Abstract
Auditory brainstem responses (ABRs), and envelope and frequency following responses (EFRs and FFRs) are widely used to study aberrant auditory processing in conditions such as aging. We have previously reported age-related deficits in auditory processing for rapid amplitude modulation (AM) frequencies using EFRs recorded from a single channel. However sensitive testing of EFRs along a wide range of modulation frequencies is required to gain a more complete understanding of the auditory processing deficits. In this study, ABRs and EFRs were recorded simultaneously from two electrode configurations in young and old Fischer-344 rats, a common auditory aging model. Analysis shows that the two channels respond most sensitively to complementary AM frequencies. Channel 1, recorded from Fz to mastoid, responds better to faster AM frequencies in the 100–700 Hz range of frequencies, while Channel 2, recorded from the inter-aural line to the mastoid, responds better to slower AM frequencies in the 16–100 Hz range. Simultaneous recording of channels 1 and 2 using AM stimuli with varying sound levels and modulation depths show that age related deficits in temporal processing are not present at slower AM frequencies but only at more rapid ones, which would not have been apparent recording from either channel alone. Comparison of EFRs between unanesthetized and isoflurane anesthetized recordings in young animals, as well as comparison with previously published ABR waveforms, suggests that the generators of Channel 1 may emphasize more caudal brainstem structures while those of Channel 2 may emphasize more rostral auditory nuclei including the inferior colliculus and the forebrain, with the boundary of separation potentially along the cochlear nucleus/superior olivary complex. Simultaneous two channel recording of EFRs help to give a more complete understanding of the properties of auditory temporal processing over a wide range of modulation frequencies which is useful in understanding neural representations of sound stimuli in normal, developmental or pathological conditions
Keywords: Envelope following response, FFR, ABR, Isoflurane, brainstem, inferior colliculus, evoked potentials
1. Introduction
Age-related declines in auditory processing are the result of combined degradation of peripheral auditory function and central auditory processing (Frisina and Frisina, 1997). In the peripheral auditory system, changes to the hair cells and the cochlea due to aging result in altered hearing sensitivities and sharpness of frequency tuning (Buckiova et al., 2007, Izquierdo et al., 2008, Chen et al., 2009). Auditory brainstem responses (ABRs) are the predominant electrophysiological measures of peripheral sensitivity and the transmission of phasic auditory information through the auditory brainstem and midbrain in response to brief click or tone stimuli. ABR thresholds increase with age in humans and animals (Boettcher et al., 2002) and ABR amplitudes decrease with age and have a shallower increase in amplitude with increasing level (Boettcher et al., 1993, Konrad-Martin et al., 2012). Wave I latencies, indicating excitation of the auditory nerve, increase with age, but the latencies of other ABR waves remain relatively unaffected (Costa et al., 1990). These results suggest that peripheral changes manifest as fewer or less synchronous auditory nerve fibers recruited in aged animals for brief sounds.
Much less is known about the degradation of neural processing in the central auditory pathway, particularly for sounds with longer durations that are relevant for processing communication sounds (hundreds of milliseconds to seconds). Deficits in temporal processing with age have been observed psychophysically in older humans (Fitzgibbons and Gordon-Salant, 1996, Frisina and Frisina, 1997), even when they have comparable hearing sensitivities to younger subjects (Dubno et al., 1984, Schneider et al., 1994, Snell et al., 1994, Mazelova et al., 2002). Some evidence for this has also been observed at the level of single neurons in the auditory pathway of animals including the cochlear nucleus (Schatteman et al., 2008), inferior colliculus (Walton et al., 1998, Palombi et al., 2001), and the auditory forebrain (Mendelson and Lui, 2004). However, despite numerous observed anatomical changes (Caspary et al., 1990, Argence et al., 2006, Tadros et al., 2007), these earlier studies have revealed relatively subtle changes in the neural responses of aged animals that do not seem to capture the extent of behavioral difficulties.
Auditory evoked potentials, such as frequency following responses (FFRs) to the carrier frequency or envelope following responses (EFRs) to the temporal modulation envelope and middle and longer latency responses from the auditory forebrain, aim at providing non-invasive neurophysiological measures of auditory temporal processing that can be directly compared to psychophysical measures in humans or invasive single neuron recordings from animals (Vander Werff and Brown, 2003, Tremblay et al., 2004, Anderson et al., 2011, Warrier et al., 2011). FFRs and EFRs have been used in a variety of animals such as gerbils (Dolphin and Mountain, 1992), dolphins (Finneran et al., 2007), birds (Lucas et al., 2007), non-human primates (Burton et al., 1992) as well as humans of all ages (Krishnan, 1999, Basu et al., 2010, Clinard et al., 2010) to assess auditory acuity and to detect changes in auditory processing. These responses are typically recorded using surface electrodes in humans and sub-dermal needle electrodes in other animals. We have previously recorded EFRs to the amplitude modulation and frequency modulation envelope under isoflurane anesthesia, with the positive electrode inserted along the midline of the skull from the Fz to Cz position, and the negative (reference) electrode under the mastoid of the stimulated (ipsilateral) ear in young and aged Fischer-344 rats (Parthasarathy et al., 2010, Parthasarathy and Bartlett, 2011). Under these experimental conditions, we demonstrated deficits in temporal processing with age for rapid modulation frequencies under degraded listening conditions. These differences were clearest for an AM frequency range of 181–512Hz.
One way to delve further into how the auditory system changes with age is to use different recording configurations that emphasize different neural generators. The most common electrode configurations for obtaining ABRs and FFRs in humans have the non-inverting or positive electrode in the Fz or Cz position on the forehead, the inverting or negative electrode at either the mastoid of the stimulated ear alone or linked mastoids, and the ground electrode either at the contralateral mastoid or the forehead (Aiken and Picton, 2008, Krishnan et al., 2010, Parbery-Clark et al., 2011). This configuration is also typically followed in animal recordings with the positive electrode in the vertex, the negative electrode in the mastoid of the stimulated ear or the nose and the ground electrode in the contralateral mastoid or nape of the neck (Burton et al., 1992, Backoff and Caspary, 1994, Lucas et al., 2007). However, various other electrode configurations have also been used in humans and animals with the aim of isolating different auditory structures within the pathway, primarily using ABRs (Galbraith et al., 2006, Ping et al., 2007). Recording between the two mastoids isolates caudal brainstem structures such as the auditory nerve better than recording from the midline (Ping et al., 2007). There are a few studies that have also obtained FFRs from humans using these different electrode configurations especially recording from the mastoids (Galbraith, 1994, Galbraith et al., 2001) and very few others that look at alternate electrode configurations, especially with the aim of emphasizing different regions of the brainstem or midbrain. Emphasis of different auditory structures can be used to focus on potential loci related to hearing loss or hearing changes. Furthermore, selective emphasis of different auditory structures by electrode configurations would be expected to produce responses with different temporal selectivities that reflect the most prominent neural generators.
