Abstract
Abnormal hyperexcitability of primary sensory neurons contributes to neuropathic pain development after nerve injury. Nerve injury profoundly reduces the expression of big conductance Ca2+-activated K+ (BK) channels in the dorsal root ganglion (DRG). However, little is known about how nerve injury affects BK channel activity in DRG neurons. In this study, we determined the changes in BK channel activity in different sizes of DRG neurons in a rat model of neuropathic pain and the contribution of brain-derived neurotrophic factor (BDNF) to reduced BK channel activity. The BK channel activity was present predominantly in small and medium DRG neurons, and ligation of L5 and L6 spinal nerves profoundly decreased the BK current density in these neurons. Blocking BK channels significantly increased the excitability of DRG neurons in sham control, but not in nerve-injured, rats. The BDNF concentration in the DRG was significantly greater in nerve-injured rats than in control rats. BDNF treatment largely reduced BK currents in DRG neurons in control rats, which was blocked by either anti-BDNF antibody or K252a, a Trk receptor inhibitor. Furthermore, either anti-BDNF antibody or K252a reversed reduction in BK currents in injured DRG neurons. BDNF treatment reduced the mRNA levels of BKα1 subunit in DRG neurons, and anti-BDNF antibody attenuated the reduction in the BKα1 mRNA level in injured DRG neurons. These findings suggest that nerve injury primarily diminishes the BK channel activity in small and medium DRG neurons. Increased BDNF levels contribute to reduced BK channel activity in DRG neurons in neuropathic pain.
Introduction
Chronic neuropathic pain is often associated with spontaneous pain and exaggerated response to either a painful stimulus (hyperalgesia) or a mild and normally nonpainful stimulus (allodynia) (Campbell and Meyer 2006). Effective treatments for neuropathic pain remain a major clinical challenge. Studies in patients with neuropathic pain have indicated that the altered central processing associated with pain is maintained dynamically by ongoing peripheral input (Gracely et al. 1992; Campero et al. 1998). Abnormal hyperactivity of damaged primary afferent fibers and neurons can induce and maintain the chronic neuropathic pain caused by nerve injury (Matzner and Devor 1994; Liu et al. 2000b; Amir et al. 2005; Ma and LaMotte 2007). Furthermore, the activity of voltage-activated Na+ channels is increased in injured dorsal root ganglion (DRG) neurons (Dib-Hajj et al. 1999; Sleeper et al. 2000). Also, nerve injury reduces the currents of several voltage-activated K+ (Kv) channels in DRG neurons (Everill and Kocsis 1999; Yang et al. 2004). However, the potential roles of other ion channels involved in increased excitability of primary sensory neurons in neuropathic pain are not fully known.
Ca2+-activated K+ channels are divided into three types on the basis of their conductance: big conductance (BK), intermediate conductance (IK), and small conductance (SK) channels (Sah 1996). BK channels can be activated by a depolarizing voltage and blocked selectively by Iberiotoxin (IbTX), whereas IK and SK channels are voltage-insensitive and can be blocked by clotrimazole and apamin, respectively. All three channels are expressed in rat DRG neurons (Zhang et al. 2003; Mongan et al. 2005; Chen et al. 2009). Activation of BK channels causes a profound feed-back inhibition of the action potential frequency and Ca2+ influx (Knaus et al. 1996; Marrion and Tavalin 1998; Furukawa et al. 2008). We have shown that spinal nerve injury causes a large and persistent reduction in the expression level of BK channels in the DRG, and blocking BK channels at the spinal level can mimic neuropathic pain in rats (Chen et al. 2009). It is not yet clear how nerve injury affects BK channel activity in DRG neurons.
The mechanisms leading to the reduction in BK channel functions in DRG neurons by nerve injury also remain unknown. Brain-derived neurotrophic factor (BDNF) belongs to the family of neurotrophins, which binds to the low-affinity p75 neurotrophin receptors and the high-affinity tyrosine kinase TrkB receptors (Barde et al. 1982; Klein et al. 1991; Chao 2003). BDNF is normally present in small and medium DRG neurons (Zhou and Rush 1996; Thompson et al. 1999). Treatment with BDNF can reduce nociceptive thresholds in rats (Pezet et al. 2002) and suppresses the activity of voltage-activated K+ channels in DRG neurons (Cao et al. 2010). Sciatic nerve ligation or axotomy increases the expression level of BDNF in the DRG (Tonra et al. 1998; Obata et al. 2003). However, little is known about whether BDNF is causally involved in the reduction in BK channel activity of DRG neurons by nerve injury. In this study, we used a rat model of neuropathic pain to determine changes in BK channel activity in different sizes of DRG neurons. Furthermore, we determined the role of BDNF in reduced BK channel activity in DRG neurons in neuropathic pain.
Materials and Methods
Animal model of neuropathic pain
Male Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN) weighing 150–180 g were used in this study. Rats were anesthetized using isoflurane (2–3%), and the left L5 and L6 spinal nerves were carefully isolated and ligated tightly (Kim and Chung 1992; Chen et al. 2009). Age-matched sham control rats were used as the control group. Before performing the terminal electrophysiological and biochemical experiments, we confirmed the presence of hyperalgesia in nerve-ligated rats by testing the nociceptive withdrawal thresholds using the paw pressure Analgesy-Meter (Ugo Basile Biological Research, Comerio, Italy) (Chen et al. 2009). All experiments were approved by the Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center and conformed to the guidelines of the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize both the suffering and number of animals used.
Dissociation of DRG neurons
Two weeks after nerve ligation, rats were anesthetized with isoflurane and then rapidly decapitated. DRG neurons were dissociated enzymatically, as we described previously (Cao et al. 2010; Cao et al. 2011). The dissociated DRG neurons were plated onto a 35-mm culture dish containing poly-L-lysine (50 μg/ml) pre-coated coverslips and incubated in 5% CO2 at 37°C for 1 h. The neurons were kept in the incubator for at least another hour before electrophysiological recordings.
Electrophysiological recordings of BK channels
Whole-cell recordings of BK channels were conducted within 2–6 h after dissociation of DRG neurons. The electrodes with tip resistance of 1–4 MΩ were pulled from glass capillaries using a micropipette puller and fire-polished. The neurons were visualized using an inverted microscope and displayed on a video monitor through a CCD camera. Whole-cell recordings were made using an EPC-10 amplifier (HEKA Instruments, Lambrecht, Germany), filtered at 2 kHz, and digitized at 10 kHz. All experiments were performed at room temperature (~25°C).
The extracellular solution consisted of (in mM) 135 NaCl, 5 KCl, 2.5 CaCl2, 1.2 MgCl2, and 5 HEPES (pH 7.4 adjusted with NaOH; osmolarity, 310 mOsm). The pipette internal solution contained (in mM) 140 KCl, 1 MgCl2, 0.1 CaCl2, 1 EGTA, 10 HEPES (pH 7.2 adjusted with KOH, osmolarity 290 mOsm) (Shieh et al. 2007). In some experiments, 1 mM EGTA was replaced by 5 mM BAPTA in the pipette solution to rule out the contribution of extracellular Ca2+ influx on BK channels. All neurons for voltage-clamp recordings were held at −80 mV, evoked by 200-ms voltage test pulses stepped from −60 mV to +80 mV, followed by a prepulse conditioning step to 0 mV for 200 ms for allowing Ca2+ influx (Neely and Lingle 1992; Li et al. 2007). Iberiotoxin (IbTX)-sensitive currents, indicative of BK channel activity, was derived by subtraction of the Kv currents recorded in the presence of 100 nM IbTX from the baseline total Kv currents. To determine the excitability of DRG neurons, neurons were voltage-clamped at −60 mV and recorded in the current-clamp mode. Current-clamp recording signals were filtered at 5 KHz and digitized at 20 KHz. The action potentials were evoked by injection of a series of depolarizing currents from 0 to 1,000 pA for 400 ms in 100 pA increments and 5 s intervals (Vydyanathan et al. 2005).
