Abstract
The sequencing of the human genome led to many insights into gene organization and structure. One interesting observation was the high frequency of bidirectional promoters characterized by two protein encoding genes whose promoters are arranged in a divergent or “head-to-head” configuration with less than 2000 base pairs of intervening sequence. Computational estimates published by various groups indicate that nearly 10% of the coding gene promoters are arranged in such a manner and the extent of this bias is a unique feature of mammalian genomes. Moreover, as a class, head-to-head promoters appear to be enriched in specific categories of gene function. Here we review the structure, composition, genomic properties and functional classifications of genes controlled by bidirectional promoters and explore the biological implication of these features.
INTRODUCTION
Janus is one of the oldest gods in Roman mythology and is depicted as, “Janus Bifrons”, an image of two faces positioned head-to-head and faced away in a divergent fashion. Janus is the god of transitions, linking all beginnings to all ends, the gateways and doorways of life. This concept of inextricably linked fates or events is well illustrated by the function and biology of the “head-to-head” or bidirectional promoters of the mammalian genome (Figure 1). The earliest recognition of this unique class of genes was first made after the initial analysis of the first draft of the human genome over a decade ago when it became clear that the positioning across the human genome was non-random with many genes arranged in a head-to-head divergent configuration [1]. We present an overview of how our understanding of this unique feature of genomic architecture has evolved since its earliest recognition, and what implications it may have for broader mechanisms through which they may be regulated.
Figure 1. Schematic of a bidirectional promoter.
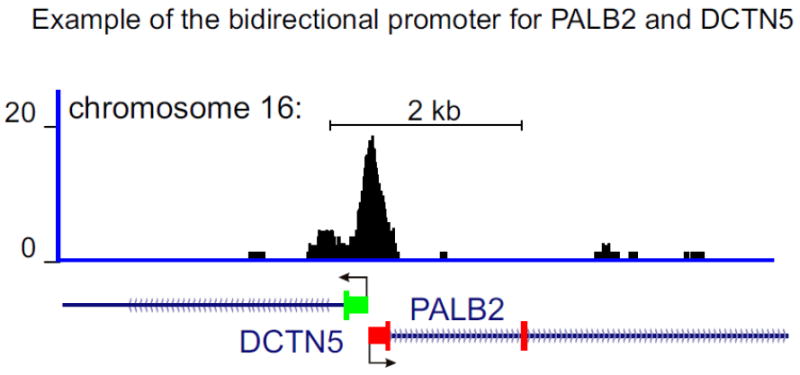
Example of a bidirectional promoter for divergently transcribed genes (head to head configuration) PALB2 and DCTN5 with TSS occurring within 2000 bp of each other. Illustrated is the tag density profile for the binding of the C-terminal binding protein (CtBP) at a bidirectional promoter.
Recognition and structural characterization of Bidirectional promoters
The work of Adachi and Lieber first summarized the early evidence of this unique arrangement of a portion of the genome where some genes could be found in a head-to-head configuration [1]. At that time it was noted that many of these genes share an intervening sequence between the transcription start sites of the divergent gene pairs that was less than 300 bp in length (Figure 2 and 3a). In addition, many of these promoters were characterized by the presence of CpG islands (regions of the genome that are devoid of DNA methylation and show a higher than average G+C content [3]). Surprisingly, it appears that many of the genes driven by bidirectional promoters share similar functions, such as DNA repair, cell cycle regulation and regulation of metabolism [1] (Figure 3b). Speculation on the teleological implication of this form of gene organization focused logically on the need for coordinated expression, that could play a role in both regulating stoichiometric levels of gene products that self-assemble (e.g. histones) or for factors that play a functional or temporally correlated role in a biological pathway or process.
Figure 2. Size distribution of bidirectional promoters in the human genome.
