Abstract
The reduction of soluble hexavalent uranium to tetravalent uranium can be catalyzed by bacteria and minerals. The end-product of this reduction is often the mineral uraninite, which was long assumed to be the only product of U(VI) reduction. However, recent studies report the formation of other species including an adsorbed U(IV) species, operationally referred to as monomeric U(IV). The discovery of monomeric U(IV) is important because the species is likely to be more labile and more susceptible to reoxidation than uraninite. Because there is a need to distinguish between these two U(IV) species, we propose here a wet chemical method of differentiating monomeric U(IV) from uraninite in environmental samples. To calibrate the method, U(IV) was extracted from known mixtures of uraninite and monomeric U(IV) and testted using X-ray absorption spectroscopy (XAS). Monomeric U(IV) was efficiently removed from biomass and Fe(II)-bearing phases by bicarbonate extraction, without affecting uraninite stability. After confirming that the method effectively separates monomeric U(IV) and uraninite, it is further evaluated for a system containing those reduced U species and adsorbed U(VI). The method provides a rapid complement, and in some cases alternative, to XAS analyses for quantifying monomeric U(IV), uraninite, and adsorbed U(VI) species in environmental samples.
Keywords: monomeric U(IV), uranium bioremediation, chemical extraction, uraninite
1. Introduction
Uranium is a redox-active metal that is used primarily to produce nuclear power and weapons. Decades of nuclear energy use and a legacy of weapons development in the U.S. and Europe have resulted in uranium contamination of some subsurface environments and have generated a need to identify repositories for storing radioactive waste. The mechanisms of uranium transport and immobilization in soils and aquifers continue to be a topic of major concern in efforts to remediate contaminated sites (1–3) and to predict the fate of uranium in subsurface nuclear repositories (4–6). Although uranium has a wide range of valence states, two states predominate in near-surface environments: hexavalent uranium, U(VI), and tetravalent uranium, U(IV) (7, 8). Under oxidizing conditions, uranium is primarily present in its hexavalent state as species of the uranyl [UO22+] cation (9), forming numerous aqueous complexes that render the U(VI) valence a soluble form of the radionuclide, especially in the presence of aqueous carbonates (10). Reductive precipitation, whereby U(VI) is transformed by abiotic and/or microbial processes to U(IV), is a promising method of in situ uranium immobilization (11–21).
While uraninite was long assumed to be the sole product of both microbial and chemical U(VI) reduction in biostimulated and naturally-reducing field sites, it is becoming increasingly clear that this assumption may not always hold. Several authors have recently reported the formation of a reduced, non-crystalline U(IV) species, referred to as monomeric or mononuclear U(IV), in systems where either biological or chemical U(VI) reduction took place (21–28). As used here, monomeric U(IV) is an operational term defining non-crystalline, disordered U(IV) species, and may in fact include polynuclear uranium species that do not exhibit regular metal-metal distances (29, 30). The formation of a non-uraninite reduced uranium product is an important development because it requires the re-evaluation of assumptions concerning the stability of reduced U(IV) in the subsurface.
The presence of multiple forms of U(IV) with varying reactivities creates a need for tests that can quantify their relative abundances in laboratory and field samples. This discrimination can be tackled by XAS if appropriate reference spectra for the end member components are available for linear combination. However, if such models are not available (and generally they may not be for natural sediments), then this approach will provide only a semi-quantitative assessment of the contribution of each species. In addition many natural samples of interest are too dilute to allow for XAS analysis and access to XAS facilities may be difficult to obtain, hindering progress on the systematic study of the conditions favoring the production of one or the other product. Hence, our goal was to develop a method to differentiate between the species that is rapid, synchrotron-independent, and quantitative. This method is intended to allow the probing of U speciation in laboratory experiments and field samples for the contribution of monomeric U(IV) to the overall U(IV) content. Because the ability to differentiate between U(IV) species and adsorbed U(VI) was deemed desirable, we also tested the method on a system containing a mixture of U(VI), uraninite, and monomeric U(IV).
2. Experimental
2.1. Media and Cultures
All reagents in this study were certified analytical grade or higher, and ultrapure water (resistivity > 18.2 MΩ cm) was used to prepare solutions. Culturing, growth and post-culture processing of Shewanella oneidensis MR-1 and Shewanella putrefaciens CN-32 was conducted as described previously (22, 23).
2.2. Iron Reduction
Iron reduction experiments to produce biogenic vivianite [Fe3(PO4)2·8H2O] or magnetite (Fe3O4) were conducted using S. putrefaciens CN-32 as described in Veeramani et al. (23).
2.3. Uranium Reduction
Unless indicated otherwise, sample preparation, experimental setup including U(VI) bioreduction and subsequent extraction procedures were conducted under strict anoxic conditions – either in serum bottles equipped with a butyl rubber stopper and an aluminum crimp or inside an anoxic chamber with an atmosphere of 2 – 5 % H2 and a balance of N2. The solutions used were filter-sterilized, and made anoxic by purging with N2 for several hours or by equilibrating the solutions inside an anoxic chamber for several days prior to the experiment. Microbial uranium reduction experiments were conducted in two growth media to favor the production of either biogenic uraninite (UO2) or monomeric U(IV) (22). To preferentially produce biogenic UO2, S. oneidensis MR-1 cells were grown in Luria Bertani (LB) medium for 12 h and washed in a simple medium (BP) containing 20 mM 1,4-piperazinediethanesulfonic acid (PIPES) buffer set to pH 6.8 and 30 mM sodium bicarbonate. The washed cells were then suspended to an optical density (OD600) of 1 in BP medium. To favor the formation of monomeric U(IV) species, washed cells were suspended at OD600 = 1 in Widdel low phosphate (WLP) medium, the composition of which is given in Supporting Information Table 1. To initiate U(VI) reduction, 20 mM L(+)-lactic acid and 0.4 mM uranyl acetate was added to each cell suspension.
