Abstract
Biologically, light including ultraviolet (UV) radiations is vital for life. However, UV exposure does not come without risk, as it is a major factor in the development of skin cancer. Natural protections against UV damage may have been affected by lifestyle changes over the past century, including changes in our sun exposure due to working environments, and the use of sunscreens. In addition, extended ‘day time’ through the use of artificial light may contribute to the disruption of our circadian rhythms; the daily cycles of changes in critical bio-factors including gene expression. Circadian disruption has been implicated in many health conditions, including cardiovascular, metabolic, and psychiatric diseases, as well as many cancers. Interestingly, the pineal hormone melatonin plays a role in both circadian regulation, as well as protection from UV skin damage, and is therefore an important factor to consider when studying the impact of UV light. This review discusses the beneficial and deleterious effects of solar exposure, including UV skin damage, Vitamin D production, circadian rhythm disruption, and the impact of melatonin. Understanding of these benefits and risks is critical for the development of protective strategies against solar radiation.
Introduction
The electromagnetic solar spectrum, which includes visible light and ultraviolet (UV) radiations among other radiations, plays a significant role in a variety of biological functions within a living system. In earth’s natural environment, we are exposed to the solar radiations in a regular 24 hour cycle which varies according to the season. These radiations may have beneficial as well as harmful effects to living organisms. For example, UV radiation has many effects on the environment and the organisms inhabiting the planet. The most beneficial impact of UV light in humans is its essential role in the production of Vitamin D3 in the skin. Calcitriol, the active form of vitamin D3, participates in a variety of the body’s protective functions, including DNA damage repair and immune function. However, excessive exposure to UV radiation can have a variety of adverse effects on the skin, including cancers of the skin. Studies have suggested that solar radiations are important regulators of ‘Circadian Rhythms’, which by definition are physical, mental and behavioral changes that follow an approximately 24 hours cycle that primarily responds to light and dark in an organism’s environment.
Unfortunately, the lifestyle factors of the modern era such as the widespread use of artificial lights to extend our ‘daylight’ time, contribute adversely to the biological processes leading to unwanted conditions and responses. For example, excessive UV exposure to skin can cause skin aging, pre-cancerous skin conditions, and melanoma and non-melanoma skin cancers. The modern research is suggesting that circadian rhythms may be involved in the development and/or progression of cancer because it is believed that approximately 10% of the genes oscillate according to the body’s circadian clock. Their functions are widely varied but are connected with the normal cell cycle, metabolic functions, and DNA damage repair. Normal circadian rhythms are therefore essential for the body’s natural defense against diseases such as cancer. It is believed that a deregulation of oscillatory expression and function of circadian rhythm regulatory genes over the 24 hour period enhances the risk of carcinogenesis. Light and dark cycles influence the circadian clock and the daily oscillations of the genes controlled by the circadian rhythms. Although artificial light can also contribute to the circadian network, the solar light is its major regulator. With increased exposure to artificial light, there is an increase in the probability of disrupting these rhythms, since circadian rhythm gene expression has been shown to be lower in artificial light relative to natural light.
Altered circadian rhythm disrupts the DNA damage responses and cell cycle regulations as well as the expression of the pineal hormone melatonin. Like other circadian factors, the circadian control of melatonin secretion is regulated by the circadian clock machinery which depends on a network of genes and their rhythmic oscillations driven by the circadian timing system located in the suprachiasmatic nucleus (SCN) of the hypothalamus as well as peripheral oscillators located in cells. Dysregulated circadian control of melatonin can contribute to the adverse effects of UV radiation on the skin, as melatonin has been shown to have a protective role against UVB skin damage. Further, melatonin is a strong anti-oxidant and can attenuate UV radiation mediated oxidative stress. Indeed, low levels of melatonin have been associated with increased risk or shown to play a role in the development of several cancers (1–5).
Light Spectrum, UV Radiation and Skin Cancer
The Earth is continuously exposed to a solar electromagnetic spectrum, irradiated by light protons ranging from infrared light at 780–5000nm, to visible light at 400–780nm, and UV light at 200–400nm. Radiation from the UV end of the solar electromagnetic spectrum provides energy that is essential for biological life, but it does not come without risk. Approximately 5% of the radiant energy from the sun is in the UV range, which consists of wavelengths that are shorter than those of the visible spectrum and longer than X-rays. There are notably 3 major subtypes of UV rays, UVA (320–400 nm), UVB (290–320 nm), and UVC (200–290 nm) (6, 7). The energy transfer of UV radiation is invaluable to life on the planet; however, it does not come without risk as this very energy is also responsible for damage to DNA and other unwanted effects (8). UV radiation is a potent mutagen which is currently accepted as being the major cause of human skin cancers (9). Both solar and indoor UVR exposures contribute to carcinogenesis of the skin. The cellular effects of UV radiation are induced primarily through a chain of events that lead to the induction of DNA lesions. The chemical nature and efficiency of the formation of DNA lesions is largely dependent on the type of UV radiation and its wavelength, along with the base composition of the DNA at the lesion site (10, 11). Different wavelengths of UV irradiance display different levels of skin penetration, resulting in a diverse set of effects (12). The absorption spectra of DNA for wavelengths greater than 300 nm increases with the number of guanine-cytosine bases, demonstrating the relationship of the DNA content and the susceptibility to UV absorption (13).
Skin, the first line of defense against environmental toxicants, is continuously exposed to the sunlight and consequently suffers directly from the deleterious effects of UV radiation (14–16). Solar UV radiation is the primary source for the development of cutaneous cancers which affect the Caucasian population more frequently. The estimated incidence rate for these cancers is approximately 1 million new cases diagnosed every year within the United States alone (17). Most skin cancers diagnosed are non-melanoma skin cancers consisting of squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) accounting for ~96% of all skin cancers, while melanoma accounts for the remaining 4% (14, 17). Most skin cancers develop on sun-exposed areas of the skin. The non-melanoma skin cancers are more easily treatable whereas melanoma, the least common form of skin cancer, is often lethal. UV radiation-induced skin cancer involves three distinct stages identified as initiation, promotion, and progression, which are mediated by alterations to cellular, molecular, and biochemical mechanisms. Initiation is the first step in the photocarcinogenesis process, involving genetic alterations which lead to DNA translational changes. Once these mutations occur, they are thought to be irreversible. These DNA mutations lead to alterations in signal transduction pathways, which in turn cause clonal expansion of initiated cells, but the clonal expansion is still considered to be reversible. If the initiated cells go through clonal expansion and the carcinogenesis processes is not halted, tumor progression begins with the malignant transformation of papillomas to carcinomas (18, 19).
It has been suggested that modern lifestyle choices such as increasing outdoor activities and sun-tanning are responsible for increasing cases of skin cancers, which has led to increased use of sunscreens. Outdoor workers get 3–9 times the amount of UV exposure compared to that of the average indoor worker (20–22). Surprisingly, although outdoor workers are much more exposed to solar UV, it is the indoor workers’ incidence of cutaneous malignant melanoma (CMM) that has increased at an exponential rate since before 1940. The lifetime risk assessments demonstrate that those with lower exposures rates have an increased risk for developing CMM (23, 24). It has also been established that outdoor workers have lower incidence rates of CMM (25–27). This does not follow the same patterns as squamous cell carcinoma (SCC) where it is the accumulated UV exposure that determines the risk for development. The difference in incidence numbers between the two groups has brought about speculation regarding the benefits of sunscreens. Most sunscreens block UVB but allow for increased penetration of UVA, leading to a reduction of cutaneous vitamin D levels with a possible inverse correlation to the increase in the incidence of melanoma (28).
Of the UV radiation emitted by the sun, UVC is effectively absorbed in the upper atmosphere preventing it from reaching the earth. On the other hand, UVA and UVB reach the earth and penetrate the skin, causing a variety of adverse effects (29, 30). UVA makes up approximately 95% of the total UV energy that reaches the surface of the Earth, while UVB makes up the remaining 5%. While UVB only makes up about 5% of the UV radiation that makes it to the earth, it is thought to be most responsible for skin cancers. UVB has been shown to have less penetrating power than UVA and mainly acts on the epidermal basal layer of the skin. It is the typical source of sunburns, inflammation, DNA damage, oxidative stress, free radical production, immunosuppression, photoaging and skin cancer (31–35). In addition to these negative effects, UVB also has an important and extremely beneficial function in the production of vitamin D3.
