Abstract
Objective
To examine the associations between dietary fat intake and ischemic stroke among postmenopausal women.
Methods
We conducted a prospective cohort study of 87,025 generally healthy postmenopausal women (age 50–79 years) enrolled in the Women’s Health Initiative Observational Study. Repeated and validated dietary assessments were done using a self-administered food frequency questionnaire. We used Cox proportional hazards models to estimate hazard ratios (HR) of ischemic stroke based on quintiles of the cumulative average of fat intake.
Results
We documented 1,049 incident cases of ischemic stroke over 663,041 person-years of follow-up. Women in the highest quintile of trans fat intake had a significantly higher incidence of ischemic stroke (HR 1.39, 95% confidence interval [CI] 1.08–1.79, P-trend = 0.048) compared with women in the lowest quintile, while controlling for multiple covariates. The observed association was modified by aspirin use (P-interaction=0.02). The HR was 1.66 (95% CI 1.21–2.36, P-trend<0.01) among baseline non-aspirin users (n=67,288) and 0.95 (95% CI 0.60–1.48, P-trend=0.43) among aspirin users (n=19,736). No significant associations were found between intakes of saturated, monounsaturated, or polyunsaturated fat and ischemic stroke or any ischemic stroke subtypes.
Interpretation
In this large cohort of postmenopausal women, higher intake of trans fat was associated with incident ischemic stroke independent of major lifestyle/dietary factors. Aspirin use may attenuate the potential adverse effect of trans fat intake on ischemic stroke.
INTRODUCTION
Trans fat intake is thought to increase incidence of cardiovascular disease through its adverse effect on serum cholesterol, inflammation and endothelial function.1 Despite this evidence, two large prospective cohort studies of ischemic stroke among healthcare professionals did not find evidence of a positive association.2, 3 The associations between other dietary fats and ischemic stroke are also inconsistent with those of coronary heart disease (CHD) even though it is generally thought that both ischemic stroke and CHD are atherosclerotic disorders.4 Contrary to expectation, epidemiologic studies have suggested that intake of saturated and animal fat intake may be inversely associated with ischemic stroke.5, 6 Data on monounsaturated and polyunsaturated fats and ischemic stroke are sparse.
Previous studies of dietary fat and ischemic stroke have included less than 500 cases of ischemic stroke, limiting statistical power.4 Exploring the interactions between dietary fat intake and aspirin use and other variables that reduce risk of ischemic stroke through their antiplatelet, cholesterol-lowering, or anti-inflammatory properties may shed light on the mechanism of ischemic stroke development, especially since there is evidence of sex differences in platelet reactivity and platelet response to aspirin.7 Finally, there have been no large-scale longitudinal studies of the association between types of fat intake and ischemic stroke among postmenopausal women who have elevated risk of stroke.
We analyzed data from the Women’s Health Initiative Observational Study (WHI-OS), a large cohort of postmenopausal US women, to prospectively examine intakes of total and specific types of fat in relation to incidence of ischemic stroke. We also examined the possible effect modification of aspirin use since both fatty acids and aspirin may influence risk of ischemic stroke through inflammatory and platelet aggregation mechanisms.
METHODS
Study Population
Full details of the WHI-OS are available at <http://whiscience.org/about/>. Between 1994 and 1998, the WHI-OS recruited women age 50 to 79 years from areas surrounding 40 clinical centers in 24 states and the District of Columbia and enrolled 93,676 women. We excluded women who reported having had a stroke (n=1,415), transient ischemic attack (n=2,250), or reported implausible daily energy intake (<600 or >5,000 kcal/d, n=3,571) at baseline, leaving 87,025 women in this analysis. All participants provided written informed consent approved by the Institutional Review Board of each participating center.
Dietary assessment
The WHI used a self-administered food frequency questionnaire (FFQ) to assess diet at enrollment and at a follow-up visit 3 years later.8 The FFQ asked about usual frequency of intake and portion size for 122 foods and food groups over the previous 3-month period, including questions about fats in meat and dairy, fats used in cooking, added fats, and reduced-fat foods. The nutrient database was derived from the University of Minnesota Nutrition Coding Center nutrient database (2005 version, Nutrition Coordinating Center, Minneapolis, MN).
The measurement properties of the WHI FFQ were evaluated in a sub-cohort of the WHI and were found to be similar to other FFQs in studies of older women.8 The Pearson correlation coefficient between the FFQ and 8 days of dietary recalls and food records were 0.64 for total fat, 0.63 for saturated fat, 0.64 for monounsaturated fat, and 0.54 for polyunsaturated fat. The correlation coefficient for trans fat was not reported in the evaluation study. However, in the WHI Dietary Modification Clinical Trial, a trend was observed toward CHD reduction among the intervention group who reached the lowest levels of trans fat and saturated fat intakes, as measured by the WHI FFQ.9 These results coupled with the validation study demonstrate that the WHI FFQ dietary trans fat intake assessment is appropriate and can successfully predict health endpoints including ischemic stroke by ranking participants according to intake.
Ascertainment of ischemic stroke outcome
Incident ischemic strokes during the follow-up period were identified through self-report during annual medical history updates through 2005. The annual response rate was 94%.10 The potential outcomes were adjudicated locally by physicians and centrally by trained neurologists using additional details from medical charts, brain imaging, or death certificates. Over 95% of WHI-OS stroke cases were classified based on brain imaging.11 Central adjudicators further classified ischemic strokes by subtypes according to the Trial of ORG 10172 Acute Stroke Trial (TOAST) Classification, based on the presumed underlying stroke etiology.12 Transient ischemic attack, hemorrhagic stroke, stroke not centrally adjudicated, strokes not requiring hospitalization, and strokes not confirmed by central adjudication were not included as a stroke outcome.