Abiotic uranium reduction experiments were conducted at an Fe:U molar ratio of 50:1 for both biogenic magnetite and vivianite. Thus, 3.86 g l−1 magnetite or 8.36 g l−1 vivianite (50 mM total Fe), were added to anoxic solutions containing 1 mM sodium bicarbonate and 20 mM PIPES buffer set to pH 7. Uranyl acetate was added to a final concentration of 1 mM to initiate uranium reduction.
Rifle Area Background Sediments (RABS), collected from a former uranium processing site at Old Rifle, CO (31), were used in laboratory uranium reduction column experiments. The details of these column studies are published in Sharp et al. (24).
2.4. Isolation of Biogenic Uraninite from biomass
To generate a biomass-free uraninite of biogenic origin, biogenic UO2(s) produced as described in Section 2.3 was separated from the cells by a NaOH treatment followed by a hexane separation, detailed in Schofield et al. (32).
2.5. Monomeric U(IV) Extraction
Monomeric U(IV) was selectively extracted from three systems: (1) prepared mechanical mixtures of monomeric U(IV) and UO2(s) formed via microbial U(VI) reduction by S. oneidensis MR-1 cells, (2) either monomeric U(IV) or UO2(s) formed via abiotic U(VI) reduction by biogenic vivianite and biogenic magnetite, respectively (23), and (3) from Rifle column sediment containing largely monomeric U(IV) species (24). The full list of samples and their description is presented in Table 1. All experiments were conducted in duplicate, and the error bars on data (Table 2, Figures 1, 3 and 4) represent the combined analytical error and replicate errors.
Table 1.
Sample descriptions, abbreviations used in text, and literature sources. Mixture ratio indicates the ratio of the volume of the uraninite-favoring system (Bio1) to the volume of the monomeric U(IV)-favoring system (Bio5) in a sample.
| Category | Name | Bio1: Bio5 | Description | Sources |
|---|---|---|---|---|
|
| ||||
| Biologically reduced systems | Bio1 | 100:0 | uraninite-favoring condition | Schofield et al., 2008 |
| Bio2 | 75:25 | |||
| Bio3 | 50:50 | Mixtures of Bio1 and Bio5 | ||
| Bio4 | 25:75 | |||
| Bio5 | 100:0 | monomeric U(IV)-favoring condition | Bernier-Latmani et al., 2010 | |
|
| ||||
| Biogenic Fe minerals | Vivianite | - | Monomeric U(IV) produced by biogenic vivianite | Veeramani et al., 2011 |
| Magnetite | - | Uraninite produced by biogenic magnetite | ||
|
| ||||
| Sediment | Sediment | - | Reduced U in biostimulated Rifle, CO sediments | Sharp et al., 2011 |
Table 2.
Chemical extraction results, calculated contributions of uraninite and monomeric U(IV) and linear combination fitting (LCF) results. All results are presented as % uraninite / % monomeric U(IV). Calculated ratios for Bio2 – Bio4 assume a linear relationship between the end-members. Uncertainty ranges for bicarbonate extraction data result from combined instrumental and replicate errors. Uncertainty for EXAFS LCF data are from scan-by-scan error analysis of XAS data, however the LCF technique is only semi-quantitative and is generally assumed to be accurate to within 10% (33). Calculated values (last column) are obtained from extraction resutls. For example, Bio2 consists of 75% Bio1 and 25% Bio5, hence it is expected to include a contribution of 34 % monomeric U(IV) from Bio1 and 23% monomeric U(IV) from Bio5, for a total of 57%.
| Category | System | Bicarbonate Extraction Result | EXAFS LCF Result | Calculated from Bio1/Bio5 extractions |
|---|---|---|---|---|
|
| ||||
| Biologically reduced systems | Bio1 | 54±3 / 46±3 | 55±2 / 45±2 | N/A |
| Bio2 | 46±3 / 54±3 | - | 43 / 57 | |
| Bio3 | 35±3 / 65±3 | 36±4 / 69±4 | 31 / 69 | |
| Bio4 | 23±3 / 77±3 | - | 20 / 80 | |
| Bio5 | 8±5 / 92±5 | N/A | N/A | |
|
| ||||
| Biogenic Fe minerals | Vivianite | 1±3 / 99±3 | N/A | N/A |
| Magnetite | 93±3 / 7±3 | 96±2 / 4±2 | N/A | |
|
| ||||
| Sediment | RABS | 2±3 / 98±3 | N/A | N/A |
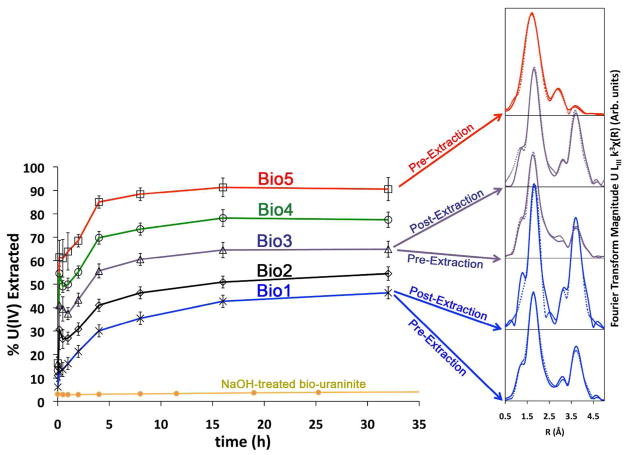
Figure 1.
Monomeric U(IV) extraction results from mixtures of Bio1 (BP medium) and Bio5 (WLP medium). For systems Bio1, Bio3, and Bio4, corresponding Fourier transforms of the EXAFS data are provided to illustrate the incresase in U-U shell magnitude (3.85Å) following extraction of monomeric U(IV) species. In effect, the removal of monomeric U(IV) results in an increase of the fractional contribution of uraninite to the overall U(IV) signal, leading to the observed increase in amplitude of the U-U shell characteristic of this product. Dashed lines indicate shell-by-shell fits (Bio5 – pre-extraction; Bio1 – post-extraction) and linear combination fits (Bio3 – pre-extraction; Bio3 – post-extraction; Bio1 – pre-extraction) of the EXAFS data (see also Supporting Information Figures 1 and 2). A control experiment using NaOH-treaded bio-uraninite, containing essentially 100% UO2 shows that little U(IV) is removed into solution from uraninite during the extraction process.