Here we report ABRs and EFRs recorded simultaneously from two channels using different configurations of positive electrode positions in young and aged Fischer-344 rats. We demonstrate that the two recording configurations used emphasize responses to different amplitude modulation frequencies, thus allowing us to test changes in temporal processing selectively across a wide range of modulation frequencies. We also compare responses from animals under isoflurane anesthesia, which should strongly dampen thalamic and cortical responses and their feedback, with responses from un-anesthetized animals. This provides insight regarding the main generators for each component of each channel, potentially isolating the caudal brainstem structures from the more rostral auditory nuclei such as the inferior colliculus and the auditory forebrain. We also demonstrate that age related deficits in temporal processing can be better understood by this simultaneous two channel recording of EFRs, compared to recording from either channel alone.
2. Materials and methods
2.1 Subjects
12 Young adult (9–12 weeks old, weighing ~275g) and 10 aged (92–95 weeks old, weighing 350–400g) Fischer-344 rats obtained from Taconic were used in this study. The animals were housed in the animal care facility for the period of the study in relatively quiet, standard conditions. All protocols are approved by the Purdue animal care and use committee (PACUC 06–106).
2.2 Experimental setup
Experiments were performed in a 9’×9’ double walled acoustic chamber (Industrial Acoustics Corporation). The animals were initially anesthetized with isoflurane gas anesthesia (4%) in an induction chamber. The animals were transferred post induction to a manifold and maintained at 1% – 1.5% isoflurane (with the aged animals maintained at a lower level of anesthesia) on a water circulated warming blanket (Gaymar) set to 37°C with the pump placed outside the recording chamber to eliminate audio and electrical interferences. The electrodes were then positioned at the different configurations (described below) and the animals were then injected intramuscularly with 0.1 –0.2mg/Kg of medetomidine (Domitor) and taken off the isoflurane. The usual duration of isoflurane anesthesia during this setup process was approximately ten minutes. Recordings were commenced 15 minutes after cessation of isoflurane, with the time window for the effects of isoflurane to wear off determined empirically as 9 minutes, based on ABRs waveforms and latencies as well as the response to foot pinch stimuli. Medetomidine is an alpha adrenergic agonist which acts as a sedative and an analgesic which is known to decrease motivation but preserve behavioral as well as neural responses in rodents (Ruotsalainen et al., 1997, Ter-Mikaelian et al., 2007). This helps to maintain animals in an un-anesthetized state, where they still respond to pain stimuli like a foot pinch, but are otherwise compliant to EFR recordings for a period of about two hours. In a subset of the young animals, the medetomidine was not administered and the entire recording was conducted under isoflurane anesthesia (1.5%) for a separate experiment described in the results section. The stimulus was presented free-field using the speaker to the right ear of the animal, at a distance of 115 cm from speaker to ear. The travel time from speaker to the ear of the animal was calculated to be 3.35ms. Impedances from the electrodes were always less than 1 kHz as tested using the head-stage (RA4LI, Tucker Davis technologies, or TDT). Sounds were generated by SigGenRP (TDT). Signal presentation and acquisition was done by BioSig software (TDT). Waveforms were converted to sounds and delivered via a multichannel processor (RX6, TDT) through a Bowers and Wilkins DM601 speaker. The output from the speakers was calibrated using a Bruel Kjaer microphone and SigCal (TDT), and was found to be within + 6dB for the frequency range tested. Digitized waveforms were recorded with a multichannel recording and stimulation system (RZ-5, TDT) and analyzed with BioSig or custom written programs in MATLAB (Mathworks).
2.3 Recording electrode configurations
Subdermal needle electrodes (Ambu) were used to obtain the responses. In the electrode configuration used in our previous studies (hereafter referred to as Channel 1), the positive electrode was placed along the midline of the head, sagitally, in the Fz to Cz position. The negative or inverting electrode was placed under the mastoid of the ear ipsilateral to the speaker, and the ground electrode was placed in the nape of the neck. In addition to this, a second positive electrode was placed horizontally, along the inter-aural line, above the location of the inferior colliculus, which allowed us to simultaneously record auditory responses from this positive electrode (hereafter referred to as Channel 2) and the same inverting electrode using the second channel of the RA4LI (TDT). The Channel 2 configuration was determined empirically to produce the greatest phase locking responses to lower modulation frequencies as reported in the results section below.
2.4 Stimulus description and recording procedures
ABRs were recorded using rectangular click stimuli of alternating polarity, 0.1ms long, presented at 26.6 clicks per second. The acquisition window was 20ms and each ABR was an average of 1500 repetitions.
Sinusoidally amplitude modulated (SAM) tones were 200 ms long with a 5ms cosine squared ramp at onset and offset and played at a rate of 3.1 per second. The acquisition window was 300ms long and each EFR was an average of 200 repetitions. The carrier frequency of the SAM stimuli was 8 kHz. This frequency is in the most sensitive region of the rat ABR audiogram (unpublished observations), and based on single neuron recordings (Hernandez et al., 2005), is also the most sensitive region of the rat IC, which is thought to be a major generator of EFRs (Kiren et al., 1994). The SAM stimuli at 45 Hz, 128 Hz and 256 Hz AM were presented in sound levels from 65dB SPL to 85 dB SPL in 5 dB steps. The sound level for presentation of the other stimuli was chosen as the lowest sound level which produced the maximum response as assessed by the amplitude of the fast Fourier transform (FFT) at the modulation frequency. This provided an animal-specific peak response while eliminating any reduction in response amplitude due to high sound levels often observed in the young. The absolute sound levels of presentation corresponded to 70–75dB SPL for the young and 80–85 dB SPL for the aged animals. The modulation frequency was systematically increased to yield the temporal modulation transfer function (tMTF). In the next experiment the 8 kHz carrier frequency tone was replaced by a broad band noise carrier (band-pass filtered between 40–40000 Hz) to isolate any frequency specific effects. The amplitude modulation depth of the SAM stimuli was also varied in the following steps, 0dB (100%), −2.5 dB (75%), −6 dB (50%), −12 dB (25%), −18 dB (12.5%), −24 dB (6.25%), and −30dB (3.125%) for three modulation frequencies, 45 Hz, 128 Hz and 256 Hz AM.
2.5 Data analysis
The ABRs were filtered from 30–3000 Hz using BioSig software (TDT). The EFRs were low pass filtered at 3000 Hz and high pass filtered below the modulation frequency for the slower MFs and from 80Hz for MFs greater than 90 Hz. Filtered data were exported to MATLAB. Fast Fourier transforms (FFT) were performed on time-domain waveforms obtained from BioSig, from 10–190ms relative to stimulus onset to exclude the ABR transients, using custom programs written in MATLAB. The maximum energy at modulation frequency or one frequency bin (3Hz) above and below it gave the peak FFT amplitude. The modulation depth detection threshold for both AM was defined as the minimum modulation depth which elicited a response which was at least twice as high (> 6dB) as the noise floor for modulation frequencies ≤ 64 Hz, where there are numerous competing generators of low frequency oscillations, and 10 dB above the noise floor for higher modulation frequencies. Similar results were found on using the average noise floor + 3 SD as the threshold amplitude. The noise floor was calculated as the average of five frequency bins above and five bins below the central three bins.
