging was first hinted at over 50 years ago in several papers documenting the marked similarities between the pathologies of stress and aging (Findley, 1949; Solez, 1952; Wexler, 1964; Wexler and Kittinger, 1965). Based in part on those findings, Landfield and colleagues went on to describe a hypothesis by which alterations in neuroendocrine control mechanisms would lead to a “runaway” positive feedback loop and the resulting accumulation of GCs could facilitate, or accelerate the aging process (Landfield et al., 1978). To support their hypothesis, they demonstrated a significant correlation between plasma CORT levels and glial reactivity in the hippocampus of aged rodents, suggesting for the first time that aging was not only associated with elevated plasma CORT, but that this hormonal increase was highly correlated with a marker of neuroinflammation. Furthermore, these findings suggested that aging was a vulnerability factor for this runaway positive feedback loop. They further showed that adrenalectomy in the aged rodent reduced hippocampal gliosis (Landfield et al., 1981). Soon thereafter, Sapolsky and colleagues reported an elevation in basal plasma CORT of aged rats and an impaired capacity for their stress-induced levels of CORT to return to basal levels (Sapolsky et al., 1983a). Taken together, these findings gave rise to the so-called “glucocorticoid cascade hypothesis” (GCH) which proposed a gradual loss of negative feedback control correlated with increased age, leading to an accumulation of GCs in the brain (Sapolsky et al., 1986). Sustained exposure to elevated GCs was reported to produce potent levels of glutamate, calcium and reactive oxygen radicals resulting in hippocampal neuron atrophy or death (Kerr et al., 1991; Magarinos and McEwen, 1995; Sapolsky et al., 1985; Watanabe et al., 1992). The hippocampus is a primary target site for GCs, possessing among the highest of concentrations of GC receptors in the brain (McEwen et al., 1968; Sapolsky et al., 1983B), and being a principle site of negative feedback control. Furthermore, the hippocampus plays a critical role in declarative forms of memory (as measured by spatial and contextual tasks) (Squire, 1992). Thus, according to the GCH, excitotoxic death of hippocampal neurons caused by a dysfunction in HPA axis negative feedback control would lead to cognitive impairments.
However, in more recent years, difficulties with the GCH have emerged. First, Alzheimer’s patients and some strains of aged rats do not show impairments of GC negative feedback control, despite exhibiting cognitive deficits (Meijer et al., 2005; Swanwick et al., 1998; Yau et al., 1994). In addition, advances in stereological techniques have revealed little or no hippocampal cell death in aged brains (Rapp and Gallagher, 1996) or brains exposed to chronic levels of CORT (Bodnoff et al., 1995; Leverenz et al., 1999; Sousa et al., 1998). Furthermore, it is questionable whether CORT levels in the brain reflect those in the plasma, and early studies measured only CORT in blood. Indeed, a recent study found that in aged Wistar rats CORT was elevated in plasma, but not in brain (Garrido et al., 2010), while another found the opposite findings in aged F344xBN rats (Barrientos et al., 2011a). Due to differences in collection and measurement techniques, the disparity in findings across laboratories is not too surprising. For example, the measurement of free vs. bound vs. total CORT can lead to very different results and interpretations, especially given that CBG levels can vary significantly across the circadian cycle (Malisch et al., 2008), with age (van Eekelen et al., 1992), and with shifts in body temperature (Cameron et al., 2010). Similarly, measurement of total GC receptors rather than the distribution of receptors between cytoplasmic and nuclear fractions can lead to varied interpretations. Moreover, without the use of automated sampling techniques, basal CORT values are often not truly basal due to increases associated with handling of subjects (Henley et al., 2009). Automated and frequent sampling is required since CORT is secreted in a pulsatile fashion (Henley et al., 2009).
Despite these inconsistencies with regard to the GCH, the preponderance of evidence does support the general idea that the aged brain is exposed to greater CORT. A recent line of research examining the enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD) further supports this notion. There are two isoforms of 11β-HSD: 11β-HSD1 is highly expressed in key metabolic tissues such as liver, adipose tissue, and brain, and functions to transform cortisone (an inactive metabolite of CORT) to the active hormone CORT. 11β-HSD2, on the other hand, is expressed in aldosterone-selective tissues, including colon, salivary and sweat glands, and placenta, and functions to oxidize CORT to cortisone and prevents activation of the MR (Seckl and Walker, 2001). Elevations in 11β-HSD1 thus amplify glucocorticoid action (Rajan et al., 1996) and interestingly, 11β-HSD1 is elevated in the hippocampus of aged animals (Holmes et al., 2010). This is relevant here because even if plasma CORT levels were equal between young and aged animals, elevated 11β-HSD1 levels in the brains of aged animals would ultimately result in greater brain CORT and gene transcription.
