Abstract
Pancreatic tumors are resistant to conventional chemotherapies. The present study was aimed at evaluating the potential of a novel plant-derived product as a therapeutic agent for pancreatic cancer (PC). The effects of an extract from the tropical tree Annona Muricata, commonly known as Graviola, was evaluated for cytotoxicity, cell metabolism, cancer-associated protein/gene expression, tumorigenicity, and metastatic properties of PC cells. Our experiments revealed that Graviola induced necrosis of PC cells by inhibiting cellular metabolism. The expression of molecules related to hypoxia and glycolysis in PC cells (i.e. HIF-1α, NF-κB, GLUT1, GLUT4, HKII, and LDHA) were downregulated in the presence of the extract. In vitro functional assays further confirmed the inhibition of tumorigenic properties of PC cells. Overall, the compounds that are naturally present in a Graviola extract inhibited multiple signaling pathways that regulate metabolism, cell cycle, survival, and metastatic properties in PC cells. Collectively, alterations in these parameters led to a decrease in tumorigenicity and metastasis of orthotopically implanted pancreatic tumors, indicating promising characteristics of the natural product against this lethal disease.
Keywords: Pancreatic cancer, therapy, cancer metabolism, natural product
1. Introduction
The overall five-year survival rate for pancreatic cancer (PC) patients was 5.5% for the period of 2001–2007, according to the National Cancer Institute (NCI), a statistic that has not varied significantly for over the last four decades [1]. In 2012, it is estimated that 43,920 new PC cases will be diagnosed and approximately 85% of these (i.e. 37,390) will succumb to the disease [2]. The main reason behind the poor prognosis of PC patients is the insidious and sporadic nature of the disease, which is often presented with no specific early clinical symptoms. By the time of diagnosis PC is already in advanced stages (i.e. III and IV) and is resistant to conventional chemotherapy and radiotherapy [3]. Interestingly, even patients diagnosed with stage I PC that have the option to undergo surgery have a 5-year overall survival of approximately 20%, a clear indication of the general failure of current standard treatments for each stage of PC [4, 5]. What is even more alarming, are the statistics that predict possible 55% increase in the expected number of new PC cases by 2030 [6]. Thus, immediate progress must be made in the prevention, early diagnosis, and systemic treatments against this lethal disease.
Gemcitabine has been the standard line of treatment for PC patients for over a decade and is associated with a median patient survival of 5.4 months [7]. Over all these years, numerous clinical efforts have been devoted to improve PC chemotherapy outcomes, but unfortunately no significant improvements have been reported apart from a clinical trial reported in May of 2011 [8]. This phase III clinical trial reported an improved overall survival of PC patients treated with a four-drug chemotherapy regimen comprising fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX). Nevertheless, a major disadvantage of this novel treatment was its related toxicity, which was noticeably high when compared to PC patients treated with gemcitabine alone. Therefore, novel, alternative PC therapeutics must not only improve the prognosis of PC patients but also minimize any possible toxicity-related side effects that will interfere with the quality of life of PC patients.
It is well known that an increased consumption of fruits and vegetables is associated with a reduced risk of most cancers, including PC [9]. For this reason, the potential of natural products in PC therapies has been widely investigated [10]. While some of these compounds have undergone clinical testing (i.e. curcumin, genistein) and have demonstrated some activity against PC, the poor bioavailability in patients minimizes their therapeutic efficacy. However, as compared with conventional chemotherapeutic drugs, the major benefit of these therapies is the apparent lack of toxicities to healthy tissues. This attracted our attention to find alternative, natural-derived chemotherapeutic drugs in order to improve the prognosis of PC patients. Traditionally, the leaves from the tropical tree Annona Muricata, also known as Graviola or Soursop, have been used for a wide range of human diseases including inflammatory conditions, rheumatism, neuralgia, diabetes, hypertension, insomnia, cystitis, parasitic infections, and cancer [11]. The major bioactive components that have been extracted from different parts of the plant are known as Annonaceous acetogenins. These are derivatives of long chain (C35 or C37) fatty acids derived from the polyketide pathway [12] that is selectively toxic to cancer cells, including multidrug-resistant cancer cell lines [13–17]. Annonaceous acetogenins induce cytotoxicity by inhibiting the mitochondrial complex I, which is involved in ATP synthesis [14]. As cancer cells have a higher demand for ATP than the normal cells, mitochondrial complex I inhibitors have potential in cancer therapeutics.
A few in vivo studies involving Annona Muricata have been reported. Among these, two reports have shown the ability of the leaf extract to regenerate pancreatic islet β cells in diabetic rats [18, 19]. These studies suggest an additional benefit of the natural product against PC given that diabetes has been classified as a risk factor of the malignant disease [20]. More recently, one study analyzing the anti-tumor efficacy of Annona Muricata was published [21]. The extract had a direct anti-tumorigenic effect on breast cancer cells by downregulating the expression of the epidermal growth factor receptor (EGFR). Although this study demonstrates the potential anti-tumorigenic properties of Graviola, the doses used in the experimental design were not properly controlled. The mice were fed with the extract mixed in the diet and the exact amount ingested by each animal could not be estimated accurately.
Although a few in vitro reports have shown the cytotoxic characteristics of Graviola against various cancer cell lines, including PC cells [12], the comprehensive in vivo effects and mechanistic scientific studies are still lacking. To our knowledge, the studies reported herein are the first to indicate that Graviola extract has promising characteristics for PC therapeutics. Comprehensive in vitro and in vivo studies in various PC cell lines revealed that the natural product inhibited multiple signaling pathways that regulate metabolism, cell cycle, survival, and metastatic properties of PC cells.
2. Materials and Methods
2.1 Graviola Extract
Graviola supplement capsules were purchased from Raintree (Carson City, NV). The capsules consisted of 100% pure, finely milled Graviola leaf/stem powder with no binders or fillers. The capsule contents were suspended in DMSO (100mg/mL). After incubating for 5min, the suspension was centrifuged and the supernatant (i.e. extract) was filtered to remove any remaining particles. Subsequent dilutions were prepared in Dulbecco’s modification of Eagle’s medium (DMEM) supplemented with 10% of fetal bovine serum (FBS). Stock solutions and respective dilutions were freshly prepared prior to treatment.
2.2 Cell Culture
The metastatic PC cell lines FG/COLO357 and CD18/HPAF were purchased from the American Type Culture Collection (ATCC). Before performing experiments, the PC cell lines were authenticated by short tandem repeat analysis. It was ensured that PC cells were used at fewer than 20 passages after purchase from ATCC. Cells were cultured in DMEM medium supplemented with 10% FBS and antibiotics (100μg/mL penicillin and 100μg/mL streptomycin). The cells were maintained at 37°C and 5% CO2 in a humidified atmosphere.
2.3 Antibodies
The antibodies for phospho-ERK1/2, total ERK, phospho-Akt (Ser 473), total Akt, NF-κB, and caspase-3 were purchased from Cell Signaling Technology (Danvers, MA). The antibodies for Cyclin-D1, phospho-FAK (Tyr 925), and total FAK were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The β-actin and β-Tubulin antibodies were obtained from Sigma Aldrich (St. Louis, MO), whereas the HIF-1α antibody was purchased from BD Biosciences (San Jose, CA). The MUC4 monoclonal antibody (8G7) used in these studies was developed by our group [22]. MMP9 antibody was obtained from a hybridoma cell supernatant kindly provided by Dr. Rakesh Singh at UNMC. The secondary antibodies used for western blot analyses were the ECL™ anti-mouse and anti-rabbit IgG conjugated to horseradish peroxidase (GE healthcare, UK). Fluorescein isothiocyanate (FITC) conjugated-anti-mouse and Alexa Fluor conjugated anti-mouse antibodies were obtained from Invitrogen (Carlsbad, CA).
2.4 Cytotoxicity Assay
To determine the cytotoxicity of Graviola extract on PC cells, 1×104 cells were seeded per well on a 96-well plate in DMEM supplemented with 10% FBS and antibiotics. After overnight incubation, different concentrations (10–200μg/mL) of the extract were added into triplicate wells. After 48hr, the media was replaced with fresh media containing thiazolyl blue tetrazolium bromide (MTT) reagent (Sigma Aldrich, St. Louis, MO). After 4hr incubation at 37°C in 5% CO2 in humidified atmosphere, the media was replaced with 100μL of DMSO and the corresponding cytotoxicity values were calculated (λ=540nm). The experiment was repeated at least three times.
2.5 Western Blot Analysis
For protein analysis, 0.5×106 of PC cells were seeded on each well of a six-well plate in DMEM supplemented with 10% FBS and antibiotics. After overnight incubation, fresh solutions of Graviola (0–200μg/mL) were prepared and added to the respective wells. Cells incubated with the corresponding amount of DMSO present in the highest concentrated solution of Graviola were used as a negative control (0μg/mL). After 48hr of incubation with the extract, protein lysates were isolated and prepared for western blot analysis, as previously described [23].
