Abstract
Synaptic transmission is divided into two broad categories on the basis of the distance over which neurotransmitters travel. Wiring transmission is the release of transmitter into synaptic clefts in close apposition to receptors. Volume transmission is the release of transmitters or modulators over varying distances before interacting with receptors. One case of volume transmission over potentially long distances involves release into cerebrospinal fluid (CSF). The CSF contains neuroactive substances that affect brain function and range in size from small molecule transmitters to peptides and large proteins. CSF-contacting neurons are a well-known and universal feature of non-mammalian vertebrates, but only supra- and subependymal serotonergic plexuses are a commonly studied feature in mammals. The origin of most other neuroactive substances in CSF is unknown. In order to determine which brain regions communicate with CSF, we describe the distribution of retrograde neuronal labeling in the rat brain following ventricular injection of Cholera toxin, β subunit (CTβ), a tracer frequently used in brain circuit analysis. Within 15 to 30 minutes following intraventricular injection, there is only diffuse, non-specific staining adjacent to the ventricular surface. Within 2 to 10 days, however, there is extensive labeling of neuronal perikarya in specific nuclear groups in the telencephalon, thalamus, hypothalamus and brainstem, many at a considerable distance from the ventricles. These observations support the view that ventricular CSF is a significant channel for volume transmission and identifies those brain regions most likely to be involved in this process.
Keywords: cerebrospinal fluid, ventricles, Cholera toxin β subunit, wiring transmission, volume transmission, suprachiasmatic nucleus
1. Introduction
Neurons of the brain generally communicate with one another through the release of transmitters and other neuroactive substances at axon terminals to affect the function of various postsynaptic elements. For example, the predominant excitatory and inhibitory transmitters, glutamate and GABA, are released into short synaptic clefts, 12–20 nm in height (Savtchenko and Rusakov, 2007) and interact with nearby postsynaptic receptors (Lopez-Munoz and Alamo, 2009, Bito, 2010). Many other neuroactive substances are also released from axon varicosities but exert their effects at a distance through volume transmission (Agnati et al., 1986, Descarries and Mechawar, 2000, Agnati et al., 2010, Fuxe et al., 2010, Okubo and Iino, 2011b). Volume transmission may take place over considerable distances, including transport via CSF, through extracellular spaces, and along fiber bundles (Bjelke et al., 1995, Fuxe et al., 2010). It is well known that normal production and flow of CSF is critical for optimal brain function (Johanson et al., 2011). Early work on CSF led to the conclusions that it is formed as a secretion by the choroid plexus in the lateral, 3rd and 4th ventricles and that it functions primarily in mechanical stabilization of the brain as well as nutrient and waste flow (Cushing, 1914, Oreskovic and Klarica, 2010). This view has been significantly revised as subsequent research conducted over many decades indicates that CSF is formed continuously by ongoing exchange with brain interstitial fluid as well as secretion from the choroid plexus and, after circulation through the ventricular compartments to subarachnoid space, is resorbed into cranial venous arachnoid villi and the olfactory lymphatic system (Pollay, 2010).
The CSF was not viewed as a conduit for neuronal communication until studies in the 1970s demonstrated both sub- and supraventricular plexuses of raphe serotonergic terminals (Aghajanian and Gallager, 1975, Lorez and Richards, 1975, Chan-Palay, 1976, Richards, 1977). The precise function of the serotonergic ependymal plexuses is unknown, but a recent report suggests that the subependymal plexus functions to modulate activity of the adult ventricular germinal epithelium (Jahanshahi et al., 2011). CSF-contacting neurons are more common in non-mammalian species (Vigh et al., 2004). On the other hand, mammalian ventricular CSF also contains a number of neuroactive substances, many of which have been shown to affect brain function, presumably by volume transmission (for reviews, see Vigh et al., 2004, Johanson et al., 2008, Veening and Barendregt, 2010, Johanson et al., 2011). The source of these substances is presumed to be brain interstitial fluid but specific sources other than the serotonergic plexuses are not known. The present study is an examination of the disposition of the well-established tracer CTβ (Trojanowski, 1983, Ericson and Blomqvist, 1988) following intraventricular infusion and therefore identifies specific areas of the brain that may provide some of these neuroactive substances in CSF.
2. Results
Six types of labeling are observed after intraventicular injections: 1) ependyma, including tanycytes; 2) perivascular spaces; 3) neuronal perikarya in nuclei with known supraependymal plexuses (dorsal raphe); 4) nuclei lying adjacent to the CSF compartment with axonal plexuses close to the ventricle (for example, the suprachiasmatic nucleus; Schwartz and Reppert, 1985); 5) neuron-containing circumventricular organs (subfornical organ); 6) neuronal perikarya in nuclei not directly adjacent to the ependymal surface but with axon terminal fields within the range of diffusion of retrograde tracers and, hence, also a potential source of CSF neuroactive substances.
2.1 Short-term versus long-term survival
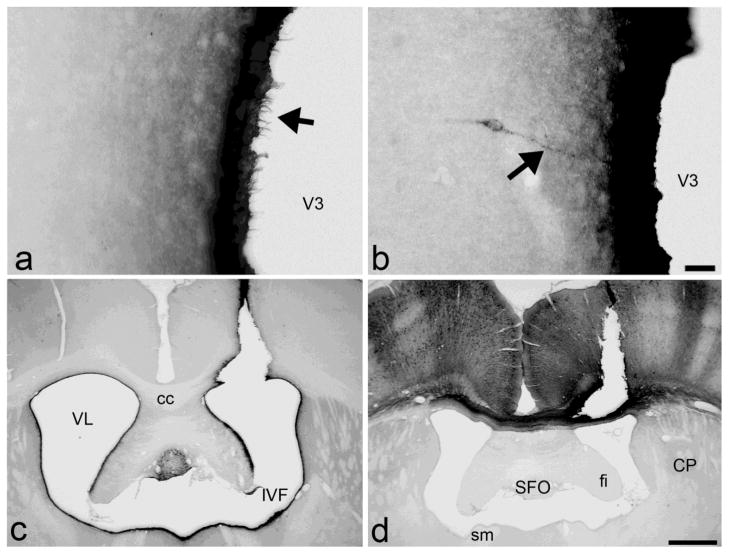
Bilateral labeling of the ependyma and associated cilia surrounding all ventricles is present within 15 minutes of injection (Figure 1a). This labeling of the ventricular lining remains unchanged over the course of a week. Diffuse reaction product also extends into brain parenchyma around the ventricular system for a distance of up to 100 μm with the density of labeling rapidly diminishing with increasing distance from the ependymal surface. Dense immunoreactivity is present in perivascular spaces, along the pial surface of the ventral and lateral surface of the brain and in a few other areas, such as the supramammillary nuclei and the hippocampus. The apical ends of tanyctes in the organum vasculosum lamina terminalis and posterior periventricular nucleus of the hypothalamus are stained. Extremely sparse and faintly labeled neurons are evident in the subfornical organ, the anterior paraventricular hypothalamic nucleus, and other medial hypothalamic nuclei such as the dorsomedial, ventromedial, and arcuate nuclei, some of which extend processes towards the ependymal wall (Figure 1b).
Figure 1.
