Abstract
To determine the number of genetic factors underlying the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for alcohol dependence (AD), we conducted structural equation twin modeling for seven AD criteria, plus two summary screening questions, in 7133 personally interviewed male and female twins from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders, who reported lifetime alcohol consumption. The best-fit twin model required three genetic and two unique environmental common factors, and criterion-specific unique environmental factors. The first genetic factor was defined by high loadings for the probe question about quantity and frequency of alcohol consumption, and tolerance criterion. The second genetic factor loaded strongly on the probe question about self-recognition of alcohol-related problems and AD criteria for loss of control, desire to quit, preoccupation and activities given up. The third genetic factor had high loadings for withdrawal and continued use despite the problems criteria. Genetic factor scores derived from these three factors differentially predicted patterns of comorbidity, educational status and other historical/clinical features of AD. The DSM-IV syndrome of AD does not reflect a single dimension of genetic liability, rather, these criteria reflect three underlying dimensions that index risk for: (i) tolerance and heavy use; (ii) loss of control with alcohol associated social dysfunction and (iii) withdrawal and continued use despite problems. While tentative and in need of replication, these results, consistent with the rodent literature, were validated by examining predictions of the genetic factor scores and have implications for gene-finding efforts in AD.
Keywords: alcohol dependence, diagnostic criteria, DSM-IV, genetics
Introduction
Alcohol dependence (AD) in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) describes a complex syndrome characterized by neurobiological, cognitive/motivational and behavioral/social symptoms. Adoption1–4 and twin studies5–11 have consistently shown that genetic factors impact substantially on risk for AD. A number of efforts are now under way to identify risk genes for AD by linkage12 and genome-wide association.13–15 The conceptual basis of these studies is that the DSM-IV syndrome of AD reflects a single dimension of genetic risk. As results from a number of studies suggested that the DSM-IV AD criteria form a single coherent phenotypic factor,16–18 the structure of the genetic risk factors for AD has not been previously examined. Multiple genetic risk factors might be expected because in rodent studies of genetic influences on a wide range of alcohol-related traits suggest that genetic contributions to each are either largely distinct or only weakly correlated.19,20 In this study, using data collected from 7548 adult male and female twins from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD),21 we perform a multivariate analysis of the seven individual DSM-IV criteria for AD. Our goal is to determine the structure of the genetic risk factors for AD.
Sample
Participants in this study derived from two interrelated studies of Caucasian same-sex twin pairs participated in VATSPSUD.21 All subjects were ascertained from the population-based Virginia Twin Registry formed from a systematic review of birth certificates in the Commonwealth of Virginia. Female-female (FF) twin pairs, born from 1934–1974, were eligible if both members responded to a mailed questionnaire in 1987–1988. Reports on symptoms of lifetime AD used in this report were collected at the fourth interview wave (FF4), conducted in 1995–1997 with cooperation in earlier waves ranging from 88 to 92%.21 For this wave, we succeeded in interviewing 85% of eligible twins. Data on the male-male and male-female pairs (MMMF) came from a sample (birth years 1940–1974) with a 72% cooperation rate, initially ascertained directly from registry records containing all twin births. The first interview (MMMF1) was completed largely through phone in 1993–1996 and was followed by a second wave of interviews (MMMF2), conducted in 1994–1998, with a response rate of 83%. Data on symptoms of AD were used from the MMMF2 wave.
Zygosity was determined by discriminate function analyses using standard twin questions validated against DNA genotyping in 496 pairs.22 The mean (s.d.) age and years of education of the twins were 36.3 (8.2) and 14.3 (2.2) at the FF4 interview, and 37.0 (9.1) and 13.6 (2.6) at the MMMF2 interview.
We used in these analyses data from 7548 twins, including both members of 3089 pairs (503 mono-zygotic (MZ) FF, 330 dizygotic (DZ) FF, 707 MZ MM, 491 DZ MM, and 1058 opposite sex DZ pairs) and 1395 twins without their co-twin. (These numbers do not exactly sum because we counted all possible pairs in our triplets and quadruplets).
The AD section of our interview was adapted from the Structured Clinical Interview for DSM disorders (SCID)23 and modified to include expanded screening questions and DSM-IV criteria.
The human subject committees at Virginia Commonwealth University approved this project. Written informed consent was obtained before face-to-face interviews and verbal consent before phone interviews. Interviewers had a master’s degree in a mental health-related field or a bachelor’s degree in this area and two years of clinical experience. At each wave, members of a twin pair were interviewed by different interviewers who were blind to clinical information about the co-twin.
Statistical methods
The twin models decompose the sources of individual differences in liability to AD into three components: additive genetic effects (A), shared family environment (C) and unique environment (E).24 Shared environment reflects family and community experiences that increase similarity in siblings who are raised together. Unique environment includes both environmental experiences not shared by siblings and random measurement error.
Our multivariate twin models estimate the degree to which genetic and environmental influences are shared across the seven DSM-IV AD criteria (and the two ‘screening criteria’ that determined if subjects were administered the alcohol section of the interview), versus those specific to each individual criterion. This is done by including in the model genetic and environmental common factors that influence risk for more than one criterion as well as criterion-specific influences.