We began by reviewing the literature on aging-related activation of hippocampal microglia and its role in cytokine-induced memory impairments. So, how is chronic elevated hippocampal CORT involved? The link is provided by a consideration of the findings of Johnson and colleagues who reported that exposure to a stressor primes or exaggerates the response to a subsequent immune challenge. In their study, they exposed young adult rats to either an acute stressor or no stress 24 h prior to a peripheral LPS injection and found that the stressed rats showed a sensitized pro-inflammatory cytokine response in plasma and brain (Johnson et al., 2002). In contrast, acute stress given immediately after LPS decreases the production of these cytokines (Goujon et al., 1995). More recently, we corroborated these findings using exogenous CORT. CORT administered to rats 2 or 24 h prior to LPS challenge potentiated IL-1β and TNFα expression in the hippocampus, while CORT administered 1 h after LPS diminished the response (Frank et al., 2010c). Hippocampal microglia are critical to this response as microglia isolated from animals pretreated with CORT and later challenged ex vivo with LPS produced greater IL-1β and TNFα expression (Frank et al., 2010c).
Taken together these findings suggest that chronic elevation of brain CORT could produce a hippocampus whose microglia are in a chronic state of activation, which upon an immune challenge would produce a potentiated pro-inflammatory response. And, as we have reviewed earlier, aged rodents have microglia with exactly this phenotype. The next section will discuss how a potentiated pro-inflammatory response, specifically in the hippocampus, can produce significant and long-lasting memory impairments.
Neuroinflammatory effects on synaptic plasticity and hippocampus-dependent memory in aging
It has been well documented that expression of pro-inflammatory cytokines above basal levels, particularly in the hippocampus, impairs both synaptic plasticity, as assessed by long-term potentiation (LTP) (Cumiskey et al., 2007; Curran and O’Connor, 2003; Tancredi et al., 2000; Vereker et al., 2000a; Vereker et al., 2000b), and hippocampus-dependent memory in adult rodents (Barrientos et al., 2002; Barrientos et al., 2003; Barrientos et al., 2004; Gibertini et al., 1995; Hauss-Wegrzyniak et al., 1998; Hauss-Wegrzyniak et al., 2002; Hauss-Wegrzyniak et al., 1999; Hein et al., 2010; Pugh et al., 1998; Pugh et al., 1999). Thus, populations or individuals that have either increased peripheral inflammatory responses to immune activating agents such as bacteria or viruses, or exaggerated brain inflammatory responses to signaling events within the brain, such as psychological stressors, are likely to be more susceptible to infection-induced memory impairments. Healthy, yet aging individuals fall into that category.
LTP is a measure of synaptic plasticity and has been widely accepted as an electrophysiological model of hippocampal learning and memory. Late-phase LTP (L-LTP) is dependent on new protein synthesis, and is thought to play a key role in producing the structural rearrangements that allow permanent storage of a memory (Alvarez and Squire, 1994; Nguyen and Kandel, 1996), as blocking L-LTP impairs long-term memory formation (reviewed in (Pang and Lu, 2004; Tyler et al., 2002)). IL-1β was first shown to completely block LTP in the CA1 region (Bellinger et al., 1993) and the dentate gyrus (Coogan and O’Connor, 1997; Coogan and O’Connor, 1999; Cunningham et al., 1996) in rat hippocampal slices, and the CA3 region of murine hippocampal slices (Katsuki et al., 1990), nearly 20 years ago. In the years since, several studies have focused on examining IL-1β-induced synaptic plasticity impairments in aging animals. Our laboratory, in collaboration with the Patterson laboratory, recently reported that L-LTP induced in area CA1 of hippocampal slices was not significantly different between healthy young and aged F344xBN rats. However, several days (4–5 days) following a peripheral E. coli infection, L-LTP induced by theta-burst stimulation was slightly reduced in young rats, but nearly obliterated in aged animals (Chapman et al., 2010). Theta-burst stimulation is a naturalistic stimulation protocol designed to mimic the burst firing of CA1 pyramidal cells at the theta frequency recorded in vivo from awake behaving animals during spatial exploration. Importantly, basal and short-term synaptic plasticity were not impaired in these animals.