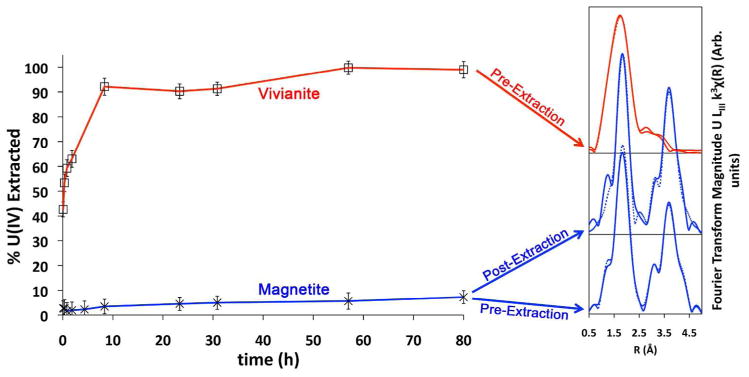
Figure 3.
Bicarbonate U(IV) extraction profiles and Fourier transforms of EXAFS data for biogenic vivianite-associated monomeric U(IV) and biogenic magnetite-associated UO2. Dotted lines in Fourier transform data indicate shell-by-shell fits (Vivianite – pre-extraction; magnetite – post-extraction) and a linear combination fit (magnetite – pre-extraction; see also Supporting Information Figures 1 and 2).
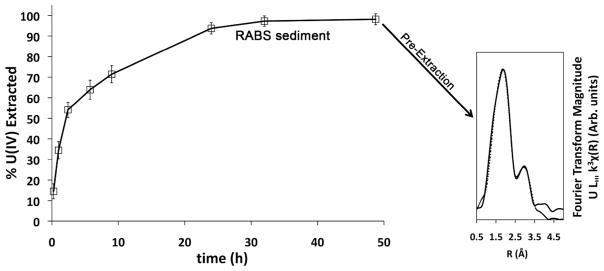
Figure 4.
Bicarbonate U(IV) extraction profile and Fourier transform of the EXAFS data for biostimulated natural sediment amended with acetate and U(VI) from Rifle, CO. Dotted line in Fourier transform indicates shell-by-shell fit of EXAFS data (see also Supporting Information Figures 1 and 2).
The extraction of monomeric U(IV) was performed by treating the various test systems with an anoxic 1.0 M sodium bicarbonate solution of pH 8.7. For the microbial systems, the cells that had preferentially produced biogenic UO2 (BP medium) or monomeric U(IV) species (WLP medium) were centrifuged for 10 min at 10,000 g. The centrifuged pellets from each system were then suspended in small volumes of BP medium to create concentrated stock cell slurry solutions. The two stock solutions were mechanically mixed at different ratios by volume: 100:0, 75:25, 50:50, 25:75, and 0:100. These samples are referred to as Bio1 to Bio5 (Table 1). For example, sample Bio2 contains 75% of the uraninite-favoring system (Bio1) and 25% of the monomeric U(IV)-favoring system (Bio5). These mixtures were diluted into an anoxic 1.0 M sodium bicarbonate solution to cell and uranium concentrations identical to those in the original U(VI) reduction experiments (OD600 = 1 and 0.4 mM U). To quantify total uranium in each experiment, a 1 mL unfiltered sample of the homogenized suspension was immediately taken, digested in oxic 15.44 M HNO3 for 48 h, and diluted in deionized water before ICP-OES (inductively coupled plasma optical emission spectrometry) analysis (ICPE-9000, Shimadzu Europa GmbH). To monitor monomeric U(IV) extraction kinetics, 1 mL samples were taken periodically and filtered through 0.2 μm membranes. To quantify extracted U, 0.5 ml of the filtrate was diluted into 4.5 ml oxic 1.0 M HNO3 and analyzed using ICP-OES. Kinetics samples were collected until steady-state U(IV) concentrations were observed.
The extraction of monomeric U(IV) from the iron mineral systems was conducted in a manner similar to the biomass systems. After U(VI) reduction was complete, concentrated suspensions of each mineral were washed with 50 mM sodium bicarbonate to remove U(VI) and concentrated to a slurry, which was then diluted to final concentrations of 50 mM Fe and 1 mM U in the 1.0 M Na-bicarbonate extraction solution. The sampling, digestion, and analysis by ICP-OES was identical to that for the biomass systems.
The biostimulated RABS column sediment, containing approximately 1 mmol U per kg sediment (24) was suspended in 1.0 M NaHCO3 to achieve a final U concentration of approximately 0.5 mM. To determine total uranium in the sediment, 0.5 g portions of the sediment were digested in aqua regia for 48 h. Aliquots of the digests were diluted in deionized water and analyzed by ICP-OES. Sampling of the bicarbonate extraction experiments was identical to that for the biomass and vivianite/magnetite experiments.
2.6. Biomass containing adsorbed U(VI)
Samples containing monomeric U(IV), uraninite, and adsorbed U(VI) were prepared to test whether the bicarbonate extraction method could effectively separate the three species. Duplicate 50 mL reduction reactors were prepared, containing 450 μM U(VI) acetate and S. oneidensis MR-1 in WLP media to favor the formation of monomeric U(IV). Following complete uranium reduction, the systems were centrifuged at 10,000 g for 10 min to remove the reduction medium, and washed in an anoxic solution containing 50 mM PIPES at pH 7.0 to wash away remaining bicarbonate and lactate. The washed bacterial pellets were suspended to an OD600 of 1 in 50 mL PIPES buffer and spiked with 450 μM anaerobic U(VI) acetate. After 2 h, more than 98% of the U(VI) was removed from solution. Following U(VI) adsorption, the bacterial pellets were suspended in anoxic 1 M NaHCO3 at pH 8.7 to initiate the extraction of the adsorbed U(VI) and monomeric U(IV). To quantify total U in the reactors, unfiltered aliquots of the homogenized suspensions were digested in 70% oxic nitric acid, diluted into oxic 0.1 M nitric acid, and analyzed using a Kinetic Phosphorescence Analyzer (KPA). KPA data reported here and in section 3.4 have total error calculated from combined analytical (instrumental) error and replicate error. The total initial U in the systems was 907±23 μM.