2.6 Statistical analysis
Significant differences are reported for the means of the FFT amplitudes using the Wilcoxon’s rank-sum test with 95 % confidence interval for differences between young and aged animals and using the Wilcoxon’s signed rank test for comparison between simultaneous two channel recordings or between isoflurane-anesthetized and un-anesthetized recordings from the same animal. The error bars reported are one standard deviation (SD) from the mean.
3. Results
3.1 Auditory Brainstem Responses
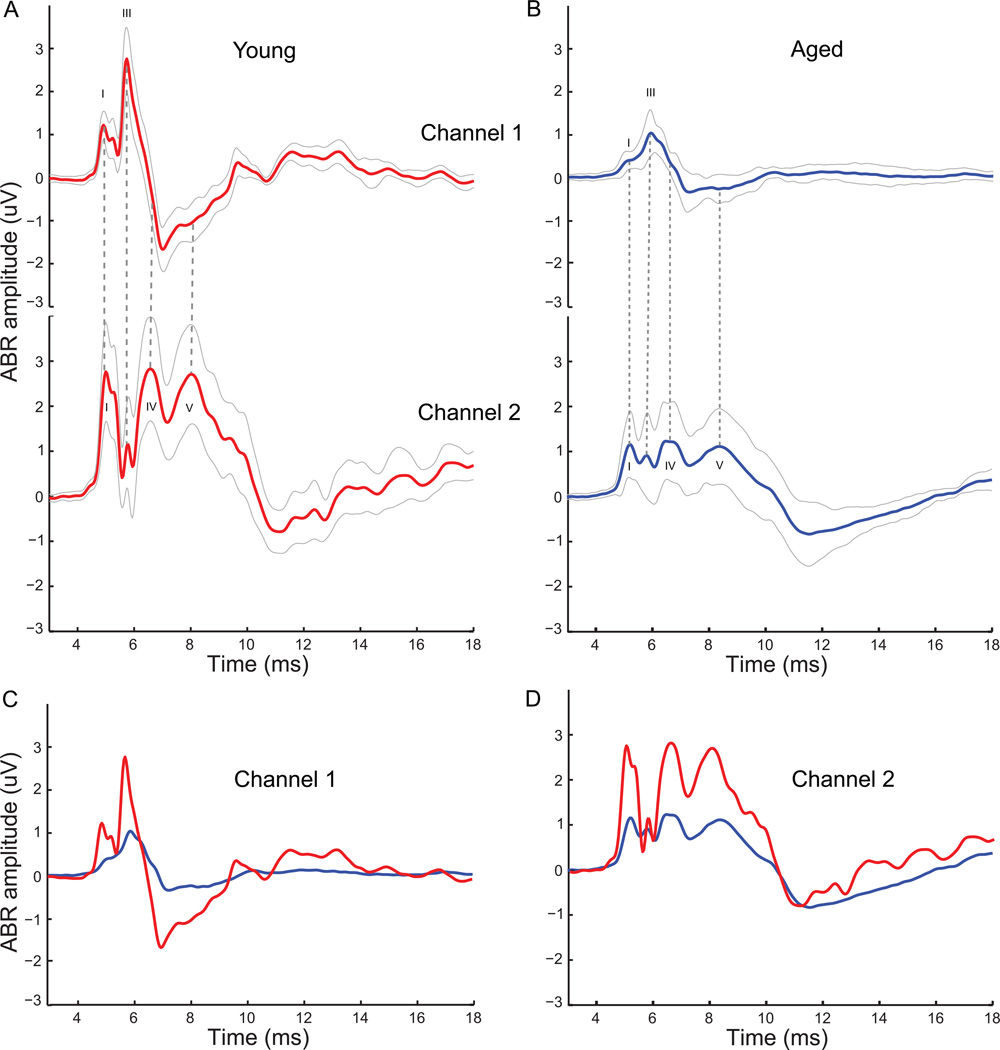
The grand averages of the auditory brainstem responses obtained from young animals at 75dB SPL (n=12) are shown in Figure1 for both channels 1 and 2. The time marked along the X-axis includes the travel time of 3.35ms to the ear. The ABR morphology indicates distinct characteristics of the waveform in each channel, plotted as the mean ABR (thick line) ± one standard deviation (thin lines) (Fig1, A). Channel 1 had a smaller wave I and a large wave III, while Channel 2 had a large wave I as well as large waves IV and V at longer latencies than wave III from ch1. The prominent wave III in Channel 1 was decreased significantly in Channel 2, while the longer latency waves IV and V observed in Channel 2 were not evident in the Channel 1 waveforms. The basic waveform morphologies for Channel 1 and Channel 2 did not differ considerably from animal to animal.
Figure 1. ABR waveforms from Channel 1 and Channel 2 show distinct and complementary waveform morphologies.
ABRs elicited to click stimuli at 75dB SPL grand-averaged across 12 young animals in red (A), and at 85dB SPL grand-averaged across 10 aged animals in blue (B) for Channel 1 (top) and Channel 2 (bottom). Waves I, III, IV and IV are marked for appropriate channels in black. Dotted gray lines compare waves between two channels at same latencies. Solid gray lines indicate standard deviation from the mean for the grand average waveforms. ABR waveforms of Channel 1 (C) and Channel 2 (D) from young (red) and aged (blue) animals superimposed to illustrate changes in ABR morphology with age. ABR amplitude in µV is marked along the y-axis and total time from stimulus onset (including travel time to the ear) is marked along the x-axis in ms.
Similar grand averages from the two channels for the aged animals are displayed in Figure 1 (n=10), for ABRs to click stimuli evoked at 85dB SPL (Fig 1, B). This higher sound level was chosen to compensate for the increased hearing threshold in the aged, so that the similarities in wave morphology can be more easily illustrated. As can be seen in the figure, the aged animals show similar wave morphology to the young in both channels. However, even with the increase in sound presentation level by 10dB, the amplitudes of the waves in the ABR were often diminished compared to the young. Furthermore, for these click stimuli, a greater variability in waveform morphology was observed in the aged, compared to the young. The trough preceding wave IV in ch2 and coinciding with wave III in ch1 was greatly reduced, and the later wave peaks (> 9 ms in Fig. 1) visible in young animals were nearly absent in aged animals.