2.6 Real-time PCR
The transcripts levels of the glucose transporters GLUT1 and GLUT4, the glycolytic enzymes hexokinase II (HKII) and lactate dehydrogenase A (LDHA), and the mucin glycoprotein MUC4 in PC cells were determined after treatment with Graviola extract by real-time PCR. 0.5×106 cells were seeded in each well of a six-well plate in complete media. After overnight incubation, fresh solutions of Graviola extract (50 and 100μg/mL) were prepared and cells were incubated for 48hr. Subsequently, cDNA was synthesized from purified RNA and real-time PCR was carried out as has been described by previous studies [23]. The sequences of the gene-specific primers used were: GLUT1: F 5′-GCCATGGAGCCCAGCAGCAA-3′; R 5′-CGGGGACTCTCGGGGCAGAA-3′ GLUT4: F 5′-GCCTGTGGCCACTGCTCCTG-3′; R 5′-GGGGTCTCTGGGCCGGGTAG-3′ HKII: F 5′-GTCATCCCCTTGTGTCAGAG-3′; R 5′-CTTCATTAGTGTCCCCATCCTG-3′ LDHA: F 5′-CCAGTGTGCCTGTATGGAGTG-3′; R 5′-GCACTCTCAACCACCTGCTTG-3′ MUC4: F 5′-GTGACCATGGAGGCCAGTG-3′; R 5′-TCATGCTCAGGTGTCCACAG-3′
2.7 Glucose Uptake
Glucose-uptake rate was assayed by utilizing [3H] 2-deoxyglucose ([3H] 2-DG). 5×104 PC cells were seeded per well in a 24-well plate. 12hr later, the cells were treated with Graviola extract (10 and 50μg/mL) for 48hr. The cells were then starved for glucose for 2hr and incubated for 20min with 2 Ci [3H] 2-DG. Subsequently, cells were lysed with 1% SDS and the lysates were counted for [3H] by utilizing a scintillation counter. Cells treated with labeled and excess unlabeled 2-DG were used as controls to set a baseline for non-specific [3H] uptake. The results were normalized to the cell counts for treated and untreated groups. Glucose uptake was normalized with that of the control cells (0μg/mL) and it is presented as the mean values ± standard error from experiments performed in triplicate.
2.8 ATP Quantification
The CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI) was used to measure the ATP content in the cells. Briefly, 1×104 PC cells were seeded in each well of an opaque 96-well plate. Cells were seeded for both ATP quantification and protein concentration estimation. Starting the next day, the cells were incubated with Graviola extract-containing media for 48hr. Subsequently, the instructions of the manufacturer for ATP quantification were followed and luminescence was measured on a Synergy™Mx Luminescent Plate Reader (BioTek, Winooski, VT). Data is presented as the mean value for samples in triplicates, normalized with the protein content for each treatment, as determined by utilizing micro-BCA protein estimation kit.
2.9 Detection and Quantification of Apoptosis and Necrosis
To quantify the number of PC cells undergoing apoptosis and necrosis after being incubated with Graviola extract, the Annexin-V-FLUOS staining kit (Roche Diagnostics, Indianapolis, IN) was used. PC cells were seeded and treated with Graviola extract as described above. After 48hr of treatment with Graviola extract, the instructions of the manufacturer were followed for staining cells for flow cytometry analysis. The experiment was repeated three times.
2.10 Cell Cycle Analysis
PC cells were synchronized at the G1/S phase using a double thymidine block. After seeding cells in 100cm2 Petri dishes, thymidine (2mM) was added for 12hr. After washing cells with serum-free media, the cells were released from thymidine block by culturing in fresh medium containing 24mM 2-deoxycytidine for 9hr. Then, cells were washed and incubated once more with thymidine (2mM) for 14hr. Subsequently, the cells were released from the second thymidine block and the respective treatment prepared in complete media was added for 48hr. For cell cycle analysis, cells were trypsinized and washed with PBS after the duration of the treatment. Cells were then fixed in 70% ethanol at 4°C for 1hr. After washing, cells were incubated with Telford reagent (EDTA, RNAse A, propidium iodide, Triton X-100 in PBS) at 4°C and analyzed by flow cytometry on the next day.
2.11 Confocal Microscopy
For confocal analysis, 2×105 PC cells were seeded on sterilized round glass cover slips. After overnight incubation, Graviola extract (0, 50 and 100μg/mL) was added to the cells, followed by a 48hr incubation. For the detection of reactive oxygen species (ROS), Graviola extract-treated PC cells were incubated with 1μM 2′-7′-Dichlorofluorescein diacetate (DCFH-DA) (Sigma Aldrich, St. Louis, MO) for 15 min. After three washes with PBS, glass cover slips were mounted on glass slides and visualized by confocal microscopy. For β-tubulin and MUC4 confocal analysis, details of the procedure are published elsewhere [23]. Finally, to visualize the arrangement of actin filaments in Graviola extract-treated cells, the cells were stained with fluorescent phallotoxins (Invitrogen, Carlsbad, CA). The instructions of the manufacturer were followed for formaldehyde-fixed cells. Post-staining, the glass cover slips were mounted with Vectashield medium (Vector Laboratories, Burlingame, CA). LSM 510 microscope, a laser scanning confocal microscope (Carl Zeiss GmbH, Thornwood, NY) was utilized to image the cells in the respective channels at a magnification of 630X.
2.12 Wound Healing Assay
For wound healing assays, 3×106 of PC cells were seeded in 60mm petri dishes in DMEM media supplemented with 10% FBS and antibiotics. After overnight incubation, an artificial wound was induced on 100% confluent PC cell monolayers using a sterile pipette tip. Graviola extract-containing (0, 50, 100μg/mL) media solutions were then added to the respective treatment plate. Images (40X) were captured immediately after adding Graviola extract (0hr) and after 24hr of treatment, by a light microscope. The motility of the cells across the wound was visualized in each treatment group.
2.13 Motility Assay
The effect of Graviola extract on the migration of PC cells was also analyzed by a transwell migration assay. FG/COLO357 cells (0.5×106) were suspended in Graviola extract-containing (0–100μg/mL) 1% FBS-DMEM media and seeded for 48hr in 8μm pore size polyethylene terephthalate (PET) membranes (Becton Dickinson, San Jose, CA). DMEM supplemented with 10% FBS was added at the bottom of each well and after 48hr of incubation, the cells that migrated to the bottom of the PET membrane were stained with Diff-Quick cell staining kit (Dade Behring Inc., Newark, DE). The number of cells migrated was quantified by performing cell counts of 10 random fields at 100X magnification. The results are presented as the average number of cells in one field.
2.14 In vivo tumorigenicity studies
The effect of Graviola extract on pancreatic tumor growth was evaluated on orthotopic tumor xenografts. 6–8 week old female athymic immunodeficient mice were purchased from the Animal Production Area of the NCI/Frederick Cancer Research and Development Center (Frederick, MD). The mice were treated in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines at UNMC and were housed in pathogen-free environment and were fed sterile water and food ad libitum.
Over 90% viable luciferase-labeled CD18/HPAF cells transduced with retroviral particles (Addgene, Cambridge, MA) were orthotopically injected into the head of the pancreas of immunodeficient mice. Details of the orthotopic implantation procedure are described elsewhere [22, 24]. After 1 week of tumor growth, oral gavage treatment of PBS-suspended Graviola extract was given daily for 35 days. The doses of Graviola extract for these studies were based on previous in vivo studies [18, 19, 25] and on the recommended dose for human consumption [11]. Treatment groups (N=8) included: PBS only (0 mg/kg), 50mg/kg, and 100mg/kg Graviola extract. Graviola extract was not dissolved in DMSO for these studies in order to demonstrate the benefit of the aqueous natural oral supplement in PC therapy. Nevertheless, the cytotoxic properties of the Graviola extract suspended in PBS were corroborated beforehand (Supplementary Fig. 1). In vivo IVIS 200 biophotonic imaging system was used to capture images (Caliper Life Sciences, Hopkinton, MA) of pancreatic tumors within every two weeks during the course of treatment with Graviola extract. Mice were sacrificed after 42 days of tumor growth and 35 days of treatment with Graviola extract. Changes in tumor growth and sites of metastasis were evaluated in each treatment group. Body weights of mice were measured before the treatment.
2.15 Analysis of pancreatic tumor tissues
On the necropsy day, pancreatic tumors from the different treatment groups were divided for protein and immunohistochemistry (IHC) analyses. The tumors were immediately frozen under liquid nitrogen for protein analysis. To prepare tumor lysates, the tumors were then suspended on radioimmunoprecipitation (RIPA) buffer and sonicated for three cycles with a Branson digital sonifier® (60% amplitude, 10s). After centrifuging the homogeneous suspension, the protein concentration in each sample was estimated and respective solutions for western blot analyses were prepared as previously described [23].