2.7. X-ray absorption spectroscopy (XAS)
Uranium X-ray absorption spectroscopy (XAS) analyses of selected samples were conducted at beamlines 4-1 and 11-2 of the Stanford Synchrotron Radiation Lightsource (SSRL) at the Stanford Linear Accelerator Laboratory (SLAC). Samples were sent from EPFL to SSRL in serum bottles sealed with butyl rubber stoppers and an aluminum seal crimped over the stopper flange and bottle. The serum bottles were shipped in a hermetically sealed stainless steel shipping can (Schuett-biotec GmbH, Göttingen, Germany) filled with N2 to a slightly positive pressure. Immediately prior to XAS analysis, samples were centrifuged to wet pellets and mounted in aluminum holders with Kapton windows inside of an anoxic chamber at SSRL containing 3 – 4% H2 and a balance of N2. During analysis, samples were mounted in a cryostat maintained at 77 K using liquid nitrogen. X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) were collected for uranium at the U LIII-edge (17.2 keV) by both transmission and fluorescence modes. A double-crystal Si (220) monochromator was used for energy selection, detuned 15 – 30% to reject higher harmonic intensities, and was initially calibrated using Y foil with the first inflection point of the Y K edge at 17038.4 eV. The Y foil was also used as an internal calibrant by simultaneously measuring the transmission spectra of the foil and each sample scan. Beam line energy resolution was controlled at less than the U LIII-edge line width (8.67 eV) by utilizing vertical slits in front of the monochromator housing and inside of the experimental hutch. EXAFS oscillations were subtracted by fitting a smoothly varying function (spline) to remove contributions below 1.4 Å, which may result in non-physical pair correlations, using the SixPACK (34) and Athena (35) analysis packages. Backscattering phase and amplitude functions used to fit the spectra were calculated using FEFF8.4 (36). Linear combination (LCF) and shell-by-shell fitting of the spectra was performed using the Athena and Artemis programs (35). Where shell-by-shell fitting was performed, extended fit results and errors are presented in Supporting Information Figures 1 and 2, and Supporting Information Table 2. A scan-by-scan analysis of error for each system fit using LCF was performed.
2.8. Electron Microscopy
The morphology and structure of particles remaining in system Bio5 following bicarbonate extraction of monomeric U(IV) species were analyzed using bright field TEM, high resolution transmission electron microscopy (HRTEM), and selected area electron diffraction (SAED) analyses on a FEI CM300UT FEG transmission electron microscope (Eindhoven, Netherlands). Details about the data collection and electron diffraction pattern fitting may be found in the Supporting Information.
3. Results and Discussion
The presence of uraninite in a sample is often determined by using electron diffraction or X-ray absorption spectroscopy (XAS) at the uranium LIII absorption edge, and examining d-spacing in case of the former and the extended X-ray absorption fine structure (EXAFS) in case of the latter. The presence of a U-U pair correlation (i.e. peak) that appears at approximately R = 3.8 Å in Fourier transforms of the EXAFS spectra (14, 28, 32) (corresponding to a U-U distance of ca. 3.85 Å) can be attributed to the presence of uraninite in a sample. Both monomeric U(IV) and uraninite exihibit ~8 O atoms in the first coordination shell. However, monomeric U(IV) lacks medium-range order, and does not include the 3.85 Å U-U pair correlation. The intrinsic strength of the characteristic U-U shell for uraninite can vary significantly from sample to sample depending upon uraninite particle size, ordering, and relative abundance. For this reason, it is difficult to assess the quantitative fractional abundance of uraninite in samples without additional information.
The extraction method presented here relies on the greater lability of monomeric U(IV) as compared to uraninite (vide infra). Indeed, it is based on the observation that a sample containing a mixture of monomeric U(IV) and uraninite that is incubated in an anoxic 1M solution of sodium bicarbonate at pH 8.7 accumulates U(IV) in solution and shows a decrease in the relative abundance of monomeric U(IV) after the incubation. This observation is supported qualitatively by comparing the EXAFS data of samples prior to and after extraction (e.g., Figure 1). Specifically, following the extraction of monomeric U(IV) from a sample, the amplitude of the U-U pair (3.85 Å) in the remaining bacterial pellet increases dramatically. This observation suggests that U(IV) present as monomeric U(IV) was preferentially complexed to carbonate and subsequently released to solution.
3.1. Biomass systems
Table 2 summarizes the results of the bicarbonate extraction method. As was established earlier, the enzymatic reduction of U(VI) generates mixtures of monomeric U(IV) and uraninite and, depending on the chemical composition of the reduction medium, the fractional contribution of one or the other varies (22). The biomass systems are labeled as Bio 1 – 5, with sample Bio1 representing the most uraninite-rich system and Bio5 representing the most monomeric-U(IV)-rich system (Table 1). For example, the sample named Bio3 contains a 50:50 physical mixture (by volume) of Bio1 and Bio5 (see Table 1). The amounts of uraninite and monomeric U(IV) in a sample are determined using two fully independent and complementary methods: (1) using the chemical extraction of monomeric U(IV) by bicarbonate: the fraction of U(IV) extracted corresponds to the fraction of U(IV) that is monomeric U(IV); and (2) using linear combination fits (LCF) of the EXAFS spectra with monomeric U(IV) and biogenic uraninite, obtained from this study, as components to the fit. In addition, for samples Bio2 – 4, we calculated the predicted contributions of uraninite and monomeric U(IV), based on the amounts present in Bio1 and Bio5 according to the extraction method.
