Abstract
Chromatin is a dynamic complex of DNA and proteins that regulates the flow of information from genome to end product. The efficient recognition and faithful repair of DNA damage, particularly double-strand damage, is essential for genomic stability and cellular homeostasis. Imperfect repair of DNA double-strand breaks (DSBs) can lead to oncogenesis. The efficient repair of DSBs relies in part on the rapid formation of foci of phosphorylated histone H2AX (γ-H2AX) at each break site, and the subsequent recruitment of repair factors. These foci can be visualized with appropriate antibodies, enabling low levels of DSB damage to be measured in samples obtained from patients. Such measurements are proving useful to optimize treatments involving ionizing radiation, to assay in vivo the efficiency of various drugs to induce DNA damage, and to help diagnose patients with a variety of syndromes involving elevated levels of γ-H2AX. We will survey the state of the art of utilizing γ-H2AX in clinical settings. We will also discuss possibilities with other histone post-translational modifications. The ability to measure in vivo the responses of individual patients to particular drugs and/or radiation may help optimize treatments and improve patient care.
Keywords: γ-H2AX foci, DNA double-strand breaks, Ionizing radiation, Biodosimeter, Chemotherapy, Clinical study
1. Introduction
Much of the research on the origins of cancer has focused on cellular DNA since alterations in its sequence, of one kind or another, appear to be central to tumorigenesis. In some cases, these changes are manifested in genomic alterations involving chromosome rearrangements, duplications, and deletions, and may have originated from misrepaired DNA double-strand breaks (DSBs). These serious ramifications make the DSB among the most serious of DNA lesions.
DNA lesions occur in the context of chromatin, an organized structure of DNA and proteins consisting of fundamental units called nucleosomes, which contain DNA coils of 145–147 bp. The nucleosomes are spaced along the continuous DNA double-helix with varying lengths of DNA linking them [1]. The nucleosome consists of an octamer of histone proteins that form a core for the DNA coil. The linker is also a complex of DNA and proteins including other histones which spans the gap between adjacent nucleosomes while maintaining a continuous DNA double helix [1]. Compared to naked DNA, the DNA in the nucleosome is compacted ~6:1. Further organization of nucleosomes into 30 nm filaments increases the packing ratio to ~40:1. Looping of the filaments brings the ratio to ~1000:1 in interphase chromatin, and mitotic condensation brings the ratio to ~10,000:1. Thus the repair of DNA lesions also occurs in the context of a highly dynamic complex structure.
The histone structures are highly conserved, consistent with the notion that these proteins play similar roles throughout evolution. Nucleosomal histones belong to four families, H2A, H2B, H3 and H4, while the linker histones are from one family, H1 [2, 3]. Each histone family contains multiple genes [4], most of which encode identical or almost identical proteins [5]. These differences are thought to reflect selective pressure rather than differential function [5]. However, in some families, several genes encode proteins with unique functions. Notably, the H2A family contains two species, H2AZ and H2AX, which are present throughout eukaryotic evolution from yeast to human [5]. Each nucleosomal octamer contains two molecules of each of the four families noted above. However, while the factors determining the relative abundance of the different gene products in each family are obscure, H2AZ and H2AX are found in a substantial minority of the H2A positions in mammals [5, 6].
2. Histone post-translational modifications (PTMs)
Histone post-translational modifications (PTMs) are dynamic biological sensors that adjust in response to endogenous and/or exogenous cellular stress [7–10]. Because some of these stresses can be associated with diseases such as cancer, identifying alterations in histone PTM homeostatic levels may yield valuable clinical information on treatment efficacy and/or the condition of a disease [10, 11] (Figure 1). An increasing body of studies suggests that these histone PTMs, also referred as “the histone code” control both chromatin functions such as DNA replication, transcription, and DNA repair, and cell cycle (reviewed in [12]). A myriad of types and numbers of PTMs are present on the N- and C-terminal tails of all the histone species [13] (see Figure 2). This myriad of histone modifications include phosphorylation, acetylation, ADP-ribosylation, deamination, methylation, proline isomerization, sumoylation, propionylation, ubiquitination, and the most recently identified O-GlcNAcylation (Figure 2) [14–19]. To further increase the complexity of histone PTMs, lysine and arginine residues can be modified with mono-, di-, or tri-methyl groups and mono- or di-methyl groups respectively (Figure 2). It may be expected that new PTM sites and/or types still await discovery. However, because of its crucial role in DSB repair and genome stability, phosphorylated H2AX (γ-H2AX) has become one of the most widely known examples of a histone PTM in recent years (Figure 2).
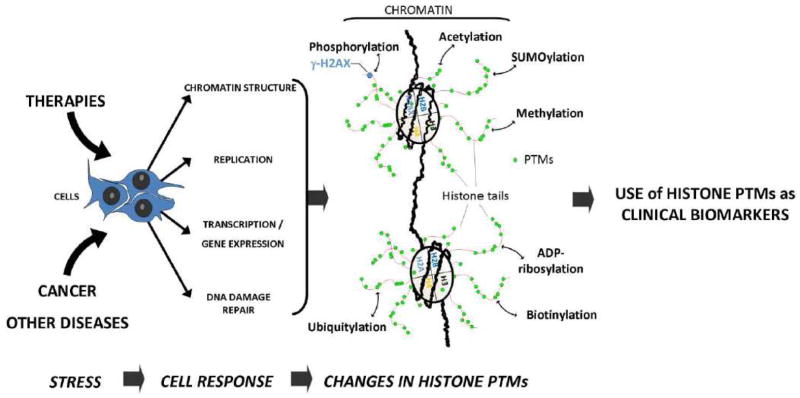
Figure 1. The rationale of using histone post-translational modifications in the clinic.
Because clinical treatments (radiotherapy and chemotherapy) and diseases such as cancer have an impact on chromatin structure, replication/transcription, and DNA repair, they will induce an alteration in histone post-translational modifications (including γ-H2AX formation). By identifying specific changes in histone PTMs, clinicians could assess a patient response to a treatment or a drug, as well as to evaluate a cancer prognosis.
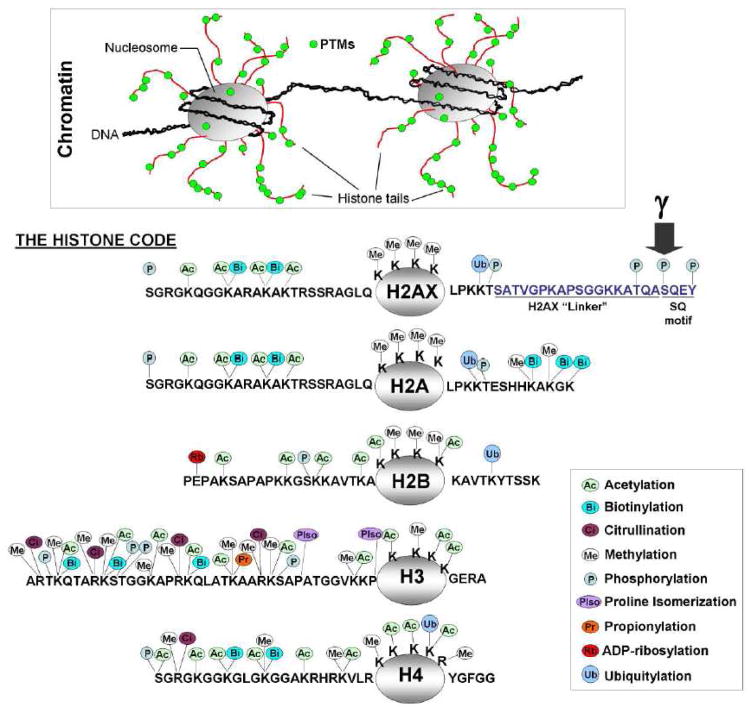
Figure 2. Core histone post-translational modifications.
The sites of post-translational modifications (PTMs) occur mostly within the histone tail domains (top inset). Specific amino acid PTM sites (acetylation, ADP-ribosylation, biotinylation, citrullination, methylation, phosphorylation, SUMOylation and ubiquitination) that are known to occur on the core histones, H2A, H2B, H3 and H4, are indicated by colored symbols (symbol key in the right inset). Some residues (Lysine, Arginine) can undergo severa,l identical or different, forms of post-translational modification (i.e. methylation or acetylation, dimethylation or trimethylation, etc …), thus increasing the complexity of the histone code. H2AX is an H2A variant with a unique longer C-terminal tail consisting of an evolutionarily conserved motif and a linker of variable sequence and length. The conserved motif contains the C-4 serine that is phosphorylated upon DNA DSB formation (arrow).
In this review, we discuss first the status of H2AX phosphorylation as a clinical tool. We then survey the clinical uses of other histone PTMs.
3. H2AX and DNA DSBs
While erroneous repair of DSBs is a major source of genomic instability and can lead ultimately to cancer, many cancer therapies induce DSBs as a way to kill cancer cells. For this reason, specific and sensitive in vivo biomarkers for DSB formation enable the responses of individual patients to these therapies to be monitored. The unique function of H2AX lies with the phosphorylation of a serine four residues from the C-terminus (C-4) [20], in a consensus sequence which has been conserved throughout eukaryotic evolution [21]. This modified form has been named γ-H2AX [20]. In mammals, many hundreds of histone H2AX molecules become phosphorylated in 10–30 minutes in the chromatin flanking each newly formed DSB [7]. These foci, named γ-H2AX foci, can be visualized with the appropriate antibody, enabling individual DSB detection by microscopy [7, 22] (Figure 3).
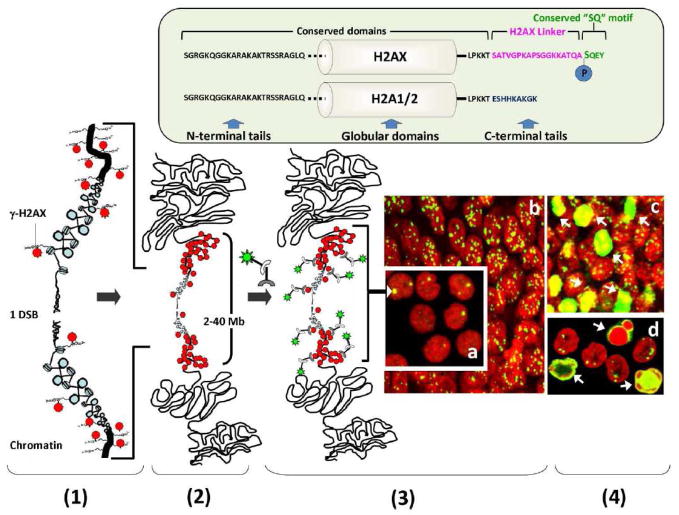
Figure 3. γ-H2AX focus formation and detection in human cells and/or tissues.
Top panel: H2AX is a variant of histone H2A that can replace other H2A subtypes in a subset of nucleosomes. Like other H2As, H2AX is composed of a central globular domain and two terminal tails. While the globular domain and the N-terminal tail are identical between H2AX and other H2As, H2AX holds a unique C-terminal tail containing an evolutionarily conserved “SQ” motif (green) connected by a “linker” that is variable, in sequence and length, through evolution (blue). The conserved “SQ” motif holds the C-4 serine that is phosphorylated upon DNA DSB formation. Bottom panel: Following exposure to radiation or genotoxic drugs and upon DSB formation, the H2AX C-4 serine is phosphorylated (γ-H2AX) (1). H2AX phosphorylation spreads away from the DNA break and can be found within a 2–40 Mb region surrounding the DSB site (2). As a result, the use of a specific anti-γ-H2AX antibody allows the visualization of DSBs as discrete foci (3) in cells (a, blood lymphocytes) as well as in tissues (b, hair bubs). In some cells, DSB induction can lead to apoptosis and accrued formation of both DSBs and γ-H2AX. Apoptosis can produce a γ-H2AX panstaining (c, d), a peripheral nuclear γ-H2AX staining (d) and/or a γ-H2AX staining of apoptotic bodies (d). Red: DNA; Green: γ-H2AX.
In human cells, H2AX abundance relative to total H2A varies from about 2% in lymphocytes to 20% in SF268 glioma cells [23], a ratio which places an H2AX molecule in every 2.5 to 25 nucleosomes on average. The rationale for these different relative amounts of H2AX between cell types is unknown; however, about 0.03% of the H2AX is phosphorylated per DSB irrespective of H2AX abundance [24]. This fraction indicates that at least 2 Mbp equivalents of chromatin are involved per DSB, but studies on metaphase chromosomes show that regions approximately ten times larger are involved, suggesting in turn that only about 10% of the H2AX molecules in a focus are phosphorylated at any one time [7, 24].