3.2 Temporal modulation transfer function (tMTF) – Change in modulation frequency
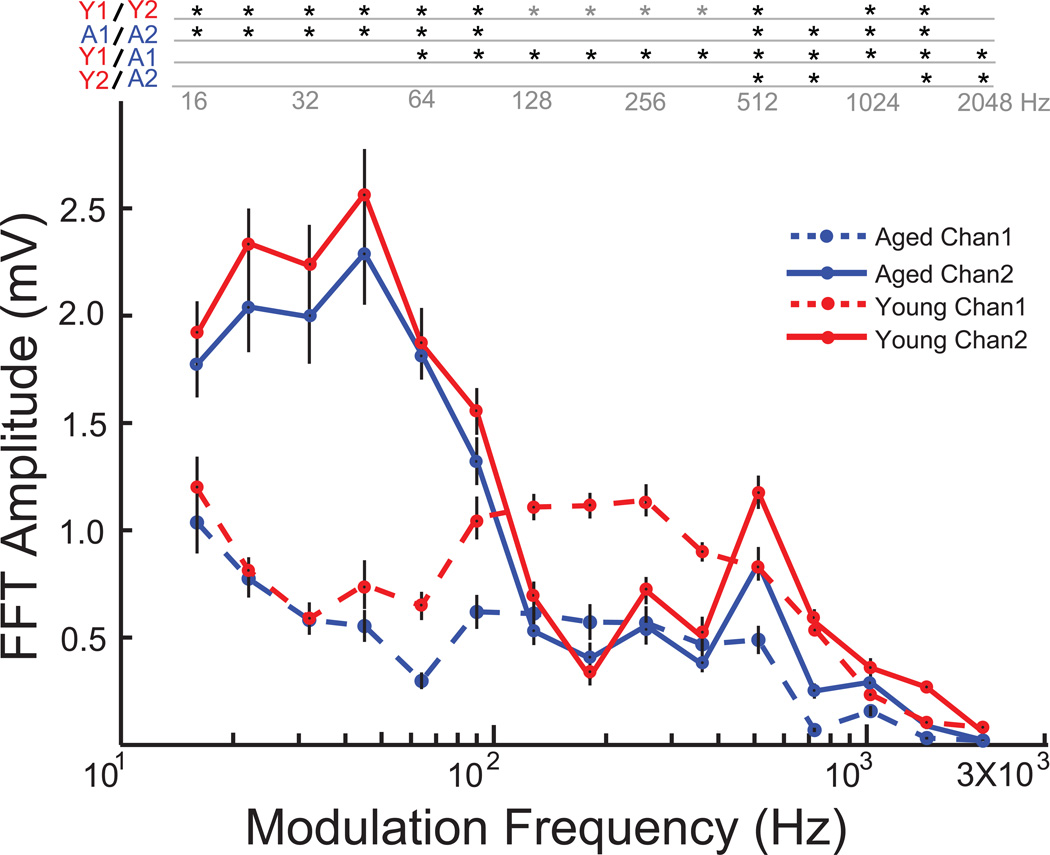
EFRs were obtained from young and aged animals, simultaneously from the two channels to a sinusoidally amplitude modulated 8 kHz carrier tone whose modulation frequency was systematically varied from 16 Hz to 2048 Hz in half octave steps. In the young animals, Channel 2 responded more robustly to the lower modulation frequencies, exhibiting a low pass nature with the amplitudes beginning to drop at 64 Hz (Fig. 2). The EFR amplitudes of Channel 2 were significantly greater than Channel 1 for modulation frequencies in the range of 16Hz to 90 Hz (p<0.01, signed rank test). Channel 1 responded better to faster modulation frequencies compared to Channel 2, exhibiting a comparatively band pass nature with frequencies in the 128Hz – 1024Hz frequency range producing the most robust responses. The EFR amplitudes of Channel 1 were significantly greater than Channel 2 for 128 Hz to 362 Hz AM frequencies (p<0.01, signed rank test). In the aged animals, the overall shapes and nature of tMTF for both the channels were similar to that of the young (Fig. 2). Channel 2 responded better to lower modulation frequencies, exhibiting a low pass nature with a similar cut off frequency of 90Hz. The amplitudes elicited by AM frequencies in the range of 16Hz to 90 Hz by Channel 2 were significantly greater than Channel 1 in the aged (p<0.01, signed rank test). There was no significant difference in EFR amplitudes between the young and the aged in Channel 2, from 16Hz AM to 512 Hz AM (p>0.05, rank-sum test). However significant age related decrease in EFR amplitudes were observed in responses from Channel 1, with aged animals eliciting lower responses than the young from 64Hz AM to 2048 Hz AM (p<0.01, rank-sum test), with no significant difference in amplitude below 64Hz AM (p>0.05, rank-sum test).
Figure 2. Temporal modulation transfer function (tMTF) for Channel 1 and Channel 2 emphasize complementary modulation frequency ranges for both young and aged.
Responses from young animals are shown in red, and aged in blue. Channel 1 responses are shown in dashed lines and Channel 2 responses in solid lines. Asterisks indicate significant differences (p<0.05, rank-sum test for young vs. aged and p<0.01, signed rank test for Channel 1 vs. Channel 2). Grey asterisks indicate Channel 1 greater than Channel 2. X-axis indicates modulation frequency in Hz and Y-axis indicates FFT amplitude at modulation frequency in mV.