Wet chemical extractions of monomeric U(IV) using the 1.0 M sodium bicarbonate treatment are presented in Figure 1 for the S. oneidensis MR-1 biomass samples. This figure plots percentage of U(IV) extracted as a function of time, which corresponds to the fractional contribution of bicarbonate labile monomeric U(IV) species to overall U(IV). As previously stated, we observe that both Bio1 and Bio5 correspond to a combination of monomeric U(IV) and uraninite. The line corresponding to Bio1 indicates the extraction of approximately 46% of the total U(IV) (Figure 1). This suggests that nearly half of the total U(IV) in the system is present as monomeric U(IV) species. This interesting result is consistent with the XAS data: the amplitude of the U-U shell is relatively low for this sample. However, the same sample after treatment with bicarbonate displays a spectrum with a significant increase in the amplitude of the U-U shell (Figure 1), qualitatively suggesting an increased fractional abundance of uraninite and concommitent decrease in monomeric U(IV).
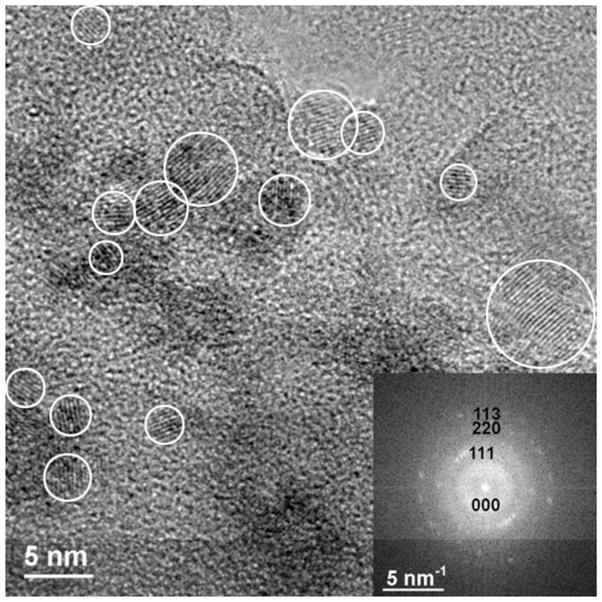
In contrast, the extraction of Bio5 plateaus at 92%, indicating that 92% of the total U(IV) in the sample is present as monomeric U(IV) (Figure 1). The corresponding XAS data show a sample with qualitatively little contribution from a U-U shell prior to bicarbonate extraction. After the bicarbonate treatment, little U remains in the sample, precluding U XAS analysis. Hence, we conclude that this sample contains approximately 92% monomeric U(IV) and that the remainder is likely uraninite that persists after the bicarbonate treatment. To investigate whether uraninite indeed remained in this sample, we performed HRTEM and SAED analyses on the post-extraction biomass (Figure 2). The image verifies the presence of crystalline nanoparticles in the range of 3 – 10 nm and the d-spacings and intensities of reflections (SAED) matched well with that of uraninite, confirming this conclusion.
Figure 2.
HRTEM image of bicarbonate-extracted biomass originally containing primarily monomeric U(IV) species (Bio5). After monomeric U(IV) extraction, removing 90% of the total uranium, fast Fourier transform (FFT) of the full HRTEM image (diffractogram; inset figure) show that remaining uranium is present as uraninite nanoparticles. White circles indicate the presence of particles (lattice fringes).
The results from the extraction method for Bio1 were compared to linear combination fitting (LCF) of EXAFS spectra (Table 2). The LCF end-members were the Bio5 sample –comprised almost entirely of monomeric U(IV)– and the treated Bio1 sample. The latter was selected as an end-member because nearly all of the monomeric U(IV) species was removed by the bicarbonate extraction, leaving bio-uraninite in the sample (Figure 1). The extraction method indicated that Bio1 was comprised of 46% monomeric U(IV). The independent LCF fit indicated that 45% of Bio1 could be accounted for by monomeric U(IV), an excellent match to the extraction result.
Additional samples consisting of physical mixtures of Bio1 and Bio5 confirmed the match between the extraction and LCF data. The amount of uranium extracted from these samples lies between that extrated from Bio1 and Bio5 at the expected ratios (Table 2). For example, for Bio3, we calculate the predicted fractional contribution of monomeric U(IV) (i.e., 69%) by using the fact that the sample is comprised of half Bio5 –which contains 92% monomeric U(IV)– and half Bio1, which contains 46% monomeric U(IV). The bicarbonate treatment actually removed 65% of the total uranium. The EXAFS LCF analysis indicates that the sample contains 69% monomeric U(IV). Thus, once again, the bicarbonate extraction method and the EXAFS results agree very well. The dramatic increase in the U-U shell seen in the treated sample as compared to the untreated is further evidence that U remaining in treated samples is largely uraninite.
We confirmed that the 1 M bicarbonate solution does not induce the dissolution of uraninite, as can occur in the presence of other ligands including citrate and EDTA (37). A chemically extracted uraninite of biogenic origin (32), known to be pure nanoparticulate uraninite, was exposed to 1 M bicarbonate as a control. The control shows little (3.8%) U(IV) extracted (Figure 1), indicating that the extracted uranium is due to the presence of monomeric U(IV) species, and not significant bicarbonate-mediated release of U(IV) from uraninite.
3.2. Vivianite and magnetite systems
Figure 3 illustrates the extraction of monomeric U(IV) from the iron oxide mineral systems. Bicarbonate extractions of U(IV) from the abiotic reduction of U(VI) by biogenic vivianite and magnetite indicate that these iron oxides produce nearly all monomeric U(IV) and uraninite, respectively. Evidencing this, approximately 99% of the total uranium is removed from the vivianite experiment, and only 7% of the total uranium is removed from the magnetite system (Table 2).
