Abstract
Aims
To elucidate how the nicotinic acetylcholine receptors expressed on bronchial and oral epithelial cells targeted by the tobacco nitrosamine (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone) (NNK) facilitate carcinogenic transformation.
Main methods
Since NNK-dependent transformation can be abolished by the nicotinergic secreted mammalian Ly-6/urokinase plasminogen activator receptor related protein-1 (SLURP-1), we compared effects of NNK and recombinant (r)SLURP-1 on the expression of genes related to tumorigenesis in human immortalized bronchial and oral epithelial cell lines BEP-2D and Het-1A, respectively.
Key findings
NNK stimulated expression of oncogenic genes, including MYB and PIK3CA in BEP2D, ETS1, NRAS and SRC in Het-1A, and AKT1, KIT and RB1 in both cell types, which could be abolished in the presence of rSLURP-1. Other cancer-related genes whose upregulation by NNK was abolishable by rSLURP-1 were the growth factors EGF in BEP2D cells and HGF in Het-1A cells, and the transcription factors CDKN2A and STAT3 (Het-1A only). NNK also upregulated the anti-apoptotic BCL2 (Het-1A) and downregulated the pro-apoptotic TNF (Het-1A), BAX and CASP8 (BEP2D), all of which could be abolished, in part, by rSLURP-1. NNK decreased expression of the CTNNB1 gene encoding the intercellular adhesion molecule β-catenin (BEP2D), as well as tumor suppressors CDKN3 and FOXD3 in BEP2D cells, SERPINB5 in Het-1A cells, and RUNX3 in both cell types. These pro-oncogenic effects of NNK were also abolished by rSLURP-1.
Significance
The obtained results identified target genes for both NNK and SLURP-1 and shed light on the molecular mechanism of their reciprocal effects on tumorigenic transformation of bronchial and oral epithelial cells.
Keywords: lung cancer, head and neck cancer, NNK (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone), SLURP-1 (secreted mammalian Ly-6/urokinase plasminogen activator receptor related protein-1), gene expression, BEP2D cells, Het-1A cells
Introduction
Tobacco nitrosamines, such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), are involved in cancers of the lung, oral cavity, esophagus, and pancreas (Hechtet al., 1993). The cells exposed to N-nitrosamines continue to divide, and display focal growth and morphologic changes suggestive of early stages of cell transformation (Murrahet al., 1993). NNK can displace the endogenous ligand acetylcholine from the nicotinic class of acetylcholine receptors (nAChRs) expressed on the plasma membrane of both bronchial and oral epithelial cells (Arredondoet al., 2006a, Arredondoet al., 2006b). Thus, in addition to a well-formulated role of genotoxic damage in the etiology of tobacco-related lung cancer, N-nitrosamines may contribute directly to tumorigenesis through the receptor-mediated mechanisms.
A novel paradigm of cell regulation via nAChRs has been discovered in studies of the cholinergic protein termed SLURP-1 (secreted mammalian Ly-6/urokinase plasminogen activator receptor related protein-1) (Chimientiet al., 2003). The amino acid composition of SLURP-1 is homologous to that of single domain frog cytotoxin and snake venom neurotoxins, such as α-bungarotoxin. SLURP-1 was detected in aerodigestive epithelia, lymphocytes, uterus, stomach, cornea, blood, saliva, sweat and urine (reviewed in Grando, 2008). Radioligand binding inhibition studies showed that SLURP-1 preferentially ligates α7 nAChR, and alters expression of cell cycle regulators, differentiation markers, and activate caspases (Arredondoet al., 2005). SLURP-1 competes with nicotine and NNK at the nAChR ligand-binding site and regulates vital function of normal and malignant cells (Grando, 2008). We have recently demonstrated that NNK-dependent transformation of immortalized human bronchial and oral epithelial cells BEP2D and Het-1A, respectively, can be abolished by recombinant (r)SLURP-1 (Chernyavskyet al., 2008).
In this study, we compared the effects of NNK and rSLURP-1 on the expression of genes related to tumorigenesis in BEP2D and Het-1A cells.
Materials and Methods
Treatment of Cells with rSLURP-1
The full length rSLURP-1 was manufactured at Virusys Corporation (Sykesville, MD), as detailed by us before (Chernyavskyet al., 2008). The BEP2D cells — an established clonal population of HPV-18-immortalized human bronchial epithelial cells—was a generous gift from Dr. Harris (NCI, NIH). The Het-1A cell line — an established clonal population of SV40-immortalized human esophageal epithelial cells — was purchased from ATCC (Manassas, VA). The BEP2D cells and Het-1A cells were grown in EpiLife® Medium containing 60 μM calcium and Human Corneal Growth Supplement (Invitrogen, Carlsbad, CA) in a humidified incubator at 5% CO2 and 37°C. At 80% confluence, experimental cells were exposed for 24 h to either 1 μM NNK alone or in combination with 1 μg/ml of rSLURP-1, and control cells were left untreated.