The synthesis of most of the histone species is tightly linked to DNA replication by means of a unique mRNA structure, while that of a few, notably H2AZ and H3.3, is independent of DNA synthesis [5, 25]. The regulation of H2AX synthesis is unusual in that it is partially linked to DNA synthesis [5]. Because H2AX is involved in DNA repair, this singular means of H2AX production may ensure that both proliferating and quiescent cells contain numbers of H2AX molecules sufficient for foci formation. The H2AX C-4 serine is phosphorylated by members of the phosphatidylinositol (PI) 3-kinase family, including ataxia telangiectasia mutated protein (ATM), AT and Rad3-related protein (ATR) and DNA-dependent protein kinase (DNA-PK) [26–28]. However, other kinases such as the c-Jun N-terminal kinase (JNK) have been shown to phosphorylate H2AX in certain situation [29]. The γ-H2AX foci serve to accumulate a myriad of types and numbers of protein species involved in DSB repair, chromatin remodeling, and cell cycle control (reviewed in [30, 31]). That these foci serve to accelerate DSB repair is evidenced by the finding that the H2AX-null mouse is sensitive to clastogenic agents and DSB repair is slower than in wild-type mice [32].
While the C-4 serine and surrounding residues of H2AX are highly conserved through evolution [21], the linker between it and the conserved globular H2A core region is of variable sequence and length in different species. One hint of a possible explanation for the variation in the linker length among organisms is that it correlates with the variation in internucleosomal length of organisms through evolution, with single-celled eukaryotes having the shortest linker and mammals the longest [33].
4. γ-H2AX as a biomarker for DNA DSBs in vivo
Cancer treatment may be improved if more information were available on the responses of individual patients to particular protocols. Currently, patient responses to treatments may be unknown for several weeks until tumor size is assessed by various imaging techniques [34]. By one criterion, RECIST, a 30% decrease in tumor diameters is considered a partial response. The Choi criterion also takes account of changes in tumor density. A more recent criterion for evaluating patient responses is disease nonprogression, useful for cytostatic drugs which may not cause tumor shrinkage but do prevent tumor progression.
In addition, tumor markers or signatures, corresponding to multiple sets of genes or mRNAs that correlate positively with tumor outcome, are being examined. However, many markers are not tumor or even cancer specific and, unless carefully vetted, outcome signatures may not correlate with prognosis any better than random signatures [35].
Of course, the ultimate criterion for treatment success is long-term survival, information which is slow to accumulate. In light of these issues, a useful adjunct to these criteria may be the capability to measure patient drug responses at the molecular level. As mentioned above, the use of specific antibodies permits the visualization of γ-H2AX foci at individual DSB sites, enabling researchers to measure the clastogenic efficiency of a particular drug or radiation protocol in a particular patient. Such information may be useful for both to improve treatment protocols and to assess patient prognosis..
5. γ-H2AX assay types
Detection of γ-H2AX, utilizing a variety of assays, is being used beyond basic research as a biomarker for cancer, a biodosimeter for radiation exposure and drug development, and a tool to identify genotoxic compounds in occupational or environmental studies [36] (Figure 4). However, microscopy is still the prevailing method for γ-H2AX detection for clinical applications since it is the most sensitive of the possible approaches, capable of detecting a single DSB [23]. Solid tissues can be processed as well as peripheral blood mononuclear cells (PBMCs) (reviewed by [23, 37, 38]). And importantly for research purposes, analysis by microscopy may discriminate between differential γ-H2AX responses with respect to drug type and cell population makeup.
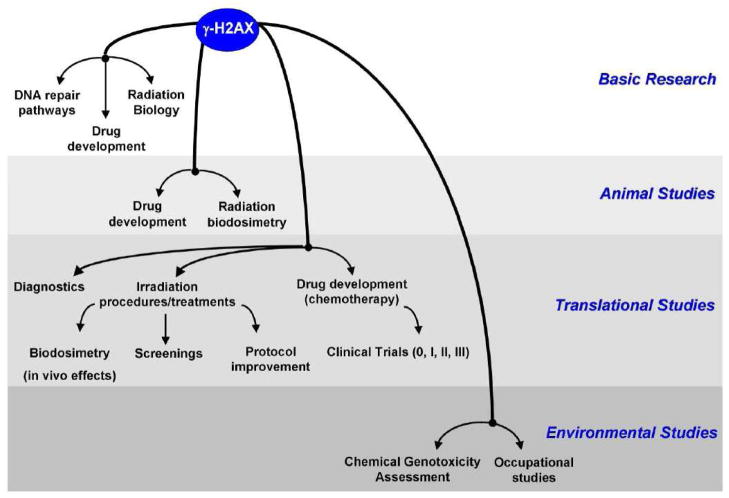
Figure 4. Clinical applications for γ-H2AX.
The γ-H2AX assay has been developed for many clinical applications. In addition to its extensive used for basic research (to study DNA repair, drug mechanistic, radiation biology, etc…), γ-H2AX has been developed in animal studies (both for radiation research and drug development) and in the clinic (diagnostics, radiation biodosimetry, drug development). Because of its sensitivity, the γ-H2AX assay has also been used in environmental studies to identify genotoxic compounds or occupational practices leading to DNA damage.
While ionizing radiation and radiomimetic chemicals induce γ-H2AX foci in virtually all cells, whether quiescent or proliferating, drugs that interfere with DNA replication induce foci primarily in proliferating cells, leaving quiescent cells with fewer foci [23]. These replication-linked foci result generally from stalled replication forks or collisions with trapped topoisomerase complexes [39]. There are also other responses. For example, imatinib mesylate, a selective small-molecule protein kinase inhibitor, induces apoptosis in gastrointestinal stromal tumor cells, which is accompanied by strong γ-H2AX staining of pyknotic nuclei [40].
There is an ongoing intensive development of high throughput γ-H2AX foci counting systems for clinical assays to automate microscopic examination, including image processing that are intended to speed up analysis while maintaining sensitivity [41–43]. Another advantage unique to microscopy is that it can discriminate objects, such as foci from the background, while flow cytometry, also used with single cell suspensions such as PBMCs, cannot. Thus, while analysis by flow cytometry may be quicker than by microscopy, it is not as sensitive. Finally, a key advantage of microscopy vs. flow cytometry comes from the fact that tissue samples, including tumor biopsies, can be analyzed by microscopy while flow cytometry analysis is restricted to single cells (PMBCs, and bone marrow cells, among others).
In addition to γ-H2AX detection by microscopy in fixed cell or tissue samples, other types of assays utilizing cell and tissue extracts are available, but are as yet not available for the clinic (i.e., electrochemoluminescent-based detection system, and whole cell ELISA) [44, 45].
6. Biological samples for γ-H2AX assays
Because cell proliferation is different among human tissues (i.e. higher proliferation rates in intestine, and bone marrow), the choice of biospecimens is critical for studies of drugs that target DNA metabolism. In contrast, protocols using radiation (i.e. radiotherapy) may produce DSBs more homogeneously throughout the human body, independently of DNA metabolism. There are several possible choices for patient tissue samples, each with its advantages and issues [38]. One option is to obtain tumor tissue samples by surgery or biopsy to assess drug impact. However, tumors collection may be difficult or unsafe for the patient, and several complexities cloud straightforward interpretation of the relationship between γ-H2AX focal incidence and tumor response to a drug. For example, tumor heterogeneity due to differences in vascularization and genetic makeup may affect γ-H2AX formation and removal, confounding the relationship of γ-H2AX formation with tumor prognosis. Thus γ-H2AX responses may differ among different metastases in the same patient as well as among different cells of the same tumor mass.
Nevertheless, being able to assess changes in γ-H2AX levels before and after drug treatment may reveal useful information. Recently, a novel method to obtain patient cancer cells by non-invasive means has been developed. Many tumors shed cells into the blood stream, cells which may be the source of future metastases. These circulating tumor cells (CTCs) are rare, ~1–10 per 7.5 ml blood, but their incidence has been correlated with progression-free, and overall survival [46]. CTCs isolated from patients after treatment exhibited increased incidence of γ-H2AX foci, indicating that they may be useful in assessing the efficacy of administered drugs [47]. Thus knowing how CTCs respond may be useful for optimizing cancer treatments.
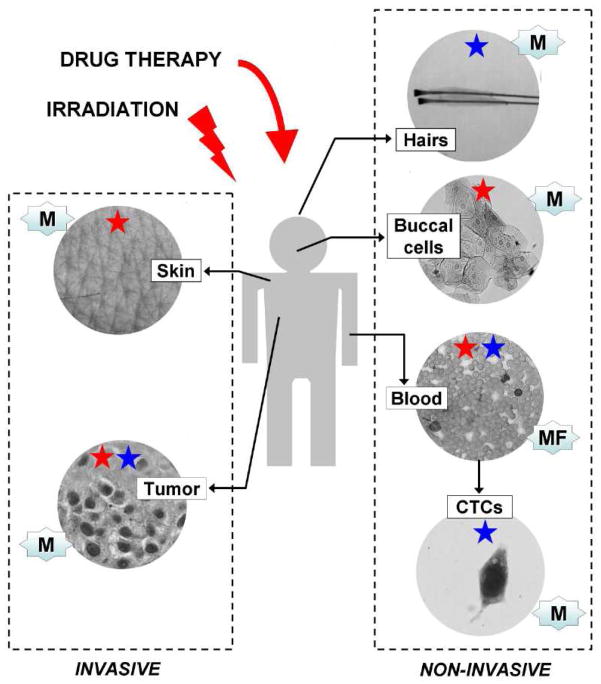
Many chemotherapeutic agents also target the patient’s normal cells. Compared to tumor cells, γ-H2AX responses in normal cells may be more uniform, reproducible, and informative. Also, several types of normal cells can be obtained non-invasively (Figure 5). Levels of γ-H2AX have been quantified by microscopy or flow cytometry in PBMCs, and by microscopy in skin biopsies, plucked hair bulbs and buccal cells [48–52]. However, the analysis of PBMCs is still by far (> 90%) the method of choice in examining γ-H2AX formation in vivo (see tables). Following blood collection, PBMCs are purified by density gradient prior to γ-H2AX staining. The use of PBMCs is already a standard procedure in many clinical protocols, and venous blood samples are commonly drawn in the clinic. In addition, PBMCs contain low γ-H2AX focal background levels, on average less than one focus per 5–10 cells [53]. Such low backgrounds improve the detection of low levels of DNA damage and enable measurement of irradiation doses as small as 1 mGy, equivalent to an average of 0.1 foci per cell [54].
Figure 5. Human samples and methods used for the γ-H2AX assay.
While tumor sampling is the most appropriate biospecimen for γ-H2AX detection to follow cancer treatments, such biopsies are often poorly accessible, particularly for repetitive sampling. For this reason, clinicians have to rely on other sources of biospecimen that can be obtained non-invasively (blood samples, circulating tumor cells (CTCs), buccal cells, plucked hairs) or invasively (skin biopsies). It should be noted that some of these samples can be used for the detection of other histone PTMs (see Table 4). M: microscopy; MF: Microscopy (i.e. immunohistochemistry or immunocytochemistry) and FACS; Red star: human samples used for radiation biodosimetry; Blue star: human samples used for drug development.
The major disadvantage of PBMCs is their state of terminal differentiation, which might make them less useful for studies of chemotherapeutic drugs that produce DSBs by interfering with DNA replication [38]. However, another accessible tissue that does contain proliferating cells is the skin. The keratinocytes proliferate from a basal layer and differentiate as they migrate toward the skin surface [55]. Skin biopsies are necessary to obtain the basal keratinocytes, an issue which limits their routine use due to its invasive nature. It is noticeable in sections of skin biopsies that hair follicle cells often exhibit the largest γ-H2AX response after drug treatment [56, 57]. An alternative, and less invasive procedure for obtaining at least some of these follicle cells, is plucking [58, 59]. Plucked hairs have been previously used as a surrogate tissue in the clinic for diagnostics [60–62] and a recent phase 1 clinical trial included the use of γ-H2AX detection in plucked eyebrow hair bulbs to confirm the effects of a PARP (Poly (ADP-ribose) polymerase) inhibitor in vivo [49]. Like plucked hairs, the use of exfoliative oral cells has been previously promoted as a non-invasive technique for cancer diagnosis [63] and for γ-H2AX detection [64].
7. Applications: γ-H2AX as a radiation biodosimeter
The most obvious application of γ-H2AX measurements is the assessment of DNA damage from ionizing radiation. The radiation can be external as during typical radiation treatments or internal as during radioisotope therapy, where radioisotopes are given by infusion or ingestion. A non-exhaustive list of clinical studies utilizing γ-H2AX as a biodosimeter is presented in Table 1.
Table 1. Non-exhaustive list of clinical studies using the γ-H2AX assay for radiation biodosimetry.