Interestingly, this pattern of electrophysiological data parallels our behavioral findings. We found that healthy aged rats performed as well as did younger rats in both a short-term and long-term test of spatial memory using the Morris water maze. Peripheral infection did not produce a short-term memory deficit in either young or aged rats, indicating that these animals were capable of learning well. In contrast, the long-term memory test revealed a large deficit in aged infected rats compared to their younger counterparts (Barrientos et al., 2006). We found this pattern to hold true for hippocampal-dependent contextual memories as well, using the contextual fear-conditioning paradigm. In addition, we demonstrated that these memory deficits were restricted to memory processes uniquely dependent on the hippocampus, as hippocampal-independent memories were spared (Barrientos et al., 2006). Buchanan and colleagues reached similar conclusions in their report in which repeated mild stress, which was shown to elevate hippocampal IL-1β mRNA in an exaggerated manner in aged mice, produced spatial memory impairments in the water maze in aged, but not young mice (Buchanan et al., 2008).
These memory deficits are consistent with the exaggerated pro-inflammatory response found in the hippocampus of aging animals (Barrientos et al., 2006). And, in addition, the duration of these deficits following infection parallels the duration of IL-1β increases in hippocampus of aged rats (Barrientos et al., 2009). That is, IL-1β protein levels remained elevated 8 days following E. coli challenge, and returned to vehicle-treated control levels at 14 days. Similarly, hippocampus-dependent memory was significantly impaired at both 4 and 8 days but not 14 days following infection.
Putative mechanisms of pro-inflammatory cytokine-induced hippocampal dysfunction
As reviewed above, pro-inflammatory cytokines such as IL-1β, when elevated above basal levels in the hippocampus, impair hippocampal-dependent memory processes. How? Many mechanisms have been implicated in the actions of IL-1β on learning and memory processes, and only some can be reviewed here.
As discussed earlier, elevations in hippocampal IL-1β have been shown to completely block LTP in hippocampus (Bellinger et al., 1993; Coogan and O’Connor, 1997; Coogan et al., 1999; Katsuki et al., 1990). In addition, blocking IL-1β action with centrally administered IL-1receptor antagonist (IL-1RA) prevents the LTP deficit induced in aged rats by peripheral infection (Chapman et al., 2010). The cellular mechanisms by which IL-1β interferes with LTP is not fully understood. Elevations in IL-1β have been demonstrated to increase reactive oxygen species (ROS) formation in the hippocampus (Vereker et al., 2000b), and in turn, these activate members of the mitogen-activated protein (MAP) kinase family, such as c-jun N-terminal kinase (JNK) (Minogue et al., 2003; Vereker et al., 2000b) and p38 (Kelly et al., 2003; Vereker et al., 2000b). While activation of other members of the MAP kinase family, such as extracellular signal-regulated protein kinase (ERK) results in neurite outgrowth, cell proliferation, or differentiation (Seger and Krebs, 1995), activation of JNK and p38 has been shown to induce cell damage or cell death (Maroney et al., 1998). Inhibition of JNK in vivo with D-JNKI1 (Minogue et al., 2003) or in vitro with SP600125 (Curran et al., 2003), blocked IL-1β-induced LTP inhibition in hippocampus. Moreover, in vivo treatment with the p38 inhibitor, SB203580, attenuated pro-inflammatory-induced inhibition of hippocampal LTP (Kelly et al., 2003). Furthermore, inhibiting ROS formation, through an antioxidant diet, reversed IL-1β-induced LTP inhibition as well as IL-1β-induced JNK and p38 activation (Vereker et al., 2000b). Together, these findings confirm that elevated IL-1β plays a prominent role in inhibiting important cellular processes associated with memory formation such as LTP, through activation of these stress-activated protein kinases.