For histopathological and IHC analyses, the tumor tissues were fixed in 10% Formalin for 48hr. The tumors were embedded in paraffin and 5μm sections were cut and stained with hematoxylin and eosin stains (H&E) and various antibodies (i.e. MMP9 and MUC4). Details of the procedure for IHC staining is described elsewhere [24]. The IHC and H&E stained slides were evaluated by pathologist at University of Nebraska Medical Centre.
2.16 Statistical Analysis
The JMP® Statistical Discovery Software (Cary, NC) was used to determine the statistical significance within the treatment replicates in each experiment. A Student’s t-test was used to calculate the corresponding p-value. All p values < 0.05 were considered statistically significant.
3. Results
3.1 Graviola extract induces cytotoxicity of pancreatic cancer cells
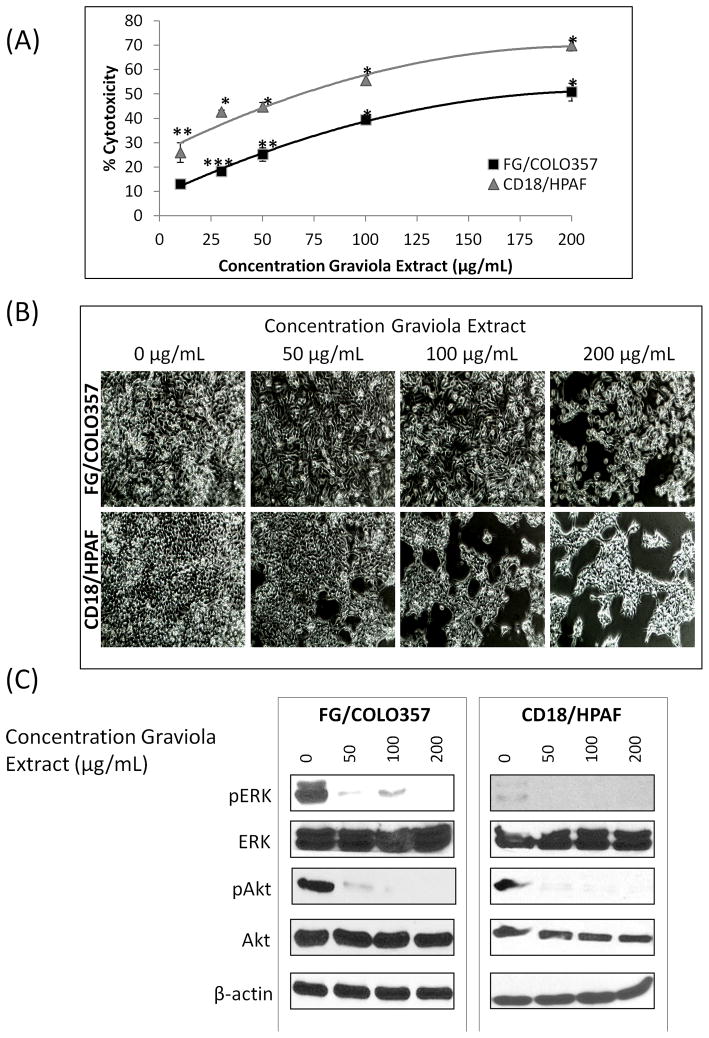
The PC cells FG/COLO357 and CD18/HPAF were incubated for 48hr with different concentrations of Graviola extract. The results from the MTT cytotoxicity assay indicated a progressive decrease in cell viability with the successive increase in the concentrations of the extract (Fig. 1A). After 48hr of treatment, the resulting IC50 of Graviola extract on FG/COLO357 and CD18/HPAF cells was 200 and 73μg/mL, respectively (Fig. 1B) and the results indicated that CD18/HPAF cell line is more sensitive to the Graviola extract than the FG/COLO357 cell line.
Figure 1. Effect of Graviola extract on pancreatic cancer cell viability.
(A) MTT cytotoxicity assay for Graviola extract-treated PC cells. Cells were incubated with different concentrations of Graviola extract and corresponding DMSO controls for 48hr. Data represents the mean value of experiments performed in triplicate ± standard error of mean. (*p-value<0.0001, **0.0001<p-value<0.001, ***p-value=0.007, compared to untreated cells); (B) Inverted microscope images (40X) of PC cells after treatment with Graviola extract for 48hr; (C) Western blot analysis of proteins involved in PC cell proliferation after 48hr treatment with Graviola extract. Protein lysates (30μg) were resolved by 10% SDS-PAGE. β-actin was used as the loading control. Each experiment was performed three times in triplicate.
It is well known that the activation of the extracellular signal-regulated kinase (ERK) and the phosphatidylinositol 3′kinase (PI3K/Akt) pathways play a crucial role in the proliferation and survival of PC [26] and inhibition of these pathways leads to the inhibition of pancreatic tumor growth [27, 28]. The present study revealed that treatment of PC cells with Graviola extract resulted in decreased activation of both ERK and Akt pathways in PC cells (Fig. 1C). Thus, the inhibition of these pathways is in agreement with the decreased viability of PC cells treated with Graviola extract.
3.2 Pancreatic cancer cell metabolism is inhibited by Graviola extract
Previous studies have shown that major bioactive components present in Graviola extract that inhibit mitochondrial complex I [13–17], suggesting their direct involvement in cell metabolism. It has already been well-documented that cancer cells undergo a metabolic shift to adapt and survive under harsh environments by enhancing aerobic glycolysis [29, 30]. Also, Akt activation leads to glycolytic ATP generation in tumor cells [31]. Hence, the effect of Graviola extract on several stages of the glycolytic pathway in PC cells was analyzed.
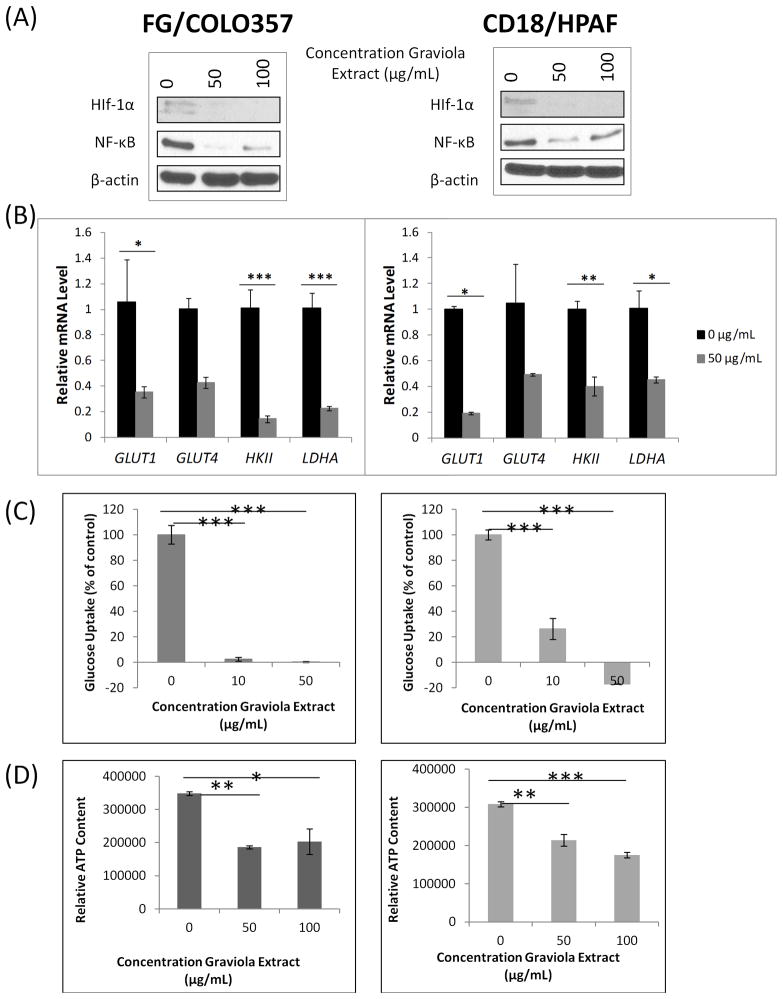
The expression of HIF-1α, a critical regulator of aerobic glycolysis in cancer cells [32], was analyzed in PC cells after incubation with Graviola extract (Fig. 2A). We observed reduced HIF-1α expression in both PC cell lines, suggesting a direct effect of this natural product on the metabolism of PC cells. Likewise, it has been previously reported that the NF-κB upregulates the expression of HIF-1α [33, 34]. Not surprisingly, the expression levels of NF-κB were also reduced in PC cells after being incubated with Graviola extract (Fig. 2A).
Figure 2. Effect of Graviola extract on the metabolism of pancreatic cancer cells.