Studies are separated in 3 major groups: x-ray examination (top), computed tomography (middle) and radiotherapy (bottom). A study illustrating the use of γ-H2AX as a biomarker to study the impact of space radiation on human health was added in the last row. All studies described in Table 1 used microscopy (i.e, immunocytochemistry or immunohistochemistry) for γ-H2AX detection.
| Procedure | Sample | Dose | Study purpose | Ref. |
|---|---|---|---|---|
| x-ray examination | PBMCs | 0.230 – 0.856 Gy cm(CT) 6.31–30.36 Gy.cm2(PTA) |
DNA damage evaluation during percutaneous transluminal angioplasty | [123] |
| x-ray examination | Oral cells | 2-3 cGy | Validation of γ-H2AX as a biomarker for low dose radiation exposure | [64] |
| x-ray examination | PBMCs | 10-3170 cGy.cm2 | DNA damage evaluation during pediatric cardiac catheterization | [71] |
| x-ray examination | PBMCs | 337-29281 μGy.m2 | DNA damage measured after angiographic procedure | [70] |
| x-ray examination | PBMCs | 112-1025 Gy. cm | DNA damage measured after coronary CT angiographic procedure | [124] |
| CT | PBMCs | 157-1.514 mGy.cm | Evaluation of DNA damage/repair during CT examinations | [53] |
| CT | PBMCs | 5.16-13.85 mGy | DNA damage measured after multi- detector row CT examinations | [125] |
| CT | PBMCs | 200-1800 mGy.cm | To compare the biological effects between helical and sequential coronary CTA as well as other CT parameters | [72] |
| CT | PBMCs | 522 to 1102 mGy.cm (Blood dose 8–20.6mGy) | To investigate the biological effects of different scanner settings | [52] |
| Radiotherapy | PBMCs | 2-2.17 Gy(SD) 72-76 Gy (CD) |
To compare the biological effects (DSBs) of 3D and SSIMRT irradiation protocols to treat prostate cancer. | [69] |
| Radionuclide therapy | PBMCs | 0.17-0.57 Gy | Measure of dose accumulation after administration of (131)I for thyroid remnant ablation | [78] |
| Radiotherapy | PBMCs | 1.6-2 Gy per fraction | Evaluation of DNA damage in different areas of the body after local radiotherapy; Estimation of the applied integral body dose | [67, 88] |
| Radiotherapy | Skin | 0.05-1 Gy | To evaluate the low-dose hypersensitivity response in skin of patients undergoing radiotherapy | [126] |
| Radiotherapy | Skin | ~1 Gy | Evaluation of DNA damage induction in prostate cancer patients undergoing radiotherapy | [48] |
| Radiotherapy | Glioma cells from cerebrospinal fluid | 24-30Gy (CD) | Evaluate radiotherapy-induced DNA damage induction in glioma cells collected in cerebrospinal fluid CSF cytological specimens | [127] |
| Space radiation | lymphoblastoid cells | 0.7 mSv per day | Evaluate the DNA damage induced by space radiation | [128] |
Abbreviations: FC, flow cytometry; PTA, percutaneous transluminal angioplasty; SD, single dose; CD, cumulated doses; CT, computed tomography; CTA, computed tomography angiography; PTA, percutaneous transluminal angioplasty; 3D, three dimensional conformal; SSIMRT, step-and-shoot-intensity modulated radiotherapy. PBMCs, peripheral blood mononuclear cells.
7.1 External irradiation
Because exposure to ionizing radiation from medical procedures has increased sharply in the last previous decades [65], radiation biodosimetry is increasingly becoming a priority in the clinic. Until recently, radiation received by patients has been quantitated solely by mathematical models and/or physical measurements with no reliable tool to measure its biological effects in patients [66].
Thus, new radiation biomarkers are needed to help evaluate the health risks from radiation exposures. Such biomarkers would help determine the doses received by patients and the biological effects of ionizing radiation on those individuals. Additionally, they would help clinicians to improve irradiation procedures used for diagnostics and/or treatments. Many studies have demonstrated the relevance of using γ-H2AX to detect and quantify DSBs. Microscopy has revealed robust γ-H2AX foci following ionizing radiation with the number of foci strongly corresponding to the expected number of DSBs [54]. Ex vivo irradiation of lymphocytes results in the appearance of 10 to 15 foci per Gy per cell within minutes after exposure [59, 67]. While γ-H2AX formation is rapid, reaching a maximum within 30 min of irradiation, some foci may remain for hours or days with the residual foci numbers also being proportional to the initial dose received [59, 68].
The first study to introduce the use of γ-H2AX in the clinic analyzed skin punch biopsies collected from prostate cancer patients undergoing radiotherapy [48] (Table 1). Microscopy revealed a linear response between γ-H2AX signals and irradiation doses in the biopsies taken at different distances from the radiation field. Their results suggested that a standard technique using γ-H2AX could be developed to assess the radiation doses in exposed individuals and to follow the biological effect in vivo of such radiation. Soon after, another study followed the formation and disappearance of γ-H2AX foci in lymphocytes of individuals subjected to small radiation doses during computed tomography examinations [53]. Similarly, the mean numbers of γ-H2AX foci in patient lymphocytes treated by radiotherapy was found to exhibit a linear dependence on the applied mean body doses [67]. However, the dependence between dose and average incidence of γ-H2AX foci in lymphocytes was found to differ by as much as four-fold depending on the location of the irradiated site on the body. Lymphocyte distribution, circulation and migration through the different organs may explain these differences. For example, lymphocytes that migrate more slowly in capillaries vs. large vessels may receive more radiation dose [67, 69].
Overall, γ-H2AX has been used as to follow the biological effects of a wide range of exposures, from a few cGy, to cumulative doses greater than 70 Gy, doses encountered during diverse clinical procedures ranging from routine examinations (x-ray examination, computed tomography (CT)), to surgical procedures using radiation-x-ray imaging (coronary angiographic procedures, cardiac catheterization), and to cancer radiotherapy (Table 1). Unexpected findings may come from these studies. In one, lymphocytes taken from individuals, adults, and children, undergoing angiographic procedures [70] or cardiac catheterization [71] exhibited generally good correlations between γ-H2AX levels and radiation doses, however in some cases with children, the incidence of foci in the low-dose range were substantially greater than expected [71]. The authors of this study concluded that pediatric radiation risks may be underestimated and suggested that further safety measures should be taken to minimize and optimize children exposure to radiation.
γ-H2AX measurements have been used to compare radiation protocols that differ in their dose-distribution for local staged prostate cancer (Table 1). Comparison of two methods, Step-and-Shoot-IMRT and conventional 3D conformal radiotherapy revealed differences in the incidence of γ-H2AX foci in the lymphocytes, leading the authors to confirm γ-H2AX measurements as a means to document the reduction of medium-dose-exposure for normal tissue by Step-and-Shoot-IMRT [69]. This finding demonstrates how the biological effects of low-dose radiation can be documented for patients in order to use the lowest dose consistent with obtaining the desired results. Another clinical study of patients subjected to a thoracic or abdominal contrast CT scan utilized two CT scanners with dose settings differing by a factor of two. The authors found twice as many foci at the higher setting, and concluded that lowering scanner settings will result in a reduction of X-ray-induced DNA damage in patients [52].
In another comparative study, patients with possible coronary artery stenoses underwent multidetector coronary computed tomography angiography using two protocols, helical data acquisition with retrospective electrocardiogram (ECG) gating, and sequential data acquisition. The authors showed that the sequential data acquisition method, which permits a relevant dose reduction, led to lower γ-H2AX foci levels [72]. Additionally, this study revealed that the addition of calcium scoring that uses a higher radiation dose (to check for the buildup of calcium in plaque on the walls of coronary arteries) led to a significant elevation of γ-H2AX foci numbers. The authors of this study concluded that clinical procedures should carefully consider the mode and adapt the CT protocols in order to avoid exposure to unnecessary radiation. In addition, individual factors, such as the body mass index, should be considered to adjust CT settings.
Apart from measuring the absorbed doses by patients in clinical procedures, γ-H2AX-based biodosimetry could be useful with victims of accidental exposure. Individuals receiving radiation doses requiring immediate life-saving medical treatments could be identified. A recent study evaluated the use of γ-H2AX as a biodosimeter, using non-human primates subjected to total-body irradiation in the non-lethal to lethal dose ranges [59]. Using pragmatic scenarios for accidental exposures, the authors showed that γ-H2AX detection in lymphocytes and plucked hair bulbs may be useful for estimation of radiation dose at times at least 4-days post-exposure at doses of 3.5 Gy and above. A robotic system using the γ-H2AX assay in lymphocytes has been developed to respond to major radiological accidents. The fully automated high-throughput system (named RABIT for Rapid Automated Biodosimetry Tool) was designed to analyze γ-H2AX fluorescence in lymphocytes present in a single drop of blood from a fingerstick. The automation of lymphocyte isolation, immunolabeling of γ-H2AX and high speed imaging allows the analysis of up to 30,000 samples per day [41, 73]. Similarly, a portable microfluidic fluorescence spectrometer, using a suspension of lymphocytes stained for γ-H2AX, is being developed and could become a portable triage tool for field use to help classify people into appropriate treatment categories based on radiation exposure levels [74].
7.2 Internal irradiation
In addition to its use as a biodosimeter for external radiation, γ-H2AX has been employed during therapy in which radioisotopes were given by infusion or oral ingestion. In cell culture, these radioisotopes were shown to generate numbers of DSBs that correlated with the levels of γ-H2AX foci [75]. The genotoxicity of several constructs containing radioisotopes, 125I, 111In, and 213Bi [75–77], were tested in cell culture utilizing γ-H2AX measurements. γ-H2AX was recently introduced in a clinical study using 131I radionuclide therapy after surgery to ablate remnant thyroid tissue and treat any iodine-avid metastases [78]. Because the blood is irradiated by β-particles from circulating 131I and from penetrating -radiation originating from activity dispersed throughout the body, γ-H2AX was quantified in leukocytes at various times after iodine administration. The peak of the γ-H2AX signal was observed at 2 hr post administration and declined thereafter; however, substantial γ-H2AX signals were still observable after 6 days. In 24 patients evaluated for physical dosimetry, there was a good correlation between γ-H2AX levels and the mean absorbed doses which ranged between 0.17 and 0.57 Gy. The authors concluded that γ-H2AX is a useful marker for detecting radiation exposure from radionuclide treatments, even for absorbed doses below 20 mGy to the blood. The authors did note, however, that there was also substantial inter-individual variation. A factor that is important with interpretation of γ-H2AX data in irradiation studies is that during internal medicine procedures, radionuclides are systemic and continuously present in the body; while during external irradiation procedures, such as CT scan, radiotherapy, angioplasty, etc, the exposures are short-term and acute, with quasi-synchronization of DSB formation and repair. Moreover, while external treatments generally involve partial-body-irradiation, individuals receiving radionuclide therapy are subjected to whole-body internal irradiation.
Another issue that remains to be resolved is the inter-individual variation often observed. This may be due to individual differences in physiology or to unnoticed differences in procedure. If the former, γ-H2AX-based biodosimetry gives researchers the tools to study the relevant physiological factors (i.e. age, alteration of genes involved in DNA repair), and if the latter, it enables researchers to determine the important variables in a procedure. Yet, one way to better appreciate the inter-variability between patients would consist in minimizing variations due to differences in sample preparation and analysis. This may be accomplished by reducing the human error factor through the development of automated sample preparation together with automated microscopy, image processing and foci quantitation [79].
8. Applications: The use of γ-H2AX in drug efficacy measurements
There are many therapeutic strategies to combat cancer. These include among others, hormonal therapy to impede growth signaling through hormone receptors on cancer cells, immunotherapy to boost the immune system to increase cancer cell killing, targeted therapy to thwart growth signaling pathways in cancer cells using small enzyme inhibitors or antibodies, and chemotherapy to interfere with cell division and DNA metabolism [80]. As with radiotherapy, chemotherapy can act by generating sufficient DSB numbers in cancer cells to induce cell death [23]. Thus monitoring DSB formation using γ-H2AX can be a sensitive indicator of drug efficiency. γ-H2AX has been used in cell culture studies [23], and animal experiments [81] as routes to measure drug toxicity, pharmacokinetics, and efficacy.
γ-H2AX was first used in a clinical study to test a combination of clofarabine and cyclophosphamide for patients with relapsed acute leukemias [82]. In 12 of 13 patient samples, γ-H2AX assays indicated that the drug combination of clofarabine and cyclophosphamide induced greater amounts of DNA damage compared to cyclophosphamide alone. To date, γ-H2AX has been or is intended to be used as a pharmacodynamic biomarker in more than three dozen clinical trials of a broad spectrum of drugs generating DNA damage, including DSBs, directly or indirectly (see Table 2 or clinicaltrials.gov). These drugs include DNA alkylating and/or cross-linking agents, topoisomerases 1 and 2 inhibitors, nucleoside analogues, a mitotic inhibitor, PARP inhibitors (involved in DNA repair [38]) and histone deacetylase (HDAC) inhibitors ([83]) (see Table 2 or clinicaltrials.gov). Many clinical protocols involving the γ-H2AX assay are investigating drug combinations of a PARP (i.e., olaparib and veliparib) or HDAC inhibitor (entinostat) with a DNA-binding antitumor drug (Table 2). The working hypothesis behind these combination protocols is that an enzyme inhibitor blocking some aspect of repair will increase the potency of the DNA damaging agent [38].
Table 2.