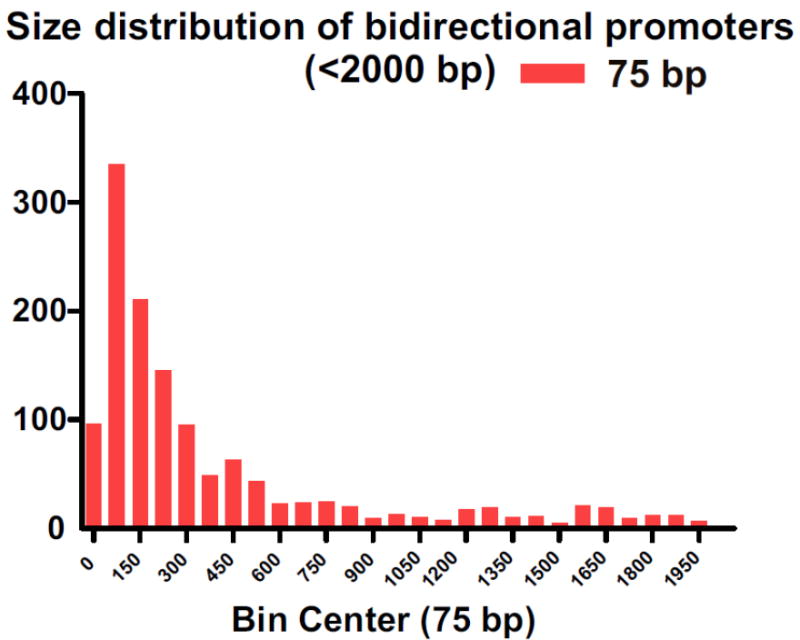
Histogram representing the size distribution of bidirectional promoters for genes (head-to-head orientation) containing TSS which occur within 2000 bp. X-axis represents an interval of 75 bp for each bin. Y-axis represents the frequency of events (bidirectional promoters) that fall into each bin. The bidirectional promoters were retrieved based on the UCSC refgene List (HG19): http://hgdownload.cse.ucsc.edu/goldenPath/hg19/database/refGene.txt.gz). A gene was determined to have a bidirectional promoter if the distance bewteen its TSS (transcriptional start site) and closest divergently transcribed TSS is less than 2000 bp in length.
Figure 3. Size and functional distribution of bidirectional promoters in the human genome.
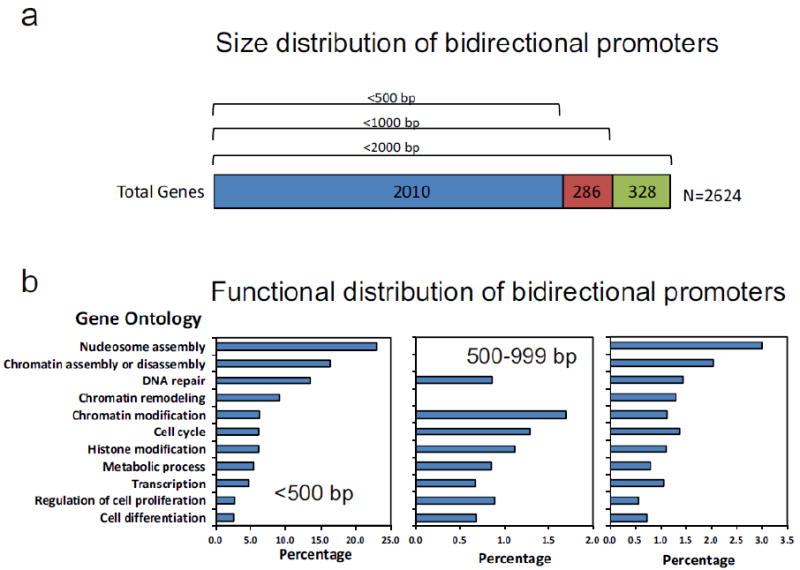
(a) Distribution of genes with bidirectional promoters according to length (bp). The majority of bidirectional promoters occur within 500 bp of the TSS of genes (head-to-head orientation). The number of genes stated is for all genes with a divergent head-to-head orientation separated by <2000 bp. (b) Gene ontology and functional distribution of genes with bidirectional promoters according to length (bp) represented in percentage of total bidirectional promoter in that category. Gene ontology analyses were performed using the GoMiner web-based software [23,35].