Another possibility is that IL-1β interferes with LTP or memory indirectly by modulating downstream mediators that in turn cause the impairment. Brain-derived neurotrophic factor (BDNF) is a plausible candidate capable of affecting memory processes due to its critical role in late synaptic plasticity processes (Pang and Lu, 2004) and long-term memory (Hall et al., 2000; Ma et al., 1998; Mizuno et al., 2000). Furthermore, BDNF is rapidly and selectively induced in the hippocampus following contextual fear conditioning (Hall et al., 2000), and the systemic administration of either IL-1β or LPS (Lapchak et al., 1993; Richwine et al., 2008) or stress-induced elevations of IL-1β in hippocampus (Nguyen et al., 1998; Pugh et al., 1999) decreases BDNF mRNA in the hippocampus (Barrientos et al., 2003; Lapchak et al., 1993; Richwine et al., 2008) and results in hippocampal-dependent memory consolidation deficits (Barrientos et al., 2004). Moreover, intra-hippocampal administration of IL-1RA prevents both the BDNF mRNA downregulation, and the memory impairments produced by the challenge (Barrientos et al., 2003). These findings have recently been confirmed at the protein level. We found that mature BDNF, but not pro BDNF was markedly reduced in hippocampal synaptoneurosomes prepared from aged animals following infection, and a central administration (intra cistern magna, icm) of IL-1RA prevented this reduction (Cortese et al., 2011). More recently, our laboratory reported that physical exercise in aged rats not only prevented E. coli-induced IL-1β elevations in the hippocampus, but also prevented BDNF reductions in CA1 of the hippocampus and reversed memory deficits caused by the infection (Barrientos et al., 2011b). Taken together, these studies provide strong evidence for the idea that pro-inflammatory cytokine-induced memory impairments may involve the downregulation of BDNF.
The immediate early gene activity-dependent cytoskeletal-associated protein (Arc) is another mediator downstream of IL-1β that has been identified as a key modulator of hippocampal memory consolidation (Bramham et al., 2008). Arc mRNA is rapidly and specifically distributed throughout the dendritic arbor of the hippocampus after neuronal activity (Link et al., 1995; Lyford et al., 1995) and localized to regions receiving direct synaptic activation (Steward et al., 1998). Inhibiting Arc protein expression with antisense oligodeoxynucleotides resulted in impaired long-term memory consolidation and L-LTP (Guzowski et al., 2000). Importantly, short-term memory and E-LTP were not impaired by Arc inhibition. This is noted because this is a pattern we have observed in E. coli-challenged aged rats who exhibit long-lasting neuroinflammatory responses (Barrientos et al., 2006; Chapman et al., 2010). In a separate study, our laboratory reported that a peripheral E. coli challenge in young and aged rats resulted in a profound suppression of hippocampal Arc mRNA expression in only aged rats (Frank et al., 2010a). This suppression correlated with long-term memory deficits, and an elevation in hippocampal IL-6 protein. Furthermore, administration of IL-1RA blocked all of these effects, underscoring the potential role of Arc in neuroinflammatory-induced memory impairments. To further support this notion, IL-1βXAT transgenic mice with sustained hippocampal IL-1β overexpression, exhibited significantly suppressed Arc mRNA expression and this was highly correlated with contextual and spatial memory impairments (Hein et al., 2010). At the protein level, Arc was recently demonstrated to be significantly reduced in aged animals that showed impairments in spatial memory consolidation (Menard and Quirion, 2012). However, neuroinflammatory markers were not measured. To the best of our knowledge, Arc protein expression has not yet been examined in aged animals following an immune challenge.
Summary
Taken together, the literature suggests that the neuroinflammatory response to a peripheral challenge is dysregulated in the aged brain, resulting in a potentiated pro-inflammatory cytokine response, whose source appears to be sensitized microglia. A dysregulated neuroendocrine response in the aged organism is skewed towards higher brain CORT levels, and this may play a critical role in priming microglia to respond in an exaggerated manner following an immune challenge. This exaggerated response appears to be most prominent in the hippocampal formation, the critical brain region mediating contextual and spatial memory consolidation, and may be the cause of hippocampal memory impairments in aged individuals. Pro-inflammatory cytokines such as IL-1β may affect cognitive processes by impairing synaptic plasticity through activation of MAP kinases JNK and p38, and/or by inhibiting downstream mediators essential to hippocampal-dependent memory processes such as BDNF and Arc. Blocking this exaggerated brain cytokine response pharmacologically, or through diet and exercise modifications may effectively block the deleterious behavioral effects, not only suggesting that these may be useful therapeutic interventions, but also supporting the view that pro-inflammatory cytokines have a causal, rather than merely correlational relationship with impaired long-term memory in older individuals.