Short-term survival cases (A and B) Within 15 minutes of injection of CTβ into the third ventricle, there is labeling of cilia along the ependymal lining (arrow in A) but only faint labeling of extremely sparse and scattered neurons, some of which extend processes towards the third ventricular wall (arrow in B). Scale bar 50 μm. Long-term survival injection sites (C and D). (C) Injection site in the lateral ventricle. Note the dense neuronal labeling in the subfornical organ and the dark ependymal immunoreactivity along the ventricular system. However, cells in the subependymal zone near the injection site are not uniformly labeled as one might expect from anterograde uptake. (D) Control injection in the cortex. Dense anterograde and retrograde labeling of ipsi- and contralateral cortex is present, with columnar organization. Unlike CSF injections, fibers of passage in the corpus callosum are also labeled and immunoreactivity is completely absent from the ependymal lining. Scale bar 1 mm. cc, corpus callosum; CP, caudoputamen; fi, fimbria; IVF, interventricular foramen; SFO, subfornical organ; sm, stria medullaris; V3, 3rd ventricle; VL, lateral ventricle.
In the long-term survival groups, diffuse, bilateral labeling of the ventricular ependyma and, in most cases, the ventral pial surface of the brain continues to be present (Figure 1c). For lateral ventricle injections, this zone of diffusion includes the septum, bed nucleus of the stria terminalis, nucleus accumbens and caudoputamen. For third ventricle injections, it includes the periventricular nucleus, anterior, dorsomedial, ventromedial, and arcuate hypothalamic nuclei. Dense retrograde neuronal labeling is present in numerous brain regions (see below). CTβ also labels what appear to be small glia within fiber tracts and nerve roots close to the ventricles or the ventral surface of the brain.
2.2 Injection sites
Long-term injection sites can be divided into three groups: 1) injections without significant parenchymal deposition of tracer along the needle track but with dense ependymal labeling, indicating that tracer was deposited predominantly into CSF (Figure 1c); 2) those with dense immunoreactivity along the needle track, indicating significant parenchymal deposition, as well as bilateral ependymal immunoreactivity (mixed cases); and 3) those with dense parenchymal immunoreactivity but without any ependymal labeling (Figure 1d, control infusions). In the mixed injection cases of group 2, the pattern of retrograde labeling differs between brains but all of these brains still show a consistently labeled set of structures that is identical to that of group 1 brains. The essential findings of group 1 are confirmed by group 2. However, only data from group 1 are described below.
2.3 Distribution of labeled neurons
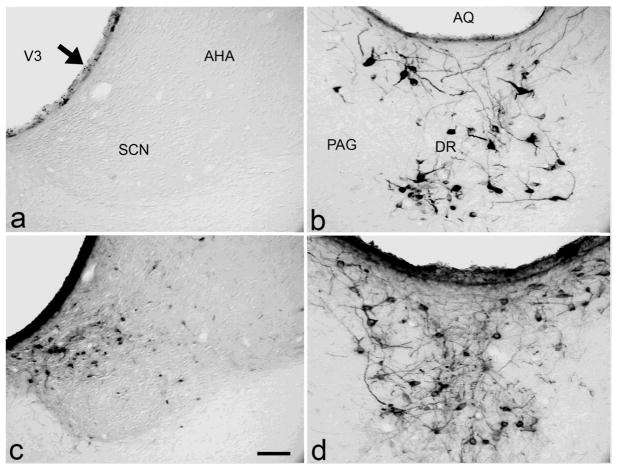
Although we do not rule out anterograde labeling, that is, uptake at the level of the cell body, it does not appear to be a primary source of neuronal label. CTβ is an antero- and retrograde tracer but it is not taken up by neurons in the subependymal zone as expected from anterograde uptake (see parenchyma near the ventricles in Figure 1c). Furthermore, anterogradely labeled CTβ fibers are observed only in cases with extensive parenchymal involvement along the needle track (see corpus callosum in control case, Figure 1d). Retrograde neuronal uptake sufficient to result in long-term labeling is likely to depend on multiple factors, including survival time, tracer concentration, affinity for various tissue components, density of axonal plexuses, and infusion parameters. For example, with different injection volumes, we noted large differences in the extent of dendritic fill (Figure 2). Nevertheless, neuronal label in select regions is present in a fairly consistent pattern in all long-term survival cases that exhibit ependymal immunoreactivity with volumes ranging between 25 and 200 nl. In contrast, with injection volumes less than 25 nl, neurons of the dorsal raphe are still labeled, but neurons of the suprachiasmatic nucleus and other brain regions are not (Figure 3).
Figure 2. Neuronal immunoreactivity increases with tracer volume.
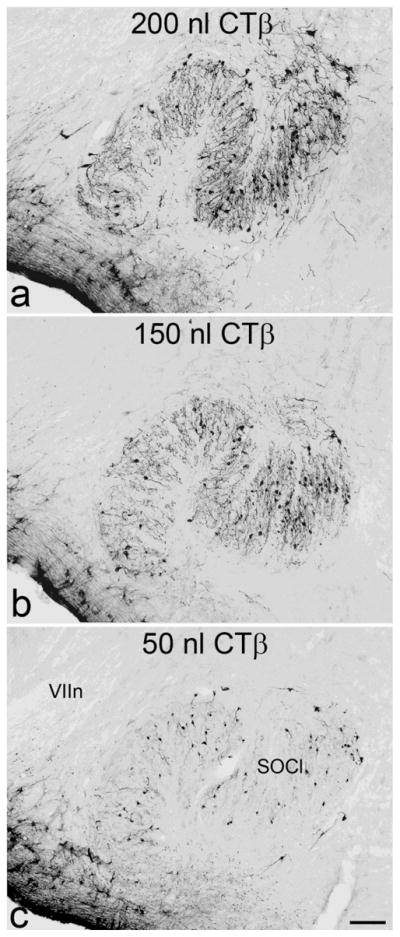
Neurons in the lateral part of the superior olivary complex are labeled with greater dendritic fill with increasing volumes of tracer (50 to 200 nl). Scale bar 100 μm. SOCl, lateral superior olive, VIIn, facial nerve.
Figure 3. Neuronal labeling in the suprachiasmatic nucleus (A, C) and dorsal raphe (B, D) with different injection volumes.
The only structures labeled following 20 nl of CTβ (A and B) are the supraependymal fiber plexus along the ventricular wall (arrow) and neurons in the dorsal raphe. Following delivery of all volumes larger than 20 nl, dense immunoreactivity is apparent in the ependymal lining and in neurons in the suprachiasmatic nucleus (C) as well as the raphe (D). Unlike third ventricle injections, most of the labeled suprachiasmatic neurons following lateral ventricle injections are located in the dorsomedial shell region (C). Scale bar 50 μm. AHA, anterior hypothalamic area; AQ, aqueduct; DR, dorsal raphe; och, optic chiasm; PAG, periaqueductal gray; SCN, suprachiasmatic nucleus.
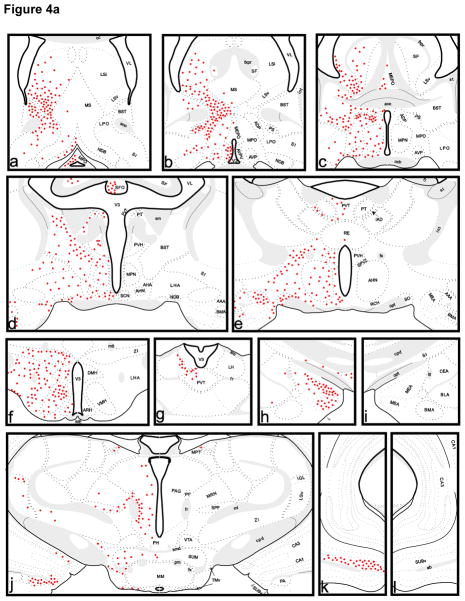
The pattern of labeling following third ventricle injections is illustrated schematically in Figure 4 and the density of label in specific brain regions is depicted in Table 1. In the telencephalon, sparse neuronal labeling is present in the intermediate (LSi) and ventral division (LSv) of the lateral septum at rostral levels. Further caudally the number of labeled neurons in the medial portions of the ventral lateral septum increases sharply. This is contiguous with labeling in the lateral preoptic area (LPO), anterodorsal preoptic nucleus (ADP), medial septum (MS), nucleus of diagonal band (NDB), substantia innominata (SI), and the septofimbrial nucleus (SF). Labeled cells are also present in the bed nucleus of stria terminalis (BST), particularly in its principal and anterodorsal divisions. There is moderate to sparse label in the medial (MEA), basomedial (BMA), basolateral (BLA), central (CEA), and posterior amygdala (PA) as well as the anterior amygdaloid area (AAA). The ventral subiculum (SUBv) contains numerous lightly labeled neurons and an occasional labeled neuron is found in CA1 and CA3 of the hippocampus.