Measurement of covariates
Participants completed physical measurements, a medication/supplement inventory, and questionnaires during baseline visits to a WHI Clinical Center. Blood pressure was measured by certified staff using standardized procedures after a 5-minute seated rest. The average of two blood pressure measurements taken with a 30-second rest in between was used in analysis. Height and weight were measured without shoes or heavy clothing and pocket contents removed. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. Participants who reported taking antihypertensive or diuretic medications were classified as antihypertensive medication users. Those who reported having high cholesterol requiring pills or taking antihyperlipidemic medication were classified as cholesterol-lowering medication users. Participants taking medications that contained aspirin as an ingredient for at least 14 days were classified as aspirin users. History of CHD was defined as having self-reported a myocardial infarction, coronary artery bypass graft, or percutaneous coronary intervention at baseline. Physical activity was assessed using information about the frequency, intensity, and duration of walking, exercise, or recreational activity and summarized into total metabolic equivalent task (MET)-hours per week.
Statistical Methods
Statistical analyses were performed using SAS software, version 9.1 (SAS Institute Inc, Cary, NC). Follow-up time was calculated in months from the date of enrollment to the date of first stroke, death, or end of the follow up, whichever came first. According to the WHI protocol, women were censored at the time of their first stroke, even if the stroke was non-ischemic.
We calculated the cumulative average of intakes for total and types of fat for each participant. If the participant was followed for the outcome past her dietary update 3 years after baseline, then we used the average of the baseline and dietary update information. Otherwise, we used the baseline dietary information only. Participants were divided into quintiles based on the cumulative average of intakes of total fat or types of fat. For descriptive statistical analysis, we examined the distribution of important covariates according to quintiles of trans fat intake, the dietary fat which has been shown to have a potent effect on increasing risk of cardiovascular disease.1
We used Cox proportional hazard models to estimate the hazard ratios (HR) and 95% confidence intervals (CI) of incident ischemic stroke for each quintile of fat intake. Because dietary fat intake and total energy intake are often highly correlated, we adjusted multivariable models using the residual regression method to obtain a measure of nutrient intake uncorrelated with total energy intake.13 Tests for trend were performed by assigning the median value of each quintile of dietary fat intake to each category and entering this variable into the model as a continuous variable. In addition, we tested for effect modification by including a multiplicative interaction term between quintiles of dietary fat intake and the modifying variable.
RESULTS
This analysis included 87,025 postmenopausal women (mean age 63.5y, standard deviation 7.3y) who had no history of stroke or TIA at baseline with an average 7.6 years of follow-up. Low socioeconomic status was associated with greater trans fat intake (Table 1). Women in the highest quintile of trans fat intake were more likely to be current smokers, have diabetes, and physically inactive, compared to those in the lower quintiles of trans fat intake. Total energy intake was higher and vitamin E intake was lower in women in the higher quintiles of trans fat intake.
Table 1.
Baseline characteristics of the study population according to quintiles of trans fat intake, WHI-OS,* 1993 to 2005†
| Characteristic | Overall (n=87,025) | Quintiles of trans fat intake
|
P-value‡ | ||||
|---|---|---|---|---|---|---|---|
| 1 (n=17,405) | 2 (n=17,405) | 3 (n=17,405) | 4 (n=17,405) | 5 (n=17,405) | |||
| Age (y) | 63.5 (7.3) | 62.9 (7.3) | 63.3 (7.4) | 63.6 (7.3) | 63.8 (7.3) | 63.8 (7.4) | <0.01 |
| Ethnicity (%) | |||||||
| White | 84.5 | 87.9 | 86.9 | 84.1 | 81.5 | 82.2 | <0.01 |
| African-American | 7.4 | 3.6 | 4.6 | 6.3 | 9.7 | 12.7 | |
| Hispanic | 3.6 | 3.4 | 3.3 | 4.4 | 4.3 | 2.6 | |
| Other | 4.5 | 5.2 | 5.3 | 5.2 | 4.5 | 2.6 | |
| Education (%) | |||||||
| Did not complete high school | 4.7 | 2.3 | 2.9 | 4.4 | 6.4 | 7.6 | <0.01 |