As subsequent and more refined drafts of the human genome became available, these observations were elegantly expanded by a series of studies by Trinklein and colleagues, who conducted the first genome-wide computational analysis of bidirectional promoters. Of a total of 23,752 annotated genes, >10% were found to be bidirectional sharing common promoter regions that were less than 1 kb apart [27]. In addition, by meta-analysis of available micro-array gene expression datasets, Trinklein et al showed that a significant number of gene pairs in bidirectional promoters showed correlated expression. Interestingly, of the gene pairs that show correlated expression 11% showed anti-correlated expression indicating the diversity of expression outcomes associated with this type of genomic architecture. A persistent theme noted by most studies is the relatively short length of a large portion of bidirectional promoters. Trinklein et al reported that nearly 67% of head-to-head promoters had intervening sequences that were less than 300 bp in length [27]. Interestingly, there was no correlation between the length of the promoter and the extent of correlated expression. However, this bimodal nature (the average of intervening space between head-to-head genes shows a second peak at <100,000 bp in Homo sapiens and <10,000 bp in Mus musculus [22]) or significant enrichment of bidirectional promoters with short intervening spacing appears to be largely a mammalian phenomena not demonstrated in flies or plants [21]. The existence of bidirectional promoter architecture is a common and conserved feature across many species, indicating a significant ancestral origin of this type of genomic organization that became more heavily utilized by higher organisms [22].
Consistent with the earlier descriptions of Adachi and Lieber [1], compared to unidirectional promoters, the median GC count of bidirectional promoters is higher (66% compared to 53% for non-bidirectional promoters); consequently, close to 77% of bidirectional promoters contain CpG islands compared to 38% of non-bidirectional promoters [27]. However, the composition of the CpG islands in bidirectional promoters did not appear to be different from non-bidirectional promoters. As might be predicted the relative level of TATA box containing bidirectional promoters was found to be significantly less than non-bidirectional promoters (8% compared to 28%) [27].
From empirical experiments using cloned regions of both unidirectional and bidirectional promoters in reporter assays, Trinklein et al suggested that some bidirectional activity may be an inherent feature of most promoters [27], an observation that has been substantiated by several recent reports of divergent transcription at gene promoters in mammalian cells [12,25,26]. However, bidirectional promoters seem to support higher levels of bidirectional activity and showed specific dependence on several features within the shared promoter regions including 1.) integrity or activity of the paired TSS, where loss of activity of one TSS could increase or decrease activity at the opposing TSS. 2.) activity of specific promoter elements, where loss of one element could impair transcription in either one or both directions and 3.) a conditional dependency on cell type for the influence of these properties of the promoters. Thus, clearly activity of bidirectional promoters will be determined by a variety of cell-type specific and other environmental influences that control the availability of trans-acting protein factors and complexes.
Similar to other studies Trinklein et al also described an over-representation of different functional classes in genes controlled by bidirectional promoters, including DNA repair genes (5-fold over-represented compared to non-bidirectional), chaperone genes (3-fold over-represented) and mitochondrial genes (2-fold over-represented) [27]. Interestingly, the work of others have found that gene pairs that are involved in similar biological functions show the highest or stronger correlations in expression [22].