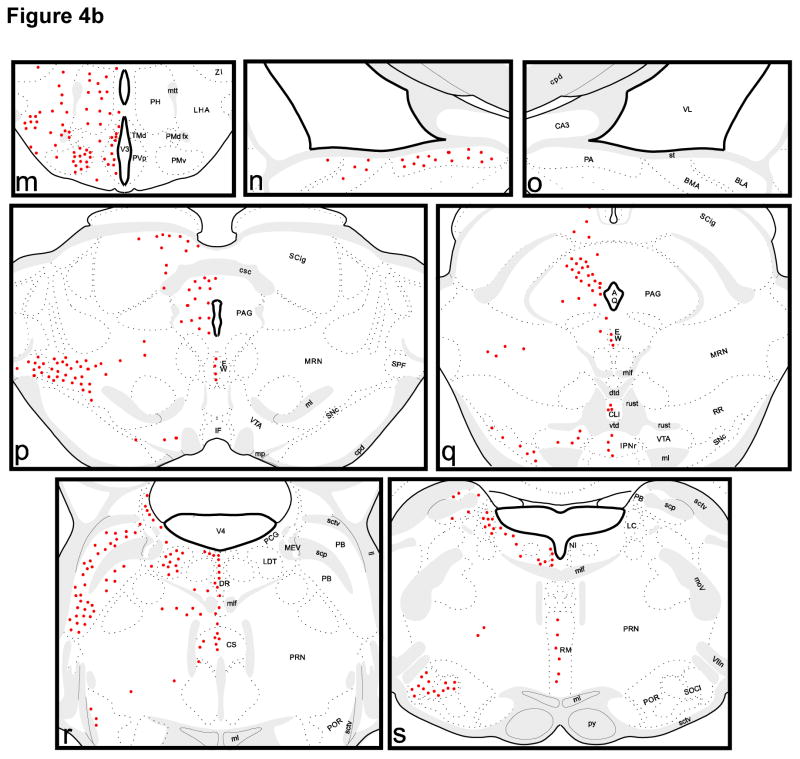
Figure 4. Pattern of neuronal labeling on coronal sections following injection of CTβ in the third ventricle (red circles).
See Section 2.3 and Table 1 for abbreviations of neuronal groups. Abbreviations of ventricles and myelinated fiber bundles are as follows: ab, angular bundle; aco, anterior commissure; cc, corpus callosum; cpd, cerebral peduncle; csc, commissure of the superior colliculus; fi, fimbria; fr, fasciculus retroflexus; fx, columns of the fornix; fxpr, precommissural fornix; int, internal capsule; mcp, middle cerebellar peduncle; ml, medial lemniscus; mlf, medial longitudinal fascicle; mov, motor root of the trigeminal nerve; mp, mammillary peduncle; mtt, mammillothalamic tract; och, optic chiasm; opt, optic tract; pm, principal mammillary tract; py, pyramidal tract; rust, rubrospinal tract; scp, superior cerebellar peduncle; sctv, ventral spinocerebellar tract; sm, stria medullaris; smd, supramammillary decussation; st, stria terminalis; VIIn, facial nerve; V3, 3rd ventricle; V4, 4th ventricle; VL, lateral ventricle; vtd, ventral tegmental decussation.
Table 1.
Extent of neuronal labeling following third ventricle injections in specific brain regions, ranging from low density label (+) to high density label (++++). See text for detailed descriptions of patterns.
| AAA | anterior amygdaloid area | ++ |
| ADP | anterodorsal preoptic nucleus | ++++ |
| AHN | anterior hypothalamic nucleus | ++ |
| ARH | arcuate hypothalamic nucleus | ++ |
| AVP | anteroventral preoptic nucleus | ++ |
| AVPV | anteroventral periventricular nucleus | ++++ |
| BLA | basolateral amygdala | + |
| BMA | basomedial amygdala | + |
| BST | bed nucleus of stria terminalis | +++ |
| CA1 | field CA1, Ammon’s horn | + |
| CA3 | field CA3, Ammon’s horn | + |
| CEA | central amygdala | + |
| CLI | central linear raphe | ++ |
| CS | central superior raphe | +++ |
| DMH | dorsomedial hypothalamic nucleus | ++ |
| DR | dorsal raphe | ++++ |
| EW | Edinger-Westphal nucleus | +++ |
| IAD | interanterodorsal thalamic nucleus | + |
| IGL | intergeniculate leaflet | +++ |
| IPNr | rostral interpeduncular nucleus | + |
| LC | locus coeruleus | ++++ |
| LDT | laterodorsal tegmental nucleus | +++ |
| LGv | ventral lateral geniculate | + |
| LH | lateral habenula | + |
| LHA | lateral hypothalamic area | +++ |
| LPO | lateral preoptic area | ++ |
| LSi | lateral septal nucleus, intermediate part | ++ |
| LSv | lateral septal nucleus, ventral part | +++ |
| MEA | medial amygdala | ++++ |
| MEPO | median preoptic nucleus | ++ |
| MPN | medial preoptic nucleus | ++ |
| MPO | medial preoptic area | ++ |
| MPT | medial pretectal area | + |
| MRN | mesencephalic reticular nucleus | ++++ |
| MS | medial septal nucleus | + |
| NDB | nucleus of the diagonal band | + |
| NI | nucleus incertus | ++ |
| PA | posterior amygdala | +++ |
| PAG | periaqueductal gray | ++ |
| PB | parabrachial nucleus | ++++ |
| PCG | pontine central gray | + |
| PF | parafascicular nucleus | ++ |
| PH | posterior hypothalamic nucleus | ++ |
| PMd | dorsal premammillary nucleus | + |
| PMv | ventral premammillary nucleus | +++ |
| POR | periolivary nucleus | + |
| PRN | pontine reticular nucleus | + |
| PS | parastrial nucleus | ++++ |
| PT | paratenial thalamic nucleus | + |
| PVH | paraventricular hypothalamic nucleus | ++ |
| PVp | periventricular preoptic nucleus | ++ |
| PVT | paraventricular thalamic nucleus | ++ |
| RCH | retrochiasmatic area | ++ |
| RE | nucleus reuniens | + |
| RM | raphe magnus | +++ |
| SCN | suprachiasmatic nucleus | +++ |
| SCig | superior colliculus, intermediate gray | ++ |
| SF | septofimbrial nucleus | + |
| SFO | subfornical organ | +++ |
| SI | substantia innominata | ++ |
| SNc | substantia nigra, pars compacta | ++ |
| SO | supraoptic nucleus | +++ |
| SOCl | superior olivary complex | ++++ |
| SPF | subparafascicular nucleus | ++ |
| SPVZ | subparaventricular zone | ++ |
| SUBv | ventral subiculum | ++++ |
| SUM | supramammillary nucleus | ++ |
| TMd | dorsal tuberomammillary nucleus | ++ |
| TMv | ventral tuberomammillary nucleus | ++ |
| VMH | ventromedial hypothalamic nucleus | +++ |
| VTA | ventral tegmental area | ++ |
| ZI | zona incerta | ++ |
In the diencephalon, there is label in the medial preoptic area (MPO), the medial preoptic nucleus (MPN), the median preoptic nucleus (MEPO), the subfornical organ (SFO), the anteroventral preoptic nucleus (AVP), and the anteroventral periventricular nucleus (AVPV). Dense label is apparent in the anterodorsal preoptic nucleus (ADP) and the parastrial nucleus (PS). Neuronal label in the caudal lateral preoptic area (LPO) is sparse to moderate in density. Dense neuronal label is present in both the shell and core of the suprachiasmatic nucleus (SCN) following third ventricle injections. The paraventricular (PVH), supraoptic (SO), anterior (AHN), dorsomedial (DMH), ventromedial (VMH), arcuate (ARH), posterior (PH), supramammillary (SUM), periventricular preoptic (PVp), dorsal (PMd) and ventral premammillary (PMv), as well as dorsal (TMd) and ventral (TMv) tuberomammillary nuclei all contain labeled neurons. Scattered label is present in the subparaventricular zone (SPVZ), dorsolateral lateral hypothalamus (LHA), and retrochiasmatic area (RCH), and a cluster of labeled cells is found ventral and lateral to the fornix (fx). In the thalamus, neuronal label is present in the paraventricular (PVT), rostral paratenial (PT), reuniens (RE) and interanterodorsal (IAD) nuclei, intergeniculate leaflet (IGL) and ventral lateral geniculate (LGv), lateral habenula (LH), the parafascicular (PF) and subparafascicular (SPF) nuclei as well as the zona incerta (ZI).