Non-exhaustive list of clinical studies using the γ-H2AX assay to measure chemotherapeutic drug effects in cancer patients.
| Condition | Drug(s) | Tissues analyzed | γ-H2AX detection | Phase | References or ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| Head and neck squamous cell carcinoma | Cisplatin/Raltegravir (MK-0518) | Tumor | M | 0 | NCT01275183 |
| Solid tumors | Veliparib (ABT-888)/irinotecan | Tumor/PBMCs | M | I | [129] |
| Her-2 negative metastatic breast cancer | Veliparib/Carboplatin | CTCs | M? | I | [130] |
| Solid tumors and lymphomas | Veliparib/Topotecan | CTCs/PBMCs | M | I | [84] |
| Breast cancer | Olaparib (AZD2281) | Eyebrows | M | I | [49] |
| MDS, CML, leukemia, and AML | 5-Azacytidine/Entinostat (MS-275) | PBMCs | IB | I | [131] |
| AML | Tipifarnib/Etoposide | AML marrow blasts | FACS | I | [132] |
| Solid tumors | SJG-136 | PBMCs/Tumor | IHC | I | [133] |
| Leukemias | Clofarabine/Cyclophosphamide | PBMCs | FACS | I | [82] |
| MDS, AML, CML | 5-Azacytidine/Entinostat | N/A | N/A | I(*) | NCT00101179 |
| Metastatic, unresectable or recurrent solid tumors | Veliparib/mitomycin C | Blood (PBMCs) | M | I | NCT01017640 |
| Solid tumors; BRCA1, BRCA2 mutations carriers | Veliparib | PBMCs/Skin/Hairs | N/A | I | NCT00892736 |
| Solid tumors or lymphomas | Veliparib/Cyclophosphamide | Blood (PBMCs)/Tumor | M | I | NCT00810966 |
| Advanced solid tumors | /Iniparib (BSI-201) | N/A | N/A | I(*) | NCT01161836 |
| Solid malignancies | 7-t-butyldimethylsilyl-10-hydroxycamptothecin | Tumor | M/IB | I | NCT01202370 |
| Lymphoma, solid tumors | Indenoisoquinolines | Tumor/Skin | N/A | I | NCT01245192 |
| Glioblastoma | Olaparib/Temozolomide | Tumor | M | I | NCT01390571 |
| Glioblastoma | Iniparib/Temozolomide | N/A | N/A | I, II | NCT00687765 |
| Uterine carcinosarcoma | Carboplatin/Paclitaxel/Iniparib | N/A | N/A | II | NCT00687687 |
| Triple negative breast cancer | Gemcitabine/Carboplatin/Iniparib | N/A | N/A | II | NCT00813956 |
| Ovarian cancer | Iniparib | N/A | N/A | II | NCT01033123 |
| Glioma | TH-302 | N/A | M TB | II | NCT01403610 |
| Breast cancer | gemcitabine/carboplatin/Iniparib | N/A | N/A | III | NCT00938652 |
| Stage IV squamous non-small-cell lung cancer | Gemicitabine/Carboplatin with or without Iniparib | N/A | N/A | III | NCT01082549 |
Abbreviations: M, microscopy (immunocytochemistry or immunohistochemistry); I, Immunoblotting; FACS, Fluorescence-Activated Cell Sorting; CTCs, Circulating Tumour Cells; PBMCs, peripheral blood mononuclear cells; N/A, not specified; (*) study completed; CML, chronic myelogenous leukemia.
There are two main motivations for the clinical use of γ-H2AX during chemotherapy. First, γ-H2AX is a pharmacodynamic biomarker which may help determine the genotoxic potential of novel anti-cancer drugs in patients. Second, γ-H2AX assays may potentially allow clinicians to tailor treatment to individuals, taking into account sensitivities and/or previous treatments. In addition to phase I, II and III clinical trials for drug development, γ-H2AX is also currently being utilized in Phase 0 studies (Table 2). Phase 0 is a recent exploratory protocol using small groups of patients designed to accelerate the development of promising drugs by testing whether a drug performs as expected from preclinical in vitro studies [84]. In Phase 0 studies, patients are treated with a potentially active agent at 10% of its expected effective dose in order to observe whether there are any molecular changes indicative of drug activity not just in vivo but in humanus. Thus, γ-H2AX is utilized to measure any DSBs in the patient’s cells at this sub-therapeutic dose.
9. Applications: γ-H2AX and clinical diagnostics
In addition to its use in the clinic as a marker for both radiation biodosimetry and drug development for cancer research, recent studies have also demonstrated the putative use of γ-H2AX for medical diagnosis (Table 3). The following section will describe how the presence of γ-H2AX residual foci have been correlated with radiosensitivity in individuals as well as the potential of γ-H2AX to analyze cancer progression or the impact of other diseases on genome integrity.
Table 3.
Non-exhaustive list of clinical studies using γ-H2AX for diagnostics
| Samples | Details of diagnostics | IR protocol | Assay | Refs. |
|---|---|---|---|---|
| RADIATION TOXICITY | ||||
| Lymphoblastoid cells | Use of γ-H2AX to discriminate cells from individuals carrying the ATM mutation | 0.1Gy/h - 24 h | M | [134] |
| G0 T cells | T cells from AT and NBS patients show impaired elimination of radiation-induced DSBs | 0.5-2Gy at 2Gy/min | FC | [135] |
| G1 Skin fibroblasts | Use of γ-H2AX to show radiation hypersensitivity in cells from parents of RB patients screened as well as in 6 of 15 from apparently normal individuals | 0.5 – 1.0 Gy at 250 cGy/min or 10 cGy/h for 24 h | M | [136] |
| T-and lymphoblastoid cells/PBMCs | Confirmation of radiosensitive A-T patients | 2 Gy | M | [137, 138] |
| PBMCs | Use of γ-H2AX to predict tissue toxicity (mucositis) in patients undergoing head-and-neck radiotherapy | 2 Gy (SD) 60–66 Gy (CD) |
FC | [139] |
| G1 Skin fibroblasts | Use of γ-H2AX to show that cells from Fanconi anemia patients display a significant delay hi the repair of radiation-induced DSBs | 1 Gy (0.45 Gy/min) | M | [140] |
| Fibroblasts | Confirmation of a novel splice variant of the DNA-PKcs gene associated with radiosensitivity | 2Gy | M | [141] |
| PBMCs | Use of γ-H2AX to identify children at risk for radiation toxicity | 1–2 Gy (1 Gy/min) | M | [142] |
| Lymphoblast cell lines | The identification of a patient with a DNA repair defect during a screening for radiosensitivity | 1, 2, 4 Gy (0.62 Gy/min) | M | [86] |
| PBMCs | Use of γ-H2AX to predict excessive normal tissue toxicity in radiotherapy patients | 2 Gy | FC | [88] |
| Blood samples | γ-H2AX as a molecular predictor of prostate cancer radiosensitivity | N/A | N/A | NCT00523471 (#) |
| OTHER DIAGNOSIS | |||
|---|---|---|---|
| Samples | Details of diagnostics | Assay | Refs. |
| Tumor biopsies | γ-H2AX as a potential cancer biomarker | M | [90] |
| Tumor biopsies | Diagnosis for metastatic renal cell carcinoma | M | [92, 143] |
| Colon biopsies | Increased DNA damage in colon of ulcerative colitis patients | M | [99] |
| Melanomas | Evaluation of the γ-H2AX assay as a marker for melauocytic lesions | M | [91] |
| Bladder urothelial carcinoma | Use of γ-H2AX to predict cancer recurrence and/or progression | M | [144] |
| PBMCs | Increased DNA damage and cell death in lymphocytes from patients with occult HBV infections | N/A | [104] |
| Immortalized lymphoblasts | Increased basal DNA damage in cells from individuals with schizophrenia | FC | [106] |
| Lung tissue (alveolar wall cells) | Increased DNA damage levels in lungs of advanced COPD patients | M | [100] |
| Malignant plasma cells | DNA damage escalation during the development of multiple myeloma | M | [95] |
| Fibroblast, T cells | Increased γ-H2AX in lymphocytes and fibroblasts of dyskeratosis congenital patients | M, FC | [102] |
| PBMCs | Increased DNA damage in lymphocytes from obese and overweight children | M | [101] |
| Curettage specimens of endometrial cancer | Use of γ-H2AX as an additional histopathological prognostic parameter in patients with endometrial cancer | M | [145] |
| Squamous epithelia of the uterine cervix | Use of γ-H2AX as a cancer biomarker in patients with cervix cancer | M | [146] |
| N/A | Use of γ-H2AX as a biomarker in women undergoing IVF Treatment | N/A | NCT00685282 (#) |
Abbreviations: M: microscopy (immunocytochemistry or immunohistochemistry); FC: flow cytometry; N/A: not applicable; PBMCs, peripheral blood mononuclear cells; SD, single dose; CD, cumulated doses; (#): ClinicalTrials.gov Identifier
9.1 Radiation hypersensitivity
Radiotherapy remains a major cancer treatment strategy, but normal tissue toxicity represents a serious limiting factor. It is estimated that between 1–5% of radiotherapy patients exhibit severe side effects, e.g., mucositis, erythema, edema, and fibrosis among others [85–87]. Therefore, there is a crucial need to find predictive markers of radiation hypersensitivity. Such markers would allow the diagnosis of radiosensitive patients and therefore allocate individualization of radiotherapy through modified irradiation protocols. Several assays, (e.g., micronucleus, colony survival, DNA pulsed-field gel electrophoresis, and comet assay), have been tested as predictors of radiosensitivity in normal tissues with modest success [86]. Because radiosensitivity is often observed in patients with genetic disorders linked to DNA damage repair defects, (e.g., ATM, retinoblastoma, Nijmegen breakage syndrome, Fanconi anemia, Ligase IV syndrome, and Seckel syndrome among others), the use of γ-H2AX is likely to be a good candidate to quickly predict DNA damage repair defects and therefore radiosensitivity [88]. A non-exhaustive list of clinical studies utilizing γ-H2AX for radiation toxicity is compiled in Table 3. The first radiosensitive patient uncovered in a clinical study using γ-H2AX showed abnormal kinetics of γ-H2AX foci after undergoing CT examinations [53].
9.2 Tumor diagnosis
γ-H2AX levels were found to be increased significantly in both precancerous and cancerous lesions, supporting the notion that genomic instability precedes cell transformation [89, 90]. On that basis, several studies have suggested that γ-H2AX may be used for the diagnostics of cancer development (Table 3). Diagnosing metastatic renal cell carcinoma (RCC) by fine-needle aspiration can be challenging. However, γ-H2AX was shown to be a useful adjunct in the diagnosis of this disease compared to other existing diagnostic antibodies [91, 92].
γ-H2AX was also found to be helpful in understanding cancer development in blood malignancies. Multiple myeloma is an aggressive and deadly hematological malignancy. Usually, disease progression is accompanied by the clonal expansion of malignant plasma cells in the bone marrow [93]. Although multiple myeloma is virtually always preceded by a stable precursor condition termed monoclonal gammopathy of undetermined significance (MGUS), the precise mechanism(s) of the disease development are still unknown [94]. A recent study showed ongoing DNA damage intensification across the disease spectrum; with low γ-H2AX levels in cells of MGUS patients compared to high γ-H2AX levels in plasma cells from patients with multiple myeloma. In addition, γ-H2AX foci were not observed in any of the normal PC or B-cell samples examined [95].
However, the use of γ-H2AX assays can have limitations. Although several studies have reported that γ-H2AX is expressed with greater intensity in a high percentage of melanoma cells compared to preneoplastic lesions (nevi) and normal melanocytes (see [91] for example), it was shown that γ-H2AX did not appear to be a prognostic indicator for melanoma. However, γ-H2AX may still have some diagnostic utility in separating melanoma from dysplastic nevi [91].
9.3 Chronic inflammation
There are multiple mechanisms involved in cancer. If a small percentage of cancers are hereditary, many factors, such as spontaneous mutations, constant exposure to exogenous and endogenous DNA damage agents, poor diet, obesity, etc, may increase cancer risk. In healthy cells, the transcription factor p53, which is activated by DNA damage, prevents cancer development by inducing senescence or apoptosis. Spontaneous or inherited mutations in p53 remove this checkpoint [96, 97]. Activated oncogenes alone or in combination to other stresses, i.e. overproduction of reactive oxygen species and chronic inflammation among others, can also lead to increased and recurrent DNA damage contributing to precancer-associated genomic instability [98] (Table 3). Therefore γ-H2AX may be a useful marker for genomic instability to assess risks of tumorigenesis in patients with chronic health problems. Recent studies show increased DSB levels in cells from patients with chronic inflammation. Colonocytes from individual experiencing ulcerative colitis, a chronic inflammatory disease that predisposes to colorectal cancer, showed that increased γ-H2AX might reflect oxidative damage due to chronic inflammation [99]. Similarly, examination of lung tissues from patients with chronic obstructive pulmonary disease (COPD) revealed high levels of γ-H2AX in alveolar wall cells compared to control individuals [100]. The authors of the study suggested that severe COPD is indeed linked to oxidative DNA damage. Thus, persistent oxidative-induced DNA damage could cause nuclear hypermutability which, in turn, would increase the risk of lung cancer and/or influence COPD pathogenesis. Childhood obesity, often linked to chronic low-grade inflammation, has been associated with an increased risk of developing some types of cancer later in life [101]. Recently, γ-H2AX was analyzed in lymphocytes from 119 children classified as normal, overweight, or obese. The study showed that both overweight and obese children had significantly higher levels of γ-H2AX when compared to children with normal weight [101]. Therefore, the results of such a study could link obesity to an increased genomic instability, making a connection for putative cancer development.