Independent-pathway structural equation twin models were fitted using the full information by maximum likelihood method in Mx.25 We tested for both quantitative sex effects (that is, is the magnitude of genetic influences different in men and women?) and qualitative sex effects (that is, do the same genetic factors influence risk to AD in the two sexes?). To obtain unbiased population-based parameter estimates, the model had to take into account the structure of the interview through which individuals were either selected into or skipped out of the AD section. Two additional binary items were created to encode our AD screening procedure. The first criterion, termed ‘excess quantity/frequency,’ was coded positive for individuals who were skipped into the AD section because they admitted that they consumed on a single day at least 13 drinks (for males) or 7 drinks (for females); or who reported at least a 4-week period of drinking ≥7 drinks per week (males) or ≥4 drinks per week (females). The second, termed ‘Perception of Alcohol Problem’, required a positive response to at least one of three questions: Has there ever been: (i) a time in your life when you drank too much? (ii) A period in your life when someone else objected to your drinking? (iii) A period in your life when you would drink instead of working or spending time with hobbies, family or friends? Subjects who were negative to all these screening criteria were skipped out of the alcohol section and no data were recorded on the presence of absence of individual AD criteria. The full information for maximum likelihood method implemented in Mx makes use of all the available twin information, and can provide asymptotically unbiased parameters when selection items are included and missingness can be assumed to be ‘at random.’26 Mx optimizations were performed using both the try hard option and different starting values to reduce the possibility that a solution found was local rather than global minimum.
We began our model fitting with a basic 1–1–1 model with specifics (where the first, second and third numbers reflect the number of genetic, shared environmental and unique environmental common factors). We then tested for quantitative and qualitative sex effects. Then, we sought to simplify the resulting model by deleting one by one all the common factors, and then the criterion-specific genetic and common environmental effects. (We did not attempt to eliminate the criteria-specific unique environmental effects as these include errors of measurement and it would be unrealistic to set these parameters to zero). Next, we made the model progressively more complex, whereas systematically searching at each step to find a ‘best-fitting’ model.
Our goal was to find the model that reflected the optimal balance between parsimony and explanatory power. This goal can be operationalized, for example, by Akaike’s information criterion (AIC),27,28 which equals χ2–2df where df equals the difference in the degrees of freedom of the two models. We seek to minimize the AIC value. After determining a best-fitting model based on AIC, the Mx estimated factor loadings for this model were extracted and rotated in SAS29 using a Varimax rotation criterion to improve interpretability.
Maximum likelihood genetic factor scores were estimated by computing the conditional likelihood of the twin pairs’ item responses, weighted by the joint likelihood of the factor score estimates. This is an application of Bayes’ theorem, in which the joint likelihood p(F,R), of the factor scores F and the item responses R, is evaluated as p(R|F)p(F). This factor score model was iteratively fitted, separately for each of the five different zygosity/sex groups, to each twin pair’s raw data to estimate genetic factor scores for each individual. To validate the genetic factors found in our best-fit twin model, we used these genetic factor scores to predict a representative group of variables unrelated to the DSM AD criteria. These measures included: one representative internalizing disorder and two representative externalizing psychiatric disorders known to be comorbid with AD;30–33 other critical clinical and historical features of alcohol use and problems and years of education (as an index of social class), which is known to be associated with AD.34 To determine if the genetic factor scores differed from each other in their prediction of the external validators, two regression analyses were performed. First, separate regressions were conducted to examine the pattern of differences in prediction for each validator. Second, a model constraining the three genetic regression coefficients to be equal within each validator variable was specified. As the outcome variables were binary or ordinal (or rescaled to be ordinal), the robust weighted least squares mean and variance adjusted estimator in Mplus version 6.035 was used to optimize models. This model estimates probit regression coefficients for each of the genetic factor scores. As the estimated genetic factor scores are calibrated on a uniform standard scale, the effect size units are more readily interpretable when comparing coefficients.
Results
Sample
The full sample (N= 7548) consisted of 55.8% males (mean age = 37.1, s.d. = 9.1) and 44.2% females (mean = 36.5, s.d. = 8.5), 5.5% of whom (N= 415) reported never having tried alcohol in their life. These subjects were excluded from all subsequent analyses so that our functional sample was n = 7133. An additional 3204 of the twins did not meet any of the screening criteria required to be given the full alcohol interview section and were therefore ‘skipped out.’ These subjects, however, were included in the analyses with values on the dependence criteria set to zero (that is, structurally missing). A total of 18.8% of the sample (N= 1416) met criteria for DSM-IV AD. The mean age at first drink for those meeting DSM-IV AD criteria was 14.7 (s.d. = 3.2).
Of the alcohol consuming subjects, 46.4% and 41% endorsed the first (excess quantity-frequency) and the second (perception of problem) screening criteria, respectively. Table 1 shows the proportion of the subjects in the alcohol consuming sample and in those that screened into the AD section, who endorsed each of the DSM-IV AD criteria. ‘Loss of control’ was the most frequently used and, ‘despite problems’, was the least frequently endorsed criterion.
Table 1.
Endorsement frequencies for the individual DSM-IV criteria for alcohol dependence
| DSM-IV criterion for alcohol dependence number | Description | Endorsement in entire samplea % | Endorsement in sample meeting screening criteria % |
|---|---|---|---|
| 1 | Tolerance | 19.6 | 37.5 |
| 2 | Withdrawal | 9.0 | 21.9 |
| 3 | Loss of control | 26.9 | 51.6 |
| 4 | Desire to quit | 20.2 | 38.7 |
| 5 | Preoccupation | 17.8 | 34.1 |
| 6 | Give up other activities | 6.8 | 13.0 |
| 7 | Used despite problem | 3.3 | 6.3 |
Minus lifetime abstainers.
Twin model fitting
We began with a baseline 111_111 model containing one common genetic, one common shared environmental and one common unique environmental factor as well as criterion-specific genetic, shared environmental and unique environmental factors (model 1 in Table 2). The results for all other models in Table 2 (Δχ2, Δdf and Δ AIC) were calculated relative to model 1. In this baseline model, thresholds were allowed to differ between the sexes, but no quantitative or qualitative sex effects were present. Models 2 and 3 introduced, respectively, quantitative and qualitative sex effects. Compared with model 1, the AIC of model 2 deteriorated but that of model 3 was improved. Therefore, all subsequent model fitting included both qualitative sex effects and thresholds that differed between men and women. In models 4, 5 and 6 we set, respectively, all shared environmental, genetic and both genetic and shared environmental criterion-specific effects to zero. Model 6 clearly fits best by AIC, indicating that no criteria-specific genetic or familial environmental effects were needed in this model.