In the midbrain, neuronal label is apparent in the medial pretectal area (MPT), rostral subnucleus of the interpenduncular nucleus (IPNr), and the Edinger-Westphal nucleus (EW). Dense neuronal label is also present in the periaqueductal gray (PAG), the intermediate gray layer of the superior colliculus (SCig), and the mesencephalic reticular nucleus (MRN) extending into the subparafascicular nucleus (SPF). In the mid to caudal periaqueductal gray, neuronal label is clustered in the dorsolateral column. Sparse to moderate neuronal label is present in the caudal ventral tegmental area (VTA) and substantia nigra, pars compacta (SNc). The dorsal raphe (DR) contains dense neuronal label both medially and in its lateral wings. In agreement with other authors (Didier-Bazes et al., 1997, Mikkelsen et al., 1997, Simpson et al., 1998) we find that the medial label is situated immediately beneath the aqueduct at mid to caudal levels of the dorsal raphe but is further ventral to the aqueduct at more anterior levels. Moderate neuronal label is also found in the raphe magnus (RM) and the central linear (CLI) and central superior raphe (CS). There is dense label in the parabrachial nucleus (PB) throughout its rostrocaudal extent. Dense neuronal label is also present in the locus coeruleus (LC), laterodorsal tegmental nucleus (LDT), and lateral part of the superior olivary complex (SOCl). Moderate to sparse neuronal label is present in the pontine central gray (PCG), pontine reticular nucleus (PRN), the nucleus incertus (NI), and the periolivary nuclei (POR).
Injections into the lateral ventricle result in a similar pattern of label as in the third ventricle, with the following exceptions. The medial septum and nucleus of diagonal band contain a greater number of labeled neurons. Dense label is also present in the hippocampus in CA1 and CA3. Unlike injections in the third ventricle, the lateral amygdala contains labeled neurons and the label in the basolateral amygdala is denser. The medial amygdala contains fewer labeled neurons and labeling is absent from the central amygdala. Occasional labeled neurons are present in the globus pallidus immediately adjacent to the internal capsule.
In the diencephalon, the subfornical organ is only labeled along its periphery. The labeling in medial hypothalamic nuclei also tends to be sparser following lateral ventricle injections. For example, label in the suprachiasmatic nucleus is sparse in the ventrolateral core (Figure 3c). No label is apparent in the supraoptic nuclei. In addition to the thalamic label following third ventricle injections, there is scattered neuronal label in the ventral anterior-lateral complex, the central medial nucleus, the medial habenula, the central linear nucleus, the rhomboid nucleus, and the reuniens. Label is also seen in the medial portion of the reticular thalamic nucleus.
In the brainstem there is sparser labeling in the mesencephalic reticular nucleus extending into the subparafascicular nucleus, the periaqueductal and pontine central gray, and the parabrachial nucleus. Unlike injections in the third ventricle, there is no label in the superior colliculus, the laterorodorsal tegmental nucleus, the central superior raphe, the raphe magnus, and the pontine reticular nucleus following lateral ventricle injections.
3. Discussion
3.1 Determinants of tracer uptake and transport
The present findings demonstrate that injection of small volumes of a tracer into ventricular CSF results in selective neuronal labeling in many areas of the brain with prominent functional roles in behavioral, neuroendocrine, and homeostatic regulation. The CTβ tracer diffuses widely across the brain parenchyma with a decreasing concentration gradient from ventricle to deep structures and specific retrograde labeling is only observed with long survival periods. The pattern of label described is in normal animals and can now be contrasted with diseased or injured conditions. Furthermore, some of the labeled neuronal groups may be the target of neuropharmacological compounds, as diffusion of these drugs is thought to mimic volume transmission (Zoli et al., 1999).
CTβ is known to be a highly sensitive retrograde tracer (for review, see Lanciego and Wouterlood, 2011). With parenchymal injections of retrograde tracers, uptake and transport resulting in perikaryal labeling is a complex function of local tracer concentration, density of subsets of specific axon terminals in the area and affinity of the tracer for uptake mechanisms (Lanciego and Wouterlood, 2011). This is exemplified by the minor differences in neuronal labeling between third and lateral ventricular injections, likely reflecting high tracer concentrations in the parenchyma close to the injection site. With such ventricular injections, proximity to the nearby ventricular surface of specific sets of axon terminals is likely to be a critical determinant of uptake and transport.
3.2 Technical concerns
Dorsal diffusion of retrograde tracers along the path of the needle was a significant technical concern of the present report, potentially leading to false positive results. Four arguments mitigate this concern. First, injections were carefully categorized by the degree of parenchymal involvement along the needle track into three groups: 1) those with extensive parenchymal involvement and no diffusion into the CSF (as evidenced by a lack of ependymal immunoreactivity), 2) those with both parenchymal and CSF diffusion, and 3) those with little parenchymal involvement at the site of injection but with extensive CSF diffusion. We observed that the pattern of retrograde labeling following purely parenchymal injections was significantly different from the last group of CSF injections. For example, widespread cortical and thalamic sites that were densely labeled following cortical injections were free of label in the third CSF injection group, supporting the notion that there was no uptake from cortical parenchyma in the latter cases.
The second observation that mitigated the concern about false positives is that in the mixed injection cases, the labeling pattern differs somewhat between brains but all of these cases still show a consistently labeled set of structures that is identical to the cases in the CSF group. Third, the majority of retrograde label was similar following injections into either the third or the lateral ventricle. If parenchymal injection of tracer arising from dorsal diffusion up the needle track led to false positive results, one would expect the injections in the third ventricle and lateral ventricle to result in widely differing patterns of retrograde labeling of afferents terminating in the overlying thalamus or cerebral cortex, respectively. Fourth, anterograde labeling of the fiber bundles above the site of injection, such as the corpus callosum, was apparent following parenchymal injections, but absent from the CSF injections, suggesting that uptake and transport of tracer was not simply the result of dorsal diffusion of CTβ.