(A) Western blot analysis of HIF-1α and NF-κB expression in PC cells after treatment with Graviola extract. Protein lysates (30μg) were resolved on 10% SDS-PAGE gels. β-actin was used as a loading control; (B) Real-time PCR-based measurement of transcript levels of glucose transporters 1 and 4 (GLUT1, GLUT4), hexokinase II (HKII), and lactate dehydrogenase A (LDHA) in PC cells after incubation with Graviola extract. Data is presented as the average fold difference in gene expression for the gene of interest in Graviola extract-treated cells versus untreated cells (0μg/mL) ± standard error of mean. The housekeeping gene β-actin was used as an internal control. (*0.01<p-value<0.05, **0.005<p-value<0.001, ***p-value<0.005); (C) Measurement of glucose uptake in PC cells after treatment with Graviola extract. Radioactive counts of cells labeled with [3H]-2-deoxyglucose were normalized with controls (***p-value<0.0001); (D) ATP quantification of PC cells after treatment with Graviola extract. A Luminescent Cell Viability assay was used to measure the ATP content in the cells. Data is presented as mean value from experiments performed in triplicates normalized with the protein content ± standard error of mean. (*p-value=0.003, **p-value=0.002, ***p-value=0.0002) Data in the left panel is from FG/COLO357 cells, whereas data in the right panel is from CD18/HPAF cells.
Subsequently, the expression of the glucose transporters 1 and 4 (GLUT1 and GLUT4), and the expression of the glycolytic enzymes hexokinase II (HKII) and lactate dehydrogenase A (LDHA), all of which are upregulated by HIF1-α in cancer cells [32, 35], were analyzed in Graviola extract-treated PC cells by real-time PCR analysis (Fig 2B). Overall, the transcript levels of GLUT1, HKII, and LDHA were significantly reduced in both PC cell lines when compared to untreated cells (i.e. 60–87% downregulation).
Cancer cells have an increased expression of glucose transporters to enhance glucose uptake, which in turn increases the glycolytic rate for an enhanced ATP production that will ultimately lead to an enhanced tumor growth [10]. Thus, based on the results discussed above, it was not surprising that PC cells treated with Graviola extract doses over 10 μg/mL had a decreased rate of glucose uptake when compared to untreated cells (0μg/mL) (Fig. 2C). Finally, to evaluate the energy outcome of the glycolytic pathway of PC cells, we measured ATP production in Graviola extract-treated PC cells (Fig. 2D), and observed significant inhibition by 42–47% and by 31–43% doses in FG/COLO357 and CD18/HPAF PC cells, respectively. Altogether, these results indicate that Graviola extract impairs the metabolism of PC cells that will ultimately lead to decreased cell viability.
3.3 Graviola extract induces necrosis of pancreatic cancer cells
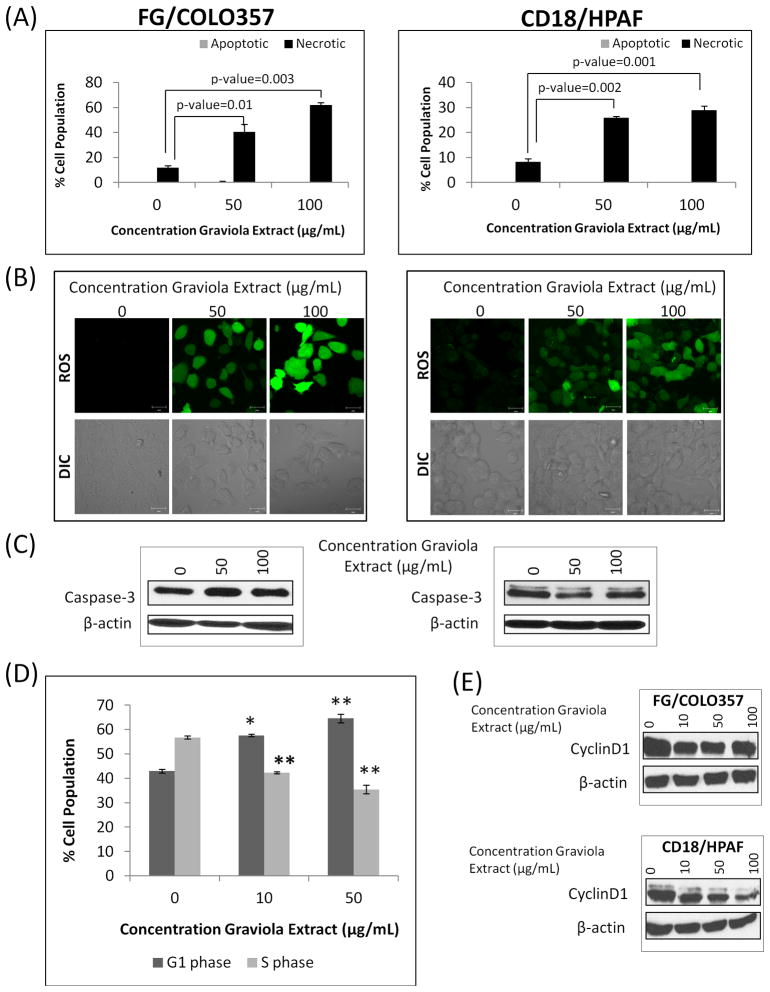
In order to evaluate the cytotoxic pathways induced by Graviola extract. PC cells were stained with annexin-V and propidium iodide (PI) staining to measure the necrotic and apoptotic cell populations by performing flow cytometry. While the necrotic cell population in both PC cell lines increased significantly after incubation with Graviola extract, the apoptotic cell population remained unchanged (Fig. 3A). Subsequently, the production of Graviola extract-induced reactive oxygen species (ROS) in FG/COLO357 and CD18/HPAF PC cells was confirmed by confocal microscopy (Fig. 3B). Additionally, it was also observed that cells incubated with Graviola extract have a gain in cell volume, a characteristic of necrotic cell death.
Figure 3. Analysis of cytotoxic mechanism of Graviola extract in pancreatic cancer cells.
(A) Quantification of apoptotic and necrotic PC cells after treatment with Graviola extract (Apoptotic cells Annexin V+/PI− staining; Necrotic cells Annexin V+/PI+staining). Data is presented as the mean value of the corresponding % cell population in duplicate samples ± standard error of mean; (B) The production of reactive oxygen species (ROS) in PC cells after treatment with Graviola extract was determined after incubating Graviola extract-treated PC cells with 2′,7′-Dichlorofluorescein diacetate (DCFH-DA). Cells were then analyzed by confocal microscopy. Scale bar represents 20μm; (C) Western blot analysis of Caspase-3 expression in PC cells after treatment with Graviola extract. Protein lysates (30μg) were resolved on 10% SDS-PAGE gels. β-actin was used as a loading control; (D) Cell cycle analysis of FG/COLO357 PC cells after treatment with Graviola extract. Cells were synchronized in the G1/S phase by thymidine block before adding Graviola extract. The effect of Graviola extract on the distribution of cells in different phases of the cell cycle was analyzed by flow cytometry. The data is presented as the mean value of the corresponding % cell population in duplicate samples ± standard error of mean. Representative flow cytometry histograms of cells treated with different concentrations of Graviola extract are shown. (*p-value=0.0001; **p-value<0.0001); (E) Western blot analysis of the expression of the cell cycle-related protein CyclinD1 in PC cells after being incubated with Graviola extract. Protein lysates (30μg) were resolved in 10% SDS-PAGE gels. β-actin was used as the loading control. In (A), (B), and (C), data in the left panel is from FG/COLO357 cells, whereas data in the right panel is from CD18/HPAF cells.
In order to confirm that Graviola extract was not inducing apoptosis of PC cells, the levels of caspase-3 expression were analyzed by western blot analysis. The Caspase-3 expression values remained statisticallyunaltered by treatment with the extract, suggesting that apoptotic pathways are not involved (Fig. 3C). Furthermore, the apoptotic cells population in Graviola extract-treated cells was also analyzed by Telford staining, and the results corroborated the findings from AnnexinV/PI staining studies, where the number of apoptotic cell population did not vary after being incubated with the natural compound (data not shown).
An analysis of the different phases of the cell cycle after treatment with Graviola extract demonstrated cell cycle arrest at G1 phase (Fig. 3D). While the G1 cell population increased from 43 to 65%, the S phase decreased from 56 to 32% with increasing concentrations of Graviola extract (0, 5, 100μg/mL). To support these results, the expression of CyclinD1 in Graviola extract-treated PC cells was analyzed (Fig. 3E). In agreement with previous studies indicating that a decreased CyclinD1 expression induces G0/G1 cell cycle arrest [36], Graviola extract-treated PC cells had also reduced expression of the cell cycle regulatory protein.