9.4 Other disorders with elevated γ-H2AX
Increased γ-H2AX was also observed in lymphocytes of individuals with Dyskeratosis congenita [102], an inherited disorder characterized by premature aging, bone marrow failure and a predisposition to cancer [103]. This increase was also observed in patients with occult HBV infections, infections which are often associated with poor therapeutic response and increased risk of developing hepatocellular carcinoma [104]. There may be an increased risk of cancer related mortality in patients with schizophrenia, especially breast cancer for women and lung cancer for men [105]. Interestingly, immortalized lymphoblasts from patients with schizophrenia, show increased spontaneous DSBs, as marked by γ-H2AX [106].
10. Other histone post-translational modifications used as biomarkers in the clinic
Deregulation in any of the histone PTMs may alter gene expression and lead to changes in these cellular processes resulting in tumorigenesis [107, 108]. Similarly, alterations in the activities of histone-modifying enzymes such as histone deacetylases and histone methyltransferases have been linked to cancer [109]. A reasonable hypothesis is that exogenous and endogenous stresses may result in the alteration of specific types of histone PTMs, facilitating oncogenesis (Figure 1). Pressure on genomic integrity would also include genotoxic traumas, such as irradiation and/or chemotherapeutic drugs used to fight cancer. For these reasons, measuring levels of histone PTMs in patient samples has been used in clinical studies for prognosis as well as to serve as predictive biomarkers to gauge patient response to new chemotherapeutic drugs (Table 4).
Table 4.
Non-exhaustive list of clinical studies using other histone PTMs.
| Application | Histone modifications | Study description | Samples | Methods | References or Clinicaltrials.gov identifier |
|---|---|---|---|---|---|
| Drug development | H3 ac | Study evaluating the effect of Romidepsin (HDAC inhibitor) in patients with T-cell lymphomas | Sézary cells | M | [115] |
| Drug development | H3 ac: H4 ac | Phase I clinical trial evaluating the effect of Romidepsin (HDAC inhibitor) in patients with chronic lymphocytic leukemia and acute myeloid lymphoma | CLL, AML | M/IB | [116] |
| Drug development | H3 ph | Phase 1 and 2 study of BI 811283 (Aurora B kinase inhibitor) in combination with cytarabine in previously untreated patients with AML | Leukaemia cells | N/A | NCT00632749 |
| Drug development | H3 ac | Phase I study using SB939 (HDAC inhibitor) in patients with advanced or metastatic solid tumors | PBMCs | IB/M/ELISA | NCT00504296 |
| Drug development | H4 ac | Study using Verinostat (HDAC inhibitor) in women with breast cancer (ductal carcinoma) | WBCs/breast tissue | M | NCT00788112 |
| Drug development | H3 ac: H4 ac | Phase II of Azacitidine (nucleoside homologue) and Entinostat (HDAC inhibitor) in patients with metatastic colorectal cancer | WBCs/Tumor biopsies | IB | NCT01105377 |
| Drug development | H3 ac: H4 ac | Phase II study using Entinostat (HDAC inhibitor) in combination with Anastrozole (aromatase inhibitor) in postmenopausal women with triple-negative breast cancer | Tumor biopsies | M | NCT01234532 |
| Diagnosis | H3 ph | Long-term follow-up in lymph node-negative invasive breast cancers in patients less than 55 years of age (adjuvant systemic chemotherapy) | Tumor biopsies | M | [121] |
| Diagnosis | H3 ac, me H4 ac, me |
Study of the prognosis and predictive value of global histone modifications in breast cancers | Tumor biopsies | M | [147] |
| Diagnosis | H3 ac, me H4 ac, me |
Prognosis and predictive value of histone modifications in pancreatic adenocarcinomas | Tumor biopsies | M | [118] |
| Diagnosis | H3 ac: H4 ac, me | Prediction of prostate cancer recurrence through global patterns of histone modification | Tumor biopsies | M | [148] |
| Diagnosis | H2A ac H3 me, ac |
Global histone modifications predict prognosis of resected non small-cell lung cancer | Tumor biopsies | M | [120] |
| Diagnosis | H4 me | Loss of histone H4K20 trimethylation influences prognosis of non-small cell lung cancer | Tumor biopsies | M | [111] |
| Diagnosis | H3 me | Evaluation of the impact of histone H3 trimethylation on the outcome of patients with breast, ovarian and pancreatic cancers | Tumor biopsies | M | [119] |
| Diagnosis | H4 ac | Evaluation of histone acetylation on the rate of complete remission, relapse-free survival and overall survival in newly diagnosed adult ALL patients | Bone marrow | M/IB | [149] |
| Diagnosis | H4 ac | Prognosis in relapsed ALL | Bone marrow | M | [150] |
Abbreviations: M, microscopy (immunocytochemistry or immunohistochemistry); IB, immunoblotting; FACS, Fluorescence-Activated Cell Sorting; WBCs, White blood cells; PBMCs, Peripheral Blood Mononuclear Cells; CLL, chronic lymphocytic leukemia; AML, acute myeloid lymphoma; ALL, acute lymphocytic leukemia; ac, acetylation; me, methylation; ph, phosphorylation; N/A, not specified
Like γ-H2AX, other histone modifications may be used as biomarkers of cancer. Indeed, the loss of H4 lysine 16 acetylation and H4 lysine 20 trimethylation was shown to be a common feature of human cancers [110]. Aberrant patterns of H4 and H3/H4 modifications were described in non-small cell lung carcinoma [111] and prostate cancer [112] respectively when compared to normal tissue. Also, compared to normal prostate tissue, prostate tumors exhibit aberrant patterns of H3 and H4 modifications [112].
10.1 Drugs targeting other histone PTMs
The potential for clinical use of histone PTMs is related to drug development. Promising compounds for modifying chromatin are being introduced as novel anticancer drugs [113]. Administered alone or in combination with DNA damaging agents (Table 4), they may alter gene expression and/or DNA damage repair pathways, thereby inhibiting uncontrolled proliferation or boosting rates of malignant cell death.
One example of the growing use of compounds for modifying chromatin is that of histone deacetylase inhibitors (HDACis) (Tables 1, 4). Treatment of cancer cells with HDACis induces histone hyperacetylation and changes in the levels of a large range of proteins associated with apoptosis and proliferation (see [114] for review). In the first study reporting a clinical response to an HDACi, romidepsin (also referred to depsipeptide) was administered to patients with T-cell lymphoma. Elevated levels of histone H3 acetylation were found in Sézary cells as soon as 4 hr after drug administration [115] (Table 4). Romidepsin was also used in a phase I clinical trial to treat acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL) [116]. Pharmacodynamic data collected by microscopy and immunoblotting showed increased levels of H4 and H3 acetylation (H4 lysines 5, 8, 12, and 16; H3 lysines 9 and 14) at 4 hr (AML) and 24 hr (both AML and CLL) after drug infusion, times that were shown to correlate with decreased HDAC activity in vivo.
10.2 Histone PTMs as prognostic indicators
In addition to their use as biomarkers for early diagnosis of cancer, histone PTMs have also been used as indicators of survival (Table 4). Several studies have shown an association between some histone PTM levels, tumor aggressiveness, and patient outcome [117]. Reduced cellular levels of H3 lysine 4 dimethylation, lysine 9 dimethylation or lysine 18 acetylation were linked to poor prognoses for pancreatic cancer [118]. Low cellular levels of H3 lysine 27 trimethylation were associated with poor outcomes in breast, ovarian and pancreatic cancers [119] while the loss of H4 lysine 20 trimethylation was found to be associated with poor survival of lung cancer patients with adenocarcinoma [111]. Patients with stage II lung carcinoma exhibiting lower levels of H2A lysine 5 acetylation were shown to have decreased survival compared to those with higher levels [120]. Analysis of H3 modifications in resected tumors from lung cancer patients showed that stage I adenocarcinoma patients with higher levels of H3 lysine dimethylation had favorable survival prognoses, while patients with higher levels of H3 lysine acetylation had poorer survival prognoses [120].
The prognostic value of histone H3 phosphorylation (PPH3) was evaluated in a homogeneous group of node-negative invasive breast cancers, from women less than 55 years of age, treated with adjuvant systemic chemotherapy [121]. PPH3 expression levels correlated with tumor diameter, estrogen receptor and carcinoma grade. The study showed that the 45% of early breast cancer patients with limited PPH3 expression had excellent prognoses (96%), in contrast to the lower survival rate (58%) of patients with strong PPH3 expression. Overall, PPH3 was pointed out as the strongest prognostic indicator, including its mitotic activity index, for operable lymph node-negative cancer patients under 55 years of age [121].
11. Concluding Remarks
The development of agents for cancer treatment is a slow and laborious process. Despite many successes in the discovery of new anti-cancer drugs, only 5% of prospective drugs gain final approval [122]. The discovery and use of new biomarkers, such as γ-H2AX, would enable clinicians to evaluate a drug’s effectiveness in a particular patient more quickly, reducing the time and cost of drug development, i.e., “fail fast”. The use of γ-H2AX has already aided studies of the effects of many drugs targeting various cancer types, e.g., leukemia, breast, colon, glioma, sarcoma, ovarian, etc. (Table 2). As a result, it is expected that an increasing number of early clinical trials will use γ-H2AX in the future because of its convenience and sensitivity.
The ability to measure the effect of a drug soon after administration to a patient promises to bring a new dimension to patient health. The inter-individual variations in treatment response could be measured and accounted for by optimizing protocols to individual patients. For cancer treatment, γ-H2AX levels taken as a measure of DNA damage may lead to one, improved treatments using ionizing radiation and other anticancer agents, two, accelerated validation of new candidate chemotherapeutic agents, and three, optimization of treatment to individual patients minimizing undesirable side-effects while maximizing treatment efficacy. Other measures, such as histone acetylation levels, are also being developed for monitoring cancer treatment. In the future, measuring alterations in histone PTMs, including but not limited to γ-H2AX, of individual patients in response to a particular drug, may become part of standard operating procedures in order to optimize individual patient treatments.
Highlights.
We review the uses of γ-H2AX and other modified histone species in the clinic.
γ-H2AX is a useful radiation biodosimeter.
γ-H2AX can be used to assay DNA damage during chemotherapy.
γ-H2AX can be used to optimize cancer treatments and other procedures.
Uses of other modified histone species are discussed.