3.3 The potential role of periventricular and ventricular innervations
CSF-contacting neurons are a phylogenetically ancient system that may detect the constituents of the internal fluid milieu (Vigh et al., 2004). The best-studied example in mammals is the dense sub- and supraependymal terminal plexus arising in the dorsal raphe (Chan-Palay, 1976, Richards, 1977). Less well-studied examples of CSF-contacting neuronal elements are a set of NADPH-diaphorase immunoreactive neurons in the subependymal layer extending dendrites into the CSF (Sancesario et al., 1996), supraependymal cells and fibers in the hypothalamus (Mitchell and Card, 1978, Card and Mitchell, 1979, Vigh et al., 2004), and spinal cord neurons extending dendrites with stereocilia into the central canal and terminal ventricle (Vigh et al., 1983). The function of these latter groups of CSF-contacting neurons is not clear. As for the serotonergic group arising in the dorsal raphe, this nucleus is involved in major depression and its neurotransmitter serotonin plays a prominent role in plasticity (Stockmeier, 1997, Michelsen et al., 2008, Hammack et al., 2012). Furthermore, serotonin has been suggested to act as a neuromodulator through volume transmission (Ciranna, 2006, Hensler, 2006). Our neuronal labeling in the medial dorsal raphe is in agreement with prior studies in which tracers were injected directly into CSF (Didier-Bazes et al., 1997, Mikkelsen et al., 1997, Simpson et al., 1998). However, we observe additional labeling of the dorsal raphe lateral wings and dense labeling in the median raphe, almost certainly reflecting a wider parenchymal distribution of tracer in the present study.
Periventricular terminals that do not contact the ventricular CSF directly are also known to influence distant targets through diffusion. For example, the suprachiasmatic nucleus, a master circadian pacemaker in the mammalian brain, is able to control the rhythmic function of its targets through volume transmission (Silver et al., 1996) and is densely labeled only with CSF injections of more than 20 nl. Furthermore, shell SCN neurons produce vasopressin that diffuses into the CSF in a circadian pattern (Schwartz and Reppert, 1985). The topography of CTβ label in the SCN shell following lateral ventricular delivery of tracer probably reflects the dense efferents of the shell in the medial periventricular zone (Watts and Swanson, 1987, Watts et al., 1987, Leak et al., 1999, Leak and Moore, 2001). Third ventricular injections, in contrast, appear to also involve the more laterally situated efferents of the core (Watts and Swanson, 1987, Watts et al., 1987, Leak et al., 1999, Leak and Moore, 2001).
Other brain regions besides the raphe and suprachiasmatic nucleus that are thought to communicate through volume transmission and were labeled in the present study include the locus coeruleus and ventral midbrain. Many cells were labeled in the locus coeruleus and a moderate number of cells were labeled in the caudal nigra and ventral tegmental area. Noradrenaline and dopamine, the predominant neurotransmitters in these two brain regions, are thought to participate in volume transmission to varying degrees and mediate aspects of arousal, reward, and motor function (Callado and Stamford, 2000, Cragg et al., 2001, Antonopoulos et al., 2004, Rice and Cragg, 2008, Fuxe et al., 2010). Other neurotransmitters that may act parasynaptically as well as synaptically include the abundant transmitters GABA and glutamate (Maldonado et al., 2011, Okubo and Iino, 2011a). Our data reveal numerous brain regions that could contribute to this extrasynaptic GABA and glutamate pool.
Many neurons in the hypothalamus were labeled in the present study. This included neurons in several preoptic regions. The medial preoptic hypothalamus has long been known to play an integral role in the control of reproduction (Simerly, 1998, Charlton, 2008). Because rat gonadotropin-releasing hormone neurons receive few synaptic inputs, they are thought to be controlled at least in part by volume transmission, specifically by somatostatin and kisspeptin (Iijima et al., 2011, Koyama et al., 2012). We also observed labeling in the tuberal hypothalamus, including in the arcuate. The arcuate nucleus houses neurons that contain β-endorphin, which is released into CSF and thought to play a role in opioid analgesia (Winther Bach et al., 1986, Bach and Yaksh, 1995, MacMillan et al., 1998). A third group of hypothalamic neurons that has been hypothesized to act through volume transmission is the hypocretin/orexin group in the lateral hypothalamus (Del Cid-Pellitero and Garzon, 2011). Hypocretin is present in the CSF (Aran et al., 2012) and is involved in the maintenance of wakefulness and the central regulation of energy homeostasis (Cao and Guilleminault, 2011, Hauw et al., 2011, Nixon et al., 2012, Tsuneki et al., 2012). We observed dense label in the perifornical lateral hypothalamus where hypocretin neurons are situated following intraventricular delivery of CTβ. Future investigations using double-label immunofluorescence and confocal microscopy to identify the phenotype of all these neurons are warranted.
3.4 Comparison to prior studies
The first studies examining the transport of proteins into the brain from the ventricular system used horseradish peroxidase (M.W. 43,000) and apoferritin (M.W. 465,000) at short survival times only and did not report significant neuronal labeling (Brightman, 1965, Wagner and Pilgrim, 1974, Borison et al., 1980). Subsequent studies with longer survival times did report neuronal label in the paraventricular nuclei of the hypothalamus, the dorsal raphe, and the hippocampal formation following intraventricular injections of horseradish peroxidase (Broadwell and Balin, 1985). Other investigators noted scattered labeled neurons adjacent to the anterior third ventricle and within the superficial brainstem reticular formation following intraventricular delivery of propidium iodide (Borges et al., 1985). Injections of DAPI into the lateral ventricle of the rat also labeled cells in the hypothalamus, thalamus, and brainstem (Chen and Su, 1989). The present study, however, is the first to systematically map out the various neuronal groups labeled following CSF tracer delivery. The previous studies and the present report suggest that molecules differ in sensitivity and specificity of neuronal labeling following intraventricular delivery, particularly those that are not designed for retrograde tracing. For example, propidium iodide, granular blue, and wheat germ agglutinin conjugated to horseradish peroxidase are taken up by Purkinje neurons in the cerebellum when injected into the lateral ventricles but unconjugated horseradish peroxidase and Evans Blue are not (Borges et al., 1985). Propidium iodide injections into the lateral ventricles resulted in labeling in areas where we observed no neuronal labeling (Chen and Su, 1989), however, molecules such as DAPI and propidium iodide are DNA intercalating agents and not generally used as retrograde tracers.
A previous study noted that the CTβ tracer labeled neurons in the dorsal raphe far better than another widely used tracer, FluoroGold (Mikkelsen et al., 1997). We confirmed this observation with injections of FluoroGold (data not shown). In the Mikkelsen study, iontophoretic delivery of CTβ into the third ventricle also labeled neurons in the medial hypothalamus, including in the paraventricular hypothalamic and suprachiasmatic nuclei. Although the emphasis of that report was on the raphe innervation of the subcommissural organ, it confirms that even tiny volumes of tracer iontophoresced into the third ventricle can result in labeling of neurons in areas other than the dorsal raphe.
4. Experimental Procedure
4.1 Animals and Surgeries
All the research for this report was performed in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and with approval of the Institutional Animal Care and Use Committee of the University of Pittsburgh. Nineteen male and female albino rats weighing between 200 and 500 grams (Harlan Sprague-Dawley) were housed in pairs in polypropylene cages and exposed to a 12:12-hour light-dark cycle. Purina Rat Chow and tap water were made available at all times.