Measurement of BDNF concentrations in DRG tissues
Left and right L5 and L6 DRGs were collected from nerve-ligated rats 14 days after surgery. Also, the left L5 and L6 DRGs from sham-operated rats were collected as the control group. After dissection, DRGs were weighed first and then homogenized in ice-cold homogenization buffer in 100 mM Tris-HCl (pH 7) containing 2% bovine serum albumin, 1 M NaCl, 4 mM EDTA-Na2, 2% Triton X-100, 0.1% sodium azide, 5 μg/ml aprotinin, 0.5 μg/ml antipain, 157 μg/ml benzamidine, 0.1 μg/ml pepstatin A, and 17 μg/ml phenylmethyl-sulphonyl fluoride. The ratio of homogenizing buffer to the DRG weight was 30 – 100 according to the actual amount of DRG tissues used. After subjecting the samples to centrifuge at 14,000 × g for 30 min, the resulting supernatants were used for the BDNF assay (Zhou et al. 2004). The BDNF concentration was measured using the Chemikine BDNF kit (Millipore, Temecula, CA) according to the manufacturer’s instruction. The absorbance at 450 nm was measured using a 96-well microplate reader (SpectraMax-M2, Molecular Devices, CA) after stopping the reaction by the stop solution. The BDNF concentration was expressed as pg BDNF/mg DRGs derived from the standard curve.
Real-time RT-PCR analysis of BK channel α1 subunit expression
Total RNA was extracted from rat lumbar DRGs at the L5–L6 level using Trizol. cDNA was prepared by using the Superscript III first-strand synthesis kit (Invitrogen, Carlsbad, CA). Quantitative PCR was performed using the iQ5 real-time PCR detection system with the SYBR Green PCR kit (Bio-Rad, Hercules, CA). All samples were analyzed in duplicate using an annealing temperature of 60°C, and each experiment was repeated at least once. The primer pairs used for Kcnma1 (BKα1, GenBank #83731) are listed as follows: forward, 5′-CCG TCC ACA GCA AAT CGG CCA-3′; reverse, 5′-CCA TGT GGG TAC TCA TGG GCT TGG-3′. To calculate the relative BKα1 subunit mRNA level in each sample, standard curves were generated using a two-fold dilution of the cDNA from the DRG as the PCR template. The relative amount of BKα1 mRNA in each sample was first normalized to the level of the housekeeping gene GAPDH and was then normalized to its expression level in control rats. The PCR product specificity was verified by melting-curve analysis and agarose gel electrophoresis.
Drug application
The drug stock solution was diluted in the appropriate external solution just before use. Each drug solution was delivered to the recording chamber at ~0.8 ml/min controlled by the positive pressure. The BDNF antibody and K252a were added to the cell culture medium after dissociation of DRG neurons. All drugs and chemicals were purchased from Sigma-Aldrich except BDNF and anti-BNDF antibody (Millipore) and IbTX (Alomone labs; Jerusalem, Israel).
Data analysis
Data are presented as means ± SEM. The current-voltage (I–V) curves for BK channels in individual neurons were generated by calculating the peak outward currents at each test potential and normalized to the cell capacitance. Parameters of action potentials were analyzed offline with the MiniAnalysis software (Synaptosoft, Fort Lee, NJ). The action potential threshold was defined as the lowest current injected that elicited an action potential with an overshoot. The action potential overshoot was determined from 0 mV to the peak of an action potential. The duration of the action potential was measured at 50% of the peak amplitude from the resting membrane potential, because the 50% amplitude was close to the base of the action potential (Vydyanathan et al. 2005). Differences between the means were tested for significance using paired and unpaired Student’s t tests, one-way ANOVA, or two-way ANOVA followed by appropriate post hoc tests. P < 0.05 was considered to be statistically significant.
Results
Sham surgery had no significant effect on the pressure withdrawal threshold of the left hindpaw (126.50 ± 3.46 g) compared with that in the right hindpaw (124.15 ± 2.60 g, n = 14, P < 0.05). Two weeks after surgery, the paw withdrawal thresholds in response to a noxious pressure stimulus applied to the left hindpaw was significantly reduced in nerve-injured rats (76.60 ± 4.22 g, n = 14) compared with that in sham control rats.
The DRG neurons were divided into three groups according to their cell diameters, which were measured with a calibrated eyepiece reticule: small (< 30 μm), medium (30–40 μm), and large (> 40 μm) (Cao et al. 2010). There were no significant differences in the capacitance of the same size of DRG neurons between nerve-injured rats (small, 26.94 ± 0.90 pF, n = 48; medium, 56.35 ± 1.85 pF, n = 55; large, 118.0 ± 7.62 pF, n = 11) and sham control rats (small, 27.91 ± 1.08 pF, n = 43; medium, 53.62 ± 1.96 pF, n = 53; large, 111.8 ± 8.55 pF, n = 13).
Nerve injury decreases BK channel activity in small and medium DRG neurons
Nerve ligation significantly decreased the total Kv current density in different sizes of DRG neurons (small: 416.19 ± 20.94 pA/pF, n = 9; medium: 238.67 ± 16.32 pA/pF, n = 9; large: 199.06 ± 10.96 pA/pF, n = 11), compared with that in the sham control group (small: 603.11 ± 64.29 pA/pF, n = 9; medium: 398.35 ± 36.12 pA/pF, n = 17; large: 295.73 ± 17.73 pA/pF, n = 9; Figs. 1–3).
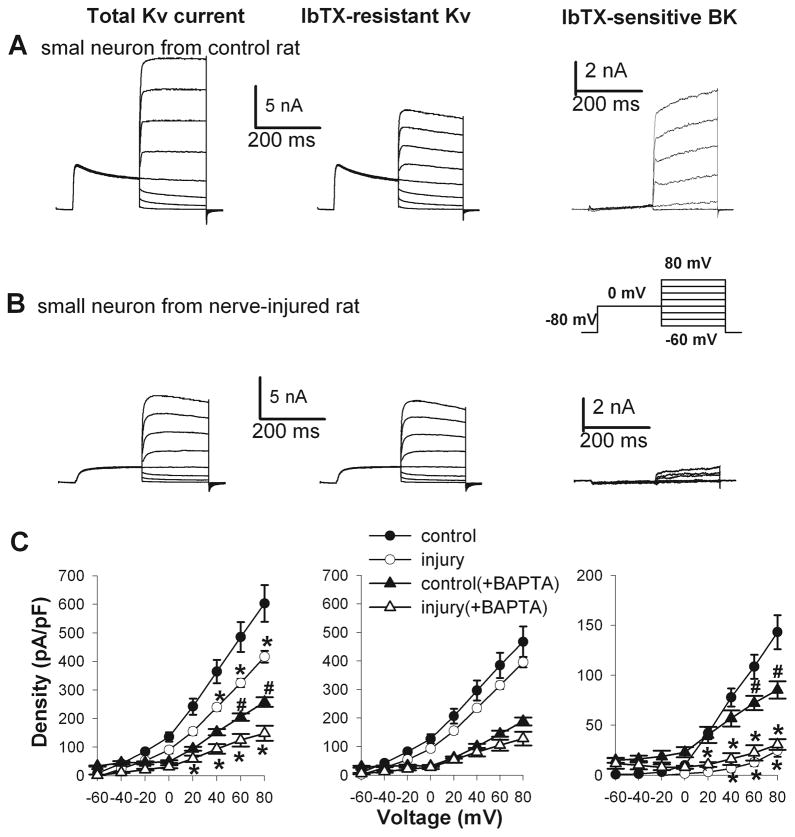
Figure 1.