Highlights.
Healthy aged individuals are vulnerable to memory deficits following immune challenge.
Following challenge, the healthy aged neuroinflammatory response is potentiated.
Sensitized microglia appears to be the source of this amplified response.
Elevated glucocorticoids may play a role in sensitizing microglia.
Potentiated neuroinflammation in the hippocampus leads to memory impairments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akana SF, Strack AM, Hanson ES, Horsley CJ, Milligan ED, Bhatnagar S, Dallman MF. Interactions among chronic cold, corticosterone and puberty on energy intake and deposition. Stress. 1999;3:131–146. doi: 10.3109/10253899909001118. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci U S A. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX(3)CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Brennan JM, Vallance KL. Adsorptive endocytosis of HIV-1gp120 by blood-brain barrier is enhanced by lipopolysaccharide. Exp Neurol. 1999;156:165–171. doi: 10.1006/exnr.1998.7011. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Crysdale NY, Amat J, Hale MW, Stamper CE, Hennessey PA, Frank MG, Watkins LR, Lowry CA, Maier SF. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011a. Characterization of hippocampal and circulating corticosterone levels of healthy aged rats. Online. [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011b;31:11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Bekker AY, Weeks EJ. Cognitive function after anaesthesia in the elderly. Best Pract Res Clin Anaesthesiol. 2003;17:259–272. doi: 10.1016/s1521-6896(03)00005-3. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Madamba S, Siggins GR. Interleukin-1 Beta inhibits synaptic stregnth and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–765. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos R, Alvarez XA, Fernandez-Novoa L, Franco A, Mangues R, Pellicer A, Nishimura T. Brain interleukin-1 beta in Alzheimer’s disease and vascular dementia. Methods Find Exp Clin Pharmacol. 1994;16:141–151. [PubMed] [Google Scholar]
- Cameron A, Henley D, Carrell R, Zhou A, Clarke A, Lightman S. Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J Clin Endocrinol Metab. 2010;95:4689–4695. doi: 10.1210/jc.2010-0942. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Maier SF, Patterson SL. Synaptic correlates of increased cognitive vulnerability with aging: peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. J Neurosci. 2010;30:7598–7603. doi: 10.1523/JNEUROSCI.5172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JK, Shchrader WT, O’Malley BW. Mechanism of steroid hormones. In: Wilson JD, Foster DW, editors. Williams’ textbook of endocrinology. 8. W.B. Saunders Co; Philadelphia: 1992. pp. 35–90. [Google Scholar]
- Coogan A, O’Connor JJ. Inhibition of NMDA receptor-mediated synaptic transmission in the rat dentate gyrus in vitro by IL-1 beta. Neuroreport. 1997;8:2107–2110. doi: 10.1097/00001756-199707070-00004. [DOI] [PubMed] [Google Scholar]
- Coogan AN, O’Connor JJ. Interleukin-1beta inhibits a tetraethylammonium-induced synaptic potentiation in the rat dentate gyrus in vitro. Eur J Pharmacol. 1999;374:197–206. doi: 10.1016/s0014-2999(99)00320-9. [DOI] [PubMed] [Google Scholar]
- Coogan AN, O’Neill LA, O’Connor JJ. The P38 mitogen-activated protein kinase inhibitor SB203580 antagonizes the inhibitory effects of interleukin-1beta on long-term potentiation in the rat dentate gyrus in vitro. Neuroscience. 1999;93:57–69. doi: 10.1016/s0306-4522(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Corona AW, Huang Y, O’Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese GP, Barrientos RM, Maier SF, Patterson SL. Aging and a Peripheral Immune Challenge Interact to Reduce Mature Brain-Derived Neurotrophic Factor and Activation of TrkB, PLC{gamma}1, and ERK in Hippocampal Synaptoneurosomes. J Neurosci. 2011;31:4274–4279. doi: 10.1523/JNEUROSCI.5818-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumiskey D, Curran BP, Herron CE, O’Connor JJ. A role for inflammatory mediators in the IL-18 mediated attenuation of LTP in the rat dentate gyrus. Neuropharmacology. 2007;52:1616–1623. doi: 10.1016/j.neuropharm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O’Neil LAJ, Lynch MA, O’Connor JJ. Interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran BP, Murray HJ, O’Connor JJ. A role for c-Jun N-terminal kinase in the inhibition of long-term potentiation by interleukin-1beta and long-term depression in the rat dentate gyrus in vitro. Neuroscience. 2003;118:347–357. doi: 10.1016/s0306-4522(02)00941-7. [DOI] [PubMed] [Google Scholar]