| High school/GED | 16.0 | 8.5 | 11.9 | 15.3 | 19.2 | 25.1 | |
| Some training after high school | 36.3 | 30.2 | 34.3 | 38.2 | 39.6 | 39.3 | |
| College graduate | 43.0 | 59.0 | 50.9 | 42.1 | 34.8 | 28.0 | |
| Family income (%) | |||||||
| <$20,000 | 15.1 | 9.8 | 12.1 | 14.4 | 17.7 | 21.6 | <0.01 |
| $20,000 to <$35,000 | 23.2 | 18.3 | 20.1 | 23.1 | 25.8 | 28.7 | |
| $35,000 to <$50,000 | 20.3 | 19.1 | 20.0 | 20.6 | 21.0 | 20.9 | |
| $50,000 to <$75,000 | 20.5 | 23.0 | 22.3 | 20.9 | 19.5 | 16.9 | |
| $75,000 or more | 20.9 | 30.0 | 25.6 | 21.1 | 16.1 | 11.9 | |
| Years as a regular smoker (%) | |||||||
| Never | 51.7 | 49.6 | 50.4 | 52.1 | 53.3 | 53.1 | <0.01 |
| <10 | 11.9 | 14.3 | 12.8 | 12.1 | 10.9 | 9.6 | |
| 10 to <20 | 10.7 | 12.6 | 12.0 | 10.6 | 9.6 | 8.7 | |
| 20 to <30 | 10.6 | 11.5 | 11.1 | 10.5 | 10.0 | 9.7 | |
| 30 or more | 15.2 | 12.0 | 13.8 | 14.8 | 16.3 | 18.9 | |
| HRT use (%) | |||||||
| Never | 40.1 | 38.9 | 39.1 | 39.1 | 40.8 | 42.9 | <0.01 |
| Former | 14.8 | 14.3 | 14.2 | 14.7 | 15.1 | 15.6 | |
| Current | 45.1 | 46.9 | 46.8 | 46.2 | 44.1 | 41.5 | |
| Physical activity (MET-hrs/wk) | 13.9 (14.4) | 19.0 (16.4) | 16.0 (14.9) | 13.7 (13.9) | 11.2 (12.5) | 9.4 (11.7) | <0.01 |
| Alcohol intake (g/d) | 5.5 (10.3) | 9.0 (14.7) | 6.4 (10.3) | 4.9 (8.6) | 3.9 (7.8) | 3.5 (7.4) | <0.01 |
| Coronary heart disease ever (%) | 3.0 | 2.7 | 2.6 | 3.0 | 3.4 | 3.5 | <0.01 |
| Atrial fibrillation ever (%) | 4.4 | 3.8 | 4.3 | 4.4 | 4.7 | 5.0 | <0.01 |
| Diabetes ever (%) | 5.3 | 3.2 | 4.2 | 5.3 | 6.3 | 7.4 | <0.01 |
| Current medication use (%) | |||||||
| Aspirin | 22.7 | 23.2 | 22.2 | 22.6 | 22.4 | 23.1 | 0.10 |
| Antihypertensive medication | 27.8 | 21.8 | 24.7 | 27.7 | 30.8 | 33.8 | <0.01 |
| Cholesterol- lowering medication | 14.6 | 13.7 | 14.3 | 14.9 | 15.4 | 14.7 | <0.01 |
| Statins | 7.8 | 7.1 | 7.6 | 8.0 | 8.6 | 8.0 | <0.01 |
| Body mass index (kg/m2) | 27.2 (5.8) | 26.1 (5.3) | 26.6 (5.4) | 27.1 (5.7) | 27.7 (6.0) | 28.5 (6.4) | <0.01 |
| Systolic blood pressure (mm Hg) | 126.7 (17.8) | 124.9 (17.8) | 125.6 (17.6) | 126.6 (17.8) | 127.5 (17.8) | 128.7 (17.8) | <0.01 |
| Diastolic blood pressure (mm Hg) | 74.7 (9.3) | 74.2 (9.2) | 74.5 (9.1) | 74.7 (9.3) | 74.8 (9.3) | 75.4 (9.4) | <0.01 |
| Hypertension (%) | 23.7 | 21.0 | 22.0 | 23.6 | 25.0 | 26.9 | <0.01 |
| Keys score§ | 34.6 (9.1) | 29.6 (8.1) | 32.6 (8.4) | 35.0 (8.7) | 37.4 (8.9) | 38.4 (8.3) | <0.01 |
| Total energy (kcal/d) | 1540.1 (549.3) | 1820.0 (499.7) | 1478.3 (443.7) | 1360.2 (474.8) | 1368.8 (518.7) | 1673.3 (637.2) | <0.01 |
| Fruits and vegetables (medium servings/d) | 4.4 (2.0) | 6.1 (2.1) | 4.8 (1.7) | 4.0 (1.6) | 3.4 (1.6) | 3.3 (1.7) | <0.01 |
| Dietary fiber (g/d) | 16.2 (6.4) | 21.9 (6.5) | 16.9 (5.1) | 14.6 (5.1) | 13.3 (5.3) | 14.2 (5.8) | <0.01 |
| Dietary vitamin E (IU/d) | 9.2 (5.2) | 11.1 (5.6) | 9.1 (5.1) | 8.2 (4.7) | 8.0 (4.7) | 9.4 (5.0) | <0.01 |
Abbreviations: WHI, Women’s Health Initiative; GED, General Equivalency Diploma; HRT, hormone replacement therapy; MET, metabolic equivalent tasks.
Data are mean (standard deviation), unless otherwise specified. Data are unadjusted.
P-values are for any difference across the quintiles, obtained by analysis of variance or Chi-square test as appropriate.
Keys Score=1.26 (2S-P)+1.5(square root(C)); S=percent of total energy from saturated fat; P=percent of total energy from polyunsaturated fat; C=daily cholesterol intake in mg/1000 kcal
Over a follow-up of 663,041 person-years, 1,049 incident cases of ischemic stroke were documented. With minimal adjustment, there was a positive relation between trans fat intake and incidence of ischemic stroke (Table 2, fifth vs. the first quintile HR 1.49, 95% CI 1.22–1.82, P for trend = 0.0002). The association became slightly attenuated after adjustment for clinical, lifestyle, and dietary factors, but remained significant. Women in the highest quintile of trans fat intake had a 39% greater incidence of ischemic stroke than women in the lowest quintile of trans fat intake (HR 1.39, 95% CI 1.08–1.79, P for trend = 0.048). We evaluated trans isomers of palmitolaidic acid (16:1), elaidic acid (18:1), and linolaidic acid (18:2) in separate models. All three were positively associated with incidence of ischemic stroke (P for trend = 0.04, 0.09, and 0.06, respectively). No significant associations were evident for total fat, other types of fat, dietary cholesterol, or Keys score.
Table 2.