The results of the bicarbonate extractions for the iron mineral systems were compared to XAS analyses of the same samples. Three samples were analyzed for each system – the uraninite-dominated magnetite samples prior to and after the bicarbonate extraction, and monomeric U(IV)-dominated vivianite sample prior to bicarbonate extraction. The low concentrations of uranium remaining in bicarbonate-extracted vivianite sample precluded XAS analyses. Uranium associated with vivianite had an EXAFS spectrum lacking a U-U shell in the Fourier transform, consistent with monomeric U(IV). Magnetite-associated U was confirmed to be largely uraninite prior to the extraction treatment, matching the results of Veeramani et al. (23). Linear combination fitting of the EXAFS was possible for the unextracted magnetite sample. The bicarbonate extraction indicates the sample contains 93% uraninite, and the LCF results indicate 96% (Table 2).
3.3. Biostimulated Sediment Extraction
Although the application of our method to biomass and Fe(II) systems containing monomeric U(IV) and uraninite is useful, a further purpose is to provide a tool to discern these species in natural sediments. Having tested the method by comparing the chemical extraction results with the EXAFS LCF results for biomass and Fe(II)-bearing mineral systems, we extended the extraction method to the RABS sediment containing reduced uranium as a product of biostimulation (Figure 4). EXAFS analysis indicated that this sample contained monomeric U(IV) and no detectable U(VI) as judged by the presence of a single U-O peak at ~1.8 Å (phase uncorrected), a U-C or U-P Fourier transform peak at R ~ 3 Å (22) and the lack of a U-U peak at 3.85 Å. The extraction method removed 98% of the total uranium pool (Table 2), verifying that monomeric U(IV) was the dominant U(IV) species. The extracted RABS sediment contained so little U as to preclude EXAFS data collection.
3.4. Samples containing adsorbed U(VI)
The samples considered above did not contain any XAS-detectable U(VI). The extraction method focused thus far on the discrimination between the two U(IV) species, monomeric U(IV) and uraninite. However, it is established that U(VI) adsorbed to solids can be extracted by bicarbonate, albeit at lower (30–100 mM) concentrations (38–42). The higher bicarbonate concentration used in U(IV) extraction is expected to also extract sorbed U(VI), if present. Hence, this method can be further extended to determine contributions of adsorbed U(VI) using a KPA. The KPA technique detects only soluble U(VI) and, when coupled to an ICP-OES or -MS to measure total U, can be used to speciate uranium oxidation state in the anaerobic extraction solutions. Hence, it should be possible to discrimate among monomeric U(IV), uraninite, and adsorbed U(VI). Hexavalent uranium in the extraction solution can be determined by anoxic analysis on the KPA while total uranium can be estimated by oxidizing the same sample, turning all U(IV) to U(VI). In this way, the difference between the total extracted uranium, and the U(VI) contribution is the extracted monomeric U(IV) in the sample. The balance of the uranium, remaining unextracted in the sample, is the uraninite contribution.
To test the above hypothesis, biomass systems containing monomeric U(IV), uraninite, and adsorbed U(VI) were prepared (see Section 2.6). Reductions were performed to favor the production of monomeric U(IV), as in sample Bio5. Thus, the reduced U species in the systems should be present as approximately 90% monomeric U(IV) and 10% uraninite according to the results of the Bio5 extraction (Table 2). After biological U(VI) reduction, an additional and equivalent amount of U(VI) was adsorbed to the same cells. Following the addition of adsorbed U(VI), U(IV) species represented 50% of the total U. Therefore, the extracted systems should contain approximately 45% monomeric U(IV), 5% uraninite, and 50% U(VI). The KPA analyses of these experiments indicate that the systems contain 49.0±0.7% monomeric U(IV), 6.2±0.6% uraninite, and 44.8±0.2% U(VI), close to the predicted ratio.
3.5. Environmental Implications
Increasingly studies in the laboratory and field are indicating that mixtures of monomeric U(IV) species and uraninite can form as the product of U(VI) reduction (14, 17). The presence of the monomeric U(IV) species, particularly at field remediation sites, is critically important because, as it lacks crystal structure, it is likely to be less stable than uraninite. Indeed, while the extraction method employed here evidences the selective remobilization of monomeric U(IV) species by aqueous carbonate species, studies over a broader range of solution chemistry are needed to affirm the relative stability of monomeric U(IV). Given their differences in mobility, knowledge of the relative contributions of monomeric U(VI), uraninite, and U(VI) in soil and aquifer matrices is likely critical in designing accurate transport models and remediation schemes.
Quantification of U species in microbe, mineral, and sediment samples is typically performed using X-ray absorption spectroscopy, a procedure that can be time-consuming, difficult to access, and requires sufficiently high uranium concentrations (> ca 50 ppm). Here we have proposed and tested a wet chemical method to quantify monomeric U(IV), uraninite, and adsorbed U(VI) in samples containing mixtures of the three. The extraction method is rapid and simple and consistent with results from X-ray absorption spectroscopy, and should provide a useful tool for scientists investigating the products of uranium reduction in the laboratory and the field.
Supplementary Material
Acknowledgments
We thank Prof. Philippe Buffat (EPFL) for designing and constructing a spray apparatus to mount the electron microscopy samples. Work carried out at EPFL was funded by Swiss NSF grants No. 20021-113784 and 200020_126821, SNSF International Cooperation grant No. IZK0Z2-12355. Work carried out at SSRL was funded by the SLAC Science Focus Area funded by the US-DOE Subsurface Biogeochemical Research program. DSA was partially supported by a Marie Curie International Incoming Fellowship from the European Commission, grant FP7-PEOPLE-2009-IIF-254143. Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy (DOE), Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program.