- Curran BP, O’Connor JJ. The inhibition of long-term potentiation in the rat dentate gyrus by pro-inflammatory cytokines is attenuated in the presence of nicotine. Neurosci Lett. 2003;344:103–106. doi: 10.1016/s0304-3940(03)00440-3. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Cannon JG, Wolff SM. New concepts on the pathogenesis of fever. Rev Infect Dis. 1988;10:168–189. doi: 10.1093/clinids/10.1.168. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley T. Role of the neurohypophysis in the pathogenesis of hypertension and some allied disorders associated with aging. Am J Med. 1949;7:70–84. doi: 10.1016/0002-9343(49)90484-2. [DOI] [PubMed] [Google Scholar]
- Foster TC. Biological markers of age-related memory deficits: treatment of senescent physiology. CNS Drugs. 2006;20:153–166. doi: 10.2165/00023210-200620020-00006. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006a;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2010a;24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol. 2010b;226:181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010c;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006b;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Garrido P, de Blas M, Del Arco A, Segovia G, Mora F. Aging increases basal but not stress-induced levels of corticosterone in the brain of the awake rat. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Goehler LE, Tilders FJH, Bol JGJM, McGorry M, Fleshner M, Maier SF, Watkins LR. Bacterial endotoxin induces fos immunoreactivity in primary afferent neurons of the vagus nerve. Neuroimmunomodulation. 1998;5:234–240. doi: 10.1159/000026343. [DOI] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, JOC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Goujon E, Parnet P, Laye S, Combe C, Kelley KW, Dantzer R. Stress downregulates lipopolysaccharide-induced expression of proinflammatory cytokines in the spleen, pituitary, and brain of mice. Brain Behav Immun. 1995;9:292–303. doi: 10.1006/brbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Lynch MA, Vraniak PD, Wenk GL. Chronic brain inflammation results in cell loss in the entorhinal cortex and impaired LTP in perforant path-granule cell synapses. Exp Neurol. 2002;176:336–341. doi: 10.1006/exnr.2002.7966. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Willard LB, Del Soldato P, Pepeu G, Wenk GL. Peripheral administration of novel anti-inflammatories can attenuate the effects of chronic inflammation within the CNS. Brain Res. 1999;815:36–43. doi: 10.1016/s0006-8993(98)01081-6. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O’Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley DE, Leendertz JA, Russell GM, Wood SA, Taheri S, Woltersdorf WW, Lightman SL. Development of an automated blood sampling system for use in humans. J Med Eng Technol. 2009;33:199–208. doi: 10.1080/03091900802185970. [DOI] [PubMed] [Google Scholar]
- Henley DE, Lightman SL. New insights into corticosteroid-binding globulin and glucocorticoid delivery. Neuroscience. 2011;180:1–8. doi: 10.1016/j.neuroscience.2011.02.053. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Carter RN, Noble J, Chitnis S, Dutia A, Paterson JM, Mullins JJ, Seckl JR, Yau JL. 11beta-hydroxysteroid dehydrogenase type 1 expression is increased in the aged mouse hippocampus and parietal cortex and causes memory impairments. J Neurosci. 2010;30:6916–6920. doi: 10.1523/JNEUROSCI.0731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper MS, Engeland WC. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol. 1991;261:R1257–1268. doi: 10.1152/ajpregu.1991.261.5.R1257. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 B inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278:19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- Kerr D, Cambell L, Appelgate M, Brodish A, Landfield P. Chronic stress-induced acceleration of electrophysiologic and morphometric biomarkers of hippocampal aging. J Neurosci. 1991;11:1316–1322. doi: 10.1523/JNEUROSCI.11-05-01316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Baskin RK, Pitler TA. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981;214:581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Waymire JC, Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978;202:1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Hefti F. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience. 1993;53:297–301. doi: 10.1016/0306-4522(93)90196-m. [DOI] [PubMed] [Google Scholar]