Animals were anesthetized with 80 mg/kg ketamine (Fort Dodge Laboratories, Fort Dodge, IA) and 20 mg/kg xylazine (Fort Dodge Laboratories) and placed in a Kopf Stereotaxic Apparatus (Tujunga, CA). Coordinates were determined with the help of the Paxinos and Watson atlas (Paxinos and Watson, 1998). Injections were aimed at the lateral ventricle at the level of the subfornical organ (DV −4.0, ML −1.8, AP −1.4) or in the third ventricle at the level of the dorsomedial hypothalamic nucleus (DV −9.0, ML 0.0, AP −3.3). Pressure injections of volumes ranging from 20 to 200 nl of CTβ (1% low salt solution, List Biologicals, Campbell, CA) were made with a syringe (external diameter 0.24 mm, Hamilton Co., Reno, NV) at a rate of 10 nanoliters per minute (Trojanowski, 1983, Ericson and Blomqvist, 1988). The cannula was left in the brain at the end of the infusion for an additional ten minutes to minimize dorsal diffusion. Three animals receiving 200 nl of CTβ were sacrificed at 15 or 30 minutes following infusion. The remaining animals (n=16) were sacrificed 2 or 7–10 days following surgery. No difference in labeling was apparent between the 2 and 7–10 day groups. The long-term injections were divisible into three groups: purely parenchymal injections with no diffusion into CSF as evidenced by lack of ependymal immunoreactivity (n=2), mixed parenchymal and CSF injections (n=8), and injections with little parenchymal immunoreactivity along the needle track but dense ependymal labeling (n=6). The pattern of labeling in the latter group was interpreted to reflect uptake of tracer from the CSF and periventricular compartments.
For sacrifice, animals were deeply anesthetized and perfused through the heart with 50 ml of saline followed by 300–400 ml of 4% paraformaldehyde in 0.1 M phosphate buffer. The brain was removed and post-fixed in the same fixative for 1 hour at 4° C. This was followed by cryoprotection in sequential changes of 20% and 30% sucrose in 0.1 M phosphate buffer for 24 hours each at 4° C. A one in six series of 30 μm sections was then cut in the coronal plane on a freezing microtome from the level of the rostral lateral septum to the caudal end of the locus coeruleus.
4.2 Immunohistochemistry and microscopy
For immunohistochemical visualization of tracer, a modification of the avidin-biotin procedure of Hsu was followed (Hsu et al., 1981). A goat polyclonal antibody generated against the β subunit of cholera toxin (List Biologicals, Campbell, CA) was used at a final dilution of 1:20,000. Reagents were diluted in 0.1 M phosphate buffered saline, which was, unless specified otherwise, also used for washes between incubations. Sections were pretreated with 0.5% sodium borohydride and 0.5% hydrogen peroxide for 15 minutes each, with several changes of buffer in between the pretreatments. Non-specific antibody binding was minimized by incubating for one hour in 10% normal donkey serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and 0.3% Triton-X 100 (Sigma) on a rotator at room temperature. This was followed by overnight primary antibody incubation with 1.5% normal donkey serum and 0.3% Triton-X 100 on a rotator at room temperature. The next day, biotinylated anti-goat secondary antibody (Jackson) was used at final dilutions of 1:1000 in the same diluent (90–120 minutes on rotator at 24° C) and was followed by Vectastain Elite avidin-biotin reagents (Vector Labs, Burlingame, CA) with 0.3% Triton-X (90–120 minutes on rotator at 24° C). Following a 5 minute preincubation in diaminobenzidine (0.05% solution in Tris Buffer, pH 7.2), hydrogen peroxide was added to begin the peroxidase reaction (final dilution 0.01%) and terminated by buffer washes within 20 minutes. Sections were then exposed to 0.04% osmium tetroxide in 0.1 M phosphate buffer for 3–5 seconds, washed, mounted, dehydrated in a graded series of ethanols, cleared in xylenes, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA). Omission of primary antibody eliminated staining.
Immunolabeling was studied using a light microscope with interference contrast optics and digitized images of select sections were recorded using a Spot-2 camera (Diagnostic Instruments) and software from Adobe Photoshop (Adobe Systems Inc., San Jose, CA). Images were uniformly contrast-enhanced. One set of immunohistochemically labeled sections from representative cases was counterstained with Neutral Red or Cresyl Violet to aid in identification of cytoarchitectonic boundaries. For ease of presentation the pattern of labeling is illustrated in Swanson’s Brain Maps with his abbreviations (Swanson, 2004).
5. Conclusion
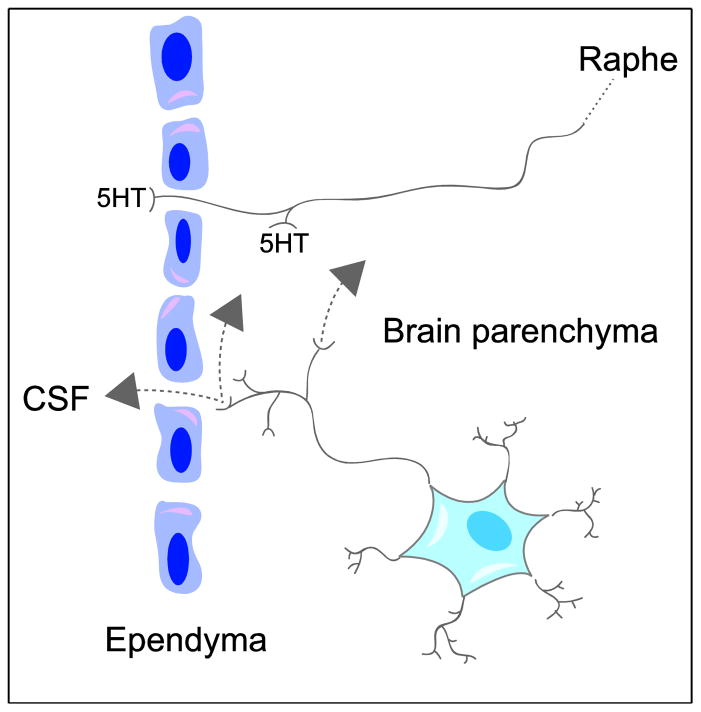
The present study demonstrates dense retrograde neuronal labeling in the telencephalon, diencephalon, midbrain, and brainstem following intraventricular delivery of a highly sensitive tracer. These data imply that substances delivered into CSF are taken up by specific groups of neurons. There are two evident interpretations of our results. One is that these neurons are specific targets for substances circulating in CSF. Although this may well be true, an additional possibility is that the neurons are an important source of neuroactive substances intended to have effects on both local and distant receptors (Figure 5). The present results thus support the concept of volume transmission and provide evidence for possible diffusion pathways in a significant number of brain regions. In this context the group of labeled cells distributed widely over the neuraxis could be viewed as another example of “indirect” CSF-contacting neurons.
Figure 5.
Schematic depicting the two types of neurons labeled by intraventricular CTβ: midbrain raphe neurons terminating both supra- and subependymally that release serotonin (5HT) into the cerebrospinal fluid (CSF) and extra-raphe neurons with axon varicosities that terminate both subependymally and deeper within brain parenchyma. The latter neurons release neuroactive substances into interstitial space that may mediate volume transmission in nearby parenchyma as well as through wider diffusion via the CSF.
Highlights.
Neurons that may release neuroactive substances into the cerebrospinal fluid were retrogradely labeled with intraventricular infusions of a tracer.
The tracer diffused into periventricular regions and was retrogradely transported by numerous afferents.
These neurons were located in regions of the telencephalon, diencephalon, midbrain, and brainstem that play important functional roles in endocrine, behavioral, and homeostatic regulation.
The data support the concept of volume transmission, whereby neuroactive substances diffuse from their original source to distant receptors via the interstitial space and the cerebrospinal fluid.
Acknowledgments
This work was supported by a startup award from Duquesne University to Rehana K. Leak and NIH Grant NS-16304 to Robert Y. Moore. There was no sponsor input into planning, execution, analysis and reporting of the data. All animal work was performed using a protocol approved by the University of Pittsburgh IACUC. We are grateful to Eric Abrahamson, Joan Speh, Nadine Suhan, and Rebecca Danchenko for helpful discussions. We are also grateful to Deb A. Wilson, Mary F. Caruso, and Jackie L. Farrer for superb administrative support.