Changes in the current density of total Kv, IbTX-resistant Kv, and IbTX-sensitive BK channels in small DRG neurons from sham control and nerve-injured rats. A and B, representative traces show Kv and BK currents in small DRG neurons from a control and a nerve-injured rat. Neurons were voltage-clamped at −80 mV and depolarized from −60 to 80 mV in 10 mV increments following a prepulse at 0 mV for 200 ms (inset). C, I–V curves show the current density of total Kv, IbTX-resistant Kv, and IbTX-sensitive BK channels in small DRG neurons from rats in the control and nerve-injured groups (n = 9 neurons in each group). Additional small DRG neurons from control and nerve-injured rats were recorded with BAPTA included in the intracellular solution (n = 10 neurons in each group). * P < 0.05 compared with the corresponding values in the sham control group. # P< 0.05 compared with the corresponding value in the sham control recorded without BAPTA.
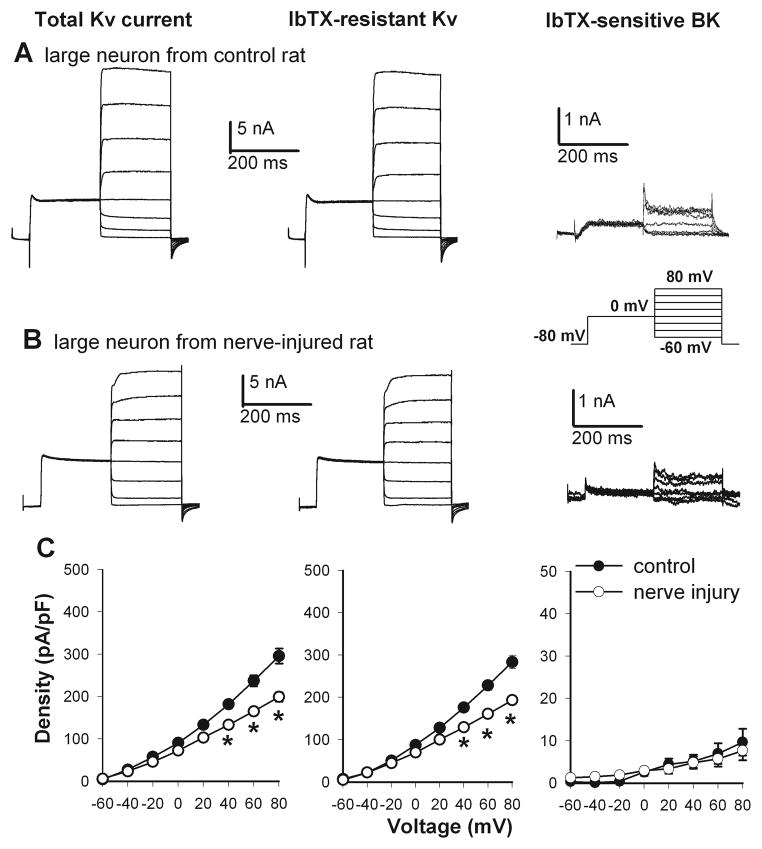
Figure 3.
Nerve injury decreased the current density of total Kv, IbTX-resistant Kv, and IbTX-sensitive BK channels in large DRG neurons. A and B, original traces show Kv and BK currents in large DRG neurons from a control and a nerve-injured rat (inset, voltage protocol). C, summary data show the current density of total Kv, IbTX-resistant, and IbTX-sensitive BK channels in large DRG neurons in the sham control (n = 9 cells) and nerve-injured (n = 11 cells) groups. * P < 0.05 compared with the corresponding value in the sham control group.
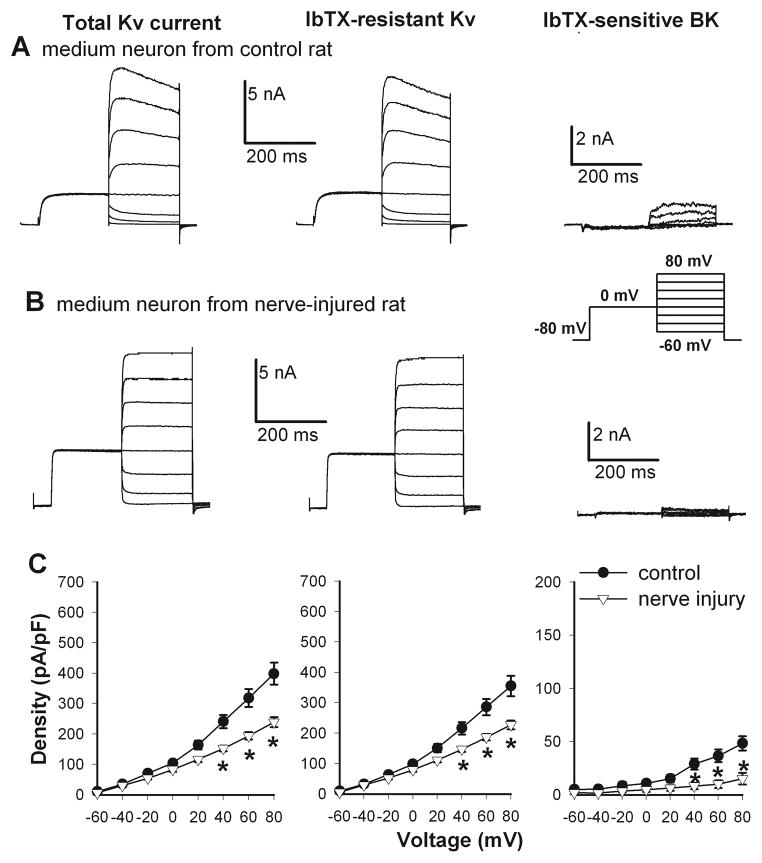
In control rats, the current density of IbTX-sensitive currents, termed BK currents, was the largest in small DRG neurons and smallest in large DRG neurons (Figs. 1–3). Nerve injury caused a profound reduction in the BK current density in small DRG neurons (28.54 ± 6.03 pA/pF, n = 9), compared with that in the control group (143.10 ± 17.02 pA/pF, n = 9, Fig. 1). Similarly, the BK current density in medium DRG neurons was also significantly decreased in nerve-injured rats (15.07 ± 5.44 pA/pF, n = 9), compared with that in sham control rats (48.31 ± 6.71 pA/pF, n = 17, Fig. 2). However, nerve injury did not significantly alter the BK current density in large DRG neurons (control: 9.65 ± 3.19 pA/pF, n = 11; nerve injury: 7.67 ± 2.24 pA/pF, n = 9; Fig. 3).
Figure 2.
Nerve injury reduced the current density of total Kv, IbTX-resistant Kv, and IbTX-sensitive BK channels in medium DRG neurons. A and B, representative traces show Kv and BK currents in medium DRG neurons from a sham control and a nerve-injured rat (inset, voltage protocol). C, I–V curves show the current density of total Kv, IbTX-resistant Kv, and IbTX-sensitive BK channels in medium DRG neurons from rats in the control (n = 17 cells) and nerve-injured (n = 9 cells) groups. * P < 0.05 compared with the corresponding value in the sham control group.
To rule out the potential contribution of Ca2+ influx to BK channel activity, we combined BAPTA and CaCl2 in the pipette solution in additional small DRG neurons from control and nerve-injured rats. Including BAPTA in the intracellular solution significantly reduced the BK current density in control DRG neurons (Fig. 1). Under this recording condition, the BK current density in small DRG neurons was still significantly lower in nerve-injured rats than in sham control rats (n = 10 neurons in each group, Fig. 1).