UV Radiation and Vitamin D
Vitamin D3 is generated in humans from photo-initiation by the action and energy supplied by UVB (36). The UVB acts on subcutaneous 7-dehydrocholestral (7-DHC) to convert it into pre-vitamin D3, after which it is thermally converted into 25-hydroxycholecalciferol (25(OH)D3) (calcifediol) (37, 38). Vitamin D3 is then converted primarily in the kidneys and liver into its most hormonally active form, 1α,25-dihydroxvitamin D3 or calcitriol, which has been shown to have an anti-tumor effect in vitro (39, 40). Calcitriol has also been shown to be formed in melanoma cells and keratinocytes where it inhibits the growth of tumors (41, 42). Calcitriol controls or eliminates melanoma cells through signaling pathways for either apoptosis or cell growth inhibition after binding to the vitamin D3 receptor on the nuclear membrane but does not affect normal melanocytes (43–47). Many types of cancers exhibit these effects when exposed to calcitriol such as melanoma, leukemia, prostate, colon, and breast (48–50). Calcitriol may regulate nearly 60 genes (49) through which it can down-regulate proto-oncogenes such as c-myc, c-fos and c-jun, as well as upregulate gene responsible for cell cycle arrest, DNA repair and affect the immune system (49, 51–53). This demonstrates the beneficial effects of UVB, but UVA can only break down vitamin D3 and do so while it is bound to vitamin D binding protein (54). This break down of vitamin D3 can also occur within the circulating plasma as UVA can penetrate to the dermal layer of the skin and reach the capillaries (55). This may be of significant interest when evaluating exposures and protection from UV radiation with indoor workers as the limited amount of vitamin D3 production they have during the workweek and UVA from window exposure is able to break down existing serum bound or newly formed vitamin D3 in the skin. Beyond the breakdown of vitamin D3, solar UVA exposure, while indoors from window penetrance, has further detrimental biological effects. Some of these include oxidative stress (56–59), damage to organelles, red blood cell lyses (57), humoral immune suppression (60) and photoaging. UVA is capable of causing DNA damage and mutations, which has led to SCC development in mice (61–64). This suggests that UVA exposure without UVB exposure will cause mutations and deplete vitamin D3 in the skin. The lack of UV exposure leaves the skin with inadequate amounts of cutaneous vitamin D3, and together these environmental stressors promote CMM (28).
In summary, UVB exposure is necessary for maintaining a healthy lifestyle due to its important role in the synthesis of vitamin D3. However, in excess, it is well known that UVB light causes a number of adverse health effects ranging from sunburn to cancer.
Effect of UV on Circadian Rhythms
Despite the fact that sunlight is a major regulator of circadian rhythms, limited information is available on the effects of UV radiation on circadian rhythms. A study on the effects of UV light on keratinocytes shows that UVB is capable of suppressing several of the genes involved in circadian rhythm regulation for a period of up to 24 hours (65). While this mechanism requires further study, it suggests that light may have more than one route of action in the maintenance of these rhythms.
A very recent study has shown that circadian rhythms may also have an impact on the skin’s ability to cope with UVB damage (66). The xeroderma pigmentosum group A (XPA) protein is critical to the body’s DNA damage repair mechanism, as it is one of several proteins which carry out DNA excision repair. Gaddameedhi and colleagues have shown that XPA oscillates in the skin with circadian rhythmicity, peaking in mice in the early evening, and reaching its lowest point of expression in the early morning. Mice subjected to UVB light in the early morning showed five-fold higher frequency and a faster growth rate of skin cancers than mice subjected to the same level of UVB light in the early evening (66). This shows that normal circadian oscillation is important in the body’s natural defense against UV-induced skin cancer, and should be considered when studying UV exposure and cancer. Based on this study, the authors suggested that the circadian rhythm could be exploited to reduce skin cancer incidence in humans. Since the core circadian clock and their outputs exhibit opposite phases in mice (being nocturnal species) versus humans (being diurnal species), the authors of this study predicted that humans will have a higher rate of repair in the morning and would be less prone to the carcinogenic effect of UV radiations early in the day. The authors further advised for humans to restrict their occupational, therapeutic, recreational, and cosmetic UVR exposure to the morning hours. However, further in-depth studies are needed in this direction.
Regulation of Circadian Rhythm
Since as many as 10% of the genes in the body are believed to have circadian oscillation, circadian rhythms impact a wide variety of physiological functions. This is important when considering the effects of UV light on the body, since the group of genes under circadian control includes those involved in the DNA damage repair response, cell proliferation, cell cycle and cell-cycle arrest, and apoptosis [reviewed in (67)]. Light also plays an important role in regulating the circadian rhythms within the body. Circadian rhythms are entrained by a central pacemaker in the SCN region of the brain when light is sensed by the retina [reviewed in (68)]. In this way, the body is able to adjust its own clock to the light/dark cycles in the environment.
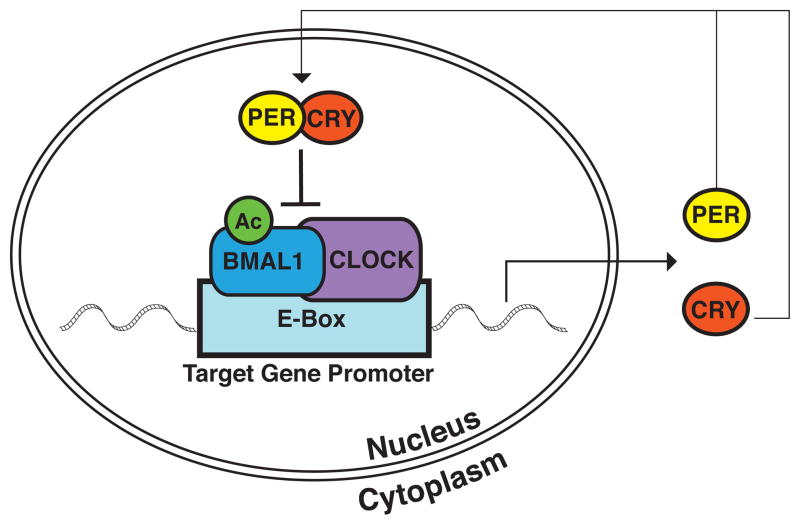
The molecular circuitry behind the oscillation of circadian rhythms is complex. However, at a basic level, several genes/proteins have been recognized as being essential to the function of the core clock mechanism in mammals (Figure 1). These proteins function in a transcriptional-translational feedback loop, the positive limb of which includes the protein CLOCK (69–71) and the aryl-hydrocarbon receptor nuclear translocator-like protein (ARNTL), also known as BMAL1(71). Both CLOCK and BMAL1 are members of the bHLH-PAS-containing family of transcription factors, containing the basic helix-loop-helix domain, which binds DNA, as well as the ligand-binding PAS domain. They begin the circadian cycle when they form a heterodimer through binding at their PAS domains (72). As a heterodimer, they bind to E-box elements in the promoters of their target genes, inducing transcription (71). Several of these target genes then function in the negative limb of the transcriptional-translational feedback loop. They are Cryptochrome 1 and 2, (CRY1 and CRY2) (73), and Period 1, 2, and 3 (PER1, PER2, and PER3) (74). During the positive phase of the circadian cycle, PER1 and PER2 are primarily cytoplasmic proteins, and require binding to the cryptochromes (73) or to PER3 (75) for nuclear translocation. Once in the nucleus, the Period/Cryptochrome complex interferes with the CLOCK/BMAL1 induced transcription (76), modulating expression of the target circadian proteins, thereby completing the cycle.
Figure 1. The Circadian Clock Network.
BMAL1 and CLOCK proteins form a heterodimer in the nucleus followed by binding to the E-Box region in the promoter of their target genes, thereby initiating transcription. Two families of target genes, the Periods (PER) and Cryptochromes (CRY), then act in a negative feedback loop by forming a heterodimer, translocating to the nucleus, and interfering with BMAL1/CLOCK-induced transcription. The acetylation state of BMAL1, which is altered by CLOCK histone acetyltransferase and SIRT1 histone deacetylase activity, plays a large role in the regulation of this system.
It is known that the expression level of BMAL1 is regulated by REV-ERB α and β, and ROR α, β, and γ, with the REV-ERBs repressing BMAL1 transcription and the RORs activating it (77, 78). Thus, the available levels of BMAL1 in the cell provide for a regulatory mechanism for the positive limb of the circadian mechanism. It is also clear that post-translational modifications play an important role in regulating this system. The phosphorylation states of PER1, PER2, and CLOCK are important for their nuclear localization. PER1 contains a nuclear localization signal (NLS) adjacent to a binding site for casein kinase 1 epsilon (CKIε). PER1 export to the cytoplasm is dependent on masking of the NLS through phosphorylation by CKIε.(79) In addition, both PER1 and PER2 have a nuclear export signal (NES) located near their PAS domains, facilitating export to the cytoplasm.(80) CLOCK’s nuclear localization oscillates with circadian rhythmicity and depends on its heterodimerization with BMAL1 and subsequent phosphorylation.(72) A second post-translational modification which plays a role in regulation of the core clock circuitry is the acetylation of BMAL1. In the normal cycle, after the formation of the CLOCK/BMAL1 heterodimer and initiation of the transcription of its target genes, CLOCK acetylates BMAL1 at its Lys537 residue, allowing for recruitment of CRY1 and subsequent repression of BMAL1-CLOCK activity (81). It has been shown that the class III histone deacetylase (HDAC) SIRT1 is responsible for de-acetylation of BMAL1 during the opposite phase of the circadian cycle, resetting the system to its original state in preparation for a new cycle (82).