3.3 Temporal modulation transfer function using a Noise carrier
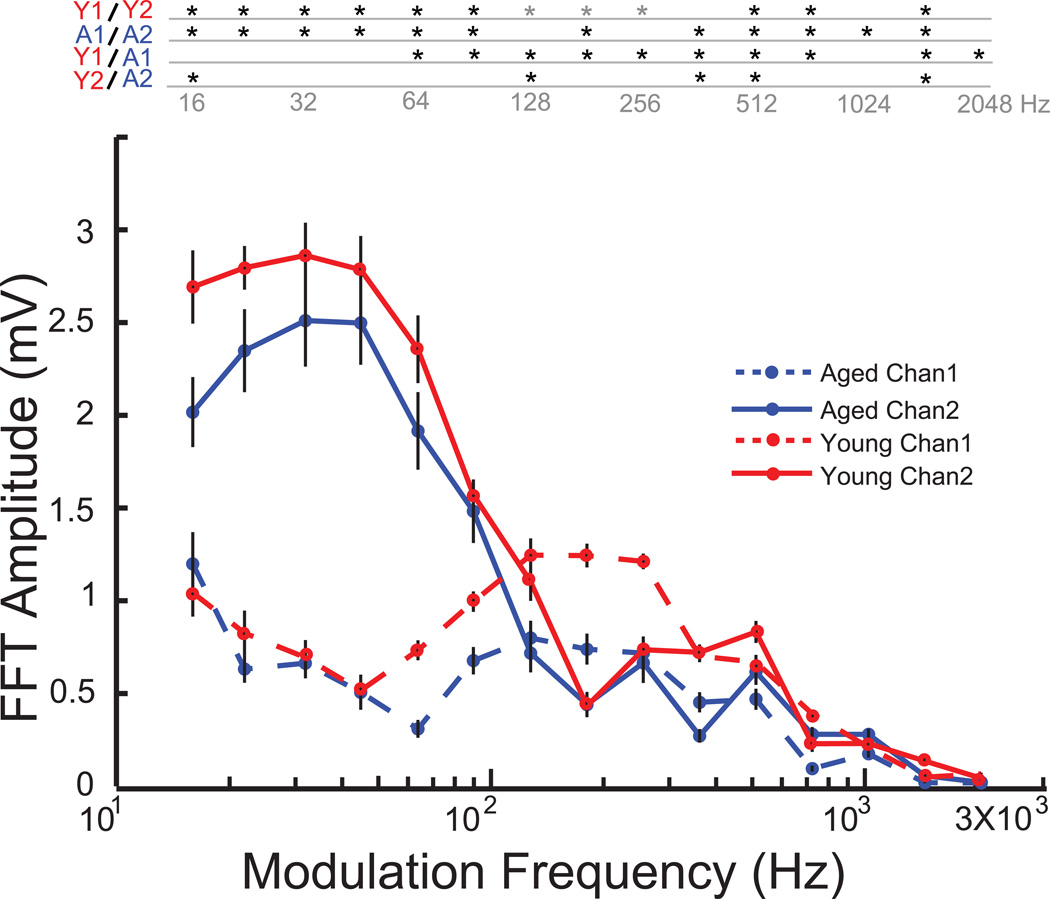
In an effort to minimize carrier frequency dependence on the amplitude modulation following responses, and to isolate the effects of temporal modulation alone, EFRs were recorded using the same modulation frequencies as for the 8 kHz carrier, but using a broad band noise carrier at the same sound level in 10 aged and 9 young animals. The results were similar to using the 8 kHz carrier frequency, with Channel 2 responding more robustly to lower modulation frequencies in the 16Hz to 90Hz AM range, and Channel 1 responding more to higher modulation frequencies in the 128Hz to 1024 Hz AM range, with the cross over at 128Hz for both young and aged animals (Fig. 3). Age related declines in EFR amplitudes were also observed in Channel 1, for AM frequencies in the 64Hz to 2048 Hz range (p<0.01, rank-sum test). Such robust decreases in FFT amplitudes with age were not observed in Channel 2, with the aged animals having a significant decrease in amplitude only at a few scattered AM frequencies (Fig. 3).
Figure 3. tMTF using noise carrier indicates characteristics for Channel 1 and Channel 2 are not dependant on carrier type for young and aged animals.
Responses from young animals are shown in red, and aged in blue. Channel 1 responses are shown in dashed lines and Channel 2 responses in solid lines. Asterisks indicate significant differences (p<0.05, rank-sum for young vs. aged and p<0.01, signed rank test for Channel 1 vs. Channel 2). Grey asterisks indicate Channel 1 greater than Channel 2. X-axis indicates modulation frequency in Hz and Y-axis indicates FFT amplitude at modulation frequency in mV.
3.4 Change in sAM sound level
To determine the rate of growth of EFR amplitudes with presentation level, the sound level of the sAM stimuli were systematically varied from 65dB SPL to 85 dB SPL in 5 dB steps for three different modulation frequencies, 45 Hz, 128 Hz and 256 Hz AM at 100 % modulation depth using an 8kHz carrier tone.
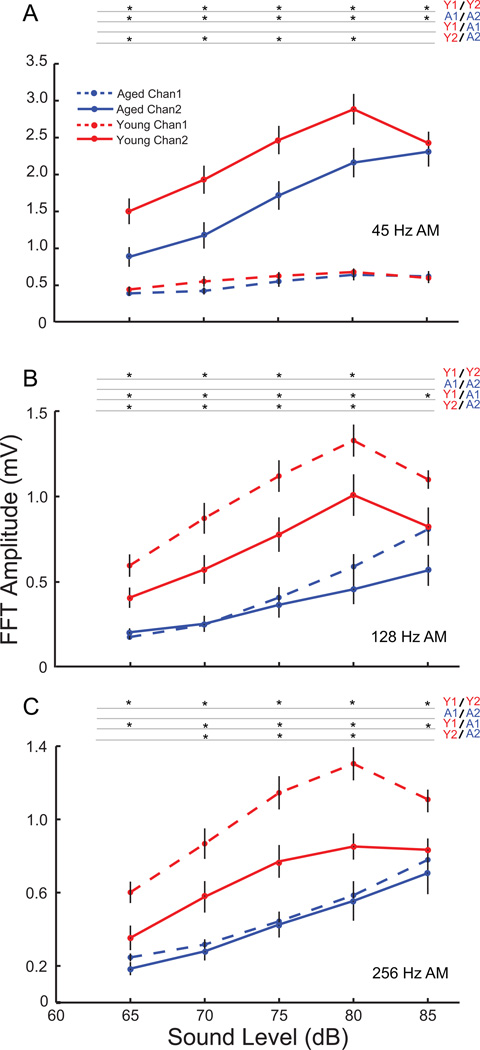
At 45 Hz AM, Channel 1 produced very low EFRs as was seen in the tMTF, even for loud sounds. This amplitude of the EFRs remained consistently low, decreasing very slightly with sound level, and with no significant differences between the young and aged at any sound level (Fig. 4A; p>0.05, rank-sum test). Channel 2 showed robust responses at 45 Hz AM for both young and aged animals. The Channel 2 amplitudes were significantly higher than the Channel 1 amplitudes for both young and aged animals at all sound levels tested (p<0.01, signed rank test). The EFRs also showed a decrease in amplitude with decrease in sound level for both young and aged animals. There was no significant difference in EFR amplitudes between the young and aged at 85 dB in Channel 2 (p>0.05, rank-sum test). However, the EFR amplitudes of the aged were significantly lower than the young, at all sound levels below 85 dB SPL (Fig. 4A, p<0.05, rank-sum test).
Figure 4. Age-related decreases in response amplitudes with decreasing sound level are evident for 45 Hz AM only in Channel 2 and for faster modulation frequencies primarily in Channel 1.
FFT amplitudes at modulation frequency with decrease in sound level for young (red) and aged (blue) animals shown for Channel 1 (dashed lines) and Channel 2 (solid lines) for 45 Hz (A), 128 Hz (B) and 256 Hz (C) AM. Asterisks indicate significant differences (p<0.01, signed rank test for Channel 1 vs. Channel 2, and p<0.05 rank-sum test for young vs. aged). X-axis indicates sound level in dB and Y-axis indicates FFT amplitude at modulation frequency in mV.