Footnotes
Disclosure Statement
The authors both have approved the final version of the article and declare that neither has a conflict of interest with other individuals or organizations.
Author Contributions
Planning of the experiments was performed jointly by Rehana K. Leak and Robert Y. Moore. The experiments were performed by Rehana K. Leak. Initial analysis of the data and preparation of the manuscript was performed by Rehana K. Leak. The final data analysis and manuscript preparation was done jointly by Rehana K. Leak and Robert Y. Moore.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Gallager DW. Raphe origin of serotonergic nerves terminating in the cerebral ventricles. Brain Res. 1975;88:221–231. doi: 10.1016/0006-8993(75)90386-8. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol Scand. 1986;128:201–207. doi: 10.1111/j.1748-1716.1986.tb07967.x. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev. 2010;64:137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Antonopoulos J, Latsari M, Dori I, Chiotelli M, Parnavelas JG, Dinopoulos A. Noradrenergic innervation of the developing and mature septal area of the rat. The Journal of comparative neurology. 2004;476:80–90. doi: 10.1002/cne.20205. [DOI] [PubMed] [Google Scholar]
- Aran A, Shors I, Lin L, Mignot E, Schimmel MS. CSF levels of hypocretin-1 (orexin-A) peak during early infancy in humans. Sleep. 2012;35:187–191. doi: 10.5665/sleep.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach FW, Yaksh TL. Release of beta-endorphin immunoreactivity into ventriculo-cisternal perfusate by lumbar intrathecal capsaicin in the rat. Brain research. 1995;701:192–200. doi: 10.1016/0006-8993(95)01003-1. [DOI] [PubMed] [Google Scholar]
- Bito H. The chemical biology of synapses and neuronal circuits. Nat Chem Biol. 2010;6:560–563. doi: 10.1038/nchembio.408. [DOI] [PubMed] [Google Scholar]
- Bjelke B, England R, Nicholson C, Rice ME, Lindberg J, Zoli M, Agnati LF, Fuxe K. Long distance pathways of diffusion for dextran along fibre bundles in brain. Relevance for volume transmission. Neuroreport. 1995;6:1005–1009. doi: 10.1097/00001756-199505090-00014. [DOI] [PubMed] [Google Scholar]
- Borges LF, Elliott PJ, Gill R, Iversen SD, Iversen LL. Selective extraction of small and large molecules from the cerebrospinal fluid by Purkinje neurons. Science. 1985;228:346–348. doi: 10.1126/science.2580350. [DOI] [PubMed] [Google Scholar]
- Borison HL, Borison R, McCarthy LE. Brain stem penetration by horseradish peroxidase from the cerebrospinal fluid spaces in the cat. Exp Neurol. 1980;69:271–289. doi: 10.1016/0014-4886(80)90211-3. [DOI] [PubMed] [Google Scholar]
- Brightman MW. The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. II. Parenchymal distribution. Am J Anat. 1965;117:193–219. doi: 10.1002/aja.1001170204. [DOI] [PubMed] [Google Scholar]
- Broadwell RD, Balin BJ. Endocytic and exocytic pathways of the neuronal secretory process and trans-synaptic transfer of wheat germ agglutinin-horseradish peroxidase in vivo. J Comp Neurol. 1985;242:632–650. doi: 10.1002/cne.902420410. [DOI] [PubMed] [Google Scholar]
- Callado LF, Stamford JA. Spatiotemporal interaction of alpha(2) autoreceptors and noradrenaline transporters in the rat locus coeruleus: implications for volume transmission. Journal of neurochemistry. 2000;74:2350–2358. doi: 10.1046/j.1471-4159.2000.0742350.x. [DOI] [PubMed] [Google Scholar]
- Cao M, Guilleminault C. Hypocretin and its emerging role as a target for treatment of sleep disorders. Curr Neurol Neurosci Rep. 2011;11:227–234. doi: 10.1007/s11910-010-0172-9. [DOI] [PubMed] [Google Scholar]
- Card JP, Mitchell JA. Further observations on the intraventricular neuronal cluster of the golden hamster brain. Scan Electron Microsc. 1979:505–510. [PubMed] [Google Scholar]
- Chan-Palay V. Serotonin axons in the supra- and subependymal plexuses and in the leptomeninges; their roles in local alterations of cerebrospinal fluid and vasomotor activity. Brain Res. 1976;102:103–130. doi: 10.1016/0006-8993(76)90578-3. [DOI] [PubMed] [Google Scholar]
- Charlton H. Hypothalamic control of anterior pituitary function: a history. J Neuroendocrinol. 2008;20:641–646. doi: 10.1111/j.1365-2826.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- Chen S, Su HS. Selective labeling by propidium iodide injected into the lateral cerebral ventricle of the rat. Brain Res. 1989;483:379–383. doi: 10.1016/0006-8993(89)90184-4. [DOI] [PubMed] [Google Scholar]
- Ciranna L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol. 2006;4:101–114. doi: 10.2174/157015906776359540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Nicholson C, Kume-Kick J, Tao L, Rice ME. Dopamine-mediated volume transmission in midbrain is regulated by distinct extracellular geometry and uptake. Journal of neurophysiology. 2001;85:1761–1771. doi: 10.1152/jn.2001.85.4.1761. [DOI] [PubMed] [Google Scholar]
- Cushing H. Studies on the Cerebro-Spinal Fluid: I. Introduction. J Med Res. 1914;31:1–19. [PMC free article] [PubMed] [Google Scholar]
- Del Cid-Pellitero E, Garzon M. Medial prefrontal cortex receives input from dorsal raphe nucleus neurons targeted by hypocretin1/orexinA-containing axons. Neuroscience. 2011;172:30–43. doi: 10.1016/j.neuroscience.2010.10.058. [DOI] [PubMed] [Google Scholar]
- Descarries L, Mechawar N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog Brain Res. 2000;125:27–47. doi: 10.1016/S0079-6123(00)25005-X. [DOI] [PubMed] [Google Scholar]
- Didier-Bazes M, Voutsinos B, Aguera M, Peyron C, Akaoka H, Belin MF. Specific potentialities of embryonic rat serotonergic neurons to innervate different periventricular targets in the adult brain. J Comp Neurol. 1997;382:29–45. [PubMed] [Google Scholar]
- Ericson H, Blomqvist A. Tracing of neuronal connections with cholera toxin subunit B: light and electron microscopic immunohistochemistry using monoclonal antibodies. J Neurosci Methods. 1988;24:225–235. doi: 10.1016/0165-0270(88)90167-7. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Dahlstrom AB, Jonsson G, Marcellino D, Guescini M, Dam M, Manger P, Agnati L. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog Neurobiol. 2010;90:82–100. doi: 10.1016/j.pneurobio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cooper MA, Lezak KR. Overlapping neurobiology of learned helplessness and conditioned defeat: implications for PTSD and mood disorders. Neuropharmacology. 2012;62:565–575. doi: 10.1016/j.neuropharm.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauw JJ, Hausser-Hauw C, De Girolami U, Hasboun D, Seilhean D. Neuropathology of sleep disorders: a review. J Neuropathol Exp Neurol. 2011;70:243–252. doi: 10.1097/NEN.0b013e318211488e. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Serotonergic modulation of the limbic system. Neurosci Biobehav Rev. 2006;30:203–214. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Iijima N, Takumi K, Sawai N, Ozawa H. An immunohistochemical study on the expressional dynamics of kisspeptin neurons relevant to GnRH neurons using a newly developed anti-kisspeptin antibody. J Mol Neurosci. 2011;43:146–154. doi: 10.1007/s12031-010-9433-y. [DOI] [PubMed] [Google Scholar]