Recently, Yang and Elnitski performed a computational study that expanded the list of bidirectional promoters by including both GenBank Expressed Sequence Tag (EST) data and the UCSC List of Known Genes and identified 3,470 bidirectional gene promoters [32]. This analysis was extended further by defining the core promoter elements and composition of this class of genes [33]. This study notes a tendency of CpG islands to extend to both TSS in the head-to-head pair. As noted above, TATA boxes were under-represented in bidirectional promoters (9% in head-to-head versus 29% in non-bidirectional) [33]. Bidirectional promoters containing TATA boxes consistently show an asymmetrical distribution favoring one TSS over the other [33]. Interestingly, histone gene pairs driven by bidirectional promoters showed higher enrichment with TATA box sequences than other functional classes. Other core elements including downstream positive elements (DPE) and initiator elements (INR) did not show biased enrichment in either non-bidirectional or bidirectional promoters. The TFIIB recognition element (BRE) showed some bias toward bidirectional promoters (16.5% versus 11.1%), however the CCAAT box sequence appeared to be nearly two-fold higher-represented in bidirectional (12.9%) as compared to non-bidirectional promoters (6.9%)[33]. Similar to the estimates by Trinklein and colleagues [27] CpG islands were found to be more prevalent in bidirectional (90%) as opposed to non-bidirectional promoters (45%) [33]. The authors in this work also correctly pointed out the large enrichment for CpG islands in bidirectional promoters is largely responsible for other features including higher basal levels of transcription, and in meta-analysis of genome-wide Pol II chromatin immuno-precipitation studies, the CpG islands of bidirectional promoters show higher Pol II occupancy [6,20,33]. The prominent role for basal transcription factors in the control of bidirectional promoters and promoters bearing CpG islands is suggested by the fact that promoters with no identifiable core promoter elements are much more enriched in non-bidirectional promoters [33]. Nonetheless bidirectional promoters still maintain unique features that are different from other non-bidirectional promoters containing CpG islands. First, they have a mirrored composition in which an identifiable center occurs where the base composition switches from G + T to A + C. Second, although bidirectional promoters have a broad range of initiation sites often seen in CpG islands, the arrangement is much more discrete than in the CpG islands of non-bidirectional promoters [33].
Features of transcription factor binding and chromatin modification at Bidirectional Promoters
The public availability of data from genome wide analyses of factor interactions with chromatin binding by ChIP-chip and ChIP-seq experiments through the ENCODE Project Consortium allows extensive validation of computational analyses of potential and putative factor binding sites enriched in bidirectional promoters across the genome [2,11,16,23]. Through a combination of computational analysis with meta-analysis of ChIP-chip experiments, Lin et al identified factor binding sites that were over-represented in bidirectional promoters [23]. This list includes binding sites for MYC, E2F1, E2F4, SP1, SP3 and STAT1 that are found to be enriched in bidirectional promoters through analysis of ChIP-chip studies and binding sites for NRF-1, CCATT boxes (NF-Y like), YY1 and GA binding protein A (GABPA) that were identified first by computational analysis [23]. Interestingly, NRF-1, CCATT, GABPA and YY1 sites are among the most conserved mammalian promoter motifs [29] suggesting that bidirectional promoters are controlled by highly conserved and ubiquitous transcriptional machinery. Though the motifs likely to be controlling bidirectional promoters are quite common, this set is limited as nearly 73% of most well characterized transcription factor binding motifs are under-represented in bidirectional promoters [23].
The Ets related factor GABPA appears to bind to more than 80% of bidirectional promoters [11]. There was also considerable overlap with binding by Ets-1 though GABPA seemed to bind a much larger fraction of bidirectional promoters than Ets-1, where Ets-1 also displayed a much greater cell type specificity [11]. GABPA is estimated to be enriched nearly 8-fold in bidirectional promoters as compared to non-bidirectional promoters [23]. CCATT enrichment in bidirectional promoters has also been studied by Hakkinen et al where it was found that stronger correlations occurred when they were presented in multiple tandem arrangements, indicating cooperative interaction between the binding sites. The Staf/ZNF143 zinc finger protein is a gene that is reportedly involved in the control of many genes that play a role in DNA repair and genome stability [19,24] and empirical studies show that it may bind to as many as 47% of bidirectional promoters with a preference for promoter pairs with smaller intervening sequences [2].
Consistent with the studies of Yang et al, the chromatin of bidirectional promoters show many characteristics commonly associated with more active promoters, this includes higher density Pol II binding, increase density of Histone H3 acetylation (H3ac) and increase promoter proximal markings with Histone H3 methylation at lysine 4 (H3K4Me2/3) [23]. Surprisingly, Histone H4 acetylation appeared to be under-represented at bidirectional promoters.