Table 2.
Model fitting results for independent-pathway models fitted to seven DSM-IV alcohol dependence criteria and two criteria that reflect screening items for the interview
| Model number | Twin Model AcCcEc_AsCsEs | Sex effects (Quant/Qual) | Δχ2 | Δdf | ΔAIC |
|---|---|---|---|---|---|
| 1 | 111_111 | −/− | — | — | — |
| 2 | 111_111 | +/− | −19.9 | −27 | +34.1 |
| 3 | 111_111 | −/+ | −9.7 | −1 | −7.7 |
| 4 | 111_101 | −/+ | −23.6 | 8 | −39.6 |
| 5 | 111_011 | −/+ | −21.5 | 8 | −37.5 |
| 6 | 111_001 | −/+ | −23.5 | 17 | −57.5 |
| 7 | 101_001 | −/+ | +83.8 | 26 | +31.8 |
| 8 | 011_001 | −/+ | +200.7 | 26 | +148.7 |
| 9 | 110_001 | −/+ | +900.7 | 26 | +848.7 |
| 10 | 211_001 | −/+ | −72.5 | 9 | −90.5 |
| 11 | 121_001 | −/+ | −54.7 | 9 | −72.7 |
| 12 | 112_001 | −/+ | −42.4 | 9 | −60.4 |
| 13 | 311_001 | −/+ | −102.4 | 2 | −106.4 |
| 14 | 221_001 | −/+ | −90.4 | 1 | −92.4 |
| 15 | 212_001 | −/+ | −108.4 | 1 | −110.4 |
| 16 | 312_001 | −/+ | −151.1 | −6 | −139.1 |
| 17 | 222_001 | −/+ | −131.1 | −7 | −117.1 |
| 18 | 213_001 | −/+ | −117.9 | −6 | −105.9 |
| 19a | 302_001 | −/+ | −150.3 | 3 | −156.3 |
| 20 | 202_001 | −/+ | −93.5 | 10 | −113.5 |
| 21 | 301_001 | −/+ | −71.6 | 11 | −93.6 |
| 22 | 303_001 | −/+ | −150.8 | −4 | −142.8 |
| 23 | 402_001 | −/+ | −145.2 | −3 | −151.3 |
| 24 | 302_011 | −/+ | −118.1 | −6 | −100.1 |
| 25 | 312_011 | −/+ | −138.5 | −15 | −96.5 |
Abbreviations: A, additive genetic effects; AIC, Akaike’s information criterion; C, shared family environment, df, degrees of freedom; E, individual specific environment; Qual, Qualitative genetic effects; Quant, quantitative genetic effects.
Best-fit model.
The subscript ‘C’ stands for ‘common’. So AC reflects the number of genetic common factors. The subscript ‘S’ stands for criterion-specific. So CS reflects whether criterion-specific shared environmental factors are included in the model (indicated by a ‘1’ in the Table) or they are absent in the model (indicated by a ‘0’ in the Table).
For all models, thresholds are allowed to differ between sexes.
Model 1 had a –2 log likelihood = 38 612.0, df = 42 267 and 72 estimated parameters.
Models 7, 8 and 9 set to zero, respectively, the single shared environmental, genetic and individual-specific environmental common factors. Models 8 and 9 fitted very poorly, indicating the need for genetic and individual-specific influences common to all the AD criteria. Surprisingly—given prior evidence of the absence of shared environmental effects for AD as a categorical diagnosis in this sample10,11,36— model 7 also fitted more poorly than model 6, suggesting the importance of shared environmental effects.
Working from model 6, we then tested, in #10, 11 and 12 with, respectively, a second genetic, shared environmental and unique environmental common factor. Of those three models, #10 fits the best by AIC by a wide margin, suggesting the existence of multiple genetic factors for the AD criteria.
We then examined models that added to model 10 a third genetic factor (#13), a second shared environmental factor (#14) or a second unique environmental factor (#15). Model 15 fits best, suggesting the need for multiple individual-specific environmental factors to explain the data.
Working from model 15, we then added a third genetic factor (#16), a second shared environmental factor (#17) or a third unique environmental factor (#18). Of these three models, model 16 fits best by a substantial margin.
At this point in the model fitting, we returned to examine the need for a shared environmental common factor. Perhaps the poor fit for model 7 reflected the need for more than one familial factor (that is, genetic or shared environmental) to explain the pattern of findings for the AD criteria rather than more specifically the need for a shared environmental common factor. Indeed, in the presence of multiple genetic factors, when we dropped the shared environmental common factor from model 16 to produce model 19, we saw less than a unit change in the –2 log likelihood and a substantial improvement in the AIC.
We tried to simplify model 19 by reducing either the number of genetic (#20) or unique environmental (#21) common factors, but the AIC deteriorated considerably for both these models. We added to model 19 a third individual-specific environmental common factor (#22) and that also failed to improve the fit. Finally, we also added to model 19 a fourth genetic factor (#23). This also did not improve the AIC. Model 19 was clearly our best-fit model.
Both shared environmental (c2) common and criteria-specific factors were eliminated early in model fitting. Perhaps this was premature and c2 variation would contribute meaningfully to our best-fit model. A model with an additional shared environmental common factor (model 16) did not improve the AIC. However, we added shared environmental criteria-specific loadings to our best-fit model (to make model 24) and to model 16 (to make model 25). Neither of these additions improved upon our best-fit model.