3.4 Motility of pancreatic cancer cells decreases after treatment with Graviola extract
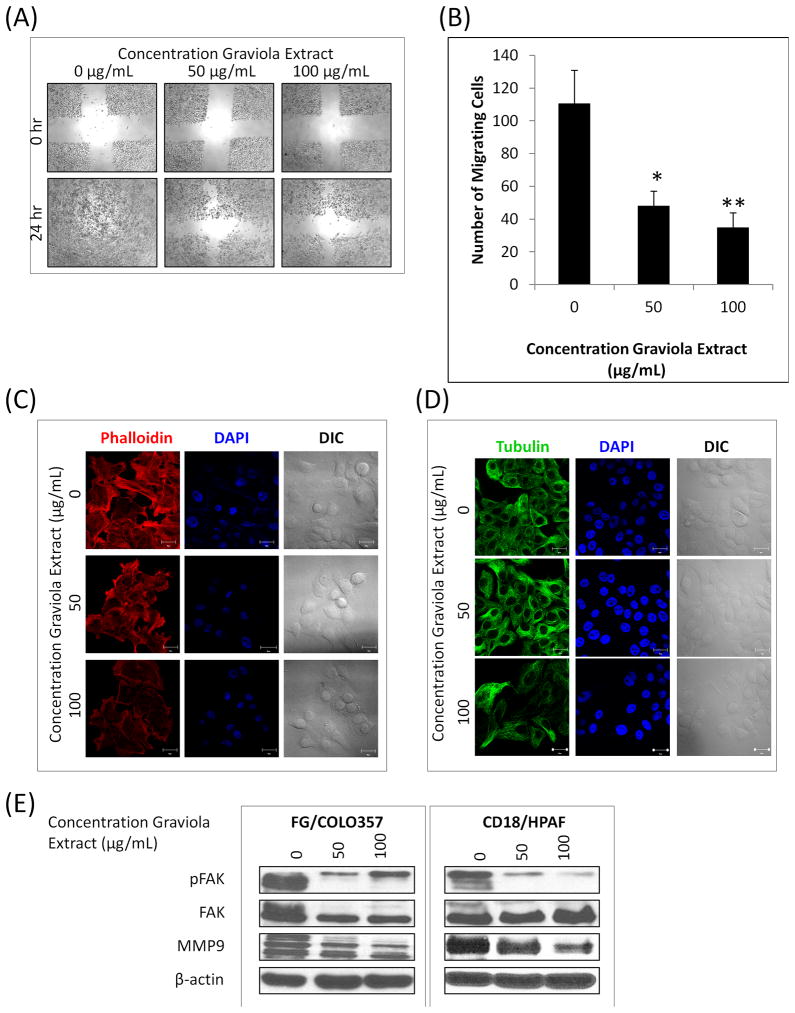
The effect of Graviola extract on the functional properties of PC cells was analyzed in vitro wound healing and migration assays (Fig. 4A, B). As it can be observed in the images from the wound healing assays, PC cells treated with Graviola extract did not close the wound even after 24hr, as opposed to untreated cells (0μg/mL), indicating reduced motility of PC cells after treatment with Graviola extract (Fig. 4A). Similarly, the migratory capacity of PC cells was also reduced after treatment with Graviola extract, as evaluated by a transwell assay (Fig. 4B), suggesting that the natural extract reduces the motility of PC cells.
Figure 4. Effect of Graviola extract in the motility, migration, and cytoskeleton of pancreatic cancer cells.
(A) Wound healing assay of FG/COLO357 PC cells after treatment with Graviola extract. Microscope images (40X) of the artificially created wound in PC cells monolayer were taken before (0hr) and after adding Graviola extract (24hr); (B) Migration of FG/COLO357 PC cells after treatment with Graviola extract. The number of cells that migrated through the 8μm pores of a polyethylene terephtalate (PET) membrane was quantified in 10 random fields. Data represent the mean value of migrating cells ± standard error of mean (*p-value = 0.0009; **p-value < 0.0001, compared to untreated control cells); (C) Actin filaments were analyzed by confocal microscopy by Rhodamine-anti-Phalloidin staining of FG/COLO357 cells after treatment with Graviola extract. Nucleus was stained with DAPI. Scale bars represent 20μm; (D) Microtubules were analyzed by confocal microscopy after FITC-anti-β Tubulin staining of FG/COLO357 cells after treatment with Graviola extract. Nucleus was stained with DAPI. Scale bars represent 20μm; (E) Expression of proteins related to migration/motility of PC cells after treatment with Graviola extract. Protein lysates (30μg) were resolved by 10% SDS-PAGE. β-actin was used as a loading control.
The motility and migration of cancer cells is associated with the rearrangements of the cortical actin and microtubules network [37, 38]. Additionally, cellular ATP depletion has been associated with reorganization of the actin cytoskeleton [39] and suppression of the dynamics of microtubules is known to induce mitotic arrest [40]. Taking this into consideration, the cytoskeleton of Graviola extract-treated PC cells was analyzed by confocal microscopy (Fig. 4C, D). The image results of phallotoxins (i.e. phalloidin) staining indicate a disruption of the cortical actin network and dissolution of stress fibers in Graviola extract-treated PC cells (Fig. 4C). Similarly, a disruption of microtubules dynamics was evident after β-tubulin staining of PC cells incubated with Graviola extract (Fig. 4D). To further analyze the effect of Graviola extract on motility and migration of PC cells, the expression levels of the phosphorylated focal adhesion kinase (pFAK), which is involved in mitogenic signaling and motility [41], and matrix metalloproteinase 9 (MMP9), which targets many extracellular proteins including adhesion molecules [42], were analyzed by western blot analysis (Fig. 4E). In agreement with the experiments discussed above, we observed that the expression levels of both pFAK and MMP9 were downregulated in Graviola extract-treated cells.
3.5 Graviola extract inhibits tumor growth and metastasis of pancreatic cancer cells
Based on the results obtained from in vitro experiments, Graviola extract has promising properties to be incorporated in PC therapeutics. Nevertheless, these anti-tumorigenic properties require further validation through in vivo experiments. In order to evaluate the therapeutic potential of Graviola extract, a more realistic situation for administering the extract was mimicked. It is recommended that Graviola extract supplement must be taken on a regular basis [11], and therefore, it was decided that the extract must be administered by oral gavage after suspending contents of the capsule in aqueous solution instead of dissolving it in DMSO. Prior to evaluating the anti-tumorigenic properties of aqueous Graviola extract suspension by in vivo experiments, pertinent in vitro experiments corroborating the cytotoxic potential of the aqueous suspensions on PC cells were completed beforehand (Supplementary Fig. 1).
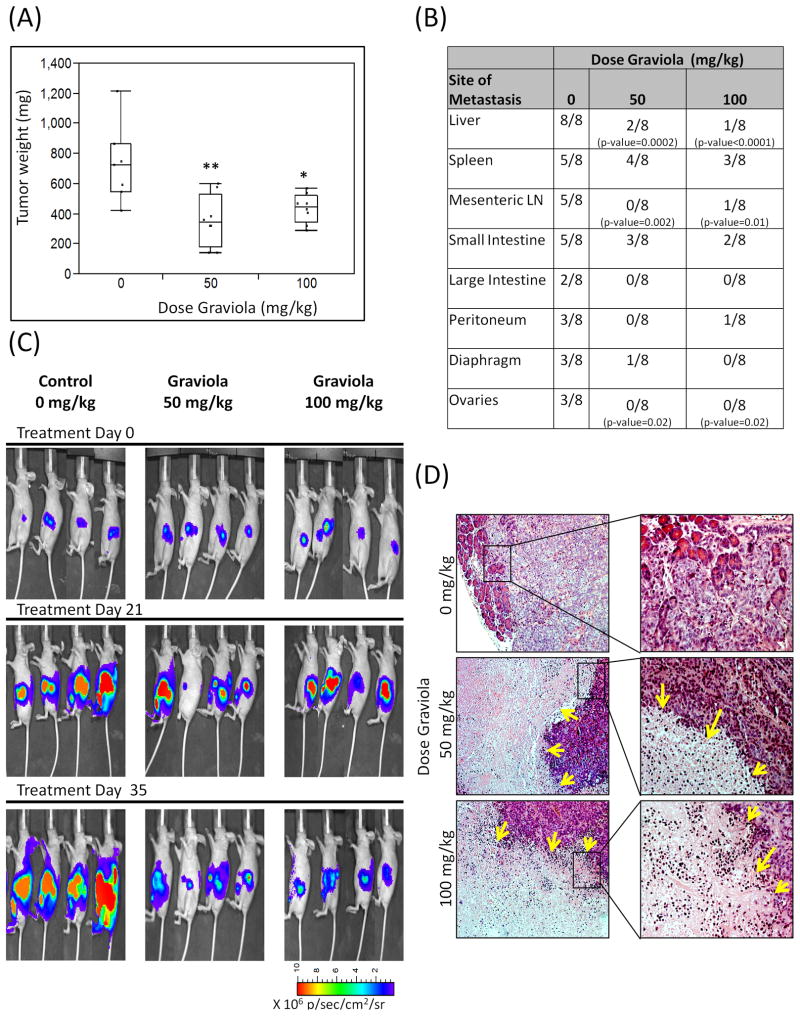
For tumorigenic studies, CD18/HPAF cells expressing luciferase were orthotopically injected into the pancreas of athymic mice. After 1 week, in vivo biophotonic imaging confirmed tumor growth in all animals and the treatment regimen was initiated. The tumor growth during the treatment was monitored by imaging every two weeks. After 35 days of treatment, the animals were euthanized and the pancreatic tumors were removed and weighed. Although pancreatic tumors were not completely eradicated, the results indicate that tumor growth decreased significantly in Graviola extract-treated mice in comparison to the control group (Fig. 5A). Specifically, the tumor growth inhibition in mice treated with a dose of 50mg/kg Graviola extract was 59.8% (p-value=0.0008) whereas in mice treated with 100 mg/kg Graviola extract the inhibition was 50.3% (p-value = 0.006), indicating the efficacy of the natural product in PC regression. The metastatic lesions in each mouse were evaluated in various vital organs including the liver, spleen, mesenteric lymph nodes (LN), small and large intestines, peritoneum, diaphragm, and ovaries (Fig. 5B). Although all the metastatic lesions were reduced in Graviola extract-treated mice in comparison to the untreated control mice, the incidence of metastasis in the liver, mesenteric LN, and ovaries was significantly reduced (p-values ≤ 0.02). Representative biophotonic tumor images illustrate the tumor growth across the different groups during the course of the treatment (Fig. 5C).