Acknowledgments
This research was supported by the NIAID Radiation/Nuclear Countermeasures Program and the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Noll M, Kornberg RD. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977;109:393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- 3.Talbert PB, Henikoff S. Histone variants--ancient wrap artists of the epigenome. Nat Rev Mol Cell Biol. 2010;11:264–275. doi: 10.1038/nrm2861. [DOI] [PubMed] [Google Scholar]
- 4.Tripputi P, Emanuel BS, Croce CM, Green LG, Stein GS, Stein JL. Human histone genes map to multiple chromosomes. Proc Natl Acad Sci U S A. 1986;83:3185–3188. doi: 10.1073/pnas.83.10.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 6.Wyrick JJ, Parra MA. The role of histone H2A and H2B post-translational modifications in transcription: a genomic perspective. Biochim Biophys Acta. 2009;1789:37–44. doi: 10.1016/j.bbagrm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains nvolved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin DM, Kucia M, Ratajczak MZ. Nuclear and chromatin reorganization during cell senescence and aging - a mini-review. Gerontology. 2011;57:76–84. doi: 10.1159/000281882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassa PO, Hottiger MO. An epigenetic code for DNA damage repair pathways? Biochem Cell Biol. 2005;83:270–285. doi: 10.1139/o05-034. [DOI] [PubMed] [Google Scholar]
- 10.Kanwal R, Gupta S. Epigenetic modifications in cancer. Clin Genet. 2011 doi: 10.1111/j.1399-0004.2011.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florean C, Schnekenburger M, Grandjenette C, Dicato M, Diederich M. Epigenomics of leukemia: from mechanisms to therapeutic applications. Epigenomics. 2011;3:581–609. doi: 10.2217/epi.11.73. [DOI] [PubMed] [Google Scholar]
- 12.Sawan C, Herceg Z. Histone modifications and cancer. Advances in genetics. 2010;70:57–85. doi: 10.1016/B978-0-12-380866-0.60003-4. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee C, Muir TW. Chemical approaches for studying histone modifications. The Journal of biological chemistry. 2010;285:11045–11050. doi: 10.1074/jbc.R109.080291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson CJ, Santos-Rosa H, Kouzarides T. Proline isomerization of histone H3 regulates lysine methylation and gene expression. Cell. 2006;126:905–916. doi: 10.1016/j.cell.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Escargueil AE, Soares DG, Salvador M, Larsen AK, Henriques JA. What histone code for DNA repair? Mutation research. 2008;658:259–270. doi: 10.1016/j.mrrev.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Liu T, Liu PY, Marshall GM. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009;69:1702–1705. doi: 10.1158/0008-5472.CAN-08-3365. [DOI] [PubMed] [Google Scholar]
- 18.Sakabe K, Hart GW. O-GlcNAc transferase regulates mitotic chromatin dynamics. The Journal of biological chemistry. 2010;285:34460–34468. doi: 10.1074/jbc.M110.158170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Wang Y. High mobility group proteins and their post-translational modifications. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics. 2008;1784:1159–1166. doi: 10.1016/j.bbapap.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. The Journal of biological chemistry. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 21.Dickey JS, Redon CE, Nakamura AJ, Baird BJ, Sedelnikova OA, Bonner WM. H2AX: functional roles and potential applications. Chromosoma. 2009;118:683–692. doi: 10.1007/s00412-009-0234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilch DR, Sedelnikova OA, Redon C, Celeste A, Nussenzweig A, Bonner WM. Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem Cell Biol. 2003;81:123–129. doi: 10.1139/o03-042. [DOI] [PubMed] [Google Scholar]
- 23.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. gammaH2AX and cancer, Nature reviews. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redon C, Pilch DR, Rogakou EP, Orr AH, Lowndes NF, Bonner WM. Yeast histone 2A serine 129 is essential for the efficient repair of checkpoint-blind DNA damage. EMBO Rep. 2003;4:678–684. doi: 10.1038/sj.embor.embor871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szenker E, Ray-Gallet D, Almouzni G. The double face of the histone variant H3.3. Cell Res. 2011;21:421–434. doi: 10.1038/cr.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. The Journal of biological chemistry. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 27.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. The Journal of biological chemistry. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 28.Park EJ, Chan DW, Park JH, Oettinger MA, Kwon J. DNA-PK is activated by nucleosomes and phosphorylates H2AX within the nucleosomes in an acetylation-dependent manner. Nucleic acids research. 2003;31:6819–6827. doi: 10.1093/nar/gkg921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu C, Zhu F, Cho YY, Tang F, Zykova T, Ma WY, Bode AM, Dong Z. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell. 2006;23:121–132. doi: 10.1016/j.molcel.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan J, Adamski R, Chen J. Focus on histone variant H2AX: to be or not to be. FEBS Lett. 2010;584:3717–3724. doi: 10.1016/j.febslet.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukas J, Lukas C, Bartek J. More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance. Nat Cell Biol. 2011;13:1161–1169. doi: 10.1038/ncb2344. [DOI] [PubMed] [Google Scholar]
- 32.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, Redon C, Pilch DR, Olaru A, Eckhaus M, Camerini-Otero RD, Tessarollo L, Livak F, Manova K, Bonner WM, Nussenzweig MC, Nussenzweig A. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinto DM, Flaus A. Structure and function of histone H2AX. Sub-cellular biochemistry. 2010;50:55–78. doi: 10.1007/978-90-481-3471-7_4. [DOI] [PubMed] [Google Scholar]
- 34.Tuma RS. Sometimes size doesn’t matter: reevaluating RECIST and tumor response rate endpoints. Journal of the National Cancer Institute. 2006;98:1272–1274. doi: 10.1093/jnci/djj403. [DOI] [PubMed] [Google Scholar]
- 35.Venet D, Dumont JE, Detours V. Most random gene expression signatures are significantly associated with breast cancer outcome. PLoS computational biology. 2011;7:e1002240. doi: 10.1371/journal.pcbi.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redon CE, Nakamura AJ, Martin OA, Parekh PR, Weyemi US, Bonner WM. Recent developments in the use of gamma-H2AX as a quantitative DNA double-strand break biomarker. Aging (Albany NY) 2011;3:168–174. doi: 10.18632/aging.100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothkamm K, Horn S. gamma-H2AX as protein biomarker for radiation exposure. Ann Ist Super Sanita. 2009;45:265–271. [PubMed] [Google Scholar]
- 38.Redon CE, Nakamura AJ, Zhang YW, Ji JJ, Bonner WM, Kinders RJ, Parchment RE, Doroshow JH, Pommier Y. Histone {gamma}H2AX and Poly(ADP-Ribose) as Clinical Pharmacodynamic Biomarkers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:4532–4542. doi: 10.1158/1078-0432.CCR-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pommier Y, Barcelo JM, Rao VA, Sordet O, Jobson AG, Thibaut L, Miao ZH, Seiler JA, Zhang H, Marchand C, Agama K, Nitiss JL, Redon C. Repair of topoisomerase I-mediated DNA damage. Prog Nucleic Acid Res Mol Biol. 2006;81:179–229. doi: 10.1016/S0079-6603(06)81005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Parry JA, Chin A, Duensing S, Duensing A. Soluble histone H2AX is induced by DNA replication stress and sensitizes cells to undergo apoptosis. Molecular cancer. 2008;7:61. doi: 10.1186/1476-4598-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner HC, Brenner DJ, Chen Y, Bertucci A, Zhang J, Wang H, Lyulko OV, Xu Y, Shuryak I, Schaefer J, Simaan N, Randers-Pehrson G, Yao YL, Amundson SA, Garty G. Adapting the gamma-H2AX assay for automated processing in human lymphocytes. 1. Technological aspects. Radiation research. 2011;175:282–290. doi: 10.1667/RR2125.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivashkevich AN, Martin OA, Smith AJ, Redon CE, Bonner WM, Martin RF, Lobachevsky PN. gammaH2AX foci as a measure of DNA damage: a computational approach to automatic analysis. Mutation research. 2011;711:49–60. doi: 10.1016/j.mrfmmm.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang MM, Mah LJ, Vasireddy RS, Georgiadis GT, El-Osta A, Royce SG, Karagiannis TC. Quantitation of gammaH2AX foci in tissue samples. J Vis Exp. 2011 doi: 10.3791/2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avondoglio D, Scott T, Kil WJ, Sproull M, Tofilon PJ, Camphausen K. High throughput evaluation of gamma-H2AX. Radiat Oncol. 2009;4:31. doi: 10.1186/1748-717X-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuzaki K, Harada A, Takeiri A, Tanaka K, Mishima M. Whole cell-ELISA to measure the gammaH2AX response of six aneugens and eight DNA-damaging chemicals. Mutation research. 2011;700:71–79. doi: 10.1016/j.mrgentox.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 47.Wang LH, Pfister TD, Parchment RE, Kummar S, Rubinstein L, Evrard YA, Gutierrez ME, Murgo AJ, Tomaszewski JE, Doroshow JH, Kinders RJ. Monitoring drug-induced gammaH2AX as a pharmacodynamic biomarker in individual circulating tumor cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:1073–1084. doi: 10.1158/1078-0432.CCR-09-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qvarnstrom OF, Simonsson M, Johansson KA, Nyman J, Turesson I. DNA double strand break quantification in skin biopsies. Radiother Oncol. 2004;72:311–317. doi: 10.1016/j.radonc.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. The New England journal of medicine. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez JE, Roch-Lefevre SH, Mandina T, Garcia O, Roy L. Induction of gamma-H2AX foci in human exfoliated buccal cells after in vitro exposure to ionising radiation. International journal of radiation biology. 2010;86:752–759. doi: 10.3109/09553002.2010.484476. [DOI] [PubMed] [Google Scholar]
- 51.Redon C, Dickey JS, Bonner WM, Sedelnikova O. gamma-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv in Space Res. 2009;43:1171–1178. doi: 10.1016/j.asr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beels L, Bacher K, Smeets P, Verstraete K, Vral A, Thierens H. Dose-length product of scanners correlates with DNA damage in patients undergoing contrast CT. European journal of radiology. 2011 doi: 10.1016/j.ejrad.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 53.Lobrich M, Rief N, Kuhne M, Heckmann M, Fleckenstein J, Rube C, Uder M. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci U S A. 2005;102:8984–8989. doi: 10.1073/pnas.0501895102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lippens S, Denecker G, Ovaere P, Vandenabeele P, Declercq W. Death penalty for keratinocytes: apoptosis versus cornification. Cell Death Differ. 2005;12(Suppl 2):1497–1508. doi: 10.1038/sj.cdd.4401722. [DOI] [PubMed] [Google Scholar]
- 56.Kinders RJ, Hollingshead M, Lawrence S, Ji J, Tabb B, Bonner WM, Pommier Y, Rubinstein L, Evrard YA, Parchment RE, Tomaszewski J, Doroshow JH. Development of a validated immunofluorescence assay for gammaH2AX as a pharmacodynamic marker of topoisomerase I inhibitor activity. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:5447–5457. doi: 10.1158/1078-0432.CCR-09-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Redon CE, Nakamura AJ, Sordet O, Dickey JS, Gouliaeva K, Tabb B, Lawrence S, Kinders RJ, Bonner WM, Sedelnikova OA. gamma-H2AX detection in peripheral blood lymphocytes, splenocytes, bone marrow, xenografts, and skin. Methods Mol Biol. 2011;682:249–270. doi: 10.1007/978-1-60327-409-8_18. [DOI] [PubMed] [Google Scholar]
- 58.Bassukas ID, Hornstein OP. Effects of plucking on the anatomy of the anagen hair bulb. A light microscopic study. Arch Dermatol Res. 1989;281:188–192. doi: 10.1007/BF00456391. [DOI] [PubMed] [Google Scholar]
- 59.Redon CE, Nakamura AJ, Gouliaeva K, Rahman A, Blakely WF, Bonner WM. The use of gamma-H2AX as a biodosimeter for total-body radiation exposure in non-human primates. PloS one. 2010;5:e15544. doi: 10.1371/journal.pone.0015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boxman IL, Hogewoning A, Mulder LH, Bouwes Bavinck JN, ter Schegget J. Detection of human papillomavirus types 6 and 11 in pubic and perianal hair from patients with genital warts. J Clin Microbiol. 1999;37:2270–2273. doi: 10.1128/jcm.37.7.2270-2273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biver-Dalle C, Gheit T, Drobacheff-Thiebaut C, Vidal C, Tommasino M, Humbert P, Pretet JL, Mougin C, Aubin F. Detection of human papillomavirus DNA in plucked eyebrow hair from HIV-infected patients. J Invest Dermatol. 2011;130:2499–2502. doi: 10.1038/jid.2010.147. [DOI] [PubMed] [Google Scholar]