Multivariable-adjusted hazard ratios (95% confidence intervals) for incident ischemic stroke by quintiles of dietary fat intake, WHI-OS,” 1993–2005†
| Dietary fat intake | Quintiles
|
P for trend | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Total fat, median intake, g/d | 40.8 | 41.9 | 43.8 | 51.3 | 75.2 | |
| Events, n | 188 | 186 | 203 | 218 | 254 | |
| Person-time, 104 yr | 13.5 | 13.5 | 13.3 | 13.1 | 12.9 | |
| Model 1§ | 1 (ref) | 0.96 (0.78, 1.18) | 1.04 (0.85, 1.27) | 1.11 (0.91, 1.35) | 1.38 (1.14, 1.66) | 0.0002 |
| Model 2|| | 1 (ref) | 0.88 (0.70, 1.10) | 0.91 (0.73, 1.14) | 0.97 (0.78, 1.22) | 1.16 (0.94, 1.44) | 0.07 |
| Model 3¶ | 1 (ref) | 0.90 (0.71, 1.13) | 0.93 (0.73, 1.19) | 1.00 (0.78, 1.28) | 1.20 (0.92, 1.57) | 0.09 |
| TFA, median intake, g/d | 2.2 | 2.3 | 2.6 | 3.4 | 6.1 | |
| Events, n | 154 | 197 | 224 | 223 | 251 | |
| Person-time, 104 yr | 13.4 | 13.4 | 13.3 | 13.2 | 13.1 | |
| Model 1 | 1 (ref) | 1.22 (0.99, 1.51) | 1.37 (1.11, 1.68) | 1.33 (1.09, 1.64) | 1.49 (1.22, 1.82) | 0.0002 |
| Model 2 | 1 (ref) | 1.23 (0.98, 1.56) | 1.30 (1.03, 1.65) | 1.16 (0.91, 1.48) | 1.38 (1.10, 1.74) | 0.02 |
| Model 3 | 1 (ref) | 1.26 (0.99, 1.61) | 1.33 (1.04, 1.71) | 1.17 (0.90, 1.51) | 1.39 (1.08, 1.79) | 0.048 |
| SFA, median intake, g/d | 12.9 | 13.4 | 14.5 | 17.0 | 26.1 | |
| Events, n | 185 | 194 | 211 | 219 | 240 | |
| Person-time, 104 yr | 13.5 | 13.4 | 13.3 | 13.1 | 12.9 | |
| Model 1 | 1 (ref) | 1.04 (0.85, 1.27) | 1.12 (0.92, 1.36) | 1.16 (0.95, 1.41) | 1.32 (1.09, 1.60) | 0.002 |
| Model 2 | 1 (ref) | 0.98 (0.78, 1.22) | 0.96 (0.77, 1.20) | 1.04 (0.84, 1.30) | 1.18 (0.95, 1.46) | 0.08 |
| Model 3 | 1 (ref) | 0.99 (0.78, 1.25) | 0.96 (0.75, 1.23) | 1.04 (0.81, 1.34) | 1.16 (0.90, 1.51) | 0.18 |
| PFA, median intake, g/d | 8.4 | 8.4 | 9.0 | 10.6 | 16.0 | |
| Events, n | 197 | 173 | 200 | 240 | 139 | |
| Person-time, 104 yr | 13.5 | 13.4 | 13.3 | 13.2 | 13.0 | |
| Model 1 | 1 (ref) | 0.85 (0.69, 1.04) | 0.98 (0.80, 1.19) | 1.15 (0.95, 1.39) | 1.18 (0.98, 1.43) | 0.005 |
| Model 2 | 1 (ref) | 0.80 (0.64, 1.00) | 0.92 (0.74, 1.15) | 1.03 (0.83, 1.27) | 1.02 (0.82, 1.26) | 0.27 |
| Model 3 | 1 (ref) | 0.81 (0.65, 1.02) | 0.94 (0.75, 1.18) | 1.04 (0.83, 1.31) | 1.05 (0.83, 1.33) | 0.22 |
| MFA, median intake, g/d | 14.9 | 15.6 | 16.7 | 19.8 | 28.9 | |
| Events, n | 191 | 192 | 195 | 228 | 243 | |
| Person-time, 104 yr | 13.6 | 13.4 | 13.3 | 13.1 | 12.9 | |
| Model 1 | 1 (ref) | 0.98 (0.80, 1.20) | 0.98 (0.80, 1.20) | 1.15 (0.95, 1.39) | 1.31 (1.08, 1.58) | 0.001 |
| Model 2 | 1 (ref) | 0.89 (0.71, 1.11) | 0.88 (0.70, 1.09) | 1.05 (0.84, 1.30) | 1.11 (0.89, 1.37) | 0.13 |
| Model 3 | 1 (ref) | 0.91 (0.72, 1.14) | 0.88 (0.69, 1.12) | 1.06 (0.83, 1.35) | 1.13 (0.87, 1.47) | 0.17 |
| Cholesterol, median intake, mg/d | 133.2 | 143.9 | 162.8 | 203.1 | 311.1 | |
| Events, n | 175 | 244 | 216 | 198 | 216 | |
| Person-time, 104 yr | 13.5 | 13.4 | 13.3 | 13.2 | 13.0 | |
| Model 1 | 1 (ref) | 1.39 (1.14, 1.69) | 1.25 (1.02, 1.52) | 1.17 (0.95, 1.43) | 1.35 (1.11, 1.65) | 0.05 |
| Model 2 | 1 (ref) | 1.33 (1.07, 1.64) | 1.18 (0.94, 1.48) | 1.09 (0.87, 1.36) | 1.16 (0.93, 1.46) | 0.72 |
| Model 3 | 1 (ref) | 1.32 (1.06, 1.65) | 1.17 (0.93, 1.47) | 1.07 (0.84, 1.35) | 1.14 (0.90, 1.44) | 0.95 |
| Keys score,‡ median score | 23.5 | 29.5 | 33.9 | 38.5 | 46.1 | |
| Events, n | 201 | 194 | 200 | 229 | 225 | |
| Person-time, 104 yr | 13.5 | 13.4 | 13.3 | 13.2 | 12.9 | |
| Model 1 | 1 (ref) | 0.99 (0.82, 1.21) | 1.02 (0.84, 1.24) | 1.19 (0.98, 1.44) | 1.19 (0.98, 1.44) | 0.02 |
| Model 2 | 1 (ref) | 0.91 (0.73, 1.13) | 0.93 (0.75, 1.16) | 1.13 (0.92, 1.39) | 1.08 (0.87, 1.34) | 0.14 |
| Model 3 | 1 (ref) | 0.91 (0.73, 1.13) | 0.92 (0.74, 1.16) | 1.11 (0.89, 1.39) | 1.04 (0.81, 1.33) | 0.35 |
Abbreviations: WHI-OS, Women’s Health Initiative Observational Study; BMI, body mass index; MFA, monounsaturated fatty acids; PFA, polyunsaturated fatty acids; SFA, saturated fatty acids; TFA, trans fatty acids.
All models were constructed using Cox proportional hazards regression analysis. Tests for tend were performed using the median value of each quintile entering this variable into the model as a continuous variable.