Figure 5. Evaluation of Graviola Extract in pancreatic cancer orthotopic xenograft model.
(A) Pancreatic tumor weight results after treatment with Graviola extract. CD18/HPAF-Luciferase cells were injected orthotopically in the pancreas of athymic nude mice. After 1 week of tumor growth, oral gavage treatment of PBS-suspended Graviola extract was given daily for 35 days (N=8). Data is presented as box plots of the mean tumor weight of mice in each treatment group. (*p-value = 0.006; **p-value=0.0008, compared to tumors of PBS-treated mice); (B) Major sites of metastasis in each treatment group. Results are presented as number of animals having metastasis out of total number of animals per group. Statistical analysis was done comparing Graviola extract-treated mice with untreated mice (0mg/kg Graviola extract); (C) In vivo biophotonic imaging of pancreatic tumors during the course of treatment with Graviola extract. Representative IVIS images of mice from different treatment groups are shown (D) Hematoxylin and Eosin (H&E) staining of paraffin embedded pancreatic tumors. Images on the right (20X) are magnified areas from the images located at the left (10X). Yellow arrows in H&E sections represent necrotic areas in tumors from mice treated with Graviola extract.
Further, tumors were evaluated by H&E (Fig. 5D) and IHC staining (Fig. 6). The H&E stained tumor sections showed necrotic cells in 20–50% of the pancreatic tumor tissues from Graviola extract-treated mice as compared with tumors from the control mice. These results further strengthen the results from in vitro experiments, which demonstrate that Graviola extract-mediated reduction in PC cell viability was through the induction of necrosis.
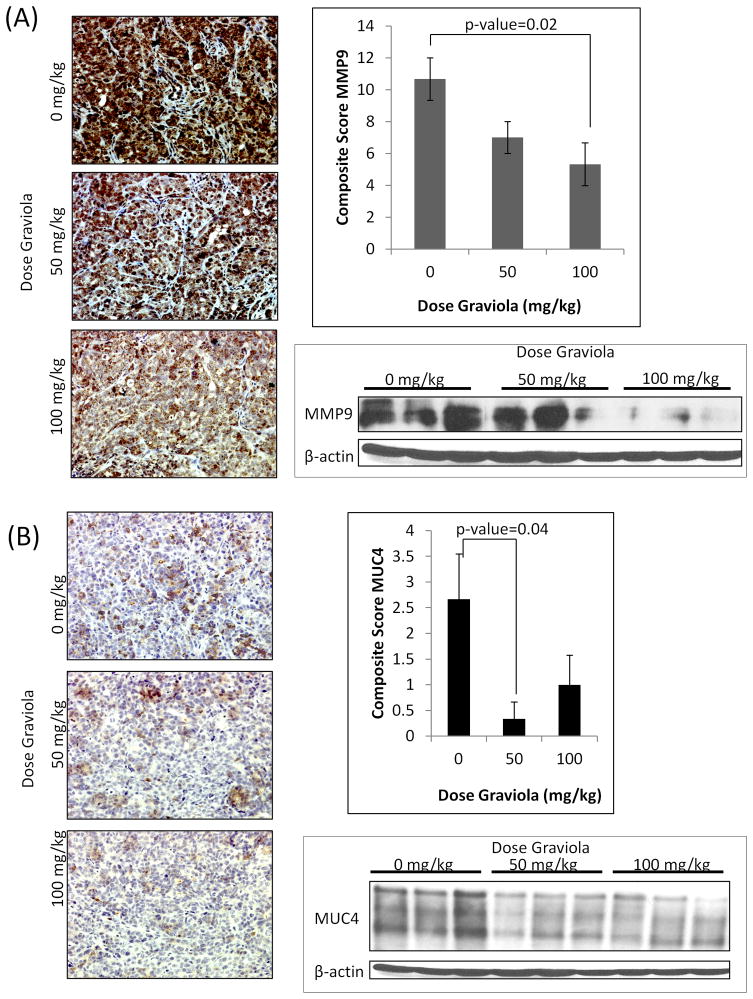
Figure 6. Immunohistochemical analyses of pancreatic tumors after treatment with Graviola extract.
(A) Immunohistochemical staining of MMP9 in paraffin-embedded pancreatic tumors. Representative images (20X) of tumors from different treatment groups are shown with the average composite score shown at the right. Data from experiments performed in triplicates is presented as the mean value of the composite score of tumors ± standard error of mean. MMP9 expression in pancreatic tumors was also assessed by western blot analysis. Homogenized protein tumor lysates (30μg) were resolved by 10% SDS-PAGE. β-actin was used as a loading control. (B) Immunohistochemistry staining of MUC4 in paraffin-embedded pancreatic tumors. Representative images (200X) of tumors from different treatment groups are shown with the average composite score shown at the right. Data from experiments performed in triplicate is presented as the mean value of the composite score of tumors ± standard error of mean. MUC4 expression in pancreatic tumors was also assessed by western blot analysis. Homogenized protein tumor lysates (30μg) were resolved by 2% agarose gels. β-actin was used as the loading control.
The tumor lysates and paraffin embedded pancreatic tumors were also evaluated by IHC for the expression of MMP9 (Fig. 6A) and MUC4 (Fig. 6B). In agreement with in vitro data, the levels of MMP9 were reduced in tumors from Graviola extract-treated mice compared to the untreated controls. As the expression of MMP9 has been related to invasion and metastasis, the reduced levels of the protein in Graviola extract-treated tumors substantiate our findings of reduced metastatic sites in these mice.
Previous studies performed by our group have established the correlation of the expression of mucin4 (MUC4) glycoprotein with progression and metastasis of PC [24, 43–45]. Therefore, we were particularly interested in evaluating the effect of Graviola extract on the expression of MUC4 in PC cells and pancreatic tumors. In vitro experiments demonstrated a significant downregulation in MUC4 expression, both at the translational (Supplementary Figs. 1C, 2A, B) and transcriptional levels (Supplementary Fig. 2C) in Graviola extract treated PC cells. Similarly, the expression of the MUC4 was reduced in pancreatic tumors from mice treated with Graviola extract as compared to the untreated mice (Fig. 6B), Further supporting our findings of reduced tumor growth and metastasis after treatment with Graviola extract.
4. Discussion
Little or no progress has been accomplished in PC treatment over the last 40 years. Novel therapeutics against this lethal malignancy must inhibit several pathways that promote survival, progression, and metastasis of PC cells. Based on the fact that cancer cells are mainly dependent on the glycolytic pathway for ATP production, glucose deprivation by anti-glycolytic drugs can induce cancer cell death [46], a pathway that can be targeted and explored in PC therapies [47].
Natural products have been investigated in PC therapeutics over several decades, but to date none has been incorporated in routine chemotherapies [10]. Traditionally, the leaves from Graviola (Annona Muricata) have been used for a wide range of human diseases including cancer [11]. The present study is the first to demonstrate that Graviola extract reduces the viability of PC cells and tumors by inducing necrosis and cell cycle arrest, and by inhibiting PC cell motility (i.e. cytoskeleton rearrangement), migration, and metabolism. Overall, in vitro experiments revealed that the compounds present in the natural extract inhibited several pathways involved in PC cell proliferation and metabolism, simultaneously. Such inhibitions ultimately led to a decrease in tumor growth and metastasis in orthotopically transplanted pancreatic tumor-bearing mice.