- Jahanshahi A, Temel Y, Lim LW, Hoogland G, Steinbusch HW. Close communication between the subependymal serotonergic plexus and the neurogenic subventricular zone. J Chem Neuroanat. 2011;42:297–303. doi: 10.1016/j.jchemneu.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Johanson C, Stopa E, McMillan P, Roth D, Funk J, Krinke G. The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol. 2011;39:186–212. doi: 10.1177/0192623310394214. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama M, Yin C, Ishii H, Sakuma Y, Kato M. Somatostatin inhibition of GnRH neuronal activity and the morphological relationship between GnRH and somatostatin neurons in rats. Endocrinology. 2012;153:806–814. doi: 10.1210/en.2011-1374. [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Wouterlood FG. A half century of experimental neuroanatomical tracing. J Chem Neuroanat. 2011;42:157–183. doi: 10.1016/j.jchemneu.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Leak RK, Card JP, Moore RY. Suprachiasmatic pacemaker organization analyzed by viral transynaptic transport. Brain Res. 1999;819:23–32. doi: 10.1016/s0006-8993(98)01317-1. [DOI] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol. 2001;433:312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- Lopez-Munoz F, Alamo C. Historical evolution of the neurotransmission concept. J Neural Transm. 2009;116:515–533. doi: 10.1007/s00702-009-0213-1. [DOI] [PubMed] [Google Scholar]
- Lorez HP, Richards JG. 5-HT nerve terminals in the fourth ventricle of the rat brain: their identification and distribution studied by fluorescence histochemistry and electron microscopy. Cell Tissue Res. 1975;165:37–48. doi: 10.1007/BF00222798. [DOI] [PubMed] [Google Scholar]
- MacMillan SJ, Mark MA, Duggan AW. The release of beta-endorphin and the neuropeptide-receptor mismatch in the brain. Brain research. 1998;794:127–136. doi: 10.1016/s0006-8993(98)00223-6. [DOI] [PubMed] [Google Scholar]
- Maldonado PP, Velez-Fort M, Angulo MC. Is neuronal communication with NG2 cells synaptic or extrasynaptic? J Anat. 2011;219:8–17. doi: 10.1111/j.1469-7580.2011.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KA, Prickaerts J, Steinbusch HW. The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer’s disease. Progress in brain research. 2008;172:233–264. doi: 10.1016/S0079-6123(08)00912-6. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Hay-Schmidt A, Larsen PJ. Central innervation of the rat ependyma and subcommissural organ with special reference to ascending serotoninergic projections from the raphe nuclei. J Comp Neurol. 1997;384:556–568. [PubMed] [Google Scholar]
- Mitchell JA, Card JP. Supraependymal neurons overlying the periventricular region of the third ventricle of the guinea pig: a correlative scanning--transmission electron microscopic study. Anat Rec. 1978;192:441–457. doi: 10.1002/ar.1091920310. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Kotz CM, Novak CM, Billington CJ, Teske JA. Neuropeptides controlling energy balance: orexins and neuromedins. Handb Exp Pharmacol. 2012:77–109. doi: 10.1007/978-3-642-24716-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y, Iino M. Visualization of glutamate as a volume transmitter. The Journal of physiology. 2011a;589:481–488. doi: 10.1113/jphysiol.2010.199539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y, Iino M. Visualization of glutamate as a volume transmitter. J Physiol. 2011b;589:481–488. doi: 10.1113/jphysiol.2010.199539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreskovic D, Klarica M. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev. 2010;64:241–262. doi: 10.1016/j.brainresrev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, Elsevier Science; 1998. [Google Scholar]
- Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010;7:9. doi: 10.1186/1743-8454-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain research reviews. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JG. Autoradiographic evidence for the selective accumulation of [3H]5-HT by supra-ependymal nerve terminals. Brain Res. 1977;134:151–157. doi: 10.1016/0006-8993(77)90933-7. [DOI] [PubMed] [Google Scholar]
- Sancesario G, Morello M, Massa R, Fusco FR, D’Angelo V, Bernardi G. NADPH-diaphorase neurons contacting the cerebrospinal fluid in the ventricles of rat brain. J Cereb Blood Flow Metab. 1996;16:517–522. doi: 10.1097/00004647-199605000-00019. [DOI] [PubMed] [Google Scholar]
- Savtchenko LP, Rusakov DA. The optimal height of the synaptic cleft. Proc Natl Acad Sci U S A. 2007;104:1823–1828. doi: 10.1073/pnas.0606636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz WJ, Reppert SM. Neural regulation of the circadian vasopressin rhythm in cerebrospinal fluid: a pre-eminent role for the suprachiasmatic nuclei. J Neurosci. 1985;5:2771–2778. doi: 10.1523/JNEUROSCI.05-10-02771.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res. 1998;92:195–203. doi: 10.1016/s0166-4328(97)00191-5. [DOI] [PubMed] [Google Scholar]
- Simpson KL, Fisher TM, Waterhouse BD, Lin RC. Projection patterns from the raphe nuclear complex to the ependymal wall of the ventricular system in the rat. J Comp Neurol. 1998;399:61–72. doi: 10.1002/(sici)1096-9861(19980914)399:1<61::aid-cne5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA. Neurobiology of serotonin in depression and suicide. Ann N Y Acad Sci. 1997;836:220–232. doi: 10.1111/j.1749-6632.1997.tb52362.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps III: structure of the rat brain: an atlas with printed and electronic templates for data, models, and schematics. Amsterdam; Boston: Elsevier, Academic Press; 2004. [Google Scholar]
- Trojanowski JQ. Native and derivatized lectins for in vivo studies of neuronal connectivity and neuronal cell biology. J Neurosci Methods. 1983;9:185–204. doi: 10.1016/0165-0270(83)90082-1. [DOI] [PubMed] [Google Scholar]
- Tsuneki H, Wada T, Sasaoka T. Role of orexin in the central regulation of glucose and energy homeostasis [Review] Endocr J. 2012 doi: 10.1507/endocrj.ej12-0030. [DOI] [PubMed] [Google Scholar]
- Veening JG, Barendregt HP. The regulation of brain states by neuroactive substances distributed via the cerebrospinal fluid; a review. Cerebrospinal Fluid Res. 2010;7:1. doi: 10.1186/1743-8454-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh B, Manzano e Silva MJ, Frank CL, Vincze C, Czirok SJ, Szabo A, Lukats A, Szel A. The system of cerebrospinal fluid-contacting neurons. Its supposed role in the nonsynaptic signal transmission of the brain. Histol Histopathol. 2004;19:607–628. doi: 10.14670/HH-19.607. [DOI] [PubMed] [Google Scholar]
- Vigh B, Vigh-Teichmann I, Manzano e Silva MJ, van den Pol AN. Cerebrospinal fluid-contacting neurons of the central canal and terminal ventricle in various vertebrates. Cell Tissue Res. 1983;231:615–621. doi: 10.1007/BF00218119. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Pilgrim C. Extracellular and transcellular transport of horseradish peroxidase (HRP) through the hypothalamic tanycyte ependyma. Cell Tissue Res. 1974;152:477–491. doi: 10.1007/BF00218933. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Winther Bach F, Ekman R, Jensen FM. Beta-endorphin-immunoreactive components in human cerebrospinal fluid. Regul Pept. 1986;16:189–198. doi: 10.1016/0167-0115(86)90018-2. [DOI] [PubMed] [Google Scholar]
- Zoli M, Jansson A, Sykova E, Agnati LF, Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol Sci. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]