References
- 1.Barnett MO, Jardine PM, Brooks SC. U(VI) Adsorption to Heterogeneous Subsurface Media: Application of a Surface Complexation Model. Environ Sci Technol. 2002;36:937–942. doi: 10.1021/es010846i. [DOI] [PubMed] [Google Scholar]
- 2.Long PE, Yabusaki SB, Meyer PD, Murray CJ, N’Guessan AL. Technical Basis for Assessing Uranium Bioremediation Performance. U. S. Nuclear Regulatory Commission; Washington, D. C: 2008. NUREG/CR-6973. [Google Scholar]
- 3.Borch T, Kretzschmar R, Kappler A, Cappellen PV, Ginder-Vogel M, Voegelin A, Campbell K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol. 2010;44:15–23. doi: 10.1021/es9026248. [DOI] [PubMed] [Google Scholar]
- 4.Arnold BW, Kuzio SP, Robinson BA. Radionuclide transport simulation and uncertainty analyses with the saturated-zone site-scale model at Yucca Mountain, Nevada. J Contam Hydrol. 2003;62–63:401–419. doi: 10.1016/s0169-7722(02)00158-4. [DOI] [PubMed] [Google Scholar]
- 5.Ewing RC, Runde W, Albrecht-Schmitt TE. Environmental impact of the nuclear fuel cycle: Fate of actinides. MRS Bull. 2011;35:859–866. [Google Scholar]
- 6.Kerr RA. Light at the end of the radwaste tunnel could be real. Science. 2011;333:150–152. doi: 10.1126/science.333.6039.150. [DOI] [PubMed] [Google Scholar]
- 7.Shock EL, Sassani DC, Betz H. Uranium in geologic fluids: Estimates of standard partial molar properties, oxidation potentials and hydrolysis constants at high temperatures and pressures. Geochim Cosmochim Acta. 1997;61:4245–4266. [Google Scholar]
- 8.Murphy WM, Shock EL. Environmental Aqueous Geochemistry of Actinides. In: Burns PC, Finch R, editors. Uranium: Mineralogy, Chemistry and the Environment. Mineralogical Society of America; Washington, D. C: 1999. pp. 221–253. [Google Scholar]
- 9.Arnold T, Utsunomiya S, Geipel G, Ewing RC, Baumann N, Brendler V. Adsorbed U(VI) surface species on muscovite identified by laser fluorescence spectroscopy and transmission electron spectroscopy. Environ Sci Technol. 2006;40:4646–4652. doi: 10.1021/es052507l. [DOI] [PubMed] [Google Scholar]
- 10.Langmuir D. Aqueous Environmental Geochemistry. Prentice Hall; Upper Saddle River, New Jersey: 1997. [Google Scholar]
- 11.Lovley DR, Phillips EJP, Gorby YA, Landa ER. Microbial reduction of uranium. Nature. 1991;350:413–416. [Google Scholar]
- 12.Lovley DR, Phillips EJP. Reduction of uranium by Desulfovibrio desulfuricans. Appl Environ Microbiol. 1992;58:850–856. doi: 10.1128/aem.58.3.850-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovley DR. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 14.O’Loughlin EJ, Kelly SD, Cook RE, Csencsits R, Kemner KM. Reduction of uranium (VI) by mixed iron(II)/iron(III) hydroxide (green rust): Formation of UO2 nanoparticles. Environ Sci Technol. 2003;37:721–727. doi: 10.1021/es0208409. [DOI] [PubMed] [Google Scholar]
- 15.Jeon B-H, Dempsey BA, Burgos WD, Barnett MO, Roden EE. Chemical reduction of U(VI) by Fe(II) at the solid – water interface using natural and synthetic Fe(III) oxides. Environ Sci Technol. 2005;39:5642–5649. doi: 10.1021/es0487527. [DOI] [PubMed] [Google Scholar]
- 16.Wall JD, Krumholz LR. Uranium reduction. Annu Rev Microbiol. 2006;60:149–166. doi: 10.1146/annurev.micro.59.030804.121357. [DOI] [PubMed] [Google Scholar]
- 17.Sheng L, Szymanowksi J, Fein JB. The effects of uranium speciation on the rate of U(VI) reduction by Shewanella oneidensis MR-1. Geochim Cosmochim Acta. 2011;75:3558–3567. [Google Scholar]
- 18.Burgos WD, McDonough JT, Senko JM, Zhang GX, Dohnalkova AC, Kelly SD, Gorby Y, Kemner KM. Characterization of uraninite nanoparticles produced by Shewanella oneidensis MR-1. Geochim Cosmochim Acta. 2008;72:4901–4915. [Google Scholar]
- 19.Zhang G, Burgos WD, Senko JM, Bishop ME, Dong H, Boyanov MI, Kemner KM. Microbial reduction of chlorite and uranium followed by air oxidation. Chem Geol. 2011;238:242–250. [Google Scholar]
- 20.Burns PC. The crystal chemistry of uranium. In: Burns PC, Finch R, editors. Uranium: Mineralogy, Chemistry and the Environment. Mineralogical Society of America; Washington, D. C: 1999. pp. 23–90. [Google Scholar]
- 21.Fletcher KE, Boyanov MI, Thomas SH, Wu QZ, Kemner KM, Löffler FE. U(VI) Reduction to Mononuclear U(IV) by Desulfitobacterium Species. Environ Sci Technol. 2010;44:4705–4709. doi: 10.1021/es903636c. [DOI] [PubMed] [Google Scholar]
- 22.Bernier-Latmani R, Veeramani H, Dalla Vecchia E, Junier P, Lezama-Pacheco JS, Suvorova EI, Sharp JO, Wigginton NS, Bargar JR. Non-uraninite products of microbial U(VI) reduction. Environ Sci Technol. 2010;44:9456–9462. doi: 10.1021/es101675a. [DOI] [PubMed] [Google Scholar]
- 23.Veeramani H, Alessi DS, Suvorova EI, Lezama-Pacheco JS, Stubbs JE, Sharp JO, Dippon U, Kappler A, Bargar JR. Bernier-Latmani RProducts of abiotic U(VI) reduction by biogenic magnetite and vivianite. Geochim Cosmochim Acta. 2011;75:2512–2528. [Google Scholar]