In PC patients, an increased metabolic activity and glucose concentration of malignant tumors has been linked to pancreatic tumor aggressiveness [47]. Additionally, the presence of hypoxia in PC has been associated with tumor growth and metastasis [48, 49]. Indeed, the presence of hypoxic environment has been linked to the oncogenic and metabolic transformation (i.e. glycolysis) of PC cells that results in resistance to conventional cancer therapeutics [48, 50]. More specifically, it has been suggested that hypoxia can induce resistance to gemcitabine through the activation of PI3K/Akt/NF-κB and MAPK/ERK pathways [51], which are also related to PC progression and survival. The activation of both of these signaling pathways was evaluated in PC cells after treatment with Graviola extract and it was found that the extract suppressed phosphorylation of the key molecules involved in these pathways, which correlated with reduced viability of PC cells. Subsequently, the expression of HIF-1α, the major transcription factor activated under hypoxic conditions, and its ensuing downstream effects on PC cell metabolism were analyzed in Graviola extract-treated cells. The results indicated the natural product inhibited PC cell metabolism by inhibiting the expression of HIF-1α, NF-κB, glucose transporters (i.e. GLUT1, GLUT4), and glycolytic enzymes (i.e. HKII, LDHA), all of which lead to the reduction of glucose uptake and ATP production by PC cells.
The overall downregulation of PC cell metabolism induced by Graviola extract resulted in PC cell death and necrosis. In agreement with previous studies of ATP reduction, the metabolic and therapeutic stress induced by Graviola extract led to an acute ATP depletion, which is accompanied by increased intracellular ROS, ultimately leading to necrosis [52–54]. While necrotic agents have not been considered beneficial in cancer therapies due to induction of local inflammation, the process itself can lead to the activation of the innate immune system capable of initiating anti-tumor immunity [52]. It makes it imperative to evaluate the effect of a necrosis-inducing product such as Graviola extract in an immune competent host. In this regard, we plan to evaluate the effect of the natural product on the progression of pancreatic adenocarcinoma in the KrasG12DPdx1-Cre spontaneous animal model, where the effect on the immune system can be evaluated.[55, 56]. In order to evaluate the potential of Graviola extract in preventing PC progression, we plan to supplement the diet of KrasG12DPdx1-Cre mice with Graviola extract after the mice start developing pancreatic intraepithelial neoplastic (PanIN) lesions. The effective concentrations of Graviola metabolites after oral absorption and effects on the immune system will be measured as well. Additional experiments will be carried out to evaluate the potential of a combination therapy of Graviola extract with the standard chemotherapeutic drug Gemcitabine. With the results discussed in the present study, it is expected that minimum doses of the chemotherapeutic drug will be needed to eradicate the malignant disease.
The major bioactive compounds identified in Annona Muricata have been classified as Annonaceous acetogenins, which inhibit mitochondrial complex I that leads to a decreased ATP production [13–17]. Although the natural extract capsules used in these studies contained numerous compounds, the presence of Annonaceous acetogenins was evident by the depletion of ATP production in PC cells after being incubated with Graviola extract. Bioactivity-guided fractionation for the identification of potent bioactive (i.e. anti-tumorigenic) compounds that are present in the Graviola extract is currently being investigated. We are also ensuring that cytotoxic effects are specific to tumorigenic cells only, by including the non-transformed immortalized pancreatic epithelial cell line HPNE, which is derived from pancreatic duct (data not shown).
Pancreatic tumors develop from a complex interplay of numerous signaling pathways and Graviola extract has shown promising anti-tumorigenic characteristics by targeting some of these pathways all at once. Although novel glycolytic inhibitors, such as Graviola extract, may have broad therapeutics applications [57], inhibition of glycolysis alone may not be sufficient to eradicate tumor cells completely. Perhaps the use of alternative medicine, like taking Graviola capsules on a regular basis, should still be considered a supplement, not a replacement for standard therapies. Currently, in vitro studies evaluating the potential of the natural product in combination with chemotherapeutic drugs are being conducted.
Supplementary Material
(A) Cytotoxicity of Graviola extract suspended in PBS. The PC cells CD18/HPAF were incubated with different doses of Graviola extract suspended in PBS for 48hr. Cytotoxicity was analyzed by MTT cytotoxic assay. Data from experiments performed in triplicate is presented as the mean value ± standard error of mean. (*p-value<0.0001, **p-value=0.0017, compared to untreated cells); (B) ATP quantification of CD18/HPAF PC cells after treatment with Graviola extract suspended in PBS. Data from experiments performed in triplicate is presented as the mean value normalized with total protein content ± standard error of mean; (C) Western blot analysis of the expression of proteins related to the proliferation, invasion, and metastasis of PC in CD18/HPAF cells after incubation with Graviola extract suspended in PBS. Protein lysates (30μg) were resolved on 2% SDS agarose gels (MUC4) and 10% SDS-PAGE (HIF-1α, Caspase3, MMP9, and β-actin). β-actin was used as a loading control.
(A) Western blot analysis of MUC4 and HER2 in PC cells after treatment with Graviola extract. Protein lysates (30μg) were resolved on 2% agarose gels for MUC4 and 10% SDS-PAGE for HER2. β-actin was used as a loading control; (B) Confocal microscope images of PC cells stained against MUC4 (anti-Alexa Fluor 594) after treatment with Graviola extract. Cell nuclei were stained with DAPI. Scale bars represent 10μm; (C) Measurement of MUC4 transcripts in PC cells incubated with Graviola extract by real-time PCR. Data is presented as the average fold difference of MUC4 levels in Graviola extract-treated cells versus untreated cells (0μg/mL) ± standard error of mean. The housekeeping gene β-actin was used as an internal control. (*p-value<0.005; **p-value<0.005, compared to untreated PC cells)
Acknowledgments
The invaluable technical support from Kavita Mallya is greatly appreciated. We would like to give special thanks to UNMC professors: Dr. Michel Ouellette for kindly providing CD18/HPAF-Luciferase and HPNE cells, Dr. Shilpa Buch for allowing us to use the Luminescence plate reader, Dr. Vimla Band for allowing us to use the microscope to image tumor H&E and IHC sections, and Dr. Steve Caplan for assisting with the analysis of confocal images and providing us the β-Tubulin antibody. We also thank Janice A. Tayor and James R. Talaska of the Confocal Laser Scanning Microscope Core Facility at UNMC, Victoria B. Smith and Megan Michalak of the UNMC Cell Analysis Core Facility, and Ms. Kristi Berger, the Eppley Cancer Center for editing this manuscript. We are also very grateful for the expertise and involvement of Drs. Amarnath Natarajan and Abijah Nyong from the Chemistry department at UNMC in the bioactivity-guided fractionation of the Graviola extract. The authors of this work are supported by grants from the National Institutes of Health: NIH-NCI Cancer Biology Training Grant UNMC T32CA009479, R01 CA78590, U01EDRN CA111294, R01 CA131944, R01 CA133774, R01 CA 138791, P50 SPORE CA127297 and U54 CA160163.