The role of higher order complexes in the control of bidirectional promoters
The recent studies described above have clearly documented the existence and regulatory relevance of bidirectional promoters and suggest ways in which this genomic configuration may contribute to the diverse mechanisms of transcriptional control. However, new efforts have to be directed at defining the mechanism through which the structure and sequence of these promoters may contribute to their regulation. Equally important is demonstrating how such modes of regulation may have relevance for different classes of biological processes.
Since evidence suggested that the number of regulatory factors that bind bidirectional promoters are much more exclusive than non-bidirectional promoters [23], one approach is to examine more carefully, the potential regulatory complexes that may be preferentially assembled or recruited at bidirectional promoters through interactions with this limited number of transcription factors.
Recently, the NADH dependent dimeric transcriptional co-repressor protein CtBP1/2 (C-terminal binding protein 1/2) was shown to be recruited to the early onset breast cancer gene, BRCA1, to repress transcription from its bidirectional promoter [14]. Interestingly, the BRCA1 promoter represents a prototypical bidirectional promoter possessing classical features of bidirectional promoters including: 1.) less than 500 bp in length [30]; 2.) regulated by GABPA proteins [4]; 3.) Bound by E2F transcription factors [7]; 4.) contains a CpG island [31]; and 5.) is a well known DNA repair protein [13]. CtBP is a dimeric complex composed of heterodimers or homodimers of CtBP1 or CtBP2. CtBP dimerization is stabilized by NADH suggesting that CtBP could function as a metabolic sensor [8,9]. One component of the CtBP dimer functions to bind to chromatin modifying complexes including histone deacetylases, histone methyltransferases and components of the polycomb complex that interact with DNA methyltransferases, suggesting that CtBP could play a major role in sculpting the epigenetic landscape of the nucleus [8,9]. The other member of the CtBP dimer serves a chromatin targeting function by binding to a number of promoter bound transcription factors or transcription factor adaptor proteins [8,9]. Notably, many of the factors bound by CtBP belong to transcription factor families that show a high frequency of binding to bidirectional promoters (See Table 1). Thus, CtBP is likely to play a major role in the transcriptional regulation of bidirectional promoters similar to BRCA1 and could conceivably have a broad influence on cellular DNA repair by targeting bidirectional promoters as a general class. Since recent studies suggest that CtBP could play a major role in tumor biology [8,9], it is possible that part of this function could be through its control of bidirectional promoters.
Table 1. DNA-binding motifs associated with CtBP and bidirectional promoters.
CtBP-interacting transcription factors and consensus sequences observed in bidirectional promoters. Consensus motifs for bidirectional promoters share similarities to the corresponding TRNASFAC DNA motifs. DNA-binding motif analyses were performed using MeMe (Multiple Em for Motif Elicitation) software [5].
| CtBP-interacting Transcription Factors and Associated DNA-binding Motifs
| ||||
|---|---|---|---|---|
| Transcription Factor | TF Family | Adaptor | Bidirectional Promoter Consensus Motif | TRANSFAC Motif |
| GATA3/4 | GATA | FOG1/2 |  |
 |
| BKLF, hkLF3/8 | Kruppel-type Zinc finger |  |
||
| ELK3 | Ets (GABPA) |  |
 |
|
| E2F4/5 | E2F | RB, p130 |  |
|
| SOX6 | SRY-box |  |
||
| BRCA 1 | Ring finger | CtlP | ||
| YY1 | Kruppel-type Zinc finger |  |
 |
|
| NRF-1 | ||||
| HIC-1 |  |
|||
The functional and biological implications of bidirectional promoters
A common finding in most studies of bidirectional promoters is that they regulate genes whose function is over-represented in specific functional groups [1,22,27,32]. In addition to DNA repair functional pathways, other common functional categories involve chromatin stability, maintenance and assembly (e.g histone genes [22]). Figures 2 and 3 show a summary of the size distribution and major functional categories enriched in genes controlled by bidirectional promoters. Notice that there is a significant bias of the functional categories, linked to genome maintenance, based on promoter length. Bidirectional promoters with head-to-head promoter intervening sequence lengths greater than 500 bp have distinctly divergent functions. The bimodal nature of the profiles is noteworthy and may reflect a length dependent selection that remains to be explored, nonetheless both show significantly decreased enrichment for categories that are over-represented in head-to-head promoters smaller than 500 bp.