Circadian Rhythms in the Skin
While the circadian rhythms within the body are thought to be synchronized through the central pacemaker in the SCN, peripheral cells have also been shown to display rhythmic oscillation on their own, both in the body and when isolated in in vitro cell culture systems (74, 83–85). This is especially important when assessing the effects of light, both visible and UV, on skin cells. The circadian genes Clock and Period 1 have been shown to be expressed in various human skin cells, including keratinocytes, melanocytes, and dermal fibroblasts (86). In addition, the core clock genes are transcribed in human skin tissue with normal circadian frequency, with Per1, Cry1, and Bmal1 levels peaking in the early morning, late afternoon, and night, respectively (87). There is some evidence that the normal levels of circadian rhythm gene expression in the skin can be suppressed by UVB radiation, as demonstrated for Per1, Clock, and Bmal1 in human keratinocytes (65). As discussed above, Gaddameedhi et al have shown that the time of UVB light exposure may dictate the tumorigenic potential and frequency of skin tumorigenesis in vivo (66). Since skin is continuously exposed to solar radiation, the regulation of cutaneous circadian rhythm in vivo needs to be carefully studied, as its disruption can lead to a variety of skin health issues.
Dysregulation of Circadian Rhythms and Health Effects
Since light has a direct and substantial impact on the entrainment of circadian rhythms, disturbances in natural light patterns, such as the increased use of artificial light at night, could potentially lead to the dysregulation of circadian rhythms. Even before the circadian molecular mechanism was elucidated, it was shown through serum cortisol and temperature measurements that light applied in irregular patterns could shift, amplify, or greatly suppress circadian oscillations in humans (88, 89). Prior to the discovery of the circadian clock network, the pineal hormone melatonin was also frequently used to study circadian rhythms, as its oscillation in response to light was known relatively early (90). Using melatonin as an output measurement, it was shown that shifting the normal light cycle by 8 hours results in a shift in circadian rhythm patterns as well. This shift also differs in length in albino rats compared to pigmented rats, as does the normal amount of time required to re-entrain the rhythms after disruption (91). This suggests that in addition to light, the degree of skin pigmentation also has significant impact on circadian rhythms. In addition, rats subjected to normal light/dark cycles in natural light expressed a higher amplitude of oscillation in their melatonin expression than rats exposed to artificial light (92), suggesting that natural light is more effective at maintaining normal circadian rhythms than artificial light, even when the light/dark cycle is not altered.
Dysregulated circadian rhythms may contribute to a variety of adverse health effects. Obesity, diabetes, and cardiovascular disease may be influenced by circadian rhythm disruptions. Shifts in eating and sleeping patterns result in increased postprandial glucose, insulin, and mean arterial pressure, as well as decreased leptin levels and inverted cortisol expression patterns (93). In addition, disruption of circadian rhythms through the shifting of normal light patterns results in an accelerated development of diabetes in diabetes-prone HIP rats (94). Epidemiologic studies also show an increased risk of metabolic disease for night shift and rotating shift workers relative to their daytime counterparts (95, 96). Studies have also suggested that many psychiatric disorders, including depression, bipolar disorder, and Alzheimer’s disease are associated with circadian disruption (97–99). However, circadian abnormalities in cardiovascular diseases and psychiatric disorders have not been well studied.
The regulation of circadian clock network in cancer is being actively investigated at present. Dysregulated expression of the core clock proteins has been shown to occur in certain cancers (100–113). The specific clock proteins that are affected have been found to vary with the type of cancer. In cancers where the expression levels of the Periods and Cryptochromes are aberrant, they have been found to be consistently down-regulated compared to normal tissues (100–104, 106, 109–111, 113). Per1 has been shown to be down-regulated in non-small cell lung cancer tissues (NSCLC) (102), colorectal cancer (106), and endometrial carcinomas (113). Lower expression of Per1 and Per2 has been shown in human gliomas (111), hepatocellular carcinomas (104), ovarian cancer (109), breast cancer (101, 110), and prostate cancer (100, 103). In addition, low expression levels of Cry2 have been demonstrated in ovarian cancer (109) and hepatocellular carcinomas (104). Per1 down-regulation could have a significant impact on tumor development, as decreased Per1 levels in breast cancer cells has been shown to result in increased cell growth in vitro and tumor growth in vivo (112). Further, forced expression of Per1 in both NSCLC and prostate cancer cell lines results in significant tumor growth reduction, and lowered survival of the cancer cells (100, 102).
In contrast to the consistently lower levels of components of the negative limb of the circadian clock, the expression levels of BMAL1 and CLOCK seem to be cancer type specific. BMAL1 has been shown to be overexpressed in ovarian and prostate cancers, whereas its expression was found to be suppressed in hematologic malignancies (103, 108, 109). The expression pattern of BMAL1 is found to be inconsistent in colorectal cancer; however, high BMAL1 coupled with low Per1 levels is shown to be associated with liver metastasis (106). CLOCK expression levels appear to be opposite of BMAL1 in at least two of these cancers, as it is down-regulated in both ovarian and prostate cancer, and up-regulated in colorectal cancer (103, 106, 109).
Circadian Rhythms and Melatonin
It is becoming clear that dysregulation of circadian rhythms can have a deleterious impact on health. Therefore, it is important to understand the mechanism of circadian rhythms’ regulation. As previously mentioned, light plays an important role in the regulation of circadian rhythms through its impact on the central pacemaker in the SCN. Interestingly, light may play a secondary role in the maintenance of circadian rhythms through its regulation of the pineal hormone melatonin. Melatonin is a phylogenetically conserved methoxyindole that was identified over five decades ago as the main secretory product of the pineal gland (114–116). Historically, melatonin has been used extensively in the study of circadian day-night rhythms and seasonal biorhythms (117, 118), as it is often considered to be a regulator of these oscillatory patterns. Melatonin is known to oscillate with circadian rhythmicity (90). It is highly expressed at night, with lower levels of expression during the daytime hours (119). As with all circadian proteins, this oscillation is sensitive to light, and any change in the normal light/dark cycle leads to consequent disturbances in the expression of melatonin production (120, 121). This can have a large impact on the expression of all circadian target genes, due to melatonin’s role in the core clock mechanism.
As previously mentioned, the acetylation state of BMAL1 plays a key role in the regulation of the circadian clock. This acetylation state is determined by the histone acetyltransferase (HAT) activity of CLOCK, as well as the histone deacetylase (HDAC) activity of SIRT1 (81, 82). Melatonin has been shown to be an inhibitor of SIRT1 HDAC activity, as well as SIRT1 expression, indicating that it plays a role in the management of circadian rhythms through its effects on SIRT1 (122). Furthermore, it has been shown that the exogenous addition of melatonin to prostate cancer cells can reestablish normal circadian oscillation (103). Thus, melatonin levels are important to consider when developing potential therapies for circadian disruption.
In recent years, melatonin has been characterized as a pleiotropic bio-regulator of numerous functions and in diverse biological systems ranging from single cell to complex organisms, including humans (123–125). It has been shown to play a role in the modulation of defense responses (126, 127), body weight and reproduction (117), tumor growth inhibitory, and anti-jet lag effects (128, 129), as well as acting as a potent antioxidant (130, 131), a chemotoxicity reducing agent (131) and a putative anti-aging substance (132–134). It is these pleiotropic characteristics that have made this endogenous factor of particular interest to researchers. The diverse effects of melatonin on cellular and organismal health have prompted investigations into mechanistic actions of melatonin against a variety of stresses including oxidative stress generated from UV exposure. When examining melatonin’s protective effects, it is important to note that while melatonin was initially identified as a pineal hormone, melatonin synthesis occurs in other tissues besides the pineal gland. The evidence for this is the presence of significant levels of melatonin, higher than that which could be achieved from melatonin levels found in plasma, in bile fluid, bone marrow, cerebrospinal fluid, ovary, eye, lymphocytes, gastral mucosa, and skin (126, 135–145). The existence of these localized melatoninergic systems suggest the importance of melatonin in biological response to specific local stressors (137, 143, 146, 147). This is especially important when studying the protective effects of melatonin against UV-induced skin damage, as the melatoninergic antioxidative system (MAS) has been described as highly differentiated in the skin (137).