Nerve injury diminishes the role of BK channels in controlling excitability of DRG neurons
Because small DRG neurons are largely endowed with BK channels and the reduction in BK channel activity by nerve injury occurred predominantly in these DRG neurons, we used small DRG neurons to study the influence of BK channels on the neuronal excitability in both control and nerve-injured rats. Injection of depolarizing currents ranging from 0 to 1,000 pA for 400 ms evoked a single action potential or repetitive firing in DRG neurons from both control and nerve-injured rats. The resting membrane potential (nerve injury: −41.62 ± 1.64 mV, n = 18; control: −53.36 ± 1.97 mV, n = 26; Fig. 4) and the amount of current injection required to elicit an action potential (nerve injury: 344.44 ± 37.24 pA, n = 18; control, 561.84 ± 46.77 pA, n = 26; Fig. 4) of DRG neurons were significant reduced in nerve-injured rats compared with those in sham control rats. The action potential duration measured at 50% of spike height (APD50) (nerve injury: 7.29 ± 0.67 ms, n = 18; control: 4.32 ± 0.35 ms, n = 26, Fig. 4) of DRG neurons was significantly increased in nerve-injured rats compared with that in control rats. However, nerve injury had no significant effect on the onset latency of action potentials elicited by current injection (nerve injury: 3.44 ± 0.27 ms, n = 18; control: 3.58 ± 0.16 ms, n = 26; Fig. 4).
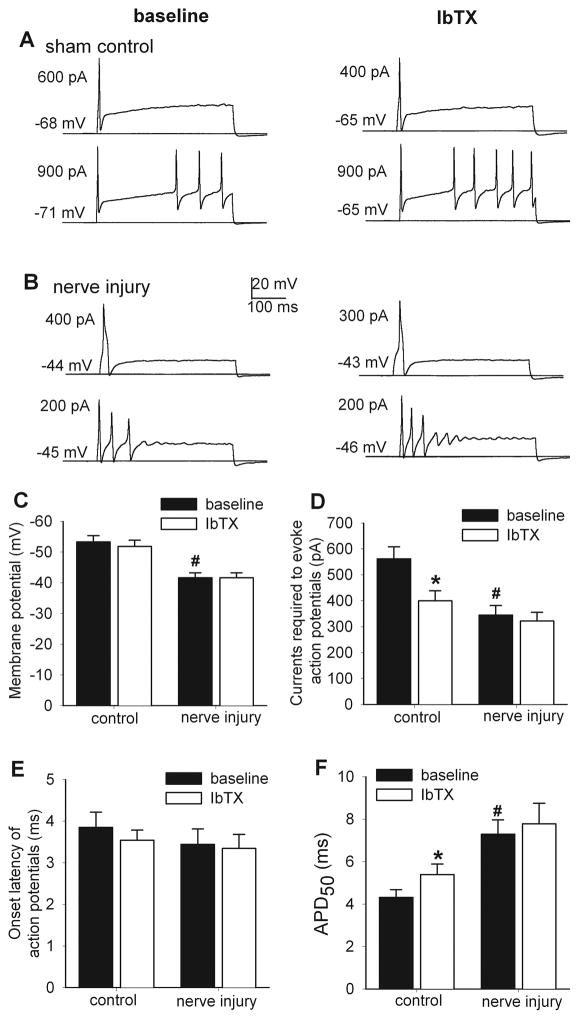
Figure 4.
Effects of IbTX on the excitability of small DRG neurons in control and nerve-injured rats. A and B, representative traces show the effect of IbTX (100 nM) on the firing activity (a neuron with single action potential vs. a neuron with repetitive action potentials) evoked by current injection in small DRG neurons from sham control and nerve-injured rats. Note that the resting membrane potential and threshold current for action potential initiation are indicated on the left of each trace. C–F, summary data show the effect of IbTX on the resting membrane potentials, amount of currents injected for action potential initiation, onset latency of action potentials, and action potential duration measured at 50% of spike height (APD50) in small DRG neurons from control (n = 26 cells) and nerve-injured (n = 18 cells) rats. * P < 0.05 compared with the baseline value. # P < 0.05 compared with the corresponding value in the sham control group.
Bath application of 100 nM IbTX significantly decreased the amount of current injection required for action potential initiation in DRG neurons obtained from control rats (baseline control, 561.54 ± 46.77 pA; IbTX, 400.00 ± 38.83 pA, n = 26) but not from nerve-injured rats (Fig. 4). Furthermore, IbTX induced a significant increase in APD50 (baseline control, 4.31 ± 0.35 ms; IbTX, 5.39 ± 0.50 ms, n = 26, Fig. 4) in small DRG neurons in sham control, but not nerve-injured, rats. However, IbTX had no significant effects on the resting membrane potential and the firing onset latency of small DRG neurons in either control or nerve-injured rats (Fig. 4).
Nerve injury increases BDNF levels in the DRG
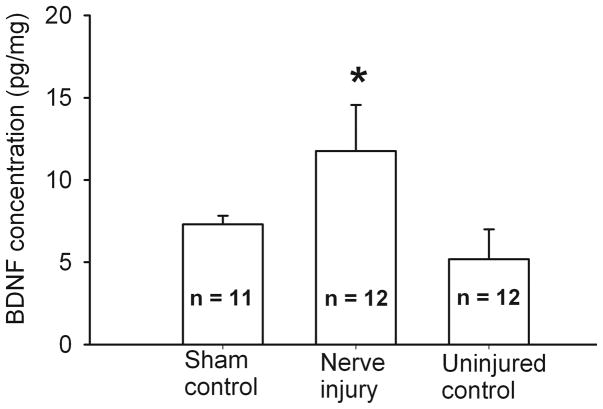
We used enzyme-linked immunosorbent assay (ELISA) to quantify changes in the BDNF level in the intact DRG caused by nerve injury. The BDNF concentration in L5 and L6 DRGs ipsilateral to nerve injury (11.76 ± 2.81 pg/mg, n = 12 rats) was significantly higher than that in the contralateral DRGs (5.19 ± 1.82 pg/mg, n = 12 rats). In the left L5 and L6 DRGs collected from sham control rats, the BDNF concentration (7.31 ± 0.53 pg/mg, n = 11 rats) was significantly lower than that in the ipsilateral (injured) DRGs of nerve-injured rats (Fig. 5).
Figure 5.
Nerve injury increased BDNF levels in DRG tissues. Summary data show changes in the BDNF level, measured with ELISA, in L5 and L6 DRG tissues from sham control (n = 11 rats) and nerve-injured (n = 12 rats) groups. Note that the DRGs contralateral to the nerve injury side were also used as uninjured controls. * P < 0.05 compared with the corresponding value in the sham group. One-way ANOVA followed by Tukey’s post hoc test.
BDNF mediates nerve injury-induced decreases in BK channel activity in DRG neurons
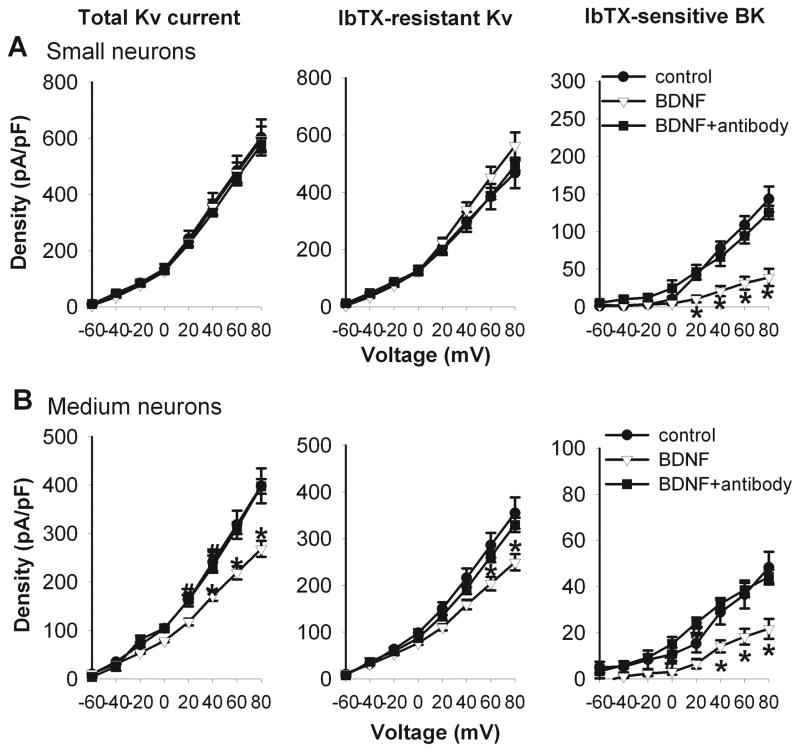
We first determined whether BDNF treatment affects the BK current density in small and medium DRG neurons obtained from sham control rats. Treatment with 50 ng/ml of BDNF for 2–4 h significantly reduced the IbTX-sensitive BK currents, but not IbTX-resistant Kv currents, in small DRG neurons (Fig. 6). Interestingly, BDNF treatment significantly decreased the BK currents and IbTX-resistant Kv currents in medium DRG neurons (Fig. 6). In the presence of anti-BDNF antibody (1:50) (Cao et al. 2010), BDNF failed to significantly alter the BK currents and total Kv currents in small and medium DRG neurons (Fig. 6).