PCR Array and Data Analysis
Total RNA was extracted using RNeasy mini kit (Qiagen, Valencia, CA). The First-Strand Kit, which contains a mixture of oligo(dT) primer and random hexamers (SABiosciences, Frederick, MD), was utilized for cDNA synthesis, using 400 ng of total RNA from experimental and control (untreated) cells. The RT2-Profiler PCR Array for human Oncogenes & Tumor Suppressor genes (PAHS-502E, SABiosciences) that profiles expression of 84 key genes involved in oncogenesis was carried out according to manufacturer’s instructions using ABI Prism 7900 HT (Applied Biosystems, Carlsbad, CA). Subsequent to amplification, characterization of products was performed by melting-curve analysis. SYBR Green fluorescence was detected in each well during the annealing step of each cycle. Analyses of the raw data were done through the SuperArray Data Analysis Web Portal (SABiosciences). Three independent experiments, each starting with fresh cells and the isolation of fresh RNA, were conducted. Each independent experiment was performed in triplicates. The Ct values for each sample corresponded to the point at which the fluorescence crosses the threshold. Results were expressed as fold change compared to intact control. The p values were calculated based on a Student’s t-test of the replicate 2−ΔCt values for the gene in each treatment group. To determine fold change in gene expression, the normalized gene expression in the experimental sample was divided by the normalized expression of the same gene in the control sample, as follows:
Results
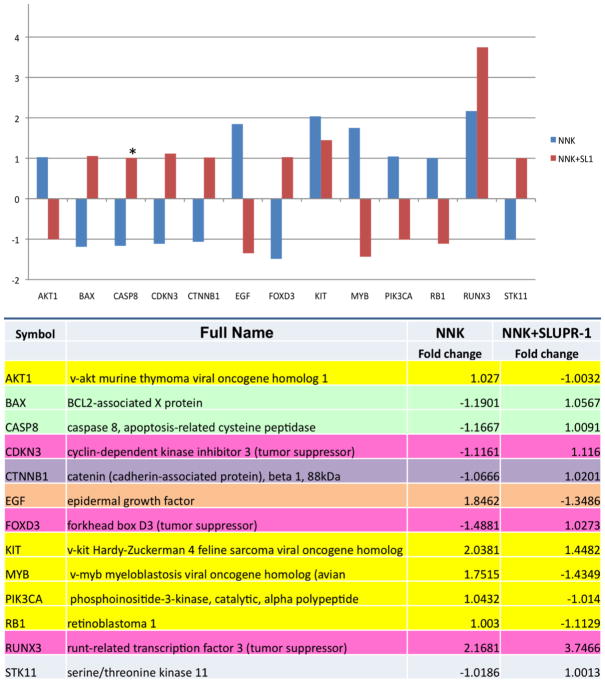
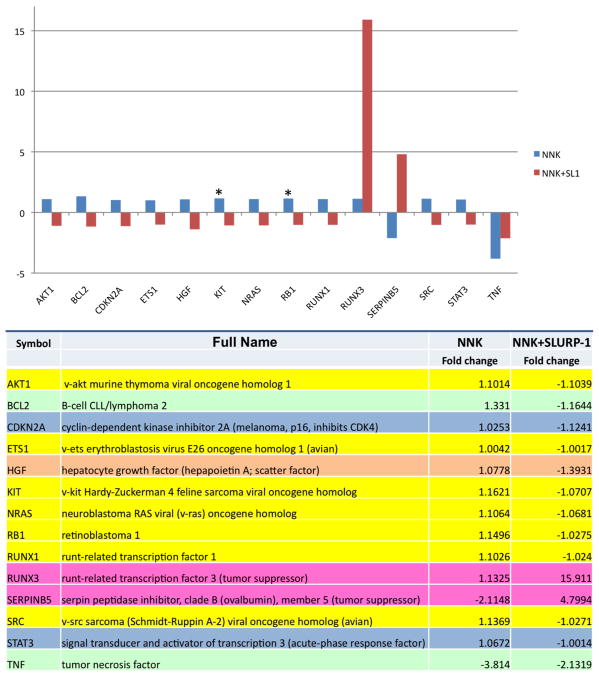
In each cell type, NNK stimulated expression of a number of oncogenic genes, such as MYB and PIK3CA in BEP2D cells (Fig. 1), ETS1, NRAS and SRC in Het-1A cells (Fig. 2), and AKT1, KIT and RB1 in both cell types, which could be abolished in the presence of rSLURP-1. Other cancer-related genes whose upregulation by NNK was abolishable by rSLURP-1 were the growth factors EGF in BEP2D cells and HGF in Het-1A cells, and the transcription factors CDKN2A and STAT3 in Het-1A cells. NNK also upregulated the anti-apoptotic BCL2 (Het-1A only) and downregulated the pro-apoptotic TNF (Het-1A), BAX and CASP8 (BEP2D) genes, all of which could be abolished, in part, in the presence of rSLURP-1. NNK decreased expression of the CTNNB1 gene encoding the intercellular adhesion molecule β-catenin (BEP2D), as well as tumor suppressors CDKN3 and FOXD3 in BEP2D cells, SERPINB5 in Het-1A cells, and RUNX3 in both cell types. These pro-oncogenic effects of NNK were also abolished by rSLURP.