Intracutaneous Synthesis of Melatonin and its Protective Role in Skin
The first evidence of melatonin synthesis in the skin was found in the Syrian gold hamster. It was found that the skin of this organism displays activity for arylalkylamine-N-acetyltransferase (AANAT), which is a key enzyme involved in melatonin biosynthesis (148, 149). Further studies demonstrated that mammalian skin has an abundant concentration of the precursor molecules required for melatonin production and a fully functioning melatoninergic system (140–142, 150, 151). Further proof of melatonin production in the skin came from organ-cultured human scalp hair follicles that not only showed melatonin production but could be stimulated to further melatonin production (152). From this, it is reasonable to assume that melatonin production does happen in the skin and is likely to provide a protective role in this organ against multiple environmental and endogenous stressors.
Melatonin has been suggested to protect the skin through several mechanisms, one of which is via its antioxidant effects (147, 153). As an antioxidant, melatonin is a strong scavenger of UV-induced ROS, preventing potential DNA damage that could lead to cancer. Melatonin has been shown to be a stronger ROS scavenger than vitamin C or vitamin E, both of which have been used therapeutically against cytotoxic events (154). Melatonin is a highly lipophilic hormone that easily gains access to the intracellular structures through its ability to cross the cellular membranes. This allows melatonin to protect intracellular structures such as mitochondria and DNA from oxidative damage. It also provides a means by which melatonin can access the DNA and promote the upregulation of other genes responsible for oxidative protection such as Cu/Zn-superoxide dismutase (CuZn-SOD), Mn-superoxide dismutase (Mn-SOD), catalase and glutathione peroxidase (GPx) (155). A second major mechanism by which melatonin protects the skin is its ability to modulate UV-induced apoptosis (147, 153). Melatonin inhibits intrinsic apoptosis pathways by antioxidant protection of mitochondrial membrane potential from UV-related formation of mitochondrial ROS (156). This shows how melatonin is itself protective and also in part responsible for activating other endogenous enzymatic protective systems against oxidative stress (137, 155). Thus, the protective effects of melatonin could potentially lead to greater cell survival in response to UV stress. Several studies have shown that cells treated with melatonin do, in fact, have a higher survival rate. Fischer et al showed that treatment of human keratinocytes with melatonin for 30 minutes prior to UV exposure resulted in higher cell viability than untreated cells (157). Pretreatment of the keratinocytes with melatonin before UV exposure has been shown to downregulate the genes associated with skin photodamage and mitogenic signaling following UV exposure (140, 158). These findings demonstrate the protective effects of melatonin against intraepidermal keratinocyte apoptosis induced by UV-irradiation or sunburns.
Conclusions
Light, melatonin, and circadian rhythms are all intertwined in a complex system (Figure 2). The balance required for proper maintenance of this system depends on the appropriate amount of exposure to light, and can have a significant impact on the normal cellular functioning. Disruption of our light – dark cycles, such as created by wide use of artificial lights, can dramatically alter the balance in circadian rhythms. The extension of our exposure to light can result in aberrant expression patterns of essential genes that are controlled by circadian rhythms, such as melatonin. This further exacerbates the irregularities, as melatonin itself feeds back into the circadian system through its role in the inhibition of the histone deacetylase, SIRT1. Melatonin also has a role in the skin as a protective agent against damaging UV light, and changes in its expression levels can therefore contribute to the development of skin cancer.
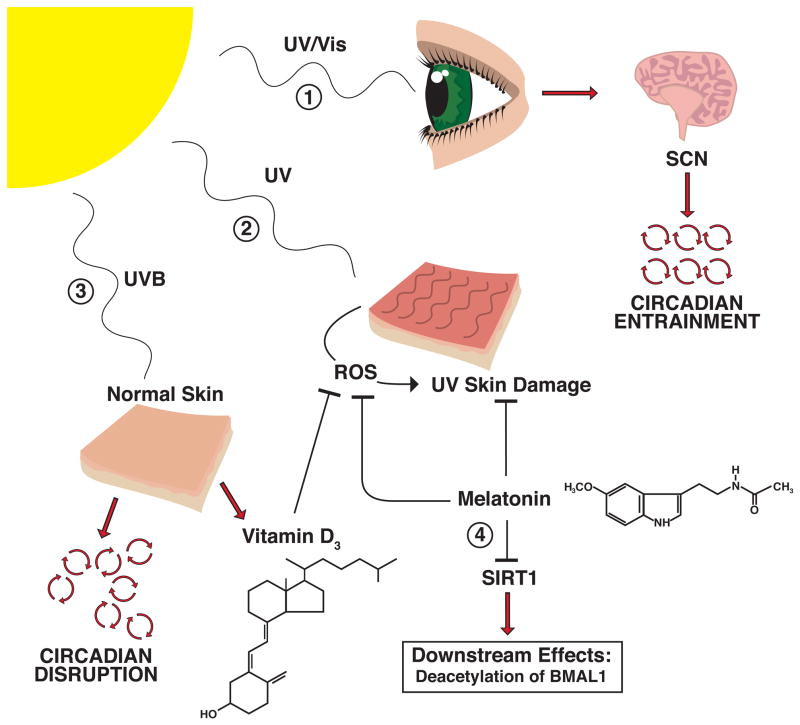
Figure 2. Light, Circadian Rhythms, and Melatonin.
1) UV or visible light from the sun is sensed by the retina, which signals the suprachiasmatic nucleus (SCN) region of the brain, enabling entrainment of circadian rhythms throughout the body. 2) UV light induces skin damage. Both Melatonin and Vitamin D3 have protective effects against this damage, through the inhibition of reactive oxygen species (ROS) formation as well as other mechanisms. 3) UVB light exposure can cause disruption of the circadian rhythms in normal skin, but also has the protective effect of producing Vitamin D3. 4) In addition to its protective effects against UVB skin damage, melatonin also plays a role in the regulation of circadian rhythms, through its inhibition of the HDAC SIRT1.
In addition to its damaging role in contributing to skin cancer, UV exposure also has positive and protective effects, necessitating a balance in the level of UV exposure required to maintain optimal health. Although UV exposure is detrimental to skin health and key to the carcinogenesis process, it does not explain all skin cancers, including the increase in melanoma incidences of indoor workers. There has been speculation to tie this in with vitamin D production and/or the breakdown of vitamin D stores within the skin which is more common in indoor workers. With all the lifestyle changes we have seen over the past century, there has also been an increase in the incidences of many cancers. Many variables could contribute to the causes of these increases. However, the role that changes in environmental stressors play cannot be ignored. Further understanding of circadian regulations is needed to develop novel strategies towards circadian related conditions and diseases which encompass a wide range from behavioral conditions to cancers.
Acknowledgments
This work was partly supported by funding from the NIH/NIAMS (T32AR055893 to Josh Desotelle and R01AR059130 to Nihal Ahmad) and the Department of Veterans Affairs (Merit Review funding to Nihal Ahmad). The authors would like to thank Mr. Corrie Busch for his help in the development of the figures for this paper.
Biographies
Joshua Desotelle received his Ph.D. degree from the University of Wisconsin – Madison in 2011. He is currently a postdoctoral fellow in a T32 Training Grant Supported by the NIH/NIAMS under the mentorship of Prof. Nihal Ahmad. His current research interest includes epigenetic regulation of genes involved in carcinogenesis. He is also interested in the regulation of oncogenes and tumor suppressing genes by microRNAs.
>Photo Dr Desotelle<
Melissa Wilking received her B.S. degree in biochemistry from the University of Wisconsin-Madison in 2003. She is currently pursuing a PhD in Cellular and Molecular Pathology at the University of Wisconsin-Madison under the mentorship of Dr. Nihal Ahmad. Her research is focused on the roles of the histone deacetylase SIRT1 and circadian rhythm disruption in the development and progression of melanoma.
>Photo Ms Wilking<
Nihal Ahmad received his PhD from University of Lucknow in Lucknow, India. Following his postdoctoral training at the Case Western Reserve University (CWRU) in Cleveland, Ohio, he joined the Department of Dermatology at the CWRU as an Assistant Professor in 2000. In 2002, he moved to the University of Wisconsin at Madison as an Assistant Professor where he is currently Professor with Tenure. The research in his laboratory is focused on three major lines of investigation; i) mechanism of cancer development and identification of molecular targets for intervention, ii) mechanism of cutaneous UV responses including photocarcinogenesis, and iii) chemoprevention and experimental therapeutics of cancer.