Figure 6.
Effects of BDNF treatment on the total Kv, Iberiotoxin (IbTX)-resistant Kv, and IbTX-sensitive BK currents in small and medium DRG neurons from sham control rats. A and B, I-V curves show that BDNF (50 ng/ml) treatment reduced the BK current density in small (n = 11) and medium (n = 13) DRG neurons from sham control rats. Note that BDNF failed to reduce BK currents in the presence of the anti-BDNF antibody. * P < 0.05 compared with the corresponding value in the control group.
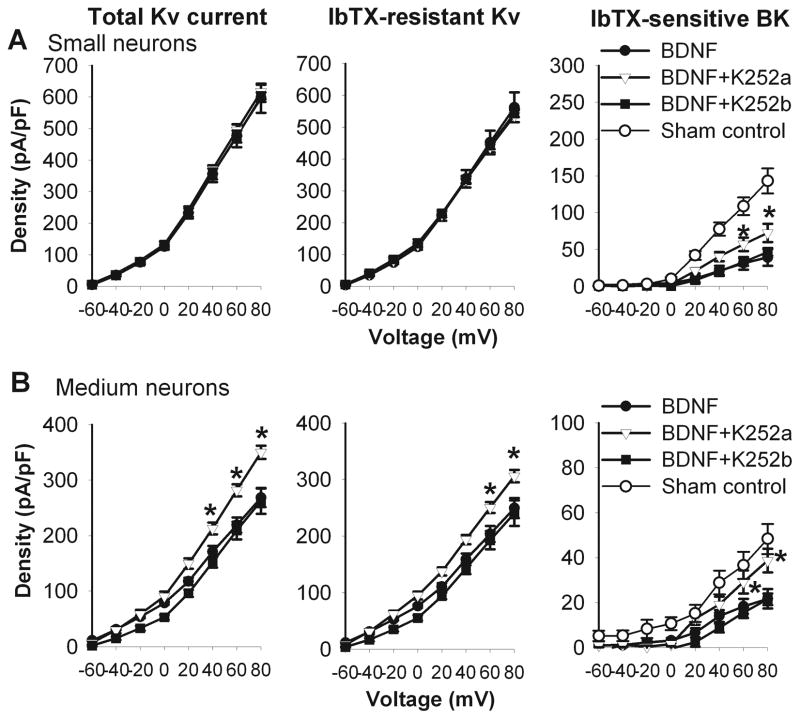
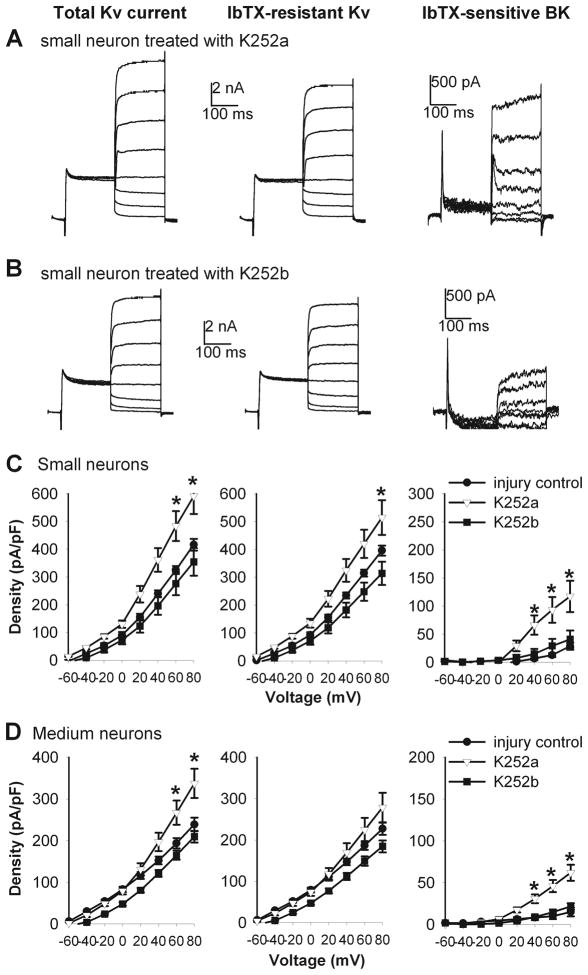
We then determined whether BDNF-induced reduction in the BK current density in small and medium DRG neurons from control rats was mediated by TrkB, the high-affinity BDNF receptor (Klein et al. 1991; Soppet et al. 1991; Squinto et al. 1991). Treatment of DRG neurons with K252a (300 nM), a Trk receptor inhibitor (Tapley et al. 1992; Bhave et al. 1999), abolished BDNF-induced reduction in the BK current density in small and medium DRG neurons (Fig. 7). However, K252b (300 nM), an inactive analogue of K252a, failed to alter the effect of BDNF on BK currents in small and medium DRG neurons from sham control rats (Fig. 7).
Figure 7.
Blocking TrkB receptors with K252a abolished the effect of BDNF on the total Kv, Iberiotoxin (IbTX)-resistant Kv, and IbTX-sensitive BK currents in small and medium DRG neurons from control rats. A and B, summary data show that K252a (n = 9), but not K252b (n = 7), abolished the effects of BDNF on the total Kv, IbTX-resistant Kv, and IbTX-sensitive BK currents in small and medium DRG neurons from sham control rats. * P < 0.05 compared with the corresponding value in the BDNF group.
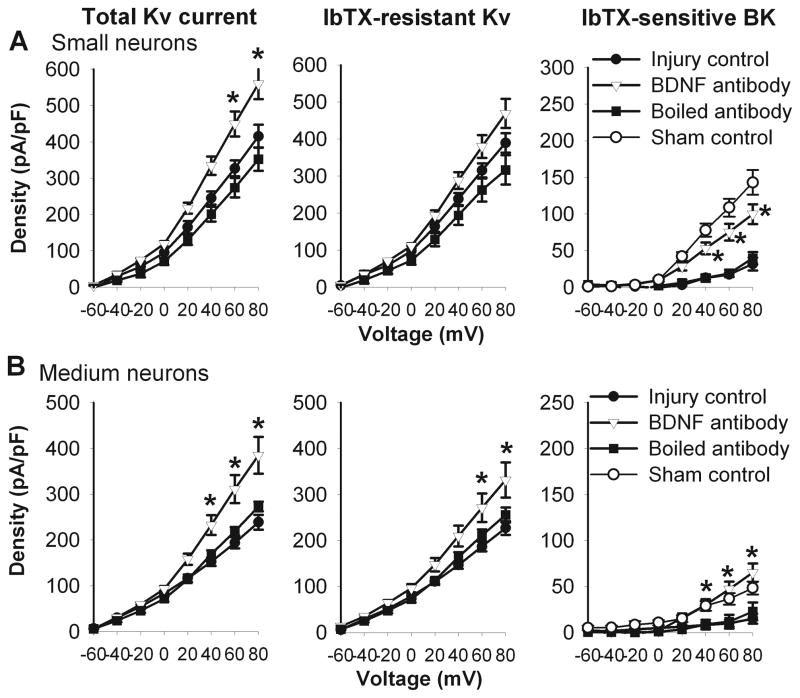
Next, we determined whether increased BDNF levels contribute to the reduction in BK currents in DRG neurons by nerve injury. To this end, we examined the effect of anti-BDNF antibody on the BK currents in small and medium DRG neurons obtained from nerve-injured rats. Treatment with anti-BDNF antibody (1:50, for 2–4 h) significantly increased the BK current density in small and medium DRG neurons (Fig. 8). However, treatment with the boiled BDNF antibody did not alter the BK current density in small and medium DRG neurons in nerve-injured rats (Fig. 8).