- 62.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H, Young M, Milam J, Cohen MH, Sharp GB, Huang Y, Greenblatt RM. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;52:1267–1275. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaugars GE, Silverman S, Jr, Ray AK, Page DG, Abbey LM, Burns JC, Svirsky JA. The use of exfoliative cytology for the early diagnosis of oral cancers: is there a role for it in education and private practice? J Cancer Educ. 1998;13:85–89. doi: 10.1080/08858199809528522. [DOI] [PubMed] [Google Scholar]
- 64.Yoon AJ, Shen J, Wu HC, Angelopoulos C, Singer SR, Chen R, Santella RM. Expression of activated checkpoint kinase 2 and histone 2AX in exfoliative oral cells after exposure to ionizing radiation. Radiation research. 2009;171:771–775. doi: 10.1667/RR1560.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. The New England journal of medicine. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 66.Monfared AS, Amiri M. Physical dosimetry and mathematical dose calculation in nuclear medicine: A comparative study. Indian J Nucl Med. 2010;25:10–11. doi: 10.4103/0972-3919.63592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sak A, Grehl S, Erichsen P, Engelhard M, Grannass A, Levegrun S, Pottgen C, Groneberg M, Stuschke M. gamma-H2AX foci formation in peripheral blood lymphocytes of tumor patients after local radiotherapy to different sites of the body: dependence on the dose-distribution, irradiated site and time from start of treatment. International journal of radiation biology. 2007;83:639–652. doi: 10.1080/09553000701596118. [DOI] [PubMed] [Google Scholar]
- 68.Lobrich M, Shibata A, Beucher A, Fisher A, Ensminger M, Goodarzi AA, Barton O, Jeggo PA. gammaH2AX foci analysis for monitoring DNA double-strand break repair: strengths, limitations and optimization. Cell Cycle. 2011;9:662–669. doi: 10.4161/cc.9.4.10764. [DOI] [PubMed] [Google Scholar]
- 69.Zwicker F, Swartman B, Sterzing F, Major G, Weber KJ, Huber PE, Thieke C, Debus J, Herfarth K. Biological in-vivo measurement of dose distribution in patients’ lymphocytes by gamma-H2AX immunofluorescence staining: 3D conformal- vs. step-and-shoot IMRT of the prostate gland. Radiat Oncol. 2011;6:62. doi: 10.1186/1748-717X-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuefner MA, Grudzenski S, Schwab SA, Wiederseiner M, Heckmann M, Bautz W, Lobrich M, Uder M. DNA double-strand breaks and their repair in blood lymphocytes of patients undergoing angiographic procedures. Investigative radiology. 2009;44:440–446. doi: 10.1097/RLI.0b013e3181a654a5. [DOI] [PubMed] [Google Scholar]
- 71.Beels L, Bacher K, De Wolf D, Werbrouck J, Thierens H. gamma-H2AX foci as a biomarker for patient X-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risks? Circulation. 2009;120:1903–1909. doi: 10.1161/CIRCULATIONAHA.109.880385. [DOI] [PubMed] [Google Scholar]
- 72.Kuefner MA, Grudzenski S, Hamann J, Achenbach S, Lell M, Anders K, Schwab SA, Haberle L, Lobrich M, Uder M. Effect of CT scan protocols on x-ray-induced DNA double-strand breaks in blood lymphocytes of patients undergoing coronary CT angiography. European radiology. 2010;20:2917–2924. doi: 10.1007/s00330-010-1873-9. [DOI] [PubMed] [Google Scholar]
- 73.Garty G, Chen Y, Salerno A, Turner H, Zhang J, Lyulko O, Bertucci A, Xu Y, Wang H, Simaan N, Randers-Pehrson G, Yao YL, Amundson SA, Brenner DJ. The RABIT: a rapid automated biodosimetry tool for radiological triage. Health Phys. 2010;98:209–217. doi: 10.1097/HP.0b013e3181ab3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pope IA, Barber PR, Horn S, Ainsbury E, Rothkamm K, Vojnovic B. A portable microfluidic fluorescence spectrophotometer device for gamma-H2AX-based biological dosimetry. Radiation Measurements. 2011;46:907–911. [Google Scholar]
- 75.Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiation research. 2002;158:486–492. doi: 10.1667/0033-7587(2002)158[0486:qdoiid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 76.Cai Z, Vallis KA, Reilly RM. Computational analysis of the number, area and density of gamma-H2AX foci in breast cancer cells exposed to (111)In-DTPA-hEGF or gamma-rays using Image-J software. International journal of radiation biology. 2009;85:262–271. doi: 10.1080/09553000902748757. [DOI] [PubMed] [Google Scholar]
- 77.Kelly MP, Lee FT, Tahtis K, Smyth FE, Brechbiel MW, Scott AM. Radioimmunotherapy with alpha-particle emitting 213Bi-C-functionalized trans-cyclohexyl-diethylenetriaminepentaacetic acid-humanized 3S193 is enhanced by combination with paclitaxel chemotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:5604s–5612s. doi: 10.1158/1078-0432.CCR-07-1071. [DOI] [PubMed] [Google Scholar]
- 78.Lassmann M, Hanscheid H, Gassen D, Biko J, Meineke V, Reiners C, Scherthan H. In vivo formation of gamma-H2AX and 53BP1 DNA repair foci in blood cells after radioiodine therapy of differentiated thyroid cancer. J Nucl Med. 2010;51:1318–1325. doi: 10.2967/jnumed.109.071357. [DOI] [PubMed] [Google Scholar]
- 79.Ivashkevich A, Redon CE, Nakamura AJ, Martin RF, Martin OA. Use of the gamma-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2011 doi: 10.1016/j.canlet.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy--revisited, Nature reviews. Drug discovery. 2011;10:591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- 81.Olive PL, Banath JP, Sinnott LT. Phosphorylated histone H2AX in spheroids, tumors, and tissues of mice exposed to etoposide and 3-amino-1,2,4-benzotriazine-1,3-dioxide. Cancer Res. 2004;64:5363–5369. doi: 10.1158/0008-5472.CAN-04-0729. [DOI] [PubMed] [Google Scholar]
- 82.Karp JE, Ricklis RM, Balakrishnan K, Briel J, Greer J, Gore SD, Smith BD, McDevitt MA, Carraway H, Levis MJ, Gandhi V. A phase 1 clinical-laboratory study of clofarabine followed by cyclophosphamide for adults with refractory acute leukemias. Blood. 2007;110:1762–1769. doi: 10.1182/blood-2007-03-081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaymes TJ, Padua RA, Pla M, Orr S, Omidvar N, Chomienne C, Mufti GJ, Rassool FV. Histone deacetylase inhibitors (HDI) cause DNA damage in leukemia cells: a mechanism for leukemia-specific HDI-dependent apoptosis? Mol Cancer Res. 2006;4:563–573. doi: 10.1158/1541-7786.MCR-06-0111. [DOI] [PubMed] [Google Scholar]
- 84.Kummar S, Chen A, Ji J, Zhang Y, Reid JM, Ames M, Jia L, Weil M, Speranza G, Murgo AJ, Kinders R, Wang L, Parchment RE, Carter J, Stotler H, Rubinstein L, Hollingshead M, Melillo G, Pommier Y, Bonner W, Tomaszewski JE, Doroshow JH. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res. 2011;71:5626–5634. doi: 10.1158/0008-5472.CAN-11-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koukourakis G. Radiation therapy for early breast cancer. Clin Transl Oncol. 2009;11:596–603. doi: 10.1007/s12094-009-0410-2. [DOI] [PubMed] [Google Scholar]
- 86.Vasireddy RS, Sprung CN, Cempaka NL, Chao M, McKay MJ. H2AX phosphorylation screen of cells from radiosensitive cancer patients reveals a novel DNA double-strand break repair cellular phenotype. Br J Cancer. 2010;102:1511–1518. doi: 10.1038/sj.bjc.6605666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harris S. Radiotherapy for early and advanced breast cancer. Int J Clin Pract. 2001;55:609–612. [PubMed] [Google Scholar]
- 88.Bourton EC, Plowman PN, Smith D, Arlett CF, Parris CN. Prolonged expression of the gamma-H2AX DNA repair biomarker correlates with excess acute and chronic toxicity from radiotherapy treatment. Int J Cancer. 2011;129:2928–2934. doi: 10.1002/ijc.25953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr, Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 90.Sedelnikova OA, Bonner WM. GammaH2AX in cancer cells: a potential biomarker for cancer diagnostics, prediction and recurrence. Cell Cycle. 2006;5:2909–2913. doi: 10.4161/cc.5.24.3569. [DOI] [PubMed] [Google Scholar]
- 91.Wasco MJ, Pu RT, Yu L, Su L, Ma L. Expression of gamma-H2AX in melanocytic lesions. Human pathology. 2008;39:1614–1620. doi: 10.1016/j.humpath.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 92.Wasco MJ, Pu RT. Comparison of PAX-2, RCC antigen, and antiphosphorylated H2AX antibody (gamma-H2AX) in diagnosing metastatic renal cell carcinoma by fine-needle aspiration. Diagnostic cytopathology. 2008;36:568–573. doi: 10.1002/dc.20839. [DOI] [PubMed] [Google Scholar]
- 93.Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol. 2011;6:249–274. doi: 10.1146/annurev-pathol-011110-130249. [DOI] [PubMed] [Google Scholar]
- 94.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, Dispenzieri A, Kumar S, Clark RJ, Baris D, Hoover R, Rajkumar SV. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walters DK, Wu X, Tschumper RC, Arendt BK, Huddleston PM, Henderson KJ, Dispenzieri A, Jelinek DF. Evidence for ongoing DNA damage in multiple myeloma cells as revealed by constitutive phosphorylation of H2AX. Leukemia. 2011;25:1344–1353. doi: 10.1038/leu.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Talos F, Moll UM. Role of the p53 family in stabilizing the genome and preventing polyploidization. Adv Exp Med Biol. 2010;676:73–91. doi: 10.1007/978-1-4419-6199-0_5. [DOI] [PubMed] [Google Scholar]
- 98.Martin OA, Redon CE, Dickey JS, Nakamura AJ, Bonner WM. Para-inflammation mediates systemic DNA damage in response to tumor growth. Commun Integr Biol. 2011;4:78–81. doi: 10.4161/cib.4.1.13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Risques RA, Lai LA, Brentnall TA, Li L, Feng Z, Gallaher J, Mandelson MT, Potter JD, Bronner MP, Rabinovitch PS. Ulcerative colitis is a disease of accelerated colon aging: evidence from telomere attrition and DNA damage. Gastroenterology. 2008;135:410–418. doi: 10.1053/j.gastro.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pastukh VM, Zhang L, Ruchko MV, Gorodnya O, Bardwell GC, Tuder RM, Gillespie MN. Oxidative DNA damage in lung tissue from patients with COPD is clustered in functionally significant sequences. Int J Chron Obstruct Pulmon Dis. 2011;6:209–217. doi: 10.2147/COPD.S15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scarpato R, Verola C, Fabiani B, Bianchi V, Saggese G, Federico G. Nuclear damage in peripheral lymphocytes of obese and overweight Italian children as evaluated by the gamma-H2AX focus assay and micronucleus test. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:685–693. doi: 10.1096/fj.10-168427. [DOI] [PubMed] [Google Scholar]
- 102.Kirwan M, Beswick R, Walne AJ, Hossain U, Casimir C, Vulliamy T, Dokal I. Dyskeratosis congenita and the DNA damage response. Br J Haematol. 2011;153:634–643. doi: 10.1111/j.1365-2141.2011.08679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dokal I. Dyskeratosis congenita. A disease of premature ageing. Lancet. 2001;358(Suppl):S27. doi: 10.1016/s0140-6736(01)07040-4. [DOI] [PubMed] [Google Scholar]
- 104.Bhargava A, Khan S, Panwar H, Pathak N, Punde RP, Varshney S, Mishra PK. Occult hepatitis B virus infection with low viremia induces DNA damage, apoptosis and oxidative stress in peripheral blood lymphocytes. Virus Res. 2010;153:143–150. doi: 10.1016/j.virusres.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 105.Tran E, Rouillon F, Loze JY, Casadebaig F, Philippe A, Vitry F, Limosin F. Cancer mortality in patients with schizophrenia: an 11-year prospective cohort study. Cancer. 2009;115:3555–3562. doi: 10.1002/cncr.24383. [DOI] [PubMed] [Google Scholar]
- 106.Catts VS, Catts SV, Jablensky A, Chandler D, Weickert CS, Lavin MF. Evidence of aberrant DNA damage response signalling but normal rates of DNA repair in dividing lymphoblasts from patients with schizophrenia. World J Biol Psychiatry. 2011 doi: 10.3109/15622975.2011.565073. [DOI] [PubMed] [Google Scholar]
- 107.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer, Nature reviews. Genetics. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 108.Feinberg AP, Tycko B. The history of cancer epigenetics, Nature reviews. Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 109.Ozdag H, Teschendorff AE, Ahmed AA, Hyland SJ, Blenkiron C, Bobrow L, Veerakumarasivam A, Burtt G, Subkhankulova T, Arends MJ, Collins VP, Bowtell D, Kouzarides T, Brenton JD, Caldas C. Differential expression of selected histone modifier genes in human solid cancers. BMC genomics. 2006;7:90. doi: 10.1186/1471-2164-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fraga MF, Esteller M. Towards the human cancer epigenome: a first draft of histone modifications. Cell Cycle. 2005;4:1377–1381. doi: 10.4161/cc.4.10.2113. [DOI] [PubMed] [Google Scholar]
- 111.Van Den Broeck A, Brambilla E, Moro-Sibilot D, Lantuejoul S, Brambilla C, Eymin B, Khochbin S, Gazzeri S. Loss of histone H4K20 trimethylation occurs in preneoplasia and influences prognosis of non-small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:7237–7245. doi: 10.1158/1078-0432.CCR-08-0869. [DOI] [PubMed] [Google Scholar]