Keys Score=1.26 (2S-P)+1.5(square root(C)); S=percent of total energy from saturated fat; P=percent of total energy from polyunsaturated fat; C=daily cholesterol intake in mg/1000 kcal
Model 1: adjusted for age and race (white, black, Hispanic, other)
Model 2: adjusted for Model 1 covariates plus education (<high school, high school diploma or GED, vocational/training school or some college or associate degree, college graduate), family income (<$20K, $20K to <$35K, $35K to <$50K, $50K to <$75K, ≥$75K), years as a regular smoker (never, <10, 10 to <20, 20 to <30, ≥30), hormone replacement therapy use (never, past, current), total MET-hours per wk, alcohol intake, history of coronary heart disease, history of atrial fibrillation, history of diabetes, aspirin use, use of antihypertensive medication, use of cholesterol-lowering medication, BMI, systolic blood pressure, and total energy intake (quntiles)
Model 3: adjusted for Model 2 covariates plus dietary vitamin E (quintiles), fruits and vegetable intake (quintiles), fiber (quintiles)
In stratified analysis, there was a 66% higher incidence of ischemic stroke for women in the highest vs. the lowest quintile of trans fat intake among non-aspirin users (n=67,288, HR 1.66, 95% CI 1.21–2.36, P for trend <0.01, Fig 1). Among aspirin users (n=19,736, 22.7%, median dose 200 mg, interquartile range 25–500 mg), the positive associations between trans fat and ischemic stroke were substantially attenuated (HR 0.95, 95% CI 0.60–1.48, P for trend = 0.43). The test for interaction was statistically significant (P for interaction = 0.02). These results were consistent for all three trans isomers: P for interaction = 0.04 for 16:1, 0.002 for 18:1, and 0.003 for 18:2. There was no evidence of interaction between trans fat intake and statin use, alcohol intake, or hormone replacement therapy use (P for interaction = 0.85, 0.60, and 0.26, respectively).
Figure 1.
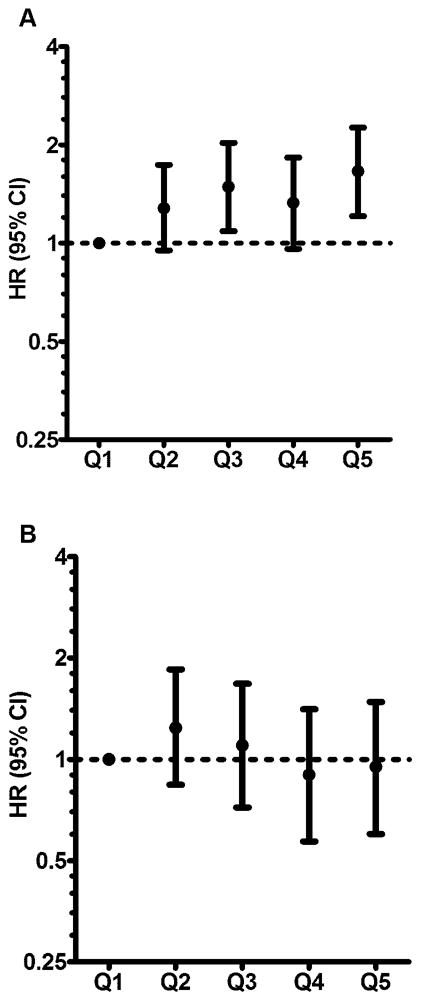
Adjusted hazard ratios (HR) and 95% confidence intervals (CI) for ischemic stroke by quintiles (Q) of trans fat intake among non-aspirin users (A) and aspirin users (B). The P-value for interaction comparing the hazard ratios with interaction present vs. hazard ratios with interaction absent = 0.02. The HRs were adjusted for covariates listed in Table 2.
The positive association between trans fat intake and ischemic stroke persisted using only baseline diet, rather than cumulative diet (highest vs. lowest quintile HR 1.28, 95% CI 1.004–1.63, P for trend = 0.14). It was robust against additional exclusions for history of CHD (highest vs. lowest quintile HR 1.42, 95% CI 1.10–1.84, P for trend = 0.04), for atrial fibrillation (HR 1.42, 95% CI 1.09–1.86, P for trend = 0.03), and for diabetes (HR 1.47, 95% CI 1.12–1.92, P for trend = 0.03). Therefore, we included individuals with these comorbidities and adjusted for them in above multivariable models. To investigate the possibility that participants changed their diet as they developed some diseases or health conditions, we stopped updating an individual’s dietary information if they reported a diagnosis of diabetes mellitus, myocardial infarction, revascularization, peripheral arterial disease, or carotid artery disease. The association was slightly attenuated, but remained significant (HR 1.37, 95% CI 1.07, 1.76, P for trend = 0.055). The positive association between trans fat intake and ischemic stroke was consistent among Caucasians only (HR 1.54, 95% CI 1.17, 2.03, P for trend = 0.02). In recent years, the definition of TIA has evolved so that some patients diagnosed with TIA may actually have ischemic stroke. When we included participants with TIAs as ischemic stroke cases, the association was attenuated, but remained positive (HR 1.11, 95% CI 0.92, 1.34, P for trend = 0.31).
Among the 1,049 incident cases of ischemic stroke, there were 101 atherotherombotic, 234 cardioembolic, and 269 lacunar infarctions; 445 were of unspecified etiology, had multiple causes, or had a negative or incomplete evaluation and were not included in subtype analysis. After adjustment for clinical, lifestyle, and dietary factors, increased trans fat intake was associated with higher risk of lacunar infarction (Table 3). However, no statistically significant associations were found between other types of fat intake and subtypes of ischemic stroke. We found no significant associations with ischemic stroke in our examination of food groups rich in fat or cholesterol after adjustment for possible confounders (Table 4).
Table 3.