Results of best-fit model
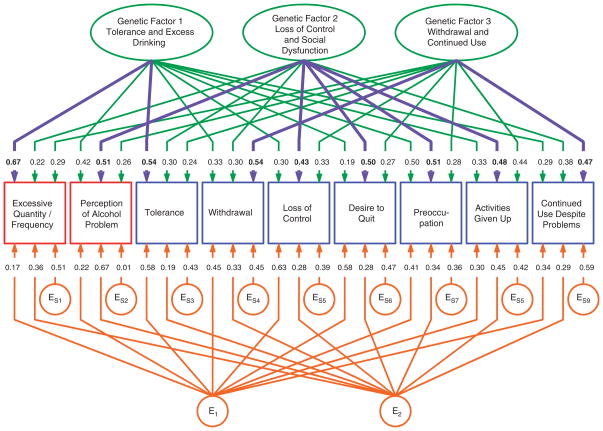
The parameter estimates for model 19 are depicted in Figure 1. Focusing first on the three genetic common factors, factor 1 had the highest loadings for the probe questions about quantity and frequency of alcohol consumption, and on the tolerance criterion. We therefore called this factor: tolerance/excess drinking. The second genetic factor loaded most strongly on the probe questions reflecting perception of having an alcohol problem and on the criteria for loss of control, desire to quit, preoccupation and activities given up. We called this factor: loss of control/social dysfunction. The highest loadings on the third genetic factor were for the withdrawal and continued use despite problems criteria. We labeled this factor: withdrawal/continued use.
Figure 1.
Parameter estimates from the best-fit multivariate twin model applied to the DSM-IV criteria for alcohol dependence. Three genetic and two individual-specific environmental common factors were identified, as well as individual-specific environmental factors unique to each criterion. The strongest genetic loading on each criterion is highlighted in blue. E for individual-specific environmental effects. Subscript numbers refer to common factors (for example, E1 refers to the first individual-specific environmental common factor), whereas subscript numbers following the letter ‘S’ (for specific) refers to criteria-specific factors (for example, ES1 refers to the individual environmental effects specific to the first criterion). Squares are observed variables and circles/ovals are latent variables.
Table 3 provides a more detailed examination of the results for the genetic risk factors in our best-fit model. The heritability of the individual AD criteria ranged from 36% (for ‘desire to quit’) to 59% (for preoccupation). The two stem items were also substantially heritable. Table 3 also presents the percentages of the total heritability of each criterion attributable to each of the three genetic common factors. For the two stem items, and five of the seven criteria, one factor clearly provides the bulk of the heritable variance. For two of the criteria, the results are less clear. Interestingly, the preoccupation criterion has very similar contributions from the factor 1 (tolerance/excess drinking) and factor 2 (loss of control/social dysfunction), whereas the ‘activities given up’ criterion has only a moderately stronger contribution from factor 2 than from factor 3 (withdrawal/continued use).
Table 3.
The total heritability of each screening item and each DSM-IV criterion for alcohol dependence and the proportion of the heritability due to the three common genetic factors
| Criteria/screening item | Total heritability | % From factor 1: tolerance/excess drinking | % From factor 2: loss of control and social dysfunction | % From factor 3: withdrawal and continued use |
|---|---|---|---|---|
| Excessive quantity/frequency | 0.58 | 77 | 8 | 15 |
| Perception of alcohol problem | 0.50 | 35 | 52 | 13 |
| Tolerance | 0.44 | 66 | 21 | 13 |
| Withdrawal | 0.49 | 22 | 18 | 60 |
| Loss of control | 0.38 | 23 | 49 | 28 |
| Desire to quit | 0.36 | 10 | 69 | 21 |
| Preoccupation | 0.59 | 42 | 44 | 14 |
| Activities given up | 0.53 | 21 | 43 | 36 |
| Continued use despite problems | 0.45 | 19 | 32 | 49 |
Bold values indicates when one factor was clearly the major contributor to the heritability of the individual criterion or screening item.
Italic values indicates when two factors made major contributions to the heritability of the individual criterion or screening item.
The pattern of loadings for the two unique environmental common factors was less distinct. Factor 1 was characterized by similarly high loadings for four of the AD criteria (‘tolerance’, ‘withdrawal’, ‘loss of control’ and ‘desire to quit’), whereas the ‘perception of problems’ stem and ‘activities given up’ criteria had a stronger relationship to factor 2.
Validation of the genetic factor scores
Based on our results from overall models and utilizing information from both the twin and the co-twin, we estimated the level of genetic liability for all subjects in our sample for each of the three identified genetic factors. Figure 2 shows the distribution of scores on these scores for all subjects in the sample meeting AD criteria. Substantial variation is seen in all three factors, which are, as expected, only weakly inter-correlated.
Figure 2.
The location in three-dimensional space defined by scores on the three genetic factors (factor 1—excess drinking and tolerance; factor 2—loss of control and social dysfunction; factor 3—withdrawal and continued use) of all individuals in the study meeting DSM-IV criteria for alcohol dependence. Circles indicate females and squares males.
We then examined whether these resulting genetic factor scores differentially predicted a representative set of external validators. If these three genetic factors reflected different aspects of the liability to AD, we reasoned that they should differ from one other in their ability to predict important variables that had no role in the assignment of the DSM AD criteria. Results are presented in Table 4. We began by analyzing AD itself as a baseline against which the other eight variables could be judged. The three factors differed significantly in their prediction of AD, with the strongest association with the second genetic factor (loss of control/social dysfunction), and the weakest with the first, or tolerance/excess drinking genetic factor. These results are expected in the first, second and third genetic factor that have strong loadings on, respectively, one, four and two of the DSM-IV criteria for AD. Of the eight external variables examined (Table 4), the three genetic factor scores differed significantly in the magnitude of their prediction for all factors but one, and for that one (conduct disorder symptoms) they differed at a trend level.