Figure 1. Alterations of the expression of oncogenesis-related genes in BEP2D cells.
The cells were treated with either 1 μM NNK alone or in combination with 1 μg/ml of rSLURP-1 (SL1) and then used in the RT2-Profiler PCR Array for human Oncogenes & Tumor Suppressor genes as detailed in Materials and Methods. The vertical axis on the graph shows the fold change values compared to intact control. Fold change values greater than one indicate an up-regulation, and fold-change values less than one indicate a down-regulation of gene expression. Asterisk represents significant (p<0.05) difference between two treatment conditions.
Figure 2. Alterations of the expression of oncogenesis-related genes in Het-1A cells.
The cells were treated, and the gene expression changes were measured and displayed as detailed in the capture to Fig. 1.
Discussion
Results of the screening experiments reported herein identify target genes for both NNK and SLURP-1 and shed light on the molecular mechanism of their reciprocal regulation of neoplastic transformation of bronchial oral and epithelial cells reported elsewhere (Chernyavskyet al., 2008). By analogy with other cell types (Faumontet al., 2009), immortalization might have affected gene expression profiles in BEP2D and Het-1A cells. This possibility, however, would not produce a confounding effect on the results of our study, because the cells treated with NNK ± rSLURP-1 were compared to untreated cells, taken as control. NNK might cause some increase of RUNX3 in both cells lines probably by mediating an indirect protective effect, whereas SLUPR-1 increased its expression substantially, probably unmasking an endogenous mechanism.
The nAChR-mediated mechanism of the initiation and progression of NNK-induced cancers has been demonstrated by our previous observations that NNK competes with nicotinic radioligands for binding to BEP2D and Het-1A cells (Arredondoet al., 2006a, Arredondoet al., 2006b). SLURP-1 is structurally similar to snake venom toxin α-bungarotoxin, which acts as α7 nAChR antagonist (Chimientiet al., 2003). Since both NNK and SLURP-1 can ligate α7 nAChR (Grando, 2008), rSLURP-1 might antagonize NNK effects on the expression of oncogenes by displacing it from the α7 nAChR ligand-binding site.
The specific role of nAChRs in lung cancer pathophysiology has been substantiated by the results of Wide Genome Association studies showing that single nucleotide polymorphisms of nAChR subunits affect carcinogenicity of nicotine and related compounds in lung cancer, in addition to being related to addiction to tobacco smoking (Amoset al., 2008, Kaur-Knudsenet al., 2011). The nAChRs are selectively overexpressed in a variety of cancers, e.g., α7 in lung cancer and α9 in breast cancer, and inhibition of nAChR protein levels attenuates nicotine- and nitrosamine-induced cell proliferation in tumor models (reviewed in (Singhet al., 2011, Wuet al., 2011)). Most recently, it has been reported that naturally occurring variants of human α9 nAChR differentially affect bronchial cell proliferation and transformation (Chikova and Grando, 2011). Therefore, it is currently believed that nAChRs in lung cells act as central mediators in the activation of cancer signaling pathways (Schuller, 2009), and epithelial nAChRs are considered as novel drug targets for prevention and treatment of cancer (Paleariet al., 2009, Russoet al., 2006).