Multivariable-adjusted hazard ratios (95% confidence intervals) of incident ischemic stroke and its subtypes according to increase in intakes of dietary fat, WHI-OS,* 1993–2005†
| Daily nutrient intake | Ischemic stroke type
|
|||
|---|---|---|---|---|
| Total | Atherothrombotic | Lacunar | Cardioembolic | |
| Total fat (per 30g/d) | ||||
| Model 1 | 1.12 (1.06, 1.20) | 1.03 (0.84, 1.26) | 1.07 (0.94, 1.21) | 1.20 (1.05, 1.37) |
| Model 2 | 1.05 (0.98, 1.13) | 0.89 (0.71, 1.12) | 1.00 (0.87, 1.15) | 1.14 (0.99, 1.32) |
| Model 3 | 1.06 (0.97, 1.16) | 0.85 (0.64, 1.12) | 1.04 (0.88, 1.24) | 1.16 (0.96, 1.39) |
| TFA (per 2 g/d) | ||||
| Model 1 | 1.13 (1.07, 1.20) | 1.02 (0.84, 1.24) | 1.18 (1.07, 1.31) | 1.15 (1.02, 1.29) |
| Model 2 | 1.08 (1.01, 1.15) | 0.88 (0.70, 1.11) | 1.13 (1.00, 1.27) | 1.07 (0.93, 1.22) |
| Model 3 | 1.08 (1.004, 1.16) | 0.87 (0.68, 1.13) | 1.16 (1.02, 1.32) | 1.04 (0.89, 1.21) |
| SFA (per 10 g/d) | ||||
| Model 1 | 1.10 (1.03, 1.17) | 0.97 (0.79, 1.18) | 1.04 (0.92, 1.17) | 1.16 (1.03, 1.32) |
| Model 2 | 1.05 (0.98, 1.12) | 0.87 (0.69, 1.10) | 0.99 (0.86, 1.14) | 1.11 (0.97, 1.28) |
| Model 3 | 1.04 (0.96, 1.13) | 0.84 (0.63, 1.11) | 1.00 (0.85, 1.18) | 1.10 (0.93, 1.30) |
| PFA (per 5 g/d) | ||||
| Model 1 | 1.10 (1.04, 1.17) | 1.12 (0.93, 1.35) | 1.10 (0.97, 1.23) | 1.11 (0.98, 1.26) |
| Model 2 | 1.03 (0.97, 1.11) | 0.98 (0.79, 1.22) | 1.02 (0.90, 1.16) | 1.05 (0.91, 1.20) |
| Model 3 | 1.05 (0.97, 1.13) | 0.95 (0.75, 1.21) | 1.08 (0.94, 1.25) | 1.04 (0.90, 1.22) |
| MFA (per 10 g/d) | ||||
| Model 1 | 1.11 (1.04, 1.18) | 1.03 (0.84, 1.26) | 1.05 (0.93, 1.19) | 1.20 (1.05, 1.37) |
| Model 2 | 1.04 (0.97, 1.12) | 0.90 (0.72, 1.14) | 0.99 (0.86, 1.14) | 1.15 (0.99, 1.33) |
| Model 3 | 1.05 (0.96, 1.14) | 0.86 (0.65, 1.14) | 1.03 (0.87, 1.22) | 1.17 (0.98, 1.40) |
| Cholesterol (per 100 mg/d) | ||||
| Model 1 | 1.04 (0.98, 1.10) | 0.95 (0.76, 1.18) | 0.98 (0.87, 1.12) | 1.10 (0.98, 1.24) |
| Model 2 | 0.99 (0.92, 1.06) | 0.85 (0.66, 1.09) | 0.92 (0.80, 1.07) | 1.06 (0.93, 1.21) |
| Model 3 | 0.98 (0.91, 1.05) | 0.84 (0.64, 1.09) | 0.92 (0.79, 1.07) | 1.05 (0.91, 1.20) |
| Keys Score (per 10 points) | ||||
| Model 1 | 1.08 (1.02, 1.15) | 0.94 (0.77, 1.14) | 1.02 (0.90, 1.15) | 1.15 (1.02, 1.30) |
| Model 2 | 1.05 (0.98, 1.12) | 0.87 (0.70, 1.09) | 0.98 (0.85, 1.12) | 1.13 (0.98, 1.31) |
| Model 3 | 1.03 (0.95, 1.12) | 0.87 (0.67, 1.13) | 0.95 (0.81, 1.12) | 1.12 (0.94, 1.33) |
Abbreviations: WHI-OS, Women’s Health Initiative Observational Study; BMI, body mass index; MFA, monounsaturated fatty acids; PFA, polyunsaturated fatty acids; SFA, saturated fatty acids; TFA, trans fatty acids.
All models were constructed using Cox proportional hazards regression analysis. Effect estimates were adjusted for covariates listed in Table 2.
Table 4.
Multivariable-adjusted hazard ratios (95% confidence intervals) of incident ischemic stroke according to intakes of red meat, beef, dairy, nuts, and eggs, WHI-OS,* 1993–2005†
| Daily nutrient intake (per 1 medium servings/d) | Ischemic stroke type
|
|||
|---|---|---|---|---|
| Total | Atherothrombotic | Lacunar | Cardioembolic | |
| Red meat | ||||
| Model 1 | 1.22 (1.08, 1.37) | 1.17 (0.78, 1.77) | 1.17 (0.92, 1.49) | 1.49 (1.18, 1.89) |
| Model 2 | 1.14 (0.97, 1.34) | 1.20 (0.73, 1.98) | 1.01 (0.73, 1.41) | 1.25 (0.90, 1.74) |
| Model 3 | 1.13 (0.95, 1.34) | 1.26 (0.75, 2.11) | 1.03 (0.74, 1.44) | 1.20 (0.85, 1.70) |
| Beef, pork, or lamb as a main dish | ||||
| Model 1 | 1.07 (0.75, 1.54) | 2.21 (0.84, 5.81) | 0.93 (0.45, 1.92) | 1.46 (0.71, 2.98) |
| Model 2 | 0.99 (0.65, 1.50) | 2.49 (0.86, 7.15) | 0.73 (0.31, 1.72) | 1.11 (0.47, 2.61) |
| Model 3 | 0.97 (0.63, 1.48) | 2.68 (0.93, 7.74) | 0.74 (0.31, 1.77) | 1.03 (0.43, 2.48) |
| Dairy | ||||
| Model 1 | 0.95 (0.90, 1.00) | 0.96 (0.81, 1.13) | 0.97 (0.88, 1.08) | 1.00 (0.90, 1.11) |
| Model 2 | 0.94 (0.88, 1.00) | 0.98 (0.79, 1.21) | 0.94 (0.82, 1.07) | 0.92 (0.80, 1.06) |
| Model 3 | 0.94 (0.87, 1.00) | 1.00 (0.81, 1.24) | 0.92 (0.81, 1.05) | 0.91 (0.79, 1.06) |
| Nuts | ||||
| Model 1 | 0.86 (0.67, 1.11) | 0.73 (0.31, 1.73) | 0.77 (0.46, 1.30) | 0.90 (0.53, 1.52) |
| Model 2 | 0.85 (0.64, 1.14) | 0.89 (0.35, 2.24) | 0.79 (0.44, 1.42) | 0.78 (0.41, 1.45) |
| Model 3 | 0.89 (0.66, 1.20) | 0.81 (0.31, 2.13) | 0.88 (0.48, 1.58) | 0.81 (0.42, 1.54) |
| Eggs | ||||
| Model 1 | 1.04 (0.72, 1.51) | 0.25 (0.05, 1.33) | 1.03 (0.49, 2.15) | 1.71 (0.89, 3.27) |
| Model 2 | 0.86 (0.55, 1.33) | 0.16 (0.02, 0.98) | 0.86 (0.37, 2.03) | 1.22 (0.55, 2.71) |
| Model 3 | 0.86 (0.55, 1.33) | 0.15 (0.02, 0.95) | 0.89 (0.38, 2.10) | 1.20 (0.53, 2.71) |
Abbreviations: WHI-OS, Women’s Health Initiative Observational Study.