The enrichment of bidirectional promoters in functional categories important in DNA repair and genome maintenance has demonstrable biological relevance since genome instability is considered a major driver of many hallmarks or common features of cancer [17]. This is particularly true for breast and ovarian cancer [18]. Recently, Yang et al explored the biological implication of the control of genome stability by bidirectional promoters and showed a high enrichment of bidirectional promoters in gene lists strongly associated with breast and ovarian cancer. This investigator correctly emphasized the importance of this association for our understanding of cancer since a major mechanism through which several DNA repair proteins and other tumor suppressor genes are inactivated are through DNA methylation and, as described above, the majority of bidirectional promoters contain CpG islands. In fact, it is well known that methylation of the binding sites for GABPA (which is highly enriched at bidirectional promoters [11]) is inhibited by DNA methylation [34], particularly at the BRCA1 promoter [4,31]. Finally, a very recent report suggests that estrogen controlled genes with estrogen receptor binding sites close to the proximal promoter are over-represented by bidirectional promoters, an observation that merits future exploration [15].
Perspectives
There still are many aspects of bidirectional promoter function that remains to be uncovered. In addition to defining and learning more about the higher order complexes that assemble at the promoter of this gene class, more must be understood about the chromatin structure at these genes. Though many aspects of head-to-head structure seem to conform to what has been described for CpG islands, the relative discrete distribution of transcription start sites in the CpG islands of bidirectional promoters compared to those found in non-bidirectional promoters suggest that distinct aspects of chromatin organization defining head-to-head promoters have yet to be defined. The high density of Pol II found at a large fraction of bidirectional promoters suggest that a major portion of head-to-head genes may be controlled by paused polymerases that remain poised for rapid gene expression. A reasonable assumption concerning bidirectional promoters is that they provide a means to co-express the paired genes flanking the shared promoter. However, it is interesting to note that, with the exception of histone genes, the majority of gene pairs shared by bidirectional promoters do not appear to participate in either similar functions or assembly pathways. It may be that the one common selective pressure for genes that are linked to bidirectional promoters is the need to insure the maintenance of high levels of basal gene expression. Thus, the placement of a gene within the context of a bidirectional promoter provides a means to guarantee expression of genes with vital function.
The sequencing of the human genome resulted in the unanticipated discovery that transcripts of coding genes account for less than 2% of the human genome compared to the 70% or more of the genome that is transcribed [28]. In this review, we focus on bidirectional promoters that transcribe coding genes in humans, however we acknowledge the importance of the existence of bidirectional promoters that initiate pervasive transcription resulting in the generation of non-coding RNA (ncRNA) transcripts with largely unknown function, encompassing nearly all organisms from bacteria to humans. A number of studies using a variety of techniques such as, GRO-seq [12], NET-seq [10], tiling arrays and RNA sequencing have identified these ncRNA transcripts and this revelation has led to a new perspective on the complexity of the transcriptome and the emergence of novel hypotheses to account for the functional properties of these transcripts. The conventional theory suggests a role for ncRNAs as regulatory components that meticulously control gene activity and sculpt the transcriptome. Such activity could explain the expansive diversity in the developmental control of different organisms despite the relatively low number of coding genes in their respective genomes. Understanding transcriptional regulation by ncRNAs is still in its infancy, but several mechanistic models have been proposed, including local, regional signal spreading, and distal effects [28]. Local effects pair sense and anti-sense transcripts to compete for a limited pool of transcription factors and polymerase or recruitment of chromatin modifying proteins as a mechanism of regulation [28]. These are likely to be interactions where the bidirectional nature of genomic configurations may have their largest effects. Regional effects constitute ncRNAs produced by bidirectional promoters that overlap sense or anti-sense transcripts resulting in either a coupling or repression of gene activity [28]. Lastly, ncRNAs can have distal effects by activating or repressing distal genes [28]. Clearly, gene regulation is a highly intricate process with a complexity that is starting to be deciphered and ncRNAs will potentially have a profound impact on the understanding of this biological process.