Figure 8.
Anti-BDNF antibody reversed nerve injury-induced reduction in the total Kv, IbTX-resistant, and IbTX-sensitive BK currents in small and medium DRG neurons. A, treatment with anti-BDNF antibody (n = 12), but not boiled anti-BDNF antibody (n = 9), increased the total Kv, IbTX-resistant Kv, and IbTX-sensitive BK currents in small DRG neurons from nerve-injured rats. B, treatment with anti-BDNF antibody (n = 12), but not boiled anti-BDNF antibody (n = 8), increased the total Kv, IbTX-resistant Kv, and IbTX-sensitive BK currents in medium DRG neurons from nerve-injured rats. * P < 0.05 compared with the corresponding value in the injury control group.
In additional experiments, we determined whether Trk receptors mediate the decrease in BK currents of DRG neurons induced by nerve injury. We examined the effect of K252a treatment (300 nM for 2–4 h) on the BK current density in DRG neurons obtained from nerve-injured rats. Treatment with K252a, but not K252b, significantly reversed the decrease in BK currents in small and medium DRG neurons in nerve-injured rats (Fig. 9).
Figure 9.
K252a reversed the effects of nerve injury on the total Kv, IbTX-resistant Kv, and IbTX-sensitive BK currents in small and medium DRG neurons. A and B, original current traces show that the differential effects of K252a and K252b on the total Kv, IbTX-resistant Kv, and IbTX-sensitive BK currents in small DRG neurons from a nerve-injured rat. C, group data show that K252a (n = 7), but not K252b (n = 11), reversed the effects of nerve injury on the total Kv, IbTX-resistant Kv, and IbTX-sensitive BK currents in small DRG neurons. D, summary data show that K252a (n = 11), but not K252b (n = 13), reversed nerve injury-induced reduction in the total Kv, IbTX-resistant Kv, and IbTX-sensitive BK currents in medium DRG neurons. * P < 0.05 compared with the corresponding value in the injury control group.
BDNF contributes to nerve injury-induced reduction in BK channel sexpression in DRG neurons
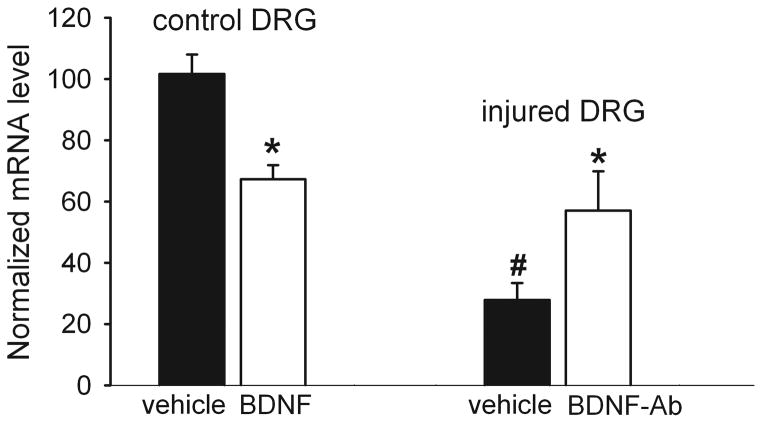
We used quantitative PCR analysis to determine the role of BDNF in nerve injury-induced reduction of BK channel expression in DRG neurons. Treatment of DRG neurons from control rats with 50 ng/ml BDNF for 2–4 h significantly reduced the mRNA level of BKα1 subunit (Fig. 10).
Figure 10.
Role of BDNF in the reduction of the BKα1 expression level in DRG neurons by nerve injury. Left, effect of treatment with BDNF (50 ng/ml for 2–4 h) or vehicle on the mRNA level of BKα1 subunit in DRG neurons in control rats (n = 6 in each group). Right, effect of treatment with the anti-BDNF antibody (BDNF-Ab, 1:50 for 2–4 h) or vehicle on the mRNA level of BKα1 subunit in DRG neurons in nerve-ligated rats (n = 6 in each group). * P < 0.05 compared with the corresponding values in the vehicle-treated group. # P < 0.05 compared with the control DRG neurons. One-way ANOVA followed by Tukey’s post hoc test.
The mRNA level of BKα1 subunit was significantly lower in nerve-injured rats than in control rats (Fig. 10). Furthermore, in DRG neurons from nerve-injured rats, treatment with anti-BDNF antibody (1:50, for 2–4 h) significantly attenuated the reduction in the mRNA level of BKα1 subunit caused by nerve injury (Fig. 10).
Discussion
BK channels are involved in the control of neuronal excitability by accelerating after-hyperpolarization, shortening the action potential duration, enhancing the repolarization, and mediating spike-frequency adaption (Zhang et al. 2003; Gu et al. 2007). BK channel activation results in the negative feedback regulation of membrane excitability, calcium influx, and neurotransmitter release (Scholz et al. 1998; Storer et al. 2009). We have shown that nerve injury causes a profound reduction in the expression level of BK channels in the DRG but not in the spinal cord (Chen et al. 2009). In the present study, we determined changes in the BK channel activity in different sizes of DRG neurons caused by nerve injury. We found that BK channel activity is predominantly present in small and medium DRG neurons. Furthermore, we showed that nerve injury caused a large reduction in the BK channel activity in these neurons. Therefore, diminished BK channel expression and function in small and medium DRG neurons can contribute to increased nociceptive input to the spinal cord and chronic pain caused by nerve injury (Chen et al. 2009).
Previous studies have shown that complete nerve transection (axotomy) causes hyperexcitability of medium and large DRG neurons (Liu et al. 2000a; Liu et al. 2002; Sapunar et al. 2005). We found that IbTX-resistant Kv currents were significantly reduced in injured small and medium DRG neurons. It has been shown that axotomy primarily reduces Kv currents in large DRG neurons (Everill and Kocsis 1999), which can account for increased excitability of these neurons. In the present study, we showed that spinal nerve ligation significantly decreased the resting membrane potential and the amount of current injection for action potential initiation and significantly increased APD50 in small DRG neurons, suggesting that the excitability of small DRG neurons is also increased in this rat model of neuropathic pain. We selected small DRG neurons to further determine the contribution of BK channels to increased neuronal excitability by nerve injury because BK channels are mostly present in these neurons. We found that blocking BK channels with IbTX reduced the amount of currents required for action potential initiation and increased APD50 in small DRG neurons from control rats, suggesting that the presence of BK channels can tonically inhibit the excitability of DRG neurons. However, these effects were not observed in DRG neurons from nerve-injured rats. Our results suggest that nerve injury-induced reduction in BK channel activity contributes critically to the hyperexcitability of primary sensory neurons in neuropathic pain. It has been reported that the activity of BK channels is also decreased in small neurons in the uninjured L4 DRG after transection of the L5 spinal nerve (Sarantopoulos et al. 2007). Thus, abnormal excitability in adjacent uninjured sensory neurons may also contribute to nerve injury-induced neuropathic pain.