>Photo Dr Ahmad<
Footnotes
This paper is part of the Special Issue in Commemoration of the 70th birthday of Dr. David R. Bickers
References
- 1.Schernhammer ES, Hankinson SE. Urinary Melatonin Levels and Breast Cancer Risk. J Natl Cancer Inst. 2005;97:1084–1087. doi: 10.1093/jnci/dji190. [DOI] [PubMed] [Google Scholar]
- 2.Schernhammer ES, Berrino F, Krogh V, Secreto G, Micheli A, Venturelli E, Sieri S, Sempos CT, Cavalleri A, Schünemann HJ, Strano S, Muti P. Urinary 6-Sulfatoxymelatonin Levels and Risk of Breast Cancer in Postmenopausal Women. J Natl Cancer Inst. 2008;100:898–905. doi: 10.1093/jnci/djn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schernhammer ES, Hankinson SE. Urinary Melatonin Levels and Postmenopausal Breast Cancer Risk in the Nurses’ Health Study Cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:74–79. doi: 10.1158/1055-9965.EPI-08-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartsch C, Bartsch H, Fluchter SH, Attanasio A, Gupta D. Evidence for modulation of melatonin secretion in men with benign and malignant tumors of the prostate: relationship with the pituitary hormones. J Pineal Res. 1985;2:121–132. doi: 10.1111/j.1600-079x.1985.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaccoli G, Carughi S, De Cata A, La Viola M, Vendemiale G. Melatonin and cortisol serum levels in lung cancer patients at different stages of disease. Med Sci Monit. 2005;11:CR284–288. [PubMed] [Google Scholar]
- 6.Timares L, Katiyar SK, Elmets CA. DNA Damage, Apoptosis and Langerhans Cells—Activators of UV-induced Immune Tolerance. Photochem Photobiol. 2008;84:422–436. doi: 10.1111/j.1751-1097.2007.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diffey BL. Sources and measurement of ultraviolet radiation. Methods. 2002;28:4–13. doi: 10.1016/s1046-2023(02)00204-9. [DOI] [PubMed] [Google Scholar]
- 8.Schuch AP, Galhardo RD, de Lima-Bessa KM, Schuch NJ, Menck CFM. Development of a DNA-dosimeter system for monitoring the effects of solar-ultraviolet radiation. Photochem Photobiol Sci. 2009;8:111–120. doi: 10.1039/b810085c. [DOI] [PubMed] [Google Scholar]
- 9.Leffell DJ, Brash DE. Sunlight and skin cancer. Sci Am. 1996;275:52–53. 56–59. doi: 10.1038/scientificamerican0796-52. [DOI] [PubMed] [Google Scholar]
- 10.Runger TM. How different wavelengths of the ultraviolet spectrum contribute to skin carcinogenesis: The role of cellular damage responses. J Invest Dermatol. 2007;127:2103–2105. doi: 10.1038/sj.jid.5700988. [DOI] [PubMed] [Google Scholar]
- 11.Yagura T, Makita K, Yamamoto H, Menck CFM, Schuch AP. Biological Sensors for Solar Ultraviolet Radiation. Sensors. 2011;11:4277–4294. doi: 10.3390/s110404277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proc Natl Acad Sci U S A. 2004;101:4954–4959. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutherland JC, Griffin KP. Absorption spectrum of DNA for wavelengths greater than 300 nm. Radiat Res. 1981;86:399–410. [PubMed] [Google Scholar]
- 14.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 15.Afaq F. Natural agents: Cellular and molecular mechanisms of photoprotection. Arch Biochem Biophys. 2011;508:144–151. doi: 10.1016/j.abb.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Xu JQ, Ward E. Cancer Statistics, 2010. CA-Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 18.Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119–1136. doi: 10.1111/j.1751-1097.1990.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 19.Melnikova VO, Ananthaswamy HN. Cellular and molecular events leading to the development of skin cancer. Mutat Res-Fundam Mol Mech Mutagen. 2005;571:91–106. doi: 10.1016/j.mrfmmm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Thieden E, Philipsen PA, Heydenreich J, Wulf HC. UV radiation exposure related to age, sex, occupation, and sun Behavior based on timestamped personal dosimeter readings. Arch Dermatol. 2004;140:197–203. doi: 10.1001/archderm.140.2.197. [DOI] [PubMed] [Google Scholar]
- 21.Godar DE. UV doses worldwide. Photochem Photobiol. 2005;81:736–749. doi: 10.1562/2004-09-07-ir-308r.1. [DOI] [PubMed] [Google Scholar]
- 22.Thieden E, Philipsen PA, Sandby-Moller J, Wulf HC. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967–973. doi: 10.1001/archderm.141.8.967. [DOI] [PubMed] [Google Scholar]
- 23.Rigel DS, Friedman RJ, Kopf AW. The incidence of malignant melanoma in the United States: Issues as we approach the 21st century. J Am Acad Dermatol. 1996;34:839–847. doi: 10.1016/s0190-9622(96)90041-9. [DOI] [PubMed] [Google Scholar]
- 24.Rigel DS, Carucci JA. Malignant melanoma: Prevention, early detection, and treatment in the 21st Century. CA-Cancer J Clin. 2000;50:215–236. doi: 10.3322/canjclin.50.4.215. [DOI] [PubMed] [Google Scholar]
- 25.Lee JAH. Melanoma and exposure to sunlight. Epidemiol Rev. 1982;4:110–136. doi: 10.1093/oxfordjournals.epirev.a036243. [DOI] [PubMed] [Google Scholar]
- 26.Vagero D, Ringback G, Kiviranta H. Melanoma and other tumors of the skin among office, other indoor and outdoor workers in Sweden 1961–1979. Br J Cancer. 1986;53:507–512. doi: 10.1038/bjc.1986.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy C, Bajdik CD, Willemze R, de Gruijl FR, Bavinck JNB S Leiden Skin Canc. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003;120:1087–1093. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- 28.Godar DE, Landry RJ, Lucas AD. Increased UVA exposures and decreased cutaneous Vitamin D(3) levels may be responsible for the increasing incidence of melanoma. Med Hypotheses. 2009;72:434–443. doi: 10.1016/j.mehy.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 29.Bachelor MA, Bowden GT. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin Cancer Biol. 2004;14:131–138. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Afaq F, Mukhtar H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp Dermatol. 2006;15:678–684. doi: 10.1111/j.1600-0625.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 31.Halliday GM, Lyons JG. Inflammatory doses of UV may not be necessary for skin carcinogenesis. Photochem Photobiol. 2008;84:272–283. doi: 10.1111/j.1751-1097.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- 32.Afaq F, V, Adhami M, Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat Res-Fundam Mol Mech Mutagen. 2005;571:153–173. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 34.Timares L, Katiyar SK, Elmets CA. DNA damage, apoptosis and langerhans cells - Activators of UV-induced immune tolerance. Photochem Photobiol. 2008;84:422–436. doi: 10.1111/j.1751-1097.2007.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katiyar SK, Bergamo BM, Vyalil PK, Elmets CA. Green tea polyphenols: DNA photodamage and photoimmunology. J Photochem Photobiol B-Biol. 2001;65:109–114. doi: 10.1016/s1011-1344(01)00248-2. [DOI] [PubMed] [Google Scholar]
- 36.Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 37.Holick MF. Skin: site of the synthesis of vitamin D and a target tissue for the active form, 1,25-dihydroxyvitamin D3. Ann N Y Acad Sci. 1988;548:14–26. doi: 10.1111/j.1749-6632.1988.tb18789.x. [DOI] [PubMed] [Google Scholar]
- 38.Maclaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–1003. doi: 10.1126/science.6281884. [DOI] [PubMed] [Google Scholar]
- 39.McGuire TF, Trump DL, Johnson CS. Vitamin D-3-induced apoptosis of murine squamous cell carcinoma cells - Selective induction of caspase-dependent MEK cleavage and up-regulation of MEKK-1. J Biol Chem. 2001;276:26365–26373. doi: 10.1074/jbc.M010101200. [DOI] [PubMed] [Google Scholar]
- 40.Danielsson C, Fehsel K, Polly P, Carlberg C. Differential apoptotic response of human melanoma cells to 1 alpha,25-dihydroxyvitamin D-3 and its analogues. Cell Death Differ. 1998;5:946–952. doi: 10.1038/sj.cdd.4400437. [DOI] [PubMed] [Google Scholar]
- 41.Chida K, Hashiba H, Fukushima M, Suda T, Kuroki T. Inhibition of tumor promotion in mouse skin by 1 alpha,25-dihydroxyvitamin D3. Cancer Res. 1985;45:5426–5430. [PubMed] [Google Scholar]