Highlights.
Bidirectional promoters encompass approximately 10% of the human genome.
Functional classes cover DNA repair, chromatin stability, maintenance and assembly.
CtBP binds to multiple transcription factors that target bidirectional promoters.
CtBP may link bidirectional promoters to multiple epigenetic regulators in cancer.
CtBP assembly at bidirectional promoters may drive multiple hallmarks of cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Adachi N, Lieber MR. Bidirectional gene organization: a common architectural feature of the human genome. Cell. 2002;109:807. doi: 10.1016/s0092-8674(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 2.Anno YN, Myslinski E, Ngondo-Mbongo RP, Krol A, Poch O, Lecompte O, Carbon P. Genome-wide evidence for an essential role of the human Staf/ZNF143 transcription factor in bidirectional transcription. Nucleic Acids Res. 2011;39:3116. doi: 10.1093/nar/gkq1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antequera F. Structure, function and evolution of CpG island promoters. Cell Mol Life Sci. 2003;60:1647. doi: 10.1007/s00018-003-3088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atlas E, Stramwasser M, Whiskin K, Mueller CR. GA-binding protein alpha/beta is a critical regulator of the BRCA1 promoter. Oncogene. 2000;19:1933. doi: 10.1038/sj.onc.1203516. [DOI] [PubMed] [Google Scholar]
- 5.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Bindra RS, Glazer PM. Basal repression of BRCA1 by multiple E2Fs and pocket proteins at adjacent E2F sites. Cancer Biol Ther. 2006;5:1400. doi: 10.4161/cbt.5.10.3454. [DOI] [PubMed] [Google Scholar]
- 8.Chinnadurai G. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol Cell. 2002;9:213. doi: 10.1016/s1097-2765(02)00443-4. [DOI] [PubMed] [Google Scholar]
- 9.Chinnadurai G. The transcriptional corepressor CtBP: a foe of multiple tumor suppressors. Cancer Res. 2009;69:731. doi: 10.1158/0008-5472.CAN-08-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churchman LS, Weissman JS. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature. 2011;469:368. doi: 10.1038/nature09652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins PJ, Kobayashi Y, Nguyen L, Trinklein ND, Myers RM. The ets-related transcription factor GABP directs bidirectional transcription. PLoS Genet. 2007;3:e208. doi: 10.1371/journal.pgen.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng CX, Wang RH. Roles of BRCA1 in DNA damage repair: a link between development and cancer. Hum Mol Genet. 2003;12(Spec No 1):R113–R123. doi: 10.1093/hmg/ddg082. [DOI] [PubMed] [Google Scholar]
- 14.Di LJ, Fernandez AG, De SA, Longo DL, Gardner K. Transcriptional regulation of BRCA1 expression by a metabolic switch. Nat Struct Mol Biol. 2010;17:1406. doi: 10.1038/nsmb.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia SA, Nagai MA. Transcriptional regulation of bidirectional gene pairs by 17-beta-estradiol in MCF-7 breast cancer cells. Braz J Med Biol Res. 2011;44:112. doi: 10.1590/s0100-879x2010007500149. [DOI] [PubMed] [Google Scholar]
- 16.Hakkinen A, Healy S, Jacobs HT, Ribeiro AS. Genome wide study of NF-Y type CCAAT boxes in unidirectional and bidirectional promoters in human and mouse. J Theor Biol. 2011;281:74. doi: 10.1016/j.jtbi.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 19.Izumi H, Wakasugi T, Shimajiri S, Tanimoto A, Sasaguri Y, Kashiwagi E, Yasuniwa Y, Akiyama M, Han B, Wu Y, Uchiumi T, Arao T, Nishio K, Yamazaki R, Kohno K. Role of ZNF143 in tumor growth through transcriptional regulation of DNA replication and cell-cycle-associated genes. Cancer Sci. 2010;101:2538. doi: 10.1111/j.1349-7006.2010.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim TH, Barrera LO, Zheng M, Qu C, Singer MA, Richmond TA, Wu Y, Green RD, Ren B. A high-resolution map of active promoters in the human genome. Nature. 