The key finding of our study is that BDNF plays a critical role in the reduction in the BK channel activity in small and medium DRG neurons induced by nerve injury. We found that nerve injury significantly increased the BDNF level (likely released from injured neurons and glia) in the DRG, which is consistent with a previous study showing that the BDNF mRNA level in the DRG is increased after nerve injury (Obata et al. 2011). BDNF treatment can reduce the mRNA level of several voltage-activated Kv channels in DRG neurons (Cao et al. 2010) and impair the chloride transporter function of spinal dorsal horn neurons (Coull et al. 2005). In this study, we showed that BDNF treatment reduced BK channel activity in small and medium DRG neurons from control rats. Also, treatment of DRG neurons from control rats with either anti-BDNF antibody or a Trk receptor inhibitor, K252a, blocked the inhibitory effect of BDNF on BK currents in DRG neurons from control rats. Importantly, treatment with either anti-BDNF antibody to neutralize BDNF or K252a reversed the reduction in BK currents of DRG neurons from nerve-injured rats. Although higher concentrations of K252a may affect other protein kinases, we used a low concentration (300 nM) of k252a, which is known to selectively inhibit the Trk receptors (Berg et al. 1992). Our findings suggest that that nerve injury-induced increases in BDNF levels suppress BK channel activity in small and medium DRG neurons through TrkB receptors.
It remains unclear how increased BDNF levels lead to the reduction in BK channel activity in DRG neurons after nerve injury. BK channels are colocalized or functionally related to Ca2+ influx sources, such as voltage-activated Ca2+ channels (Marrion and Tavalin 1998; Muller et al. 2010), NMDA receptors (Isaacson and Murphy 2001), and ryanodine receptors (Chavis et al. 1998). In this study, we included EGTA in the pipette solution and used a prepulse conditioning step to 0 mV for allowing Ca2+ influx to facilitate BK channel activation (Neely and Lingle 1992; Li et al. 2007). BDNF can suppress N-type Ca2+ channels (Bouron et al. 2006) and may reduce BK channel activity by decreasing Ca2+ influx via these Ca2+ channels. Also, complete nerve transection can reduce the voltage-activated Ca2+ channel function in DRG neurons (McCallum et al. 2006), although ligation of L5 and L6 spinal nerves potentiates the activity of voltage-activated Ca2+ channels in small DRG neurons (Li et al. 2012). To further exclude the contribution of reduced Ca2+ influx to the reduction in BK channel activity, we clamped the intracellular Ca2+ level by combining BAPTA and CaCl2 in the pipette solution. We found that intracellular application of BAPTA decreased BK channel function. Under this recording condition, nerve injury still significantly reduced BK channel activity in small DRG neurons, suggesting that reduced BK channel activity in DRG neurons by nerve injury is unlikely caused by reduced Ca2+ influx. Moreover, because an increase in the intracellular Na+ concentration can suppress BK channel activity (Liang et al. 2008), BDNF may stimulate Na+ channels to increase the intracellular Na+ concentration via TrkB receptors (Kafitz et al. 1999).
In the present study, we found that BDNF treatment significantly decreased the mRNA level of BKα1 subunit in control DRG neurons and that treatment with anti-BDNF antibody attenuated the decrease in the mRNA level of BKα1 subunit in injured DRG neurons. Our results suggest that BDNF can suppress BK channel expression in injured DRG neurons through epigenetic and transcriptional mechanisms. However, neutralizing BDNF only partially reversed the decrease in the mRNA level of BKα1 subunit in DRG neurons by nerve injury. In addition to the transcription mechanism, BDNF may affect BK channel functions through other mechanisms such as BK channel trafficking and modulation. We have shown that BDNF contributes to the reduction in voltage-activated K+ channels in DRG neurons in painful diabetic neuropathy (Cao et al. 2010). Thus, BDNF seems to be involved in diminished activity of both voltage-activated K+ channels and BK channels in primary sensory neurons caused by nerve injury and diabetic neuropathy. The importance of BDNF in the regulation of BK channels in vivo still needs to be determined in future studies.
In summary, our findings indicate that nerve injury diminishes the activity of BK channels present in small and medium DRG neurons. Reduced BK channel function plays an important role in increased excitability of these DRG neurons after nerve injury. Furthermore, our study suggests that increased BDNF levels in the DRG after nerve injury contributes to reduced BK channel activity through TrkB receptors in neuropathic pain. Because activation of BK channels normally dampens the excitability of primary afferent neurons to limit nociceptive input to the spinal cord (Furukawa et al. 2008; Chen et al. 2009), BK channels serve as an important feedback controller of the nociceptive system. Our findings provide new information for our understanding of the mechanisms underlying increased excitability of primary sensory neurons in neuropathic pain.
Acknowledgments
This study was supported by the National Institutes of Health grants GM064830, DE022015, and NS073935 and the N.G. and Helen T. Hawkins Endowment to H.L.P. The authors declare that they have no conflict of interest regarding the work reported here.
References
- Amir R, Kocsis JD, Devor M. Multiple interacting sites of ectopic spike electrogenesis in primary sensory neurons. J Neurosci. 2005;25:2576–2585. doi: 10.1523/JNEUROSCI.4118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. Embo J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg MM, Sternberg DW, Parada LF, Chao MV. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. The Journal of biological chemistry. 1992;267:13–16. [PubMed] [Google Scholar]
- Bhave SV, Ghoda L, Hoffman PL. Brain-derived neurotrophic factor mediates the anti-apoptotic effect of NMDA in cerebellar granule neurons: signal transduction cascades and site of ethanol action. J Neurosci. 1999;19:3277–3286. doi: 10.1523/JNEUROSCI.19-09-03277.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouron A, Boisseau S, De Waard M, Peris L. Differential down-regulation of voltage-gated calcium channel currents by glutamate and BDNF in embryonic cortical neurons. Eur J Neurosci. 2006;24:699–708. doi: 10.1111/j.1460-9568.2006.04946.x. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campero M, Serra J, Marchettini P, Ochoa JL. Ectopic impulse generation and autoexcitation in single myelinated afferent fibers in patients with peripheral neuropathy and positive sensory symptoms. Muscle Nerve. 1998;21:1661–1667. doi: 10.1002/(sici)1097-4598(199812)21:12<1661::aid-mus6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Cao XH, Byun HS, Chen SR, Pan HL. Diabetic neuropathy enhances voltage-activated Ca2+ channel activity and its control by M4 muscarinic receptors in primary sensory neurons. J Neurochem. 2011;119:594–603. doi: 10.1111/j.1471-4159.2011.07456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XH, Byun HS, Chen SR, Cai YQ, Pan HL. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem. 2010;114:1460–1475. doi: 10.1111/j.1471-4159.2010.06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chavis P, Ango F, Michel JM, Bockaert J, Fagni L. Modulation of big K+ channel activity by ryanodine receptors and L-type Ca2+ channels in neurons. Eur J Neurosci. 1998;10:2322–2327. doi: 10.1046/j.1460-9568.1998.00243.x. [DOI] [PubMed] [Google Scholar]
- Chen SR, Cai YQ, Pan HL. Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain. J Neurochem. 2009;110:352–362. doi: 10.1111/j.1471-4159.2009.06138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Fjell J, Cummins TR, Zheng Z, Fried K, LaMotte R, Black JA, Waxman SG. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain. 1999;83:591–600. doi: 10.1016/S0304-3959(99)00169-4. [DOI] [PubMed] [Google Scholar]
- Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol. 1999;82:700–708. doi: 10.1152/jn.1999.82.2.700. [DOI] [PubMed] [Google Scholar]