At 128 Hz AM, evidence of the complementary frequency preferences of the channels was seen, with Channel 1 responding more robustly, while the amplitudes of Channel 2 began to decline. The amplitudes of responses from Channel 2 were similar to Channel 1 at 85 dB SPL but were significantly lower than Channel 1 at all lower sound levels (Fig. 4B; p<0.01, signed rank test). In the aged, there was no significant difference between the EFR amplitudes obtained from Channel 1 and Channel 2 except at 85 dB SPL (p<0.05, signed rank test) Evidence of age related declines in EFR amplitudes was seen in the responses from Channel 1, with aged animals having significantly lower responses than the young animals for all sound levels tested (p<0.05, rank-sum test). Age related differences were also found in Channel 2, with aged animals showing significantly lower responses than the young at sound levels of 80dB SPL and below (Fig. 4B; p<0.05, rank-sum test).
The trend of Channel 1 showing stronger responses to faster modulation frequencies was more pronounced at 256 Hz AM, with Channel 1 showing significantly higher responses than Channel 2 at all sound levels tested in the young (Fig 4C; p<0.01, signed rank test). There were no significant differences in amplitudes between the two channels in the aged. Age related decreases in response amplitude were observed for all presentation levels in Channel 1 and in the 70db – 80dB SPL range in Channel 2 (p<0.05, rank-sum test).
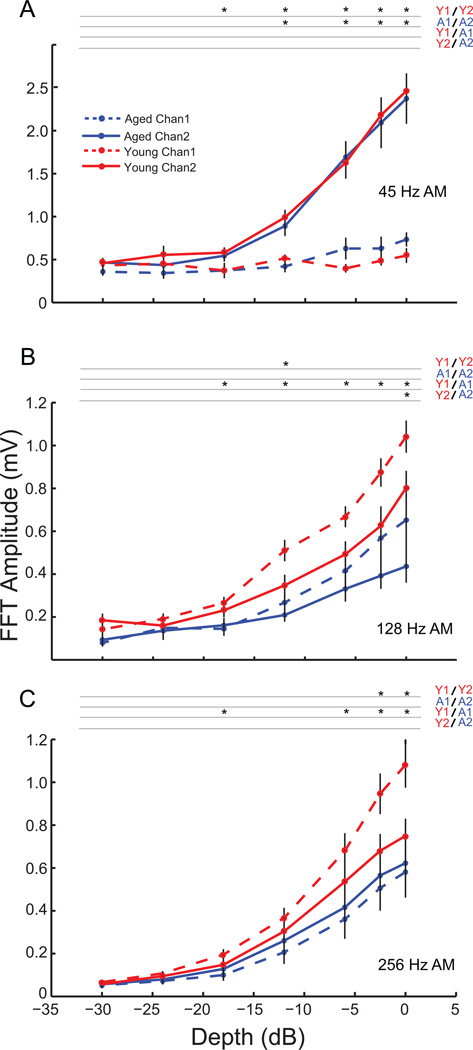
3.5 Varying depth of amplitude modulation
In a previous study, we demonstrated age-related differences in modulation depth sensitivity for Channel 1 at 256 Hz and 512 Hz AM in isoflurane anesthetized rats (Parthasarathy and Bartlett, 2011). To compare those findings with recordings from Channel 2, and from Channel 1 in un-anesthetized animals, EFRs were obtained to sAM stimuli using 8 kHz carrier frequency, modulated at 45 Hz, 128 Hz and 256 Hz AM, and varying in AM depth from 0 db (100%) to – 30dB (3.125 %).
At 45 Hz AM, similar to the change in sound level, Channel 2 responded more robustly than Channel 1 for both young and aged animals. Channel 2 responses decreased monotonically with decreasing depth for both young and aged animals. Responses from Channel 1 were significantly lower than Channel 2 for young animals from 100% down to 12.5% for the young, and to 25 % for the aged (Fig. 5A; p<0.01, signed rank test). There were no significant differences between responses from young and aged animals in Channel 2 or Channel 1 for any of the modulation depths tested at 45 Hz AM.
Figure 5. Differential age-related sensitivity to modulation depth was only seen in Channel 1 for 128 Hz and 256 Hz.
FFT amplitudes at modulation frequency with decrease in AM depth for young (red) and aged (blue) animals shown for Channel 1 (dashed lines) and Channel 2 (solid lines) for 45 Hz (A), 128 Hz (B) and 256 Hz (C) AM. Asterisks indicate significant differences (p<0.05, signed rank test for Channel 1 vs. Channel 2, rank-sum test for young vs. aged). X-axis indicates modulation depth in dB and Y-axis indicates FFT amplitude at modulation frequency in mV.
At 128 Hz AM, the Channel 1 and Channel 2 responses were comparable. The responses of the young and aged animals in both channels decreased monotonically with decreases in depth. Though the responses of Channel 1 generally seemed larger than Channel 2, especially at high modulation depths, there were no significant differences between Channel 1 and Channel 2 responses for any modulation depths in the aged, or for most modulation depths in the young (Fig. 5B). For Channel 2, the young animals had significantly higher responses than the aged only at 100 % modulation depth (p<0.01, rank-sum test). Age related decreases in temporal processing were more evident in Channel 1, with the younger animals having significantly higher responses than the aged at most of the modulation depths (p<0.05, rank-sum test, Fig 5B).
This trend was further strengthened at 256 Hz AM, with the young animals having significantly greater responses than the aged at most modulation depths in Channel 1 (Fig. 5C; p<0.05, rank-sum test). However there were no significant differences between the responses of the young and the aged at any modulation depths tested in Channel 2.
In order to illustrate differences in detection thresholds between the two channels for young and aged animals at the modulation frequencies tested, the detection threshold as a difference of Channel 1 to Channel 2 is provided in Table 1. A positive number indicates that the detection threshold of Channel 2 is lower than Channel 1 and a negative number indicates that the detection threshold of Channel 1 is lower. As can be seen from the table, at 45 Hz AM, the difference is strongly positive for both young and aged animals and is significantly different between Channel 1 and Channel 2 for young and aged animals (p<0.05, signed rank test). At 128 Hz AM, Channel 1 thresholds are significantly lower in young animals (p<0.05, signed rank test). Channel 1 thresholds were also lower at 256 Hz AM, but the trend was not statistically significant. There were basically no differences between Channel 1 and Channel 2 thresholds in the aged animals at 128 and 256 Hz AM. This illustrates that the channel and modulation frequency specific decreases in temporal processing are evident in the response amplitudes as well as the detection thresholds with age. Therefore, in the case of modulation depths, recording from only one channel would miss a clear age-related difference in auditory-evoked responses.
Table 1.
Differences in AM depth detection thresholds between channel 1 and channel 2 for young and aged animals at various modulation frequencies.
| Young (dB) | Aged (dB) | |
|---|---|---|
| 45 Hz AM | 10.6 ± 3.5 | 9.4 ± 3.0 |
| 128 Hz AM | −6.0 ± 4.2 | −0.75 ± 5.9 |
| 256 Hz AM | −7.5 ± 8.9 | −0.75 ± 3.9 |
Values indicate difference of channel 2 and channel 1 detection thresholds ± 1 standard deviation in dB. Positive values indicate that channel 2 has a lower threshold than channel 1.