Table 4.
Prediction of patterns of comorbidity and features of alcohol drinking and problem by the three additive genetic factors scores with age and sex as covariates
| Validator variable | Effect size | Constrained model setting factor 1 = factor 2 = factor 3 | |||
|---|---|---|---|---|---|
| Factor 1–Tolerance/excess drinking | Factor 2–loss of control and social dysfunction | Factor 3 –withdrawal and continued use | χ2 (df = 2) | P-value | |
| Alcohol dependence | 0.96# | 2.51# | 1.66# | 299.1 | < 0.001 |
| Conduct disorder Sxs | 0.15# | 0.26# | 0.25# | 5.7 | 0.059 |
| Major depression | 0.17# | 0.41# | 0.25# | 15.2 | < 0.001 |
| Cannabis dependence | 0.22 | 0.57# | 0.35# | 9.9 | 0.007 |
| Age at first drink | −0.41# | −0.33# | −0.27# | 8.3 | 0.015 |
| Maximum drinksa | 1.20# | 0.66# | 0.63# | 173.2 | < 0.001 |
| Treatment seeking | 0.53# | 0.95# | 0.57# | 27.4 | < 0.001 |
| Number of episodes | −0.04 | 0.21# | −0.02 | 14.1 | 0.001 |
| Years of education | −0.19# | −0.25# | 0.05 | 36.2 | < 0.001 |
Effect size: probit regression coefficients (see Statistical methods).
P < 0.01;
P < 0.001.
In a 24-h period.
The pattern of results is of interest. The tolerance/excess drinking genetic factor most strongly predicted age at first drink and maximal drinks. The loss of control/social dysfunction genetic factor more strongly predicted conduct disorder symptoms, major depression, cannabis dependence, treatment seeking and fewer years of education. The withdrawal/continued use genetic factor was the only factor unrelated to years of education, and predicted conduct disorder symptoms nearly as strongly as factor 2. The results for number of episodes were of particular interest—as this was relatively strongly predicted by the loss of control/social dysfunction genetic factor but unrelated to the levels of the two other genetic factors.
Comment
The major goal of this report was to determine the structure of the genetic risk factors for the DSM-IV diagnostic criteria for AD. Three genetic factors were required to explain the pattern of occurrence of individual DSM-IV criteria for AD in male and female alcohol-consuming MZ and DZ twin pairs from a population-based register. We labeled these three genetic factors: tolerance/excess drinking, loss of control/social dysfunction, and withdrawal and continued use.
We also assessed whether the three genetic factor scores underlying AD were differentially associated with putative external validators. The three factors differed significantly in their prediction of nearly all of these validators and formed a coherent pattern of associations. The loss of control/social dysfunction genetic factor was most strongly related to other psychopathology and treatment seeking for AD, whereas the tolerance/excess drinking factor predicted age of drinking onset and maximum daily consumption. Their ability to differentially predict a set of relevant external validators supports the validity of these three genetic factors.
Congruent with our previous findings in this sample, when AD was analyzed as a dichotomous trait,36 we detected evidence for qualitative but not quantitative sex effects—meaning that the magnitude of genetic influences on AD criteria was equal for men and women, but that the genetic sources of liability were partially, but not completely, overlapping in the two sexes.
Our findings do not fit closely into the large prior literature on AD typologies37–40 for two reasons. First, our modeling focused solely on the clinical criteria for AD. Other features of AD not examined here (for example, comorbidities, age at onset, course of illness, and personality type) typically have a prominent role in prior typologies. Second, typological analysis is person-focused, sorting affected individuals into different subgroups. Our analyses were criteria-focused, determining how different criteria for AUDs clustered together in alcohol consuming individuals.
Some might find it surprising that we detected evidence for multiple genetic dimensions for DSM-IV AD given the prior consistent evidence for the unidimensionality of the AD syndrome.16–18 These results suggest the problems inherent in extrapolating from the phenotypic structure of a diagnostic category to the underlying structure of its genetic risk factors.
To our knowledge, this is one of the first demonstrations that DSM criteria for a major psychiatric disorder may be genetically complex and reflect multiple dimensions of liability. Such results are, however, not surprising given that DSM disorders reflect clinical–historical syndromes of unknown etiology rather than discrete pathophysiologically defined diseases.
What parallels can be drawn between our results and the extensive rodent literature on genetic influences on alcohol-related traits?41,42 It is difficult to determine definitively how many of the DSM-IV AD criteria can be meaningfully measured in rodents. As some (for example, ‘a persistent desire … to cut down’) clearly cannot,43 two DSM-IV AD criteria—tolerance and withdrawal—can be evaluated in rodents using methods broadly congruent with the human phenotypes.43,44 An examination of Figure 1 would lead to the prediction that if rodents are like humans, the relationship between genetic risk factors for tolerance and withdrawal should be modest and positive. As the two criteria do not load strongly on the same common factor, they both have positive loadings on all three factors.