Footnotes
Conflicts of Interest Statement
There are no potential conflicts of interest involved with this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SEER Stat Fact Sheets:Pancreas. National Cancer Institute; Oct 28, 2011. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Chakraborty S, Baine MJ, Sasson AR, Batra SK. Current status of molecular markers for early detection of sporadic pancreatic cancer. Biochim Biophys Acta. 2011;1815:44–64. doi: 10.1016/j.bbcan.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, Talamonti MS. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110:1227–1234. doi: 10.1002/cncr.22916. [DOI] [PubMed] [Google Scholar]
- 6.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 7.Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 8.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 9.Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, Bamlet WR, de Andrade M, Oberg AL, Hammer TJ, Rabe KG, Anderson KE, Olson JE, Sinha R, Petersen GM. Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer Causes Control. 2011;22:1613–1625. doi: 10.1007/s10552-011-9838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stan SD, Singh SV, Brand RE. Chemoprevention strategies for pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2010;7:347–356. doi: 10.1038/nrgastro.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor L. Herbal Secrets of the Rainforest. 2 Sage Press, Inc; 2002. Technical Data Report for Graviola: Annona Muricata. [Google Scholar]
- 12.Kim GS, Zeng L, Alali F, Rogers LL, Wu FE, Sastrodihardjo S, McLaughlin JL. Muricoreacin and murihexocin C, mono-tetrahydrofuran acetogenins, from the leaves of Annona muricata. Phytochemistry. 1998;49:565–571. doi: 10.1016/s0031-9422(98)00172-1. [DOI] [PubMed] [Google Scholar]
- 13.Oberlies NH, Jones JL, Corbett TH, Fotopoulos SS, McLaughlin JL. Tumor cell growth inhibition by several Annonaceous acetogenins in an in vitro disk diffusion assay. Cancer Lett. 1995;96:55–62. doi: 10.1016/0304-3835(95)92759-7. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin JL. Paw paw and cancer: annonaceous acetogenins from discovery to commercial products. J Nat Prod. 2008;71:1311–1321. doi: 10.1021/np800191t. [DOI] [PubMed] [Google Scholar]
- 15.Tormo JR, Royo I, Gallardo T, Zafra-Polo MC, Hernandez P, Cortes D, Pelaez F. In vitro antitumor structure-activity relationships of threo/trans/threo mono-tetrahydrofuranic acetogenins: correlations with their inhibition of mitochondrial complex I. Oncol Res. 2003;14:147–154. doi: 10.3727/000000003771013099. [DOI] [PubMed] [Google Scholar]
- 16.Chang FR, Wu YC. Novel cytotoxic annonaceous acetogenins from Annona muricata. J Nat Prod. 2001;64:925–931. doi: 10.1021/np010035s. [DOI] [PubMed] [Google Scholar]
- 17.Liaw CC, Chang FR, Lin CY, Chou CJ, Chiu HF, Wu MJ, Wu YC. New cytotoxic monotetrahydrofuran annonaceous acetogenins from Annona muricata. J Nat Prod. 2002;65:470–475. doi: 10.1021/np0105578. [DOI] [PubMed] [Google Scholar]
- 18.Adewole SO, Caxton-Martins EA. Morphological Changes and Hypoglycemic Effects of Annona Muricata Linn. (Annonaceae) Leaf Aqueous Extract on Pancreatic B- Cells of Streptozotocin-Treated Diabetic Rats. African J Biomed Res. 2006;9:173–180. [Google Scholar]
- 19.Adeyemi DO, Komolafe OA, Adewole SO, Obuotor EM, Abiodum AA, Adenowo TK. Histomorphological and morphometric studies of the pancreatic islet cells of diabetic rats treated with extracts of Annona Muricata. Folia Morphol. 2010;69:92–100. [PubMed] [Google Scholar]
- 20.Magruder JT, Elahi D, Andersen DK. Diabetes and pancreatic cancer: chicken or egg? Pancreas. 2011;40:339–351. doi: 10.1097/MPA.0b013e318209e05d. [DOI] [PubMed] [Google Scholar]
- 21.Dai Y, Hogan S, Schmelz EM, Ju YH, Canning C, Zhou K. Selective growth inhibition of human breast cancer cells by graviola fruit extract in vitro and in vivo involving downregulation of EGFR expression. Nutr Cancer. 2011;63:795–801. doi: 10.1080/01635581.2011.563027. [DOI] [PubMed] [Google Scholar]
- 22.Moniaux N, Varshney GC, Chauhan SC, Copin MC, Jain M, Wittel UA, Andrianifahanana M, Aubert JP, Batra SK. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52:253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- 23.Torres MP, Ponnusamy MP, Chakraborty S, Smith LM, Das S, Arafat HA, Batra SK. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol Cancer Ther. 2010;9:1419–1431. doi: 10.1158/1535-7163.MCT-10-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–630. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 25.de Sousa OV, Vieira GD, de Jesus RG, Yamamoto CH, Alves MS. Antinociceptive and Anti-Inflammatory Activities of the Ethanol Extract of Annona muricata L. Leaves in Animal Models. Int J Mol Sci. 2010;11:2067–2078. doi: 10.3390/ijms11052067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seufferlein T. Novel protein kinases in pancreatic cell growth and cancer. Int J Gastrointest Cancer. 2002;31:15–21. doi: 10.1385/IJGC:31:1-3:15. [DOI] [PubMed] [Google Scholar]
- 27.Chang Q, Chapman MS, Miner JN, Hedley DW. Antitumour activity of a potent MEK inhibitor RDEA119/BAY 869766 combined with rapamycin in human orthotopic primary pancreatic cancer xenografts. BMC Cancer. 2010;10:515. doi: 10.1186/1471-2407-10-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei WT, Chen H, Ni ZL, Liu HB, Tong HF, Fan L, Liu A, Qiu MX, Liu DL, Guo HC, Wang ZH, Lin SZ. Antitumor and apoptosis-promoting properties of emodin, an anthraquinone derivative from Rheum officinale Baill, against pancreatic cancer in mice via inhibition of Akt activation. Int J Oncol. 2011;39:1381–1390. doi: 10.3892/ijo.2011.1147. [DOI] [PubMed] [Google Scholar]
- 29.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 32.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick SF, Tambuwala MM, Bruning U, Schaible B, Scholz CC, Byrne A, O’Connor A, Gallagher WM, Lenihan CR, Garvey JF, Howell K, Fallon PG, Cummins EP, Taylor CT. An intact canonical NF-kappaB pathway is required for inflammatory gene expression in response to hypoxia. J Immunol. 2011;186:1091–1096. doi: 10.4049/jimmunol.1002256. [DOI] [PubMed] [Google Scholar]
- 34.Nam SY, Ko YS, Jung J, Yoon J, Kim YH, Choi YJ, Park JW, Chang MS, Kim WH, Lee BL. A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-kappaB promotes gastric tumour growth and angiogenesis. Br J Cancer. 2011;104:166–174. doi: 10.1038/sj.bjc.6606020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 36.Masamha CP, Benbrook DM. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 2009;69:6565–6572. doi: 10.1158/0008-5472.CAN-09-0913. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham CC. Actin structural proteins in cell motility. Cancer Metastasis Rev. 1992;11:69–77. doi: 10.1007/BF00047604. [DOI] [PubMed] [Google Scholar]
- 38.Kaverina I, Straube A. Regulation of cell migration by dynamic microtubules. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bacallao R, Garfinkel A, Monke S, Zampighi G, Mandel LJ. ATP depletion: a novel method to study junctional properties in epithelial tissues. I. Rearrangement of the actin cytoskeleton. J Cell Sci. 1994;107(Pt 12):3301–3313. doi: 10.1242/jcs.107.12.3301. [DOI] [PubMed] [Google Scholar]
- 40.Jordan MA, Horwitz SB, Lobert S, Correia JJ. Exploring the mechanisms of action of the novel microtubule inhibitor vinflunine. Semin Oncol. 2008;35:S6–S12. doi: 10.1053/j.seminoncol.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63:610–615. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 43.Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br J Cancer. 2009;101:1155–1161. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, Batra SK. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–320. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 45.Swartz MJ, Batra SK, Varshney GC, Hollingsworth MA, Yeo CJ, Cameron JL, Wilentz RE, Hruban RH, Argani P. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791–796. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 46.El MN, Caro-Maldonado A, Ramirez-Peinado S, Munoz-Pinedo C. Sugar-free approaches to cancer cell killing. Oncogene. 2011;30:253–264. doi: 10.1038/onc.2010.466. [DOI] [PubMed] [Google Scholar]
- 47.Komar G, Kauhanen S, Liukko K, Seppanen M, Kajander S, Ovaska J, Nuutila P, Minn H. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin Cancer Res. 2009;15:5511–5517. doi: 10.1158/1078-0432.CCR-09-0414. [DOI] [PubMed] [Google Scholar]
- 48.Vasseur S, Tomasini R, Tournaire R, Iovanna JL. Hypoxia Induced Tumor Metabolic Switch Contributes to Pancreatic Cancer Aggressiveness. Cancers. 2010;2:2138–2152. doi: 10.3390/cancers2042138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duffy JP, Eibl G, Reber HA, Hines OJ. Influence of hypoxia and neoangiogenesis on the growth of pancreatic cancer. Mol Cancer. 2003;2:12. doi: 10.1186/1476-4598-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yokoi K, Fidler IJ. Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clin Cancer Res. 2004;10:2299–2306. doi: 10.1158/1078-0432.ccr-03-0488. [DOI] [PubMed] [Google Scholar]
- 51.Chen EY, Mazure NM, Cooper JA, Giaccia AJ. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 2001;61:2429–2433. [PubMed] [Google Scholar]
- 52.Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–7279. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 53.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 54.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 56.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Cytotoxicity of Graviola extract suspended in PBS. The PC cells CD18/HPAF were incubated with different doses of Graviola extract suspended in PBS for 48hr. Cytotoxicity was analyzed by MTT cytotoxic assay. Data from experiments performed in triplicate is presented as the mean value ± standard error of mean. (*p-value<0.0001, **p-value=0.0017, compared to untreated cells); (B) ATP quantification of CD18/HPAF PC cells after treatment with Graviola extract suspended in PBS. Data from experiments performed in triplicate is presented as the mean value normalized with total protein content ± standard error of mean; (C) Western blot analysis of the expression of proteins related to the proliferation, invasion, and metastasis of PC in CD18/HPAF cells after incubation with Graviola extract suspended in PBS. Protein lysates (30μg) were resolved on 2% SDS agarose gels (MUC4) and 10% SDS-PAGE (HIF-1α, Caspase3, MMP9, and β-actin). β-actin was used as a loading control.
(A) Western blot analysis of MUC4 and HER2 in PC cells after treatment with Graviola extract. Protein lysates (30μg) were resolved on 2% agarose gels for MUC4 and 10% SDS-PAGE for HER2. β-actin was used as a loading control; (B) Confocal microscope images of PC cells stained against MUC4 (anti-Alexa Fluor 594) after treatment with Graviola extract. Cell nuclei were stained with DAPI. Scale bars represent 10μm; (C) Measurement of MUC4 transcripts in PC cells incubated with Graviola extract by real-time PCR. Data is presented as the average fold difference of MUC4 levels in Graviola extract-treated cells versus untreated cells (0μg/mL) ± standard error of mean. The housekeeping gene β-actin was used as an internal control. (*p-value<0.005; **p-value<0.005, compared to untreated PC cells)