3.6 Comparison of responses from anesthetized and un-anesthetized animals
Multiple neural generators are known to contribute to the ABR and EFRs, including contributions from the inferior colliculus (Kiren et al., 1994) and potentially the auditory thalamus and auditory cortex (Arnold and Burkard, 2001). In order to drastically reduce the contributions of thalamus and cortex, ABRs and EFRs were recorded from nine young animals, simultaneously from Channel 1 and Channel 2, using isoflurane anesthesia. Isoflurane anesthesia is known to have a drastic effect on responses in the auditory forebrain (Tennigkeit et al., 1997, Cheung et al., 2001), while it is thought to affect auditory responses in the auditory midbrain or brainstem to a much lesser degree (Santarelli et al., 2003, Anderson and Young, 2004). This strategy of using anesthesia to isolate generators of EFRs has been previously used for single channel EFR recordings (Kuwada et al., 2002, Szalda and Burkard, 2005). Responses were compared to those from the same nine young animals sedated with Domitor, whose forebrain contributions should be largely intact.
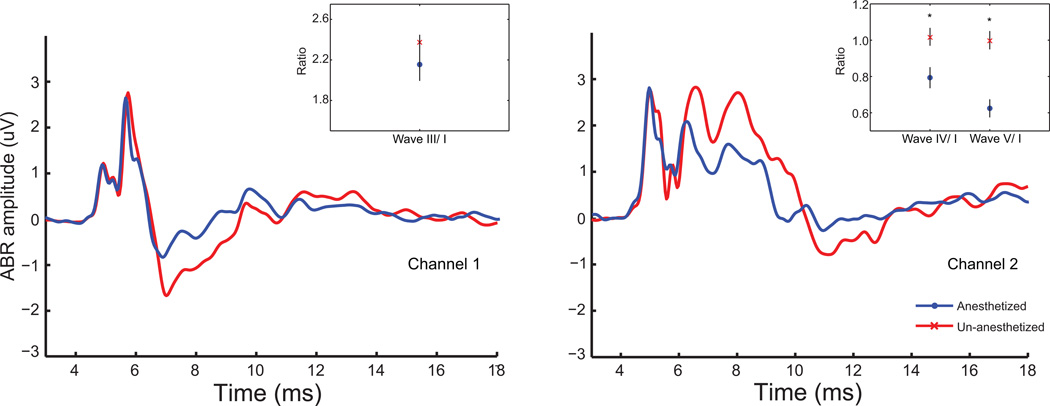
Figure 6 shows the comparison of the grand averages of the ABRs from Channel 1 and Channel 2, obtained from 9 animals to click stimuli presented at 75dB SPL from anesthetized as well as un-anesthetized conditions. As can be observed from the grand averages, in Channel 1, wave I and wave III are minimally affected by anesthesia, either in amplitude or latency, while the negativity following wave III is significantly affected. In Channel 2, the effects were clearer than in Channel 1 and became evident at approximately the same latencies (6.5 ms post-stimulus onset). Reduced amplitudes in waves IV, V, and the components through 13 ms post-stimulus onset were observed.
Figure 6. Isoflurane affects amplitudes of later but not earlier ABR waves.
Grand-averaged ABRs elicited to click stimuli at 75 dB SPL from the same nine young animals under un-anesthetized conditions (red) as well as under isoflurane anesthesia (blue) simultaneously from Channel 1 (left) and Channel 2 (right). Total time from stimulus onset is marked along the X-axis in ms, and ABR amplitudes in µV are marked along the Y-axis. Inset shows ratio of wave III/wave I amplitudes for Channel 1 (left) and wave IV/wave I and wave V/wave I amplitudes for Channel 2 (right). Ratio is marked along the Y-axis and wave type is marked along the X-axis. Asterisks indicate significant differences (p<0.05, signed rank test).
A quantitative analysis of the ABR amplitudes revealed that there was no significant difference in wave I amplitudes with anesthesia in Channel 1 or Channel 2 (p>0.05, signed rank test). The inset of Figure 6 shows wave III amplitudes in Channel 1, as well as wave IV and V amplitudes in Channel 2 as a ratio relative to their respective wave I amplitudes. In Channel 1, there was no significant decrease in wave III amplitudes with anesthesia (p>0.05, signed rank test). In Channel 2, anesthesia had a more profound effect on the later waves, with a significant decrease in the amplitudes of waves IV and V, relative to wave I in the anesthetized animals (p<0.05, signed rank test).
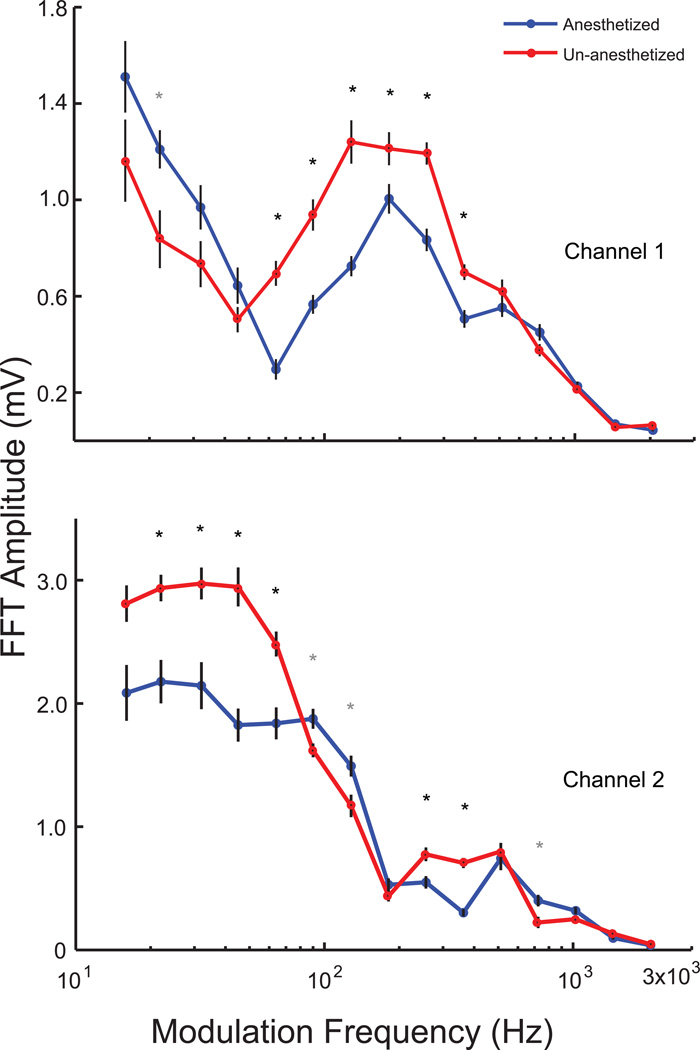
EFRs were obtained from 9 young animals to different modulation frequencies, at 100 % modulation depth, using a broadband noise carrier. Thus tMTFs, simultaneously from both channels, were generated similar to Figure 3, for the same animals from both un-anesthetized and anesthetized conditions. In both channels there seemed to be an overall shift in the tMTF towards the right i.e. towards the higher modulation frequencies, and a reduction of responses in the lower modulation frequencies, with anesthesia. Statistical analysis revealed that anesthesia significantly decreases the amplitudes of the responses from 90Hz to 362 Hz in Channel 1, and from 22 Hz to 90 Hz in Channel 2 (p<0.01, signed rank test, Figure 7A, B).