The relationship between genetic liability to alcohol tolerance and alcohol withdrawal has been evaluated in rodents in three ways (see ref. 45 for a detailed review). First, two pairs of mouse lines have been selectively bred to be sensitive to alcohol withdrawal, as measured by the emergence of mild or severe handling-induced convulsions after a period of chronic alcohol vapor inhalation.46 Despite a more than 10-fold difference in alcohol withdrawal severity in the withdrawal seizure-prone and -resistant selected lines, these lines do not differ in the magnitude of their ethanol tolerance, as assessed by chronic ethanol-induced hypothermia or by loss of righting reflex duration.47 Second, the correlation between mean strain values for alcohol tolerance and withdrawal severity has been assessed in a range of inbred mouse strains. Acute alcohol withdrawal severity48 did not correlate significantly across these inbred strains with the magnitude of tolerance to ethanol hypothermia49 or to the duration of loss of righting reflex.50 Third, 20–27 recombinant inbred strains created from the DBA/2J and C57BL/6J mouse strains have been examined for several phenotypes that reflect alcohol tolerance and severity of withdrawal. Severity of acute withdrawal (Metten and Belknap, unpublished data) was not correlated significantly with tolerance to ethanol-induced hypothermia,51,52 ataxia in the grid test,53 or two different measures of acute functional tolerance on a dowel balancing test.54,55 For chronic withdrawal, however, there were significant positive correlations with both dowel test tolerance outcomes but not with the other measures of tolerance. In summary, across the three approaches that have examined this question, the rodent literature is congruent with our findings in humans that genetic risk factors for tolerance to alcohol and alcohol-induced withdrawal are weakly inter-related.45
Limitations
These results should be interpreted in the context of seven major methodological limitations. First, these twins were all white and born in Virginia. Whether these results would be replicated in other samples remains to be seen. Second, as the AIC performs well in simulations at identifying the true model,28 a variety of other fit indices for structural models have been proposed. Whereas correlated, their performance is not identical. The current version of Mx produces three additional indices: the Bayesian Information Criterion (BIC),56 the sample size adjusted BIC (aBIC)57 and the Deviance Information Criterion (DIC).58 Of all the models we examined (Table 2), the model that fits best by AIC (#19) also produced the best value for the aBIC and the DIC, and the second best-fit (by a single unit) for the BIC. Thus our conclusion that model 19 reflected the best balance of parsimony and explanatory power was not a result of idiosyncratic features of our preferred fit index—the AIC.
Third, to obtain unbiased parameter estimates, we needed to include the screening items to ‘correct’ for those missing individuals who were skipped out of the AD interview section. This reflects an inherent problem in the diagnosis of AD, as many of the questions assessing AD criteria are inappropriate for individuals who are only social drinkers. We cannot rule out the possibility that our modeling results might have differed had we used a different set of screening items.
Fourth, our assessment was solely by self-report and we cannot rule out the possibility that our results were influenced by poor or biased recall. However, prior analyses in this sample, including both self and co-twin reports, indicated that self-report diagnoses of AD assessed the ‘latent’ liability to AD with substantial accuracy.59
Fifth, as pointed out by Lessov et al,60 correlated measurement errors could bias our parameter estimates. To examine this possibility, we replaced in our best-fit model the two unique environmental-common factors, with a triangular decomposition (Cholesky) model as per Lessov.60 Inconsistent with the hypothesis that our results were biased, the fit of this Cholesky was much worse than our best-fit model by AIC, BIC, aBIC and DIC criteria.
Sixth, the total number of items available (seven criteria and two screening items) is small to define multiple factors. This may result in reduced cross-sample stability and further emphasizes the need for replication of these findings.
Finally, despite much effort it was not, for technical reasons, possible to obtain 95% confidence intervals on all the parameters in our best-fit model. Plausible confidence intervals were obtainable by permutation for the first genetic factor and the criterion-specific environmental loadings. As expected, these parameters were known with modest accuracy, with a mean span of confidence intervals of 0.25 and 0.19, respectively.
Conclusion
These results, as tentative and in need of replication, if correct have implication for efforts to study genetic risk factors for AD. Prior twin or adoption studies have looked at the magnitude of aggregate genetic effects, developmental processes or patterns of comorbidity assuming that AD reflected a single dimension of genetic liability. These results will need to be reconsidered in light of evidence for multiple genetic factors underlying AD. Molecular genetic studies—particularly candidate gene and genome-wide association studies—have similarly focused almost exclusively on the comparison with subjects meeting the criteria for AD with matched controls. If correct, the results reviewed herein suggest that this approach would be at best inefficient. Cases and controls would likely differ on three relatively independent dimensions of genetic risk with the pattern and degree of difference varying considerably across individuals. As these results need replication before they should lead to widespread changes in analytic strategy, they highlight the assumption, widely accepted but rarely tested, that psychiatric and substance use disorders as described by current diagnostic systems reflect a single dimension of genetic risk. This assumption is unwarranted and should not continue to be accepted before being subjected to empirical test.