Conclusions
rSLURP-1 and NNK produce reciprocal effect on gene expression in human bronchial and oral epithelial cells that may mediate their pro- and anti-oncogenic effects, respectively. The pro-oncogenic effects of NNK can be abolished, in part, by rSLURP-1. The cellular nAChRs that mediate biologic effects of NNK and SLURP-1, α7 and α9 in particular, may provide a novel molecular target to prevent, reverse, or retard progression of lung and oral and cancers by nicotinergic ligands. Future studies should ultimately establish the nAChR subtype(s) mediating the reciprocal effects of NNK and SLURP-1, and identify the downstream signaling pathways linking the nAChR subtypes to the tumorigenic transformation of epithelial cells leading to lung and oral cancers.
Acknowledgments
This work was supported by the NIH grants ES017009 and DE14173, and a grant from Lung Cancer Research Foundation to S.A.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–22. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Grando SA. The nicotinic receptor antagonists abolish pathobiologic effects of tobacco-derived nitrosamines on BEP2D cells. J Cancer Res Clin Oncol. 2006a;132:653–63. doi: 10.1007/s00432-006-0113-9. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Grando SA. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol Ther. 2006b;5:511–7. doi: 10.4161/cbt.5.5.2601. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Webber RJ, Grando SA. Biological effects of SLURP-1 on human keratinocytes. J Invest Dermatol. 2005;125:1236–41. doi: 10.1111/j.0022-202X.2005.23973.x. [DOI] [PubMed] [Google Scholar]
- Chernyavsky AI, Arredondo J, Putney DJ, Marsh JS, Grando SA. Potential role for epithelial nicotinic receptors in tobacco related oral and lung cancers. J Stomatol Invest. 2008;2:5–14. [Google Scholar]
- Chikova A, Grando SA. Naturally occurring variants of human α9 nicotinic receptor differentially affect bronchial cell proliferation and transformation. PLoS One. 2011;6:e27978. doi: 10.1371/journal.pone.0027978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimienti F, Hogg RC, Plantard L, Lehmann C, Brakch N, Fischer J, et al. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet. 2003;12:3017–24. doi: 10.1093/hmg/ddg320. [DOI] [PubMed] [Google Scholar]
- Faumont N, Durand-Panteix S, Schlee M, Gromminger S, Schuhmacher M, Holzel M, et al. c-Myc and Rel/NF-kappaB are the two master transcriptional systems activated in the latency III program of Epstein-Barr virus-immortalized B cells. J Virol. 2009;83:5014–27. doi: 10.1128/JVI.02264-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grando SA. Basic and clinical aspects of non-neuronal acetylcholine: biological and clinical significance of non-canonical ligands of epithelial nicotinic acetylcholine receptors. J Pharmacol Sci. 2008;106:174–9. doi: 10.1254/jphs.fm0070087. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Foiles PG, Murphy SE, Peterson LA. Tobacco-specific nitrosamine adducts: studies in laboratory animals and humans. Environ Health Perspect. 1993;99:57–63. doi: 10.1289/ehp.939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur-Knudsen D, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. Nicotinic Acetylcholine Receptor Polymorphism, Smoking Behavior, and Tobacco-Related Cancer and Lung and Cardiovascular Diseases: A Cohort Study. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.32.9870. [DOI] [PubMed] [Google Scholar]
- Murrah VA, Gilchrist EP, Moyer MP. Morphologic and growth effects of tobacco-associated chemical carcinogens and smokeless tobacco extracts on human oral epithelial cells in culture. Oral Sur Oral Med Oral Pathol. 1993;75:323–32. doi: 10.1016/0030-4220(93)90145-t. [DOI] [PubMed] [Google Scholar]
- Paleari L, Negri E, Catassi A, Cilli M, Servent D, D’Angelillo R, et al. Inhibition of nonneuronal alpha7-nicotinic receptor for lung cancer treatment. Am J Respir Crit Care Med. 2009;179:1141–50. doi: 10.1164/rccm.200806-908OC. [DOI] [PubMed] [Google Scholar]
- Russo P, Catassi A, Cesario A, Servent D. Development of novel therapeutic strategies for lung cancer: targeting the cholinergic system. Curr Med Chem. 2006;13:3493–512. doi: 10.2174/092986706779026192. [DOI] [PubMed] [Google Scholar]
- Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011:456743. doi: 10.1155/2011/456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Lee CH, Ho YS. Nicotinic acetylcholine receptor-based blockade: applications of molecular targets for cancer therapy. Clin Cancer Res. 2011;17:3533–41. doi: 10.1158/1078-0432.CCR-10-2434. [DOI] [PubMed] [Google Scholar]