All models were constructed using Cox proportional hazards regression analysis. Effect estimates were adjusted for covariates listed in Table 2.
DISCUSSION
In the largest cohort study of strokes among postmenopausal women, we observed a positive association between trans fat intake and incidence of ischemic stroke that persisted after adjustment for major lifestyle, cardiovascular, and dietary factors. This association was substantially attenuated among women using aspirin. The WHI FFQ dietary assessment, designed specifically to measure fat intake, was repeated and validated. In addition, stroke was ascertained rigorously in the WHI.
Two prospective studies of male and female healthcare professionals, the Health Professionals Follow-up Study and the Nurses’ Health Study with 455 and 385 cases of ischemic stroke, respectively, did not find significant associations for total fat, cholesterol, or specific types of fat, including trans fat, and ischemic stroke after adjustment for potential confounders and major lifestyle variables.2, 3
Our unique study base of older women may have contributed to our ability to detect the association between trans fat intake and ischemic stroke among non-users of aspirin. In a trial of platelet reactivity, women’s platelets were more reactive than men’s to most agonists and platelet response after taking 81-mg/d aspirin decreased the same or more in women than men.14 A sex-specific meta-analysis of randomized trials found that aspirin therapy (75–500 mg/d) reduced risk of cardiovascular disease by 12% mainly due to a 24% reduction in risk of ischemic stroke among women but not men.7 Increased platelet response to an agonist and inhibition after aspirin intake may at least in part explain the attenuated association between trans fat intake and ischemic stroke among aspirin users in this cohort.
Platelet aggregability increases with advancing age, possibly more so among women than men.15 A case-control study of serum saturated fatty acids and ischemic stroke reported a positive association among middle-aged men, but not younger men, suggesting that these associations may vary by age.16 The older age range of women in WHI-OS could explain why the association between trans fat intake and ischemic stroke was found in the older, but not the younger Nurses’ Health Study, cohort.
In addition to its function as an anti-platelet, aspirin reduces inflammation by blocking prostaglandin synthesis at higher doses. While less than 1% of WHI-OS aspirin users reported taking high-dose (≥4 g/d) aspirin, there is evidence that lower doses of aspirin are also anti-inflammatory. The Physicians’ Health Study reported that the risk reduction of myocardial infarction decreased in a linear fashion across quintiles of CRP in the 325-mg aspirin treatment group, suggesting that low-dose aspirin may have anti-inflammatory effects.17 Also, aspirin use has been linked with attenuated associations in studies of trans fat intake and inflammatory diseases, such as colorectal and prostate cancer.18, 19 Ischemic stroke prevention guidelines recommend that aspirin be used among women with sufficient risk of stroke to outweigh possible harm due to bleeding.20
Women with higher trans fat intake had elevated incidence of lacunar and cardioembolic, but not atherothrombotic infarction, the subtype of ischemic stroke that has been associated with elevated total cholesterol and decreased HDL-C.21 In addition, we found no statistically significant associations between other types of fat and incidence of ischemic stroke, highlighting the uncertainty that dietary fat intake acts through dyslipidemia to cause cerebral infarction.4 While dyslipidemia is an established risk factor for CHD, accumulated evidence on the association between cholesterol and ischemic stroke revealed null or weak associations. A meta-analysis of randomized trials of nonstatin lipid lowering did not translate to decreased risk of stroke, even though the trials were successful in lowering mean cholesterol level in the treatment versus placebo groups by 6%-23%22. In addition, a meta-analysis of 450,000 individuals from 45 prospective cohorts reported that there was no association between blood cholesterol and stroke after standardizing by age.23 However, recent trials of cholesterol-lowering using statin drugs suggested that incidence of ischemic stroke may be reduced, but these effects are likely independent of reduction in serum total cholesterol.24, 25 Statins are thought to have diverse beneficial effects on vascular risk factors that extend beyond its cholesterol-lowering properties.26 Despite the clear benefit of statin therapy in high-risk individuals, there is limited evidence showing the benefit of statin therapy among low-risk individuals for the primary prevention of cardiovascular disease.27 In our study, statistical power for comparisons by statin use was lower than for aspirin use. It could have limited our capability of examining statin’s effect modification.
To our knowledge, only one prospective study has reported significant associations between types of fat and stroke subtypes.28 Iso et al. reported that serum saturated fatty acids were positively associated with incidence of ischemic stroke, while serum linoleic acid was associated with decreased incidence of ischemic stroke. These associations were largely driven by lacunar stroke, this subtype comprising 95 of the 122 ischemic strokes. Investigators did not examine serum trans fat and acknowledged the uncertainty of the associations for other ischemic stroke subtypes. Further exploration is needed on the association between types of fat and ischemic stroke subtypes.