- 42.Eisman JA, Barkla DH, Tutton PJM. Suppression of in vivo growth of human cancer solid tumor xenografts by 1,25-dihydroxyvitamin D3. Cancer Res. 1987;47:21–25. [PubMed] [Google Scholar]
- 43.Lehmann B, Pietzsch J, Kampf A, Meurer M. Human keratinocyte line HaCaT metabolizes 1 alpha-hydroxyvitamin D-3 and vitamin D-3 to 1 alpha,25-dihydroxyvitamin D-3 (calcitriol) J Dermatol Sci. 1998;18:118–127. doi: 10.1016/s0923-1811(98)00033-4. [DOI] [PubMed] [Google Scholar]
- 44.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–1086. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- 45.Frampton RJ, Omond SA, Eisman JA. Inhibition of human cancer cell growth by 1,25-dihydroxyvitamin D3 metabolites. Cancer Res. 1983;43:4443–4447. [PubMed] [Google Scholar]
- 46.Evans SRT, Houghton AM, Schumaker L, Brenner RV, Buras RR, Davoodi F, Nauta RJ, Shabahang M. Vitamin D receptor and growth inhibition by 1,25-dihydroxyvitamin D-3 in human malignant melanoma cell lines. J Surg Res. 1996;61:127–133. doi: 10.1006/jsre.1996.0092. [DOI] [PubMed] [Google Scholar]
- 47.Sauer B, Ruwisch L, Kleuser B. Antiapoptotic action of 1 alpha,25-dihydroxyvitamin D-3 in primary human melanocytes. Melanoma Res. 2003;13:339–347. doi: 10.1097/00008390-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Minghetti PP, Norman AW. 1,25(OH)2-vitamin D3 receptors: gene regulation and genetic circuitry. Faseb J. 1988;2:3043–3053. doi: 10.1096/fasebj.2.15.2847948. [DOI] [PubMed] [Google Scholar]
- 49.Studzinski GP, Moore DC. Sunlight—Can It Prevent as well as Cause Cancer? Cancer Res. 1995;55:4014–4022. [PubMed] [Google Scholar]
- 50.van der Rhee H, Coebergh JW, de Vries E. Sunlight, vitamin D and the prevention of cancer: a systematic review of epidemiological studies. Eur J Cancer Prev. 2009;18:458–475. doi: 10.1097/CEJ.0b013e32832f9bb1. [DOI] [PubMed] [Google Scholar]
- 51.May E, Asadullah K, Zugel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377–393. doi: 10.2174/1568010042634596. [DOI] [PubMed] [Google Scholar]
- 52.Wang QM, Jones JB, Studzinski GP. Cyclin-dependent kinase inhibitor p27 as a mediator of the G(1)-S phase block induced by 1,25-dihydroxyvitamin D-3 in HL60 cells. Cancer Res. 1996;56:264–267. [PubMed] [Google Scholar]
- 53.Wong G, Gupta R, Dixon KM, Deo SS, Choong SM, Halliday GM, Bishop JE, Ishizuka S, Norman AW, Posner GH, Mason RS. 1,25-dihydroxyvitamin D and three low-calcemic analogs decrease UV-induced DNA damage via the rapid response pathway. J Steroid Biochem Mol Biol. 2004;89–90:567–570. doi: 10.1016/j.jsbmb.2004.03.072. [DOI] [PubMed] [Google Scholar]
- 54.Webb AR, Decosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. 1989;68:882–887. doi: 10.1210/jcem-68-5-882. [DOI] [PubMed] [Google Scholar]
- 55.Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol. 1981;77:13–19. doi: 10.1111/1523-1747.ep12479191. [DOI] [PubMed] [Google Scholar]
- 56.Tyrrell RM, Keyse SM. New trends in photobiology. The interaction of UVA radiation with cultured cells. J Photochem Photobiol B-Biol. 1990;4:349–361. doi: 10.1016/1011-1344(90)85014-n. [DOI] [PubMed] [Google Scholar]
- 57.Godar DE, Thomas DP, Miller SA, Lee W. Long-wavelength UVA radiation induces oxidative stress, cytoskeletal damage and hemolysis. Photochem Photobiol. 1993;57:1018–1026. doi: 10.1111/j.1751-1097.1993.tb02965.x. [DOI] [PubMed] [Google Scholar]
- 58.Godar DE. Light and death: Photons and apoptosis. J Invest Dermatol Symp Proc. 1999;4:17–23. doi: 10.1038/sj.jidsp.5640175. [DOI] [PubMed] [Google Scholar]
- 59.Godar DE. Singlet oxygen-triggered immediate preprogrammed apoptosis. Methods Enzymol. 2000;319:309–330. doi: 10.1016/s0076-6879(00)19032-9. [DOI] [PubMed] [Google Scholar]
- 60.Matthews YJ, Halliday GM, Phan TA, Damian DL. Wavelength dependency for UVA-induced suppression of recall immunity in humans. J Dermatol Sci. 2010;59:192–197. doi: 10.1016/j.jdermsci.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Peak JG, Peak MJ. Comparison of initial yields of DNA-to-protein crosslinks and single-strand breaks induced in cultured human cells by far- and near-ultraviolet light, blue light and X-rays. Mutat Res. 1991;246:187–191. doi: 10.1016/0027-5107(91)90121-4. [DOI] [PubMed] [Google Scholar]
- 62.Jones CA, Huberman E, Cunningham ML, Peak MJ. Mutagenesis and cytotoxicity in human epithelial cells by far- and near-ultraviolet radiations: action spectra. Radiat Res. 1987;110:244–254. [PubMed] [Google Scholar]
- 63.Halliday GM, Agar NS, Barnetson RSC, Ananthaswamy HN, Jones AM. UV-A fingerprint mutations in human skin cancer. Photochem Photobiol. 2005;81:3–8. doi: 10.1562/2004-07-27-IR-247. [DOI] [PubMed] [Google Scholar]
- 64.Sterenborg H, Vanderleun JC. Tumorigenesis by a long wavelength UV-A source. Photochem Photobiol. 1990;51:325–330. doi: 10.1111/j.1751-1097.1990.tb01718.x. [DOI] [PubMed] [Google Scholar]
- 65.Kawara S, Mydlarski R, Mamelak AJ, Freed I, Wang B, Watanabe H, Shivji G, Tavadia SK, Suzuki H, Bjarnason GA, Jordan RCK, Sauder DN. Low-dose Ultraviolet B Rays Alter the mRNA Expression of the Circadian Clock Genes in Cultured Human Keratinocytes. J Invest Dermatol. 2002;119:1220–1223. doi: 10.1046/j.1523-1747.2002.19619.x. [DOI] [PubMed] [Google Scholar]
- 66.Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci U S A. 2011;108:18790–18795. doi: 10.1073/pnas.1115249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sancar A, Lindsey-Boltz LA, Kang TH, Reardon JT, Lee JH, Ozturk N. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 2010;584:2618–2625. doi: 10.1016/j.febslet.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 69.Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional Identification of the Mouse Circadian Clock Gene by Transgenic BAC Rescue. Cell. 1997;89:655–667. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TDL, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional Cloning of the Mouse Circadian Clock Gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK Protein in the Mammalian Circadian Mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 72.Kondratov RV, Chernov MV, Kondratova AA, Gorbacheva VY, Gudkov AV, Antoch MP. BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 2003;17:1921–1932. doi: 10.1101/gad.1099503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 Are Essential Components of the Negative Limb of the Circadian Clock Feedback Loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 74.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period Homologs in Mammals: Differential Light Responses in the Suprachiasmatic Circadian Clock and Oscillating Transcripts Outside of Brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 75.Yagita K, Yamaguchi S, Tamanini F, van der Horst GTJ, Hoeijmakers JHJ, Yasui A, Loros JJ, Dunlap JC, Okamura H. Dimerization and nuclear entry of mPER proteins in mammalian cells. Genes Dev. 2000;14:1353–1363. [PMC free article] [PubMed] [Google Scholar]
- 76.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der GTJ, Horst, Hastings MH, Reppert SM. Interacting Molecular Loops in the Mammalian Circadian Clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 77.Guillaumond F, Dardente H, Giguère V, Cermakian N. Differential Control of Bmal1 Circadian Transcription by REV-ERB and ROR Nuclear Receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 78.Preitner N, Damiola F, Luis Lopez M, Zakany J, Duboule D, Albrecht U, Schibler U. The Orphan Nuclear Receptor REV-ERBα Controls Circadian Transcription within the Positive Limb of the Mammalian Circadian Oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 79.Vielhaber E, Eide E, Rivers A, Gao ZH, Virshup DM. Nuclear Entry of the Circadian Regulator mPER1 Is Controlled by Mammalian Casein Kinase I varepsilon. Mol Cell Biol. 2000;20:4888–4899. doi: 10.1128/mcb.20.13.4888-4899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vielhaber EL, Duricka D, Ullman KS, Virshup DM. Nuclear Export of Mammalian PERIOD Proteins. J Biol Chem. 2001;276:45921–45927. doi: 10.1074/jbc.M107726200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- 82.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 84.Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence Imaging of Individual Fibroblasts Reveals Persistent, Independently Phased Circadian Rhythms of Clock Gene Expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zanello SB, Jackson DM, Holick MF. Expression of the circadian clock genes clock and period1 in human skin. J Invest Dermatol. 2000;115:757–760. doi: 10.1046/j.1523-1747.2000.00121.x. [DOI] [PubMed] [Google Scholar]