- Laursen P. The impact of aging on cognitive functions. An 11 year follow-up study of four age cohorts. Acta Neurol Scand Suppl. 1997;172:7–86. [PubMed] [Google Scholar]
- Laye S, Goujon E, Combe C, VanHoy R, Kelley KW, Parnet P, Dantzer R. Effects of lipopolysaccharide and glucocorticoids on expression of interleukin-1 beta converting enzyme in the pituitary and brain of mice. J Neuroimmunol. 1996;68:61–66. doi: 10.1016/0165-5728(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, Peskind ER. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J Neurosci. 1999;19:2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19:497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Luterman JD, Haroutunian V, Yemul S, Ho L, Purohit D, Aisen PS, Mohs R, Pasinetti GM. Cytokine gene expression as a function of the clinical progression of Alzheimer disease dementia. Arch Neurol. 2000;57:1153–1160. doi: 10.1001/archneur.57.8.1153. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3 neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–95. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to- brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland T., Jr Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen Comp Endocrinol. 2008;156:210–217. doi: 10.1016/j.ygcen.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Maroney AC, Glicksman MA, Basma AN, Walton KM, Knight E, Jr, Murphy CA, Bartlett BA, Finn JP, Angeles T, Matsuda Y, Neff NT, Dionne CA. Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J Neurosci. 1998;18:104–111. doi: 10.1523/JNEUROSCI.18-01-00104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Weiss J, Schwartz L. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- Meijer OC, Topic B, Steenbergen PJ, Jocham G, Huston JP, Oitzl MS. Correlations between hypothalamus-pituitary-adrenal axis parameters depend on age and learning capacity. Endocrinology. 2005;146:1372–1381. doi: 10.1210/en.2004-0416. [DOI] [PubMed] [Google Scholar]
- Menard C, Quirion R. Successful Cognitive Aging in Rats: A Role for mGluR5 Glutamate Receptors, Homer 1 Proteins and Downstream Signaling Pathways. PLoS One. 2012;7:e28666. doi: 10.1371/journal.pone.0028666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue AM, Schmid AW, Fogarty MP, Moore AC, Campbell VA, Herron CE, Lynch MA. Activation of the c-Jun N-terminal kinase signaling cascade mediates the effect of amyloid-beta on long term potentiation and cell death in hippocampus: a role for interleukin-1beta? J Biol Chem. 2003;278:27971–27980. doi: 10.1074/jbc.M302530200. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Wakins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Rajan V, Edwards CR, Seckl JR. 11 beta-Hydroxysteroid dehydrogenase in cultured hippocampal cells reactivates inert 11-dehydrocorticosterone, potentiating neurotoxicity. J Neurosci. 1996;16:65–70. doi: 10.1523/JNEUROSCI.16-01-00065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remarque EJ, Bollen EL, Weverling-Rijnsburger AW, Laterveer JC, Blauw GJ, Westendorp RG. Patients with Alzheimer’s disease display a pro-inflammatory phenotype. Exp Gerontol. 2001;36:171–176. doi: 10.1016/s0531-5565(00)00176-5. [DOI] [PubMed] [Google Scholar]
- Reul JM, Sutanto W, van Eekelen JA, Rothuizen J, de Kloet ER. Central action of adrenal steroids during stress and adaptation. Adv Exp Med Biol. 1990;274:243–256. doi: 10.1007/978-1-4684-5799-5_15. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology. 2008;33:1369–1377. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging. 1998;19:97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The adrenocortical stress-response in the aged male rat: impairment of recovery from stress. Exp Gerontol. 1983a;18:55–64. doi: 10.1016/0531-5565(83)90051-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. The Journal of Neuroscience. 1985;5:1121–1127. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, McEwen BS, Rainbow TC. Quantitative autoradiography of [3H]corticosterone receptors in rat brain. Brain Res. 1983b;271:331–334. doi: 10.1016/0006-8993(83)90295-0. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Walker BR. Minireview: 11beta-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142:1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. Faseb J. 1995;9:726–735. [PubMed] [Google Scholar]
- Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Solez C. Aging and adrenal cortical hormones. Geriatrics. 1952;7:241–245. contd. [PubMed] [Google Scholar]
- Sousa N, Almeida OF, Holsboer F, Paula-Barbosa MM, Madeira MD. Maintenance of hippocampal cell numbers in young and aged rats submitted to chronic unpredictable stress. Comparison with the effects of corticosterone treatment. Stress. 1998;2:237–249. doi: 10.3109/10253899809167288. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Martin LA, Calvert WS, Boehm GW. Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behav Brain Res. 2005;159:145–151. doi: 10.1016/j.bbr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Swanwick GR, Kirby M, Bruce I, Buggy F, Coen RF, Coakley D, Lawlor BA. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer’s disease: lack of association between longitudinal and cross-sectional findings. Am J Psychiatry. 1998;155:286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- Tafti M, Nishino S, Aldrich MS, Liao W, Dement WC, Mignot E. Major histocompatibility class II molecules in the CNS: increased microglial expression at the onset of narcolepsy in canine model. J Neurosci. 1996;16:4588–4595. doi: 10.1523/JNEUROSCI.16-15-04588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancredi V, D’Antuono M, Cafe C, Giovedi S, Bue MC, D’Arcangelo G, Onofri F, Benfenati F. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- Tsolaki M, Drevelegas A, Karachristianou S, Kapinas K, Divanoglou D, Routsonis K. Correlation of dementia, neuropsychological and MRI findings in multiple sclerosis. Dementia. 1994;5:48–52. doi: 10.1159/000106694. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Gayle D, Ilyin SE, Flynn MC, Langhans W, Schwartz GJ, Plata-Salaman CR. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res Bull. 2001;54:443–453. doi: 10.1016/s0361-9230(01)00445-2. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt FW, Gao S, Baiyewu O, Ogunniyi AO, Gureje O, Perkins A, Emsley CL, Dickens J, Evans R, Musick B, Hall KS, Hui SL, Hendrie HC. Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- Van Dam AM, Bauer J, Tilders FJ, Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1 beta in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65:815–826. doi: 10.1016/0306-4522(94)00549-k. [DOI] [PubMed] [Google Scholar]
- van Eekelen JA, Rots NY, Sutanto W, de Kloet ER. The effect of aging on stress responsiveness and central corticosteroid receptors in the brown Norway rat. Neurobiol Aging. 1992;13:159–170. doi: 10.1016/0197-4580(92)90024-r. [DOI] [PubMed] [Google Scholar]
- Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000a;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- Vereker E, O’Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000b;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent GK, Velkoff VA United States Department of Commerce, C.B. The Next Four Decades: The older population in the United States: 2010 to 2050. Washington, DC: 2010. [Google Scholar]
- VonDras DD, Powless MR, Olson AK, Wheeler D, Snudden AL. Differential effects of everyday stress on the episodic memory test performances of young, mid-life, and older adults. Aging Ment Health. 2005;9:60–70. doi: 10.1080/13607860412331323782. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Exposure to excess glucocorticoids atlers dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1992;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Wexler BC. Correlation of Adrenocortical Histopathology with Arteriosclerosis in Breeder Rats. Acta Endocrinol (Copenh) 1964;46:613–631. doi: 10.1530/acta.0.0460613. [DOI] [PubMed] [Google Scholar]
- Wexler BC, Kittinger GW. Adrenocortical Function in Arteriosclerotic Female Breeder Rats. J Atheroscler Res. 1965;5:317–329. doi: 10.1016/s0368-1319(65)80047-3. [DOI] [PubMed] [Google Scholar]
- Wofford JL, Loehr LR, Schwartz E. Acute cognitive impairment in elderly ED patients: etiologies and outcomes. Am J Emerg Med. 1996;14:649–653. doi: 10.1016/S0735-6757(96)90080-7. [DOI] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Brain Behav Immun. 2010. Protracted downregulation of CX(3)CR1 on microglia of aged mice after lipopolysaccharide challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Morris RG, Seckl JR. Hippocampal corticosteroid receptor mRNA expression and spatial learning in the aged Wistar rat. Brain Res. 1994;657:59–64. doi: 10.1016/0006-8993(94)90953-9. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]