The WHI FFQ, which was designed to emphasize measurement of fatty acid intakes because it was a key measure in the WHI Dietary Modification Trial, is a strength of the present study. However, the WHI FFQ is subject to the same challenges as other self-reported dietary assessment methods commonly used in large-scale prospective cohort studies. The WHI FFQ performed similar to or better than others in similar populations.8 We reduced error in the dietary assessment and accounted for possible changes in diet during follow-up by using the cumulative average of two dietary measurements. The results are unlikely to be due to confounding, as the results remained consistent through several sensitivity analyses and we considered numerous dietary and lifestyle variables. However, in any observational study the possibility of confounding by unknown factors cannot be completely excluded.
The WHI-OS includes by far the largest number of ischemic strokes studied to date with respect to dietary fat intake with a long follow-up of an average of 8 years. Importantly, the ischemic stroke and subtype classifications in the WHI were centrally adjudicated, reducing the likelihood of misclassification. One concern of the study is the statistical power for the atherothrombotic infarction, the least prevalent ischemic stroke subtype in the US. However, the number of atherothrombotic infarction cases in this study exceeds the number of total ischemic stroke cases in many previous studies.
In summary, a significant and positive association between trans fat intake and ischemic stroke was present among non-users of aspirin and absent among users of aspirin. Our results highlight the importance of limiting the amount of dietary trans fat intake and using aspirin for primary ischemic stroke prevention among women, specifically postmenopausal women who have elevated risk of ischemic stroke.
Acknowledgments
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles Kooperberg; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Haleh Sangi-Haghpeykar; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence S. Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Erin LeBlanc; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael S. Simon.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. This study was supported by a grant (R21NS056445) from the National Institute of Neurological Disorders and Stroke. Ms. Yaemsiri was supported by the American Heart Association Mid-Atlantic Predoctoral Fellowship.
References
- 1.Mozaffarian D, Aro A, Willett WC. Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr. 2009;63 (Suppl 2):S5–21. doi: 10.1038/sj.ejcn.1602973. [DOI] [PubMed] [Google Scholar]
- 2.He K, Merchant A, Rimm EB, et al. Dietary fat intake and risk of stroke in male US healthcare professionals: 14 year prospective cohort study. BMJ. 2003;327:777–782. doi: 10.1136/bmj.327.7418.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iso H, Stampfer MJ, Manson JE, et al. Prospective study of fat and protein intake and risk of intraparenchymal hemorrhage in women. Circulation. 2001;103:856–863. doi: 10.1161/01.cir.103.6.856. [DOI] [PubMed] [Google Scholar]
- 4.He K, Xu Y, Van Horn L. The puzzle of dietary fat intake and risk of ischemic stroke: a brief review of epidemiologic data. J Am Diet Assoc. 2007;107:287–295. doi: 10.1016/j.jada.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Renaud SC. Diet and stroke. J Nutr Health Aging. 2001;5:167–172. [PubMed] [Google Scholar]
- 6.Sauvaget C, Nagano J, Hayashi M, Yamada M. Animal protein, animal fat, and cholesterol intakes and risk of cerebral infarction mortality in the adult health study. Stroke. 2004;35:1531–1537. doi: 10.1161/01.STR.0000130426.52064.09. [DOI] [PubMed] [Google Scholar]
- 7.Berger JS, Roncaglioni MC, Avanzini F, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 8.Patterson RE, Kristal AR, Tinker LF, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 9.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 10.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 11.Chen JC, Brunner RL, Ren H, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008;39:3185–3192. doi: 10.1161/STROKEAHA.108.521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein LB, Jones MR, Matchar DB, et al. Improving the reliability of stroke subgroup classification using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria. Stroke. 2001;32:1091–1098. doi: 10.1161/01.str.32.5.1091. [DOI] [PubMed] [Google Scholar]
- 13.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 14.Becker DM, Segal J, Vaidya D, et al. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA. 2006;295:1420–1427. doi: 10.1001/jama.295.12.1420. [DOI] [PubMed] [Google Scholar]
- 15.Meade TW, Vickers MV, Thompson SG, et al. Epidemiological characteristics of platelet aggregability. Br Med J (Clin Res Ed) 1985;290:428–432. doi: 10.1136/bmj.290.6466.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tilvis RS, Erkinjuntti T, Sulkava R, et al. Serum lipids and fatty acids in ischemic strokes. Am Heart J. 1987;113:615–619. doi: 10.1016/0002-8703(87)90642-9. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 18.Vinikoor LC, Schroeder JC, Millikan RC, et al. Consumption of trans-fatty acid and its association with colorectal adenomas. Am J Epidemiol. 2008;168:289–297. doi: 10.1093/aje/kwn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavarro JE, Stampfer MJ, Campos H, et al. A prospective study of trans-fatty acid levels in blood and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:95–101. doi: 10.1158/1055-9965.EPI-07-0673. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873–923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 21.Tirschwell DL, Smith NL, Heckbert SR, et al. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63:1868–1875. doi: 10.1212/01.wnl.0000144282.42222.da. [DOI] [PubMed] [Google Scholar]
- 22.Corvol JC, Bouzamondo A, Sirol M, et al. Differential effects of lipid-lowering therapies on stroke prevention: a meta-analysis of randomized trials. Arch Intern Med. 2003;163:669–676. doi: 10.1001/archinte.163.6.669. [DOI] [PubMed] [Google Scholar]
- 23.Cholesterol diastolic blood pressure, and stroke: 13, 000 strokes in 450, 000 people in 45 prospective cohorts. Prospective studies collaboration. Lancet. 1995;346:1647–1653. [PubMed] [Google Scholar]
- 24.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 25.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 26.Di Napoli P, Taccardi AA, Oliver M, De Caterina R. Statins and stroke: evidence for cholesterol-independent effects. Eur Heart J. 2002;23:1908–1921. doi: 10.1053/euhj.2002.3236. [DOI] [PubMed] [Google Scholar]
- 27.Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. :CD004816. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iso H, Sato S, Umemura U, et al. Linoleic acid, other fatty acids, and the risk of stroke. Stroke. 2002;33:2086–2093. doi: 10.1161/01.str.0000023890.25066.50. [DOI] [PubMed] [Google Scholar]