Acknowledgments
This study was supported in part by Grants AA011408, AA017828, AA10760 and AA13519 from the National Institute of Health (NIH) and a Grant from the US Department of Veterans Affairs. The NIH and the US Department of Veterans Affairs had no direct role in the design or conduct of the study or in the collection, management, analysis and interpretation of the data, and did not review or approve this manuscript. KSK had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Linda Corey, PhD, provided assistance with the ascertainment of twins from the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry (MATR). The MATR, now directed by Judy Silberg, PhD, has received support from the NIH, the Carman Trust, the WM Keck, John Templeton and Robert Wood Johnson Foundations, and Grant UL1RR031990 from the National Center for Research Resources. Pamela Metten, PhD, and Andy Cameron BS provided assistance with the rodent correlational analyses. We thank Pamela Metten and John Belknap, PhD, for access to unpublished data.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- 1.Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Arch Gen Psychiatry. 1973;28:238–243. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- 2.Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- 3.Cadoret RJ, O’Gorman TW, Troughton E, Heywood E. Alcoholism and antisocial personality. Interrelationships, genetic and environmental factors. Arch Gen Psychiatry. 1985;42:161–167. doi: 10.1001/archpsyc.1985.01790250055007. [DOI] [PubMed] [Google Scholar]
- 4.Cadoret RJ, Troughton E, O’Gorman TW. Genetic and environmental factors in alcohol abuse and antisocial personality. J Stud Alcohol. 1987;48:1–8. doi: 10.15288/jsa.1987.48.1. [DOI] [PubMed] [Google Scholar]
- 5.Hrubec Z, Omenn GS. Evidence of genetic predisposition to alcoholic cirrhosis and psychosis: twin concordances for alcoholism and its biological end points by zygosity among male veterans. Alcohol Clin Exp Res. 1981;5:207–215. doi: 10.1111/j.1530-0277.1981.tb04890.x. [DOI] [PubMed] [Google Scholar]
- 6.Pickens RW, Svikis DS, McGue M, Lykken DT, Heston LL, Clayton PJ. Heterogeneity in the inheritance of alcoholism. A study of male and female twins. Arch Gen Psychiatry. 1991;48:19–28. doi: 10.1001/archpsyc.1991.01810250021002. [DOI] [PubMed] [Google Scholar]
- 7.McGue M, Pickens RW, Svikis DS. Sex and age effects on the inheritance of alcohol problems: a twin study. J Abnorm Psychol. 1992;101:3–17. doi: 10.1037//0021-843x.101.1.3. [DOI] [PubMed] [Google Scholar]
- 8.Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- 9.Kendler KS, Prescott CA, Neale MC, Pedersen NL. Temperance board registration for alcohol abuse in a national sample of Swedish male twins, born 1902 to 1949. Arch Gen Psychiatry. 1997;54:178–184. doi: 10.1001/archpsyc.1997.01830140090015. [DOI] [PubMed] [Google Scholar]
- 10.Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. JAMA. 1992;268:1877–1882. [PubMed] [Google Scholar]
- 11.Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. AJP. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- 12.Prescott CA, Sullivan PF, Kuo P, Webb BT, Vittum J, Patterson DG, et al. Genome-wide linkage study in the Irish Affected Sib Pair Study of Alcohol Dependence: evidence for a susceptibility region for symptoms of alcohol dependence on chromosome 4. Mol Psychiatry. 2006;11:603–611. doi: 10.1038/sj.mp.4001811. [DOI] [PubMed] [Google Scholar]
- 13.Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L, et al. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcohol Clin Exp Res. 2010;34:840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, et al. A genome-wide association study of alcohol dependence. Proc Nat Acad Sci USA. 2010;107:5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendler KS, Kalsi G, Holmans PA, Sanders AR, Aggen SH, Dick DM, et al. Genomewide association analysis of symptoms of alcohol dependence in the molecular genetics of schizophrenia (mgs2) control sample. Alcohol Clin Exp Res. 2011;35:963–975. doi: 10.1111/j.1530-0277.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borges G, Ye Y, Bond J, Cherpitel CJ, Cremonte M, Moskalewicz J, et al. The dimensionality of alcohol use disorders and alcohol consumption in a cross-national perspective. Addiction. 2010;105:240–254. doi: 10.1111/j.1360-0443.2009.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beseler CL, Taylor LA, Leeman RF. An item-response theory analysis of DSM-IV alcohol-use disorder criteria and ‘binge’ drinking in undergraduates. J Stud Alcohol Drugs. 2010;71:418–423. doi: 10.15288/jsad.2010.71.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha TD, Chou SP, Grant BF. Toward an alcohol use disorder continuum using item response theory: results from the National Epidemiologic Survey on alcohol and related conditions. Psychol Med. 2006;36:931–941. doi: 10.1017/S003329170600746X. [DOI] [PubMed] [Google Scholar]
- 19.Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK. Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends Neurosci. 1999;22:173–179. doi: 10.1016/s0166-2236(99)01393-4. [DOI] [PubMed] [Google Scholar]
- 20.Crabbe JC, Metten P, Cameron AJ, Wahlsten D. An analysis of the genetics of alcohol intoxication in inbred mice. Neurosci Biobehav Rev. 2005;28:785–802. doi: 10.1016/j.neubiorev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. 1. Guilford Press; New York: 2006. [Google Scholar]
- 22.Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry. 1999;56:39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- 23.Spitzer RL, Williams JB, Gibbon J. Structured Clinical Interview for DSM-III-R-Patient Version (SCID-P, 4/1/87) New York State Psychiatric Institute; New York: 1987. [Google Scholar]
- 24.Guerrini I, Cook CC, Kest W, Devitgh A, McQuillin A, Curtis D, et al. Genetic linkage analysis supports the presence of two susceptibility loci for alcoholism and heavy drinking on chromosome 1p22.1-11.2 and 1q21.3-24. 2. BMC Genet. 2005;6:11. doi: 10.1186/1471-2156-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6. Department of Psychiatry Virginia Commonwealth University Medical School; Richmond, VA: 2003. [Google Scholar]
- 26.Little RJA, Rubin DB. Wiley Series in Probability and Mathematical Statistics: Applied Probability and Statistics. JohnWiley & Sons, Inc; New York: 1987. Statistical Analysis with Missing Data. [Google Scholar]
- 27.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- 28.Williams L, Holahan P. Parsimony-based fit indices for multiple-indicator models: do they work? Struct Equation Model. 1994;1:161–189. [Google Scholar]