2005;436:876. doi: 10.1038/nature03877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koyanagi KO, Hagiwara M, Itoh T, Gojobori T, Imanishi T. Comparative genomics of bidirectional gene pairs and its implications for the evolution of a transcriptional regulation system. Gene. 2005;353:169. doi: 10.1016/j.gene.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Li YY, Yu H, Guo ZM, Guo TQ, Tu K, Li YX. Systematic analysis of head-to-head gene organization: evolutionary conservation and potential biological relevance. PLoS Comput Biol. 2006;2:e74. doi: 10.1371/journal.pcbi.0020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin JM, Collins PJ, Trinklein ND, Fu Y, Xi H, Myers RM, Weng Z. Transcription factor binding and modified histones in human bidirectional promoters. Genome Res. 2007;17:818. doi: 10.1101/gr.5623407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myslinski E, Gerard MA, Krol A, Carbon P. A genome scale location analysis of human Staf/ZNF143-binding sites suggests a widespread role for human Staf/ZNF143 in mammalian promoters. J Biol Chem. 2006;281:39953. doi: 10.1074/jbc.M608507200. [DOI] [PubMed] [Google Scholar]
- 25.Seila AC, Calabrese JM, Levine SS, Yeo GW, Rahl PB, Flynn RA, Young RA, Sharp PA. Divergent transcription from active promoters. Science. 2008;322:1849. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seila AC, Core LJ, Lis JT, Sharp PA. Divergent transcription: a new feature of active promoters. Cell Cycle. 2009;8:2557. doi: 10.4161/cc.8.16.9305. [DOI] [PubMed] [Google Scholar]
- 27.Trinklein ND, Aldred SF, Hartman SJ, Schroeder DI, Otillar RP, Myers RM. An abundance of bidirectional promoters in the human genome. Genome Res. 2004;14:62. doi: 10.1101/gr.1982804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei W, Pelechano V, Jarvelin AI, Steinmetz LM. Functional consequences of bidirectional promoters. Trends Genet. 2011;27:267. doi: 10.1016/j.tig.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, Lander ES, Kellis M. Systematic discovery of regulatory motifs in human promoters and 3’ UTRs by comparison of several mammals. Nature. 2005;434:338. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu CF, Brown MA, Nicolai H, Chambers JA, Griffiths BL, Solomon E. Isolation and characterisation of the NBR2 gene which lies head to head with the human BRCA1 gene. Hum Mol Genet. 1997;6:1057. doi: 10.1093/hmg/6.7.1057. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Huo D, Chen Y, Nwachukwu C, Collins C, Rowell J, Slamon DJ, Olopade OI. CpG island methylation affects accessibility of the proximal BRCA1 promoter to transcription factors. Breast Cancer Res Treat. 2010;120:593. doi: 10.1007/s10549-009-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang MQ, Elnitski LL. A computational study of bidirectional promoters in the human genome. Lect Notes Comput Sci. 2007;4463:361. [Google Scholar]
- 33.Yang MQ, Elnitski LL. Diversity of core promoter elements comprising human bidirectional promoters. BMC Genomics. 2008;9(Suppl 2):S3. doi: 10.1186/1471-2164-9-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokomori N, Tawata M, Saito T, Shimura H, Onaya T. Regulation of the rat thyrotropin receptor gene by the methylation-sensitive transcription factor GA-binding protein. Mol Endocrinol. 1998;12:1241. doi: 10.1210/mend.12.8.0142. [DOI] [PubMed] [Google Scholar]
- 35.Zeeberg BR, Feng W, Wang G, Wang MD, Fojo AT, Sunshine M, Narasimhan S, Kane DW, Reinhold WC, Lababidi S, Bussey KJ, Riss J, Barrett JC, Weinstein JN. GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biol. 2003;4:R28. doi: 10.1186/gb-2003-4-4-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]