- Furukawa N, Takasusuki T, Fukushima T, Hori Y. Presynaptic large-conductance calcium-activated potassium channels control synaptic transmission in the superficial dorsal horn of the mouse. Neurosci Lett. 2008;444:79–82. doi: 10.1016/j.neulet.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580:859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Murphy GJ. Glutamate-mediated extrasynaptic inhibition: direct coupling of NMDA receptors to Ca(2+)-activated K+ channels. Neuron. 2001;31:1027–1034. doi: 10.1016/s0896-6273(01)00428-7. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Klein R, Nanduri V, Jing SA, Lamballe F, Tapley P, Bryant S, Cordon-Cardo C, Jones KR, Reichardt LF, Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991;66:395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus HG, Schwarzer C, Koch RO, Eberhart A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML, Sperk G. Distribution of high-conductance Ca(2+)-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci. 1996;16:955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Cao XH, Chen SR, Han HD, Lopez-Berestein G, Sood AK, Pan HL. Upregulation of Cav-beta3 subunit in primary sensory neurons increases voltage-activated Ca2+ channel activity and nociceptive input in neuropathic pain. J Biol Chem. 2012;287:6002–6013. doi: 10.1074/jbc.M111.310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Gao SB, Lv CX, Wu Y, Guo ZH, Ding JP, Xu T. Characterization of voltage-and Ca2+-activated K+ channels in rat dorsal root ganglion neurons. J Cell Physiol. 2007;212:348–357. doi: 10.1002/jcp.21007. [DOI] [PubMed] [Google Scholar]
- Liang GH, Kim MY, Park S, Kim JA, Choi S, Suh SH. Intracellular Na(+) modulates large conductance Ca(2+)-activated K (+) currents in human umbilical vein endothelial cells. Pflugers Arch. 2008;457:67–75. doi: 10.1007/s00424-008-0490-9. [DOI] [PubMed] [Google Scholar]
- Liu CN, Devor M, Waxman SG, Kocsis JD. Subthreshold oscillations induced by spinal nerve injury in dissociated muscle and cutaneous afferents of mouse DRG. J Neurophysiol. 2002;87:2009–2017. doi: 10.1152/jn.00705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000a;85:503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- Liu X, Eschenfelder S, Blenk KH, Janig W, Habler H. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain. 2000b;84:309–318. doi: 10.1016/s0304-3959(99)00211-0. [DOI] [PubMed] [Google Scholar]
- Ma C, LaMotte RH. Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. J Neurosci. 2007;27:14059–14068. doi: 10.1523/JNEUROSCI.3699-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Matzner O, Devor M. Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol. 1994;72:349–359. doi: 10.1152/jn.1994.72.1.349. [DOI] [PubMed] [Google Scholar]
- McCallum JB, Kwok WM, Sapunar D, Fuchs A, Hogan QH. Anesthesiology. Vol. 105. United States: 2006. Painful peripheral nerve injury decreases calcium current in axotomized sensory neurons; pp. 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongan LC, Hill MJ, Chen MX, Tate SN, Collins SD, Buckby L, Grubb BD. The distribution of small and intermediate conductance calcium-activated potassium channels in the rat sensory nervous system. Neuroscience. 2005;131:161–175. doi: 10.1016/j.neuroscience.2004.09.062. [DOI] [PubMed] [Google Scholar]
- Muller CS, Haupt A, Bildl W, Schindler J, Knaus HG, Meissner M, Rammner B, Striessnig J, Flockerzi V, Fakler B, Schulte U. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc Natl Acad Sci U S A. 2010;107:14950–14957. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely A, Lingle CJ. Two components of calcium-activated potassium current in rat adrenal chromaffin cells. J Physiol. 1992;453:97–131. doi: 10.1113/jphysiol.1992.sp019220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Fukuoka T, Yi D, Tokunaga A, Hashimoto N, Yoshikawa H, Noguchi K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 2003;101:65–77. doi: 10.1016/s0304-3959(02)00296-8. [DOI] [PubMed] [Google Scholar]
- Obata N, Mizobuchi S, Itano Y, Matsuoka Y, Kaku R, Tomotsuka N, Morita K, Kanzaki H, Ouchida M, Yokoyama M. Decoy strategy targeting the brain-derived neurotrophic factor exon I to attenuate tactile allodynia in the neuropathic pain model of rats. Biochem Biophys Res Commun. 2011;408:139–144. doi: 10.1016/j.bbrc.2011.03.137. [DOI] [PubMed] [Google Scholar]
- Pezet S, Cunningham J, Patel J, Grist J, Gavazzi I, Lever IJ, Malcangio M. BDNF modulates sensory neuron synaptic activity by a facilitation of GABA transmission in the dorsal horn. Mol Cell Neurosci. 2002;21:51–62. doi: 10.1006/mcne.2002.1166. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sapunar D, Ljubkovic M, Lirk P, McCallum JB, Hogan QH. Distinct membrane effects of spinal nerve ligation on injured and adjacent dorsal root ganglion neurons in rats. Anesthesiology. 2005;103:360–376. doi: 10.1097/00000542-200508000-00020. [DOI] [PubMed] [Google Scholar]
- Sarantopoulos CD, McCallum JB, Rigaud M, Fuchs A, Kwok WM, Hogan QH. Opposing effects of spinal nerve ligation on calcium-activated potassium currents in axotomized and adjacent mammalian primary afferent neurons. Brain Res. 2007;1132:84–99. doi: 10.1016/j.brainres.2006.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Gruss M, Vogel W. Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. J Physiol. 1998;513:55–69. doi: 10.1111/j.1469-7793.1998.055by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh CC, Turner SC, Zhang XF, Milicic I, Parihar A, Jinkerson T, Wilkins J, Buckner SA, Gopalakrishnan M. A-272651, a nonpeptidic blocker of large-conductance Ca2+-activated K+ channels, modulates bladder smooth muscle contractility and neuronal action potentials. Br J Pharmacol. 2007;151:798–806. doi: 10.1038/sj.bjp.0707278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeper AA, Cummins TR, Dib-Hajj SD, Hormuzdiar W, Tyrrell L, Waxman SG, Black JA. Changes in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomy. J Neurosci. 2000;20:7279–7289. doi: 10.1523/JNEUROSCI.20-19-07279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppet D, Escandon E, Maragos J, Middlemas DS, Reid SW, Blair J, Burton LE, Stanton BR, Kaplan DR, Hunter T, Nikolics K, Parada LF. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991;65:895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- Squinto SP, Stitt TN, Aldrich TH, Davis S, Bianco SM, Radziejewski C, Glass DJ, Masiakowski P, Furth ME, Valenzuela DM, et al. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991;65:885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- Storer RJ, Immke DC, Goadsby PJ. Large conductance calcium-activated potassium channels (BKCa) modulate trigeminovascular nociceptive transmission. Cephalalgia. 2009;29:1242–1258. doi: 10.1111/j.1468-2982.2009.01849.x. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- Thompson SW, Bennett DL, Kerr BJ, Bradbury EJ, McMahon SB. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci U S A. 1999;96:7714–7718. doi: 10.1073/pnas.96.14.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonra JR, Curtis R, Wong V, Cliffer KD, Park JS, Timmes A, Nguyen T, Lindsay RM, Acheson A, DiStefano PS. Axotomy upregulates the anterograde transport and expression of brain-derived neurotrophic factor by sensory neurons. J Neurosci. 1998;18:4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vydyanathan A, Wu ZZ, Chen SR, Pan HL. A-type voltage-gated K+ currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons. J Neurophysiol. 2005;93:3401–3409. doi: 10.1152/jn.01267.2004. [DOI] [PubMed] [Google Scholar]
- Yang EK, Takimoto K, Hayashi Y, de Groat WC, Yoshimura N. Altered expression of potassium channel subunit mRNA and alpha-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neuroscience. 2004;123:867–874. doi: 10.1016/j.neuroscience.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Gopalakrishnan M, Shieh CC. Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience. 2003;122:1003–1011. doi: 10.1016/j.neuroscience.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nai Q, Chen M, Dittus JD, Howard MJ, Margiotta JF. Brain-derived neurotrophic factor and trkB signaling in parasympathetic neurons: relevance to regulating alpha7-containing nicotinic receptors and synaptic function. J Neurosci. 2004;24:4340–4350. doi: 10.1523/JNEUROSCI.0055-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XF, Rush RA. Endogenous brain-derived neurotrophic factor is anterogradely transported in primary sensory neurons. Neuroscience. 1996;74:945–953. doi: 10.1016/0306-4522(96)00237-0. [DOI] [PubMed] [Google Scholar]