- 29.SAS Institute Inc. SAS OnlineDoc Version 9.1.3. SAS Institute Inc; Cary, NC: 2002–2005. [Google Scholar]
- 30.Merikangas KR, Gelernter CS. Comorbidity for alcoholism and depression. Psychiatr Clin North Am. 1990;13:613–632. [PubMed] [Google Scholar]
- 31.Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. Alcoholism and major depression in women. A twin study of the causes of comorbidity. Arch Gen Psychiatry. 1993;50:690–698. doi: 10.1001/archpsyc.1993.01820210024003. [DOI] [PubMed] [Google Scholar]
- 32.Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Arch Gen Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- 33.Kendler KS, Aggen S, Knudsen GP, Roysamb E, Neale M, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II personality disorders. AJP. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annu Rev Clin Psychol. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- 35.Muthen LK, Muthen BO. Mplus User’s Guide: Sixth Edition; version 6.0. 6. Muthen & Muthen; Los Angeles, CA: 2010. version 6.0. [Google Scholar]
- 36.Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of US twins. Alcohol Clin Exp Res. 1999;23:1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- 37.Babor TF. The classification of alcoholics: typology theories from the 19th century to the present. Alcohol Health Res World. 1996;20:14. [PMC free article] [PubMed] [Google Scholar]
- 38.Morey LC, Blashfield RK. Empirical classifications of alcoholism: a review. J Stud Alcohol. 1981;42:925–937. doi: 10.15288/jsa.1981.42.925. [DOI] [PubMed] [Google Scholar]
- 39.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 40.Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, et al. Types of alcoholics I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- 41.Bjork K, Hansson AC, Sommer WH. Genetic variation and brain gene expression in rodent models of alcoholism implications for medication development. Int Rev Neurobiol. 2010;91:129–171. doi: 10.1016/S0074-7742(10)91005-2. [DOI] [PubMed] [Google Scholar]
- 42.Crabbe JC. Review. Neurogenetic studies of alcohol addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3201–3211. doi: 10.1098/rstb.2008.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovinger DM, Crabbe JC. Laboratory models of alcoholism: treatment target identification and insight into mechanisms. Nat Neurosci. 2005;8:1471–1480. doi: 10.1038/nn1581. [DOI] [PubMed] [Google Scholar]
- 44.Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crabbe JC, Kendler S, Hjaltason O. Current Topics in Behavioral Neuroscience (CTN); Behavioural Neurobiology of Alcohol Addiction. Heidelberg: Springer, Germany; 2010. Modeling the diagnostic criteria for alcohol dependence with genetic animal models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crabbe JC, Kosobud A, Young ER, Tam BR, McSwigan JD. Bidirectional selection for susceptibility to ethanol withdrawal seizures in Mus musculus. Behav Genet. 1985;15:521–536. doi: 10.1007/BF01065448. [DOI] [PubMed] [Google Scholar]
- 47.Crabbe JC, Kosobud A. Sensitivity and tolerance to ethanol in mice bred to be genetically prone or resistant to ethanol withdrawal seizures. J Pharmacol Exp Ther. 1986;239:327–333. [PubMed] [Google Scholar]
- 48.Metten P, Crabbe JC. Common genetic determinants of severity of acute withdrawal from ethanol, pentobarbital and diazepam in inbred mice. Behav Pharmacol. 1994;5:533–547. doi: 10.1097/00008877-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 49.Crabbe JC, Janowsky JS, Young ER, Kosobud A, Stack J, Rigter H. Tolerance to ethanol hypothermia in inbred mice: genotypic correlations with behavioral responses. Alcohol Clin Exp Res. 1982;6:446–458. doi: 10.1111/j.1530-0277.1982.tb05007.x. [DOI] [PubMed] [Google Scholar]
- 50.Ponomarev I, Crabbe JC. Characterization of acute functional tolerance to the hypnotic effects of ethanol in mice. Alcohol Clin Exp Res. 2004;28:991–997. doi: 10.1097/01.alc.0000131978.79857.5e. [DOI] [PubMed] [Google Scholar]
- 51.Crabbe JC, Belknap JK, Mitchell SR, Crawshaw LI. Quantitative trait loci mapping of genes that influence the sensitivity and tolerance to ethanol-induced hypothermia in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1994;269:184–192. [PubMed] [Google Scholar]
- 52.Crabbe JC, Phillips TJ, Gallaher EJ, Crawshaw LI, Mitchell SR. Common genetic determinants of the ataxic and hypothermic effects of ethanol in BXD/Ty recombinant inbred mice: genetic correlations and quantitative trait loci. J Pharmacol Exp Ther. 1996;277:624–632. [PubMed] [Google Scholar]
- 53.Phillips TJ, Lessov CN, Harland RD, Mitchell SR. Evaluation of potential genetic associations between ethanol tolerance and sensitization in BXD/Ty recombinant inbred mice. J Pharmacol Exp Ther. 1996;277:613–623. [PubMed] [Google Scholar]
- 54.Gallaher EJ, Jones GE, Belknap JK, Crabbe JC. Identification of genetic markers for initial sensitivity and rapid tolerance to ethanol-induced ataxia using quantitative trait locus analysis in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1996;277:604–612. [PubMed] [Google Scholar]
- 55.Kirstein SL, Davidson KL, Ehringer MA, Sikela JM, Erwin VG, Tabakoff B. Quantitative trait loci affecting initial sensitivity and acute functional tolerance to ethanol-induced ataxia and brain cAMP signaling in BXD recombinant inbred mice. J Pharmacol Exp Ther. 2002;302:1238–1245. doi: 10.1124/jpet.302.3.1238. [DOI] [PubMed] [Google Scholar]
- 56.Schwarz G. Estimating the dimension of a model. Annual Statistics. 1978;6:461–464. [Google Scholar]
- 57.Draper D. Assessment and propagation of model uncertainty. J R Stat Soc Ser B-Methodological. 1995;57:45–97. [Google Scholar]
- 58.Raftery AE. Bayesian model selection in structural equation models. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. 1. Sage: Newbury Park, CA; 1993. pp. 163–180. [Google Scholar]
- 59.Kendler KS, Prescott CA, Jacobson K, Myers J, Neale MC. The joint analysis of personal interview and family history diagnoses: evidence for validity of diagnosis and increased heritability estimates. Psychol Med. 2002;32:829–842. doi: 10.1017/s0033291702005858. [DOI] [PubMed] [Google Scholar]
- 60.Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, Bucholz KK, et al. Defining nicotine dependence for genetic research: evidence from Australian twins. Psychol Med. 2004;34:865–879. doi: 10.1017/s0033291703001582. [DOI] [PubMed] [Google Scholar]