- 24.Sharp JO, Lezama-Pacheco JS, Schofield EJ, Junier P, Ulrich KU, Chinni S, Veeramani H, Margot-Roquier C, Webb SM, Tebo BM, Giammar DE, Bargar JR, Bernier-Latmani R. Uranium speciation and stability after reductive immobilization in aquifer sediments. Geochim Cosmochim Acta. 2011;75:6497–6510. [Google Scholar]
- 25.Campbell KM, Davis JA, Bargar J, Giammar D, Bernier-Latmani R, Kukkadapu R, Williams KH, Veeramani H, Ulrich K-U, Stubbs J, Yabusaki S, Figueroa L, Lesher E, Wilkins MJ, Peacock A, Long PE. Composition, stability, and measurement of reduced uranium phases for groundwater bioremediation at Old Rifle, CO. Appl Geochem. 2011;26:S167–S169. [Google Scholar]
- 26.Ray AE, Bargar JR, Sivaswamy V, Dohnalkova A, Fujita Y, Peyton BM, Magnuson TS. Evidence for multiple modes of uranium immobilization by an anaerobic bacterium. Geochim Cosmochim Acta. 2011;75:2684–2695. [Google Scholar]
- 27.Sivaswamy V, Boyanov MI, Peyton BM, Viamajala S, Gerlach R, Apel WA, Sani RK, Dohnalkova A, Kemner KM, Borch T. Multiple mechanisms of uranium immobilization by Cellulomonas sp. strain ES6. Biotechnol Bioeng. 2011;108:264–276. doi: 10.1002/bit.22956. [DOI] [PubMed] [Google Scholar]
- 28.Boyanov MI, Fletcher KE, Kwon MJ, Rui X, O’Loughlin EJ, Löffler FE, Kemner KM. Solution and microbial controls on the formation of reduced U(IV) species. Environ Sci Technol. 2011;45:8336–8344. doi: 10.1021/es2014049. [DOI] [PubMed] [Google Scholar]
- 29.Walther C, Fuss M, Büchner S. Formation and hydrolysis of polynuclear Th(IV) complexes – a nano-electrospray mass-spectrometry study. Radiochim Acta. 2008;96:411–425. [Google Scholar]
- 30.Walther C, Rothe J, Brendebach B, Fuss M, Altmaier M, Marquardt CM, Büchner S, Cho H-R, Yun J-I, Seibert A. New insights in the formation processes of Pu(IV) colloids. Radiochim Acta. 2009;97:199–207. [Google Scholar]
- 31.Anderson RT, Vrionis HA, Ortiz-Bernad I, Resch CT, Long PE, Dayvault R, Karp K, Marutzky S, Metzler DR, Peacock A, White DC, Lowe M, Lovley DR. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl Environ Microbiol. 2003;69:5884–5891. doi: 10.1128/AEM.69.10.5884-5891.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schofield EJ, Veeramani H, Sharp JO, Suvorova E, Bernier-Latmani R, Mehta A, Stahlman J, Webb SM, Clark DL, Conradson SD, Ilton ES, Bargar JR. Structure of biogenic uraninite produced by Shewanella oneidensis strain MR-1. Environ Sci Technol. 2008;42:7898–7904. doi: 10.1021/es800579g. [DOI] [PubMed] [Google Scholar]
- 33.Kelly SD, Hesterberg D, Ravel B. Analysis of soils and minerals using X-ray absorption spectroscopy. In: Ulery AL, Drees R, editors. Methods of Soil Analysis. Part 5. Mineralogical Methods. Soil Sci. Soc. Am; Madison, WI: 2008. pp. 387–463. [Google Scholar]
- 34.Webb SM. SIXPACK: a graphical user interface for XAS analysis using IFEFFIT. Phys Scripta. 2005;T115:1011–1014. [Google Scholar]
- 35.Ravel B, Newville M. Athena, Artemis, Hephaestus: Data Analysis for X-Ray Absorption Spectroscopy Using Ifeffit. J Synchrotron Radiat. 2005;12:537–541. doi: 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- 36.Rehr JJ, Albers RC, Zabinsky SI. High-order multiple-scattering calculations of X-ray-absorption fine-structure. Phys Rev Lett. 1992;69:3397–3400. doi: 10.1103/PhysRevLett.69.3397. [DOI] [PubMed] [Google Scholar]
- 37.Luo W, Gu B. Dissolution of uranium-bearing minerals and mobilization of uranium by organic ligands in a biologically reduced sediment. Environ Sci Technol. 2011;45:2994–2999. doi: 10.1021/es103073u. [DOI] [PubMed] [Google Scholar]
- 38.Phillips EJP, Landa ER, Lovley DR. Remediation of uranium contaminated soils with bicarbonate extraction and microbial U(VI) reduction. J Ind Microbiol. 1995;14:203–207. [Google Scholar]
- 39.Fredrickson JK, Zachara JM, Kennedy DW, Duff MC, Gorby YA, Li SW, Krupka KM. Reduction of U(VI) in goethite (α-FeOOH) suspensions by a dissimilatory metal-reducing bacterium. Geochim Cosmochim Acta. 2000;64:3085–3098. [Google Scholar]
- 40.Senko JM, Istok JD, Suflita JM, Krumholz LR. In-situ evidence for uranium immobilization and remobilization. Environ Sci Technol. 2002;36:1491–1496. doi: 10.1021/es011240x. [DOI] [PubMed] [Google Scholar]
- 41.Elias DA, Senko JM, Krumholz LR. A procedure for quantitation of total oxidized uranium for bioremediation studies. J Microbiol Meth. 2003;53:343–353. doi: 10.1016/s0167-7012(02)00252-x. [DOI] [PubMed] [Google Scholar]
- 42.Zhou P, Gu B. Extraction of oxidized and reduced forms of uranium from contaminated soils: Effects of carbonate concentration and pH. Environ Sci Technol. 2005;39:4435–4440. doi: 10.1021/es0483443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.