- 87.Bjarnason GA, Jordan RC, Wood PA, Li Q, Lincoln DW, Sothern RB, Hrushesky WJ, Ben-David Y. Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am J Pathol. 2001;158:1793–1801. doi: 10.1016/S0002-9440(10)64135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Czeisler C, Kronauer R, Allan J, Duffy J, Jewett M, Brown E, Ronda J. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 89.Jewett ME, Kronauer RE, Czeisler CA. Light-induced suppression of endogenous circadian amplitude in humans. Nature. 1991;350:59–62. doi: 10.1038/350059a0. [DOI] [PubMed] [Google Scholar]
- 90.Quay WB. Circadian and Estrous Rhythms in Pineal Melatonin and 5-Hydroxy Indole-3-Acetic Acid. Proc Soc Exp Biol Med. 1964;115:710–713. doi: 10.3181/00379727-115-29014. [DOI] [PubMed] [Google Scholar]
- 91.Kennaway DJ. Effect of a phase advance of the light/dark cycle on pineal function and circadian running activity in individual rats. Brain Res Bull. 1994;33:639–644. doi: 10.1016/0361-9230(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 92.Laakso ML, Porkka-Heiskanen T, Alila A, Peder M, Johansson G. Twenty-four-hour patterns of pineal melatonin and pituitary and plasma prolactin in male rats under ‘natural’ and artificial lighting conditions. Neuroendocrinology. 1988;48:308–313. doi: 10.1159/000125027. [DOI] [PubMed] [Google Scholar]
- 93.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Nat Acad Sci. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of Circadian Rhythms Accelerates Development of Diabetes through Pancreatic Beta-Cell Loss and Dysfunction. J Biol Rhythms. 2011;26:423–433. doi: 10.1177/0748730411416341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38:848–854. doi: 10.1093/ije/dyn360. [DOI] [PubMed] [Google Scholar]
- 96.Pietroiusti A, Neri A, Somma G, Coppeta L, Iavicoli I, Bergamaschi A, Magrini A. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med. 2010;67:54–57. doi: 10.1136/oem.2009.046797. [DOI] [PubMed] [Google Scholar]
- 97.Weldemichael DA, Grossberg GT. Circadian rhythm disturbances in patients with Alzheimer’s disease: a review. Int J Alzheimers Dis. 2010;2010:716453. doi: 10.4061/2010/716453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Westrich L, Sprouse J. Circadian rhythm dysregulation in bipolar disorder. Curr Opin Investig Drugs. 2010;11:779–87. [PubMed] [Google Scholar]
- 99.Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 100.Cao Q, Gery S, Dashti A, Yin D, Zhou Y, Gu J, Koeffler HP. A Role for the Clock Gene Per1 in Prostate Cancer. Cancer Res. 2009;69:7619–7625. doi: 10.1158/0008-5472.CAN-08-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen ST, Choo KB, Hou MF, Yeh KT, Kuo SJ, Chang JG. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis. 2005;26:1241–1246. doi: 10.1093/carcin/bgi075. [DOI] [PubMed] [Google Scholar]
- 102.Gery S, Komatsu N, Kawamata N, Miller CW, Desmond J, Virk RK, Marchevsky A, Mckenna R, Taguchi H, Koeffler HP. Epigenetic Silencing of the Candidate Tumor Suppressor Gene Per1 in Non–Small Cell Lung Cancer. Clin Cancer Res. 2007;13:1399–1404. doi: 10.1158/1078-0432.CCR-06-1730. [DOI] [PubMed] [Google Scholar]
- 103.Jung-Hynes B, Huang W, Reiter RJ, Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J Pineal Res. 2010;49:60–68. doi: 10.1111/j.1600-079X.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin YM, Chang JH, Yeh KT, Yang MY, Liu TC, Lin SF, Su WW, Chang JG. Disturbance of circadian gene expression in hepatocellular carcinoma. Mol Carcinog. 2008;47:925–933. doi: 10.1002/mc.20446. [DOI] [PubMed] [Google Scholar]
- 105.Mullenders J, Fabius AWM, Madiredjo M, Bernards R, Beijersbergen RL. A Large Scale shRNA Barcode Screen Identifies the Circadian Clock Component ARNTL as Putative Regulator of the p53 Tumor Suppressor Pathway. PLoS ONE. 2009;4:e4798. doi: 10.1371/journal.pone.0004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oshima T, Takenoshita S, Akaike M, Kunisaki C, Fujii S, Nozaki A, Numata K, Shiozawa M, Rino Y, Tanaka K, Masuda M, Imada T. Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer. Oncol Rep. 2011;25:1439–1446. doi: 10.3892/or.2011.1207. [DOI] [PubMed] [Google Scholar]
- 107.Shih HC, Choo KB, Chang TJ, Yang MY, Shih MC, Yeh KT, Liu TC, Lin SF, Chang JG. Disturbance of circadian gene expression in endometrial cancer: detection by real-time quantitative RT-PCR. Oncol Rep. 2005;14:1533–1538. [PubMed] [Google Scholar]
- 108.Taniguchi H, Fernández AF, Setién F, Ropero S, Ballestar E, Villanueva A, Yamamoto H, Imai K, Shinomura Y, Esteller M. Epigenetic Inactivation of the Circadian Clock Gene BMAL1 in Hematologic Malignancies. Cancer Res. 2009;69:8447–8454. doi: 10.1158/0008-5472.CAN-09-0551. [DOI] [PubMed] [Google Scholar]
- 109.Tokunaga H, Takebayashi Y, Utsunomiya H, akahira JI, higashimoto M, mashiko M, Ito K, Niikura H, Takenoshita SI, Yaegashi N. Clinicopathological significance of circadian rhythm-related gene expression levels in patients with epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2008;87:1060–1070. doi: 10.1080/00016340802348286. [DOI] [PubMed] [Google Scholar]
- 110.Winter SL, Bosnoyan-Collins L, Pinnaduwage D, Andrulis IL. Expression of the circadian clock genes Per1 and Per2 in sporadic and familial breast tumors. Neoplasia. 2007;9:797–800. doi: 10.1593/neo.07595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xia HC, Niu ZF, Ma H, Cao SZ, Hao SC, Liu ZT, Wang F. Deregulated expression of the Per1 and Per2 in human gliomas. Can J Neurol Sci. 2010;37:365–370. doi: 10.1017/s031716710001026x. [DOI] [PubMed] [Google Scholar]
- 112.Yang X, Wood PA, Ansell CM, Quiton DFT, Oh EY, Du-Quiton J, Hrushesky WJM. The circadian clock gene Per1 suppresses cancer cell proliferation and tumor growth at specific times of day. Chronobiol Int. 2009;26:1323–1339. doi: 10.3109/07420520903431301. [DOI] [PubMed] [Google Scholar]
- 113.Yeh KT, Yang MY, Liu TC, Chen JC, Chan WL, Lin SF, Chang JG. Abnormal expression of period 1 (PER1) in endometrial carcinoma. J Pathol. 2005;206:111–120. doi: 10.1002/path.1756. [DOI] [PubMed] [Google Scholar]
- 114.Lerner AB, Case JD, Heinzelman RV. Structure of melatonin. J Am Chem Soc. 1959;81:6084–6085. [Google Scholar]
- 115.Lerner AB, Case JD, Mori W, Wright MR. Melatonin in Peripheral Nerve. Nature. 1959;183:1821–1821. doi: 10.1038/1831821a0. [DOI] [PubMed] [Google Scholar]
- 116.Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc. 1958;80:2587–2587. [Google Scholar]
- 117.Lerchl A, Schlatt S. Influence of photoperiod on pineal melatonin synthesis, fur color, body weight, and reproductive function in the female Djungarian hamster, Phodopus sungorus. Neuroendocrinology. 1993;57:359–364. doi: 10.1159/000126380. [DOI] [PubMed] [Google Scholar]
- 118.Bubenik GA, Smith PS. Circadian and circannual rhythms of melatonin in plasma of male white-tailed deer and the effect of oral administration of melatonin. J Exp Zool. 1987;241:81–89. doi: 10.1002/jez.1402410110. [DOI] [PubMed] [Google Scholar]
- 119.Hofman MA, Skene DJ, Swaab DF. Effect of photoperiod on the diurnal melatonin and 5-methoxytryptophol rhythms in the human pineal gland. Brain Res. 1995;671:254–260. doi: 10.1016/0006-8993(94)01339-j. [DOI] [PubMed] [Google Scholar]