- 112.Ellinger J, Kahl P, von der Gathen J, Rogenhofer S, Heukamp LC, Gutgemann I, Walter B, Hofstadter F, Buttner R, Muller SC, Bastian PJ, von Ruecker A. Global levels of histone modifications predict prostate cancer recurrence. The Prostate. 2010;70:61–69. doi: 10.1002/pros.21038. [DOI] [PubMed] [Google Scholar]
- 113.Kwa FA, Balcerczyk A, Licciardi P, El-Osta A, Karagiannis TC. Chromatin modifying agents - the cutting edge of anticancer therapy. Drug Discov Today. 2011;16:543–547. doi: 10.1016/j.drudis.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 114.Epping MT, Bernards R. Molecular basis of the anti-cancer effects of histone deacetylase inhibitors. The international journal of biochemistry & cell biology. 2009;41:16–20. doi: 10.1016/j.biocel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 115.Piekarz RL, Robey R, Sandor V, Bakke S, Wilson WH, Dahmoush L, Kingma DM, Turner ML, Altemus R, Bates SE. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood. 2001;98:2865–2868. doi: 10.1182/blood.v98.9.2865. [DOI] [PubMed] [Google Scholar]
- 116.Byrd JC, Marcucci G, Parthun MR, Xiao JJ, Klisovic RB, Moran M, Lin TS, Liu S, Sklenar AR, Davis ME, Lucas DM, Fischer B, Shank R, Tejaswi SL, Binkley P, Wright J, Chan KK, Grever MR. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood. 2005;105:959–967. doi: 10.1182/blood-2004-05-1693. [DOI] [PubMed] [Google Scholar]
- 117.Kurdistani SK. Histone modifications in cancer biology and prognosis, Progress in drug research. Fortschritte der Arzneimittelforschung. Progres des recherches pharmaceutiques. 2011;67:91–106. doi: 10.1007/978-3-7643-8989-5_5. [DOI] [PubMed] [Google Scholar]
- 118.Manuyakorn A, Paulus R, Farrell J, Dawson NA, Tze S, Cheung-Lau G, Hines OJ, Reber H, Seligson DB, Horvath S, Kurdistani SK, Guha C, Dawson DW. Cellular histone modification patterns predict prognosis and treatment response in resectable pancreatic adenocarcinoma: results from RTOG 9704. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:1358–1365. doi: 10.1200/JCO.2009.24.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wei Y, Xia W, Zhang Z, Liu J, Wang H, Adsay NV, Albarracin C, Yu D, Abbruzzese JL, Mills GB, Bast RC, Jr, Hortobagyi GN, Hung MC. Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian, Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian,and pancreatic cancers. Mol Carcinog. 2008;47:701–706. doi: 10.1002/mc.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barlesi F, Giaccone G, Gallegos-Ruiz MI, Loundou A, Span SW, Lefesvre P, Kruyt FA, Rodriguez JA. Global histone modifications predict prognosis of resected non small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:4358–4364. doi: 10.1200/JCO.2007.11.2599. [DOI] [PubMed] [Google Scholar]
- 121.Skaland I, Janssen EA, Gudlaugsson E, Klos J, Kjellevold KH, Soiland H, Baak JP. Phosphohistone H3 expression has much stronger prognostic value than classical prognosticators in invasive lymph node-negative breast cancer patients less than 55 years of age. Mod Pathol. 2007;20:1307–1315. doi: 10.1038/modpathol.3800972. [DOI] [PubMed] [Google Scholar]
- 122.Sarker D, Workman P. Pharmacodynamic biomarkers for molecular cancer therapeutics. Advances in cancer research. 2007;96:213–268. doi: 10.1016/S0065-230X(06)96008-4. [DOI] [PubMed] [Google Scholar]
- 123.Geisel D, Heverhagen JT, Kalinowski M, Wagner HJ. DNA double-strand breaks after percutaneous transluminal angioplasty. Radiology. 2008;248:852–859. doi: 10.1148/radiol.2483071686. [DOI] [PubMed] [Google Scholar]
- 124.Kuefner MA, Hinkmann FM, Alibek S, Azoulay S, Anders K, Kalender WA, Achenbach S, Grudzenski S, Lobrich M, Uder M. Reduction of X-ray induced DNA double-strand breaks in blood lymphocytes during coronary CT angiography using high-pitch spiral data acquisition with prospective ECG-triggering. Investigative radiology. 2010;45:182–187. doi: 10.1097/RLI.0b013e3181d3eddf. [DOI] [PubMed] [Google Scholar]
- 125.Rothkamm K, Balroop S, Shekhdar J, Fernie P, Goh V. Leukocyte DNA damage after multi-detector row CT: a quantitative biomarker of low-level radiation exposure. Radiology. 2007;242:244–251. doi: 10.1148/radiol.2421060171. [DOI] [PubMed] [Google Scholar]
- 126.Simonsson M, Qvarnstrom F, Nyman J, Johansson KA, Garmo H, Turesson I. Low-dose hypersensitive gammaH2AX response and infrequent apoptosis in epidermis from radiotherapy patients. Radiother Oncol. 2008;88:388–397. doi: 10.1016/j.radonc.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 127.Abe H, Kawahara A, Sugita Y, Yamaguchi T, Terasaki M, Kage M. Follow-up evaluation of radiation-induced DNA damage in CSF disseminated high-grade glioma using phospho-histone H2AX antibody. Diagnostic cytopathology. 2011 doi: 10.1002/dc.21696. [DOI] [PubMed] [Google Scholar]
- 128.Ohnishi T, Takahashi A, Nagamatsu A, Omori K, Suzuki H, Shimazu T, Ishioka N. Detection of space radiation-induced double strand breaks as a track in cell nucleus. Biochemical and biophysical research communications. 2009;390:485–488. doi: 10.1016/j.bbrc.2009.09.114. [DOI] [PubMed] [Google Scholar]
- 129.LoRusso P, Ji JJ, Li J, Heilbrun LK, Shapiro G, SEABSA, Smith DW, Pilat MJ, Zhang J, Chen AP, Nechiporchik N, RE Parchment Phase I study of the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of the poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888; V) in combination with irinotecan (CPT-11; Ir) in patients (pts) with advanced solid tumors. 2011 doi: 10.1158/1078-0432.CCR-15-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Viswanathan S, Wesolowski R, Layman RM, Alejandra G, Miller B, Chalmers JJ, Bejastani S, Zhao W, Pierluigu G, Cotrill J, Phelps MA, Schaaf LJ, Geyer SM, Hall N, Knopp MV, Shapiro CL, Villalona-Calero MA, Chen A, Grever MR, Ramaswamy B. A phase I dose-escalation study of ABT-888 (veliparib) in combination with carboplatin in HER2-negative metastatic breast cancer (MBC) 2011 ASCO Annual Meeting Abstract No: TPS106. 2011 [Google Scholar]
- 131.Fandy TE, Herman JG, Kerns P, Jiemjit A, Sugar EA, Choi SH, Yang AS, Aucott T, Dauses T, Odchimar-Reissig R, Licht J, McConnell MJ, Nasrallah C, Kim MK, Zhang W, Sun Y, Murgo A, Espinoza-Delgado I, Oteiza K, Owoeye I, Silverman LR, Gore SD, Carraway HE. Early epigenetic changes and DNA damage do not predict clinical response in an overlapping schedule of 5-azacytidine and entinostat in patients with myeloid malignancies. Blood. 2009;114:2764–2773. doi: 10.1182/blood-2009-02-203547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Karp JE, Flatten K, Feldman EJ, Greer JM, Loegering DA, Ricklis RM, Morris LE, Ritchie E, Smith BD, Ironside V, Talbott T, Roboz G, Le SB, Meng XW, Schneider PA, Dai NT, Adjei AA, Gore SD, Levis MJ, Wright JJ, Garrett-Mayer E, Kaufmann SH. Active oral regimen for elderly adults with newly diagnosed acute myelogenous leukemia: a preclinical, phase 1 trial of the farnesyltransferase inhibitor tipifarnib (R115777, Zarnestra) combined with etoposide. Blood. 2009;113:4841–4852. doi: 10.1182/blood-2008-08-172726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hochhauser D, Meyer T, Spanswick VJ, Wu J, Clingen PH, Loadman P, Cobb M, Gumbrell L, Begent RH, Hartley JA, Jodrell D. Phase I study of sequence-selective minor groove DNA binding agent SJG-136 in patients with advanced solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:2140–2147. doi: 10.1158/1078-0432.CCR-08-1315. [DOI] [PubMed] [Google Scholar]
- 134.Kato TA, Nagasawa H, Weil MM, Little JB, Bedford JS. Levels of gamma-H2AX Foci after low-dose-rate irradiation reveal a DNA DSB rejoining defect in cells from human ATM heterozygotes in two at families and in another apparently normal individual. Radiation research. 2006;166:443–453. doi: 10.1667/RR3604.1. [DOI] [PubMed] [Google Scholar]
- 135.Porcedda P, Turinetto V, Lantelme E, Fontanella E, Chrzanowska K, Ragona R, De Marchi M, Delia D, Giachino C. Impaired elimination of DNA double-strand break-containing lymphocytes in ataxia telangiectasia and Nijmegen breakage syndrome. DNA Repair (Amst) 2006;5:904–913. doi: 10.1016/j.dnarep.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 136.Kato TA, Wilson PF, Nagasawa H, Fitzek MM, Weil MM, Little JB, Bedford JS. A defect in DNA double strand break processing in cells from unaffected parents of retinoblastoma patients and other apparently normal humans. DNA Repair (Amst) 2007;6:818–829. doi: 10.1016/j.dnarep.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 137.Porcedda P, Turinetto V, Brusco A, Cavalieri S, Lantelme E, Orlando L, Ricardi U, Amoroso A, Gregori D, Giachino C. A rapid flow cytometry test based on histone H2AX phosphorylation for the sensitive and specific diagnosis of ataxia telangiectasia. Cytometry A. 2008;73:508–516. doi: 10.1002/cyto.a.20566. [DOI] [PubMed] [Google Scholar]
- 138.Porcedda P, Turinetto V, Orlando L, Lantelme E, Brusco A, De Marchi M, Amoroso A, Ricardi U, Gregori D, Giachino C. Two-tier analysis of histone H2AX phosphorylation allows the identification of Ataxia Telangiectasia heterozygotes. Radiother Oncol. 2009 doi: 10.1016/j.radonc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 139.Fleckenstein J, Kuhne M, Seegmuller K, Derschang S, Melchior P, Graber S, Fricke A, Rube CE, Rube C. The Impact of Individual In Vivo Repair of DNA Double-Strand Breaks on Oral Mucositis in Adjuvant Radiotherapy of Head-and-Neck Cancer. International journal of radiation oncology, biology, physics. 2011;81:1465–1472. doi: 10.1016/j.ijrobp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 140.Leskovac A, Vujic D, Guc-Scekic M, Petrovic S, Joksic I, Slijepcevic P, Joksic G. Fanconi anemia is characterized by delayed repair kinetics of DNA double-strand breaks. Tohoku J Exp Med. 2010;221:69–76. doi: 10.1620/tjem.221.69. [DOI] [PubMed] [Google Scholar]
- 141.Abbaszadeh F, Clingen PH, Arlett CF, Plowman PN, Bourton EC, Themis M, Makarov EM, Newbold RF, Green MH, Parris CN. A novel splice variant of the DNA-PKcs gene is associated with clinical and cellular radiosensitivity in a patient with xeroderma pigmentosum. J Med Genet. 2010;47:176–181. doi: 10.1136/jmg.2009.068866. [DOI] [PubMed] [Google Scholar]
- 142.Rube CE, Fricke A, Schneider R, Simon K, Kuhne M, Fleckenstein J, Graber S, Graf N, Rube C. DNA repair alterations in children with pediatric malignancies: novel opportunities to identify patients at risk for high-grade toxicities. International journal of radiation oncology, biology, physics. 2010;78:359–369. doi: 10.1016/j.ijrobp.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 143.Wasco MJ, Pu RT. Utility of antiphosphorylated H2AX antibody (gamma-H2AX) in diagnosing metastatic renal cell carcinoma. Applied immunohistochemistry & molecular morphology: AIMM/official publication of the Society for Applied Immunohistochemistry. 2008;16:349–356. doi: 10.1097/PAI.0b013e3181577993. [DOI] [PubMed] [Google Scholar]
- 144.Cheung WL, Albadine R, Chan T, Sharma R, Netto GJ. Phosphorylated H2AX in noninvasive low grade urothelial carcinoma of the bladder: correlation with tumor recurrence. J Urol. 2009;181:1387–1392. doi: 10.1016/j.juro.2008.10.146. [DOI] [PubMed] [Google Scholar]
- 145.Brunner AH, Hinterholzer S, Riss P, Heinze G, Weiss K, Brustmann H. Expression of gamma-H2AX in endometrial carcinomas: an immunohistochemical study with p53. Gynecol Oncol. 2011;121:206–211. doi: 10.1016/j.ygyno.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 146.Brustmann H, Hinterholzer S, Brunner A. Expression of phosphorylated histone H2AX (gamma-H2AX) in normal and neoplastic squamous epithelia of the uterine cervix: an immunohistochemical study with epidermal growth factor receptor. Int J Gynecol Pathol. 2011;30:76–83. doi: 10.1097/PGP.0b013e3181eb2fcb. [DOI] [PubMed] [Google Scholar]
- 147.Elsheikh SE, Green AR, Rakha EA, Powe DG, Ahmed RA, Collins HM, Soria D, Garibaldi JM, Paish CE, Ammar AA, Grainge MJ, Ball GR, Abdelghany MK, Martinez-Pomares L, Heery DM, Ellis IO. Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Res. 2009;69:3802–3809. doi: 10.1158/0008-5472.CAN-08-3907. [DOI] [PubMed] [Google Scholar]
- 148.Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262–1266. doi: 10.1038/nature03672. [DOI] [PubMed] [Google Scholar]
- 149.Advani AS, Gibson SE, Douglas E, Jin T, Zhao X, Kalaycio M, Copelan E, Sobecks R, Sekeres M, Sungren S, Hsi ED. Histone H4 acetylation by immunohistochemistry and prognosis in newly diagnosed adult acute lymphoblastic leukemia (ALL) patients. BMC Cancer. 2010;10:387. doi: 10.1186/1471-2407-10-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Advani AS, Gibson S, Douglas E, Diacovo J, Elson P, Kalaycio M, Copelan E, Sekeres M, Sobecks R, Sungren S, Lagoo A, Rizzieri D, Hsi E. Histone H4 acetylation by immunohistochemistry and prognosis in relapsed acute lymphocytic leukaemia (ALL) Br J Haematol. 2011;153:504–507. doi: 10.1111/j.1365-2141.2011.08607.x. [DOI] [PubMed] [Google Scholar]