- 120.Lewy A, Wehr T, Goodwin F, Newsome D, Markey S. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 121.Bojkowski CJ, Aldhous ME, English J, Franey C, Poulton AL, Skene DJ, Arendt J. Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Horm Metab Res. 1987;19:437–440. doi: 10.1055/s-2007-1011846. [DOI] [PubMed] [Google Scholar]
- 122.Jung-Hynes B, Schmit TL, Reagan-Shaw SR, Siddiqui IA, Mukhtar H, Ahmad N. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J Pineal Res. 2011;50:140–149. doi: 10.1111/j.1600-079X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. Melatonin - Nature’s most versatile biological signal? Febs J. 2006;273:2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 124.Hardeland R, Pandi-Perumal SR. Melatonin, a potent agent in antioxidative defense: actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr Metab (Lond) 2005;2:22. doi: 10.1186/1743-7075-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hardeland R. Antioxidative protection by melatonin - Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119–130. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- 126.Carrillo-Vico A, Calvo JR, Abreu P, Lardone PJ, Garcia-Maurino S, Reiter RJ, Guerrero JM. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. Faseb J. 2004;18:537–539. doi: 10.1096/fj.03-0694fje. [DOI] [PubMed] [Google Scholar]
- 127.Guerrero JM, Reiter RJ. Melatonin-immune system relationships. Curr Top Med Chem. 2002;2:167–179. doi: 10.2174/1568026023394335. [DOI] [PubMed] [Google Scholar]
- 128.Jung B, Ahmad N. Melatonin in cancer management: Progress and promise. Cancer Res. 2006;66:9789–9793. doi: 10.1158/0008-5472.CAN-06-1776. [DOI] [PubMed] [Google Scholar]
- 129.Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD001520. CD001520.doi:10.1002/14651858.CD001520. [DOI] [PubMed] [Google Scholar]
- 130.Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res. 1993;14:151–68. doi: 10.1111/j.1600-079x.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 131.Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–97. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 132.Karasek M, Reiter RJ. Melatonin and aging. Neuroendocrinol Lett. 2002;23:14–16. [PubMed] [Google Scholar]
- 133.Reiter RJ, Tan DX, Kim SJ, Manchester LC, Qi WB, Garcia JJ, Cabrera JC, El-Sokkary G, Rouvier-Garay V. Augmentation of indices of oxidative damage in life-long melatonin-deficient rats. Mech Ageing Dev. 1999;110:157–173. doi: 10.1016/s0047-6374(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 134.Reiter RJ, Tan DX, Manchester LC, El-Sawi MR. Melatonin Reduces Oxidant Damage and Promotes Mitochondrial Respiration. Ann N Y Acad Sci. 2002;959:238–250. doi: 10.1111/j.1749-6632.2002.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 135.Bubenik GA, Hacker RR, Brown GM, Bartos L. Melatonin concentrations in the luminal fluid, mucosa, and muscularis of the bovine and porcine gastrointestinal tract. J Pineal Res. 1999;26:56–63. doi: 10.1111/j.1600-079x.1999.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 136.Cahill GM, Besharse JC. Light-sensitive melatonin synthesis by Xenopus photoreceptors after destruction of the inner retina. Vis Neurosci. 1992;8:487–490. doi: 10.1017/s0952523800005009. [DOI] [PubMed] [Google Scholar]
- 137.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. Faseb J. 2006;20:1564–1566. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 138.Itoh MT, Ishizuka B, Kuribayashi Y, Amemiya A, Sumi Y. Melatonin, its precursors, and synthesizing enzyme activities in the human ovary. Mol Hum Reprod. 1999;5:402–408. doi: 10.1093/molehr/5.5.402. [DOI] [PubMed] [Google Scholar]
- 139.Slominski A, Baker J, Rosano TG, Guisti LW, Ermak G, Grande M, Gaudet SJ. Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. J Biol Chem. 1996;271:12281–12286. doi: 10.1074/jbc.271.21.12281. [DOI] [PubMed] [Google Scholar]
- 140.Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, Slominski RM, Tobin DJ. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27:137–147. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, Slugocki G, McNulty J, Kauser S, Tobin DJ, Jing C, Johansson O. Serotoninergic and melatoninergic systems are fully expressed in human skin. Faseb J. 2002;16:896–898. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- 142.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. Faseb J. 2005;19:176–194. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 143.Tan DX, Manchester LC, Reiter RJ, Qi WB, Hanes MA, Farley NJ. High physiological levels of melatonin in the bile of mammals. Life Sci. 1999;65:2523–2529. doi: 10.1016/s0024-3205(99)00519-6. [DOI] [PubMed] [Google Scholar]
- 144.Tan DX, Manchester LC, Reiter RJ, Qi WB, Zhang M, Weintraub ST, Cabrera J, Sainz RM, Mayo JC. Identification of highly elevated levels of melatonin in bone marrow: its origin and significance. Biochim Biophys Acta-Gen Subj. 1999;1472:206–214. doi: 10.1016/s0304-4165(99)00125-7. [DOI] [PubMed] [Google Scholar]
- 145.Tan DX, Manchester LC, Sanchez-Barcelo E, Mediavilla MD, Reiter RJ. Significance of High Levels of Endogenous Melatonin in Mammalian Cerebrospinal Fluid and in the Central Nervous System. Curr Neuropharmacol. 2010;8:162–167. doi: 10.2174/157015910792246182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Reiter RJ, Tan DX. What constitutes a physiological concentration of melatonin? J Pineal Res. 2003;34:79–80. doi: 10.1034/j.1600-079x.2003.2e114.x. [DOI] [PubMed] [Google Scholar]
- 147.Reiter RJ, Tan DX, Maldonado MD. Melatonin as an antioxidant: physiology versus pharmacology. J Pineal Res. 2005;39:215–216. doi: 10.1111/j.1600-079X.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 148.Slominski A, Pisarchik A, Semak I, Sweatman T, Szczesniewski A, Wortsman J. Serotoninergic system in hamster skin. J Invest Dermatol. 2002;119:934–942. doi: 10.1046/j.1523-1747.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 149.Gaudet SJ, Slominski A, Etminan M, Pruski D, Paus R, Namboodiri MAA. Identification and characterization of two isozymic forms of arylamine N-acetyltransferase in Syrian hamster skin. J Invest Dermatol. 1993;101:660–665. doi: 10.1111/1523-1747.ep12371672. [DOI] [PubMed] [Google Scholar]
- 150.Maurer M, Metz M. The status quo and quo vadis of mast cells. Exp Dermatol. 2005;14:923–929. doi: 10.1111/j.1600-0625.2005.00369.x. [DOI] [PubMed] [Google Scholar]
- 151.Slominski A, Wortsman J. Neuroendocrinology of the Skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 152.Kobayashi H, Kromminga A, Dunlop TW, Tychsen B, Conrad F, Suzuki N, Memezawa A, Bettermann A, Aiba S, Carlberg C, Paus R. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. Faseb J. 2005;19:1710–1712. doi: 10.1096/fj.04-2293fje. [DOI] [PubMed] [Google Scholar]
- 153.Fischer TW, Scholz G, Knoll B, Hipler UC, Elsner P. Melatonin suppresses reactive oxygen species in UV-irradiated leukocytes more than vitamin C and trolox. Skin Pharmacol Appl Skin Physiol. 2002;15:367–373. doi: 10.1159/000064543. [DOI] [PubMed] [Google Scholar]
- 154.Fischer TW, Slominski A, Zmijewski MA, Reiter RJ, Paus R. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol. 2008;17:713–730. doi: 10.1111/j.1600-0625.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 155.Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 156.Fischer TW, Zmijewski MA, Wortsman J, Slominski A. Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J Pineal Res. 2008;44:397–407. doi: 10.1111/j.1600-079X.2007.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fischer TW, Zbytek B, Sayre RM, Apostolov EO, Basnakian AG, Sweatman TW, Wortsman J, Elsner P, Slominski A. Melatonin increases survival of HaCaT keratinocytes by suppressing UV-induced apoptosis. J Pineal Res. 2006;40:18–26. doi: 10.1111/j.1600-079X.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 158.Cho JW, Kim CW, Lee KS. Modification of gene expression by melatonin in UVB-irradiated HaCaT keratinocyte cell lines using a cDNA microarray. Oncol Rep. 2007;17:573–577. [PubMed] [Google Scholar]