Figure 7. tMTF from young animals indicates that isoflurane decreases responses up to 90 Hz in Channel 2 and from 90–362 Hz in Channel 1.
Responses from un-anesthetized animals are shown in red, and isoflurane anesthetized animals in blue for Channel 1 (top) and Channel 2 (bottom) from the same nine young animals. Asterisks indicate significant differences (p<0.01, signed rank test). Grey asterisks indicate anesthetized responses greater than un-anesthetized responses. X-axis indicates modulation frequency in Hz and Y-axis indicates FFT amplitude at modulation frequency in mV.
4. Discussion
In this paper we have recorded ABRs and FFRs simultaneously from two channels in un-anesthetized young and aged Fischer-344 rats. These two channels produced distinct ABR morphologies (Fig. 1A, B), suggesting that they emphasized different neural generators, which was supported by differential effects of isoflurane on the two ABR waveforms (Fig. 6). Channel 1 had a larger wave III amplitude in the ABR, while Channel 2 had larger waves I, IV and V (Fig. 1). Although the overall morphologies were similar in aged animals, the amplitudes of all components were significantly reduced (Fig 1C, D). This was true even when threshold differences were accounted for, similar to previous studies in gerbils (Boettcher et al., 1993) and humans (Konrad-Martin et al., 2012).
Using the FFRs to sustained sound stimuli, the two channels were found to be sensitive to a complementary range of modulation frequencies, and enabled a more precise understanding of temporal processing deficits in aged animals. Channel 1 was more sensitive to faster modulation frequencies, in the range of 128 Hz to 1024 Hz, whereas Channel 2 was more sensitive to slower modulation frequencies, in the range of 16Hz to 128 Hz. Hence, recording EFRs from both channels simultaneously allows testing of a more complete range of AM frequencies with higher sensitivity than would be possible recording from either channel alone. This can facilitate understanding of deficits in temporal processing which can occur not only in aging but also in pathological conditions such as hearing loss, dyslexia, or central auditory processing disorder (CAPD). This was also evident from our data. Aged animals showed a similar shape of the tMTF and similar preference for frequency ranges as the young in both Channel 1 and Channel 2 (Fig 2). There was no significant decrease in EFR amplitudes due to an increase in modulation frequency, for any of the frequencies up to 512Hz AM in Channel 2 for the aged. However, looking at Channel 1, significant age related decrease in EFR amplitudes emerged for ≥ 64Hz AM, which would not be detected by looking at Channel 2 alone (Fig 2). Similar trends were observed in the tMTF while using a broad band noise carrier (Fig 3). Regardless of carrier type, two channel recording shows that neural representations of higher modulation frequencies are strongly affected by aging.
EFR amplitudes were sensitive to sound level only in Channel 2 but not in Channel 1 for 45 Hz AM, mainly because Channel 1 was minimally responsive at 45 Hz AM at all sound levels tested. EFR amplitudes were significantly lower in aged animals in Channel 2 from 65–80 dB (Fig 4A). Aged animals had significantly lower response amplitudes than the young for most sound levels in Channel 1 for 128 Hz and 256 Hz AM which was not observed in Channel 2 (Fig 4B, C). This further demonstrates that age related deficits in auditory processing can be studied over a wider range of modulation frequencies by recording from two channels simultaneously.
We have previously shown age related decreases in response amplitudes with decreases in modulation depth for the aged at 128 Hz and 256 Hz AM frequencies under isoflurane using Channel 1 (Parthasarathy and Bartlett, 2011). This was reproduced using medetomidine to a similar degree (Fig 5 B, C). However there are no age related deficits in temporal processing with decrease in depth at 45 Hz AM from Channel 2 recordings (Fig 5A). Thus it can be seen that age related loss in temporal processing is mainly evident at faster modulation frequencies, and not the slower ones, using evoked-potential recordings. This has been observed in EFR studies in humans where AM frequencies of 40 Hz showed very little age related differences (Boettcher et al., 2001), while age related loss of temporal processing was observed for higher modulation frequencies (He et al., 2008). This is also consistent with single unit studies in the IC of rodents, where age related changes in gap detection thresholds were only present for gaps less than 10ms (corresponding to a period of 100 Hz or more) and not for larger gaps (Walton et al., 1998).
The electrode placements in this study were determined empirically. Previous studies have primarily recorded FFRs or ABRs using a vertical versus horizontal configuration to emphasize the brainstem or the auditory nerve respectively in humans (Galbraith, 1994, Galbraith et al., 2000) as well as rats (Galbraith et al., 2006). The intention in the present study was to find an electrode position that would yield more robust responses to lower modulation frequencies than the configuration used in our previous studies. Based on published waveform morphologies of previous studies, the Channel 2 ABRs (Figure 1A, bottom) are more typical of responses obtained from rodents using sub-dermal needle electrodes (Backoff and Caspary, 1994) or surface electrodes (Funai and Funasaka, 1983) at similar configurations while the waveform morphologies of Channel 1 (Figure 1A, top) are similar to those reported before from similar positions using surface electrodes (Chen and Chen, 1991). Our previous studies demonstrated temporal processing deficits with age in the 128–512 Hz AM range using the Channel 1 configuration and isoflurane anesthesia (Parthasarathy et al., 2010, Parthasarathy and Bartlett, 2011). In the current study, the differences observed between young and aged animals to changes in sound level, modulation frequency and SAM depth are essentially similar between anesthetized and un-anesthetized recordings (Figs 2, 4, 6). However there is a significant decrease in EFR amplitudes with anesthesia in the young in both Channel 1 (64–362 Hz) and Channel 2 (22–64 Hz) (Figure 7). Whether the aged animals are affected in a similar fashion to anesthesia warrants further study. The responses from the anesthetized animals also seem to be greater than the un-anesthetized animals for a small range of modulation frequencies in both Channel 1 (22–32 Hz) and Channel 2 (90–128 Hz). Whether this phenomenon is of physiological relevance also requires further study, since isoflurane is known to modulate the GABA-ergic system (Banks and Pearce, 1999, Benkwitz et al., 2003) which is essential for temporal processing of auditory stimuli (Caspary et al., 2008), as well as induce changes in the membrane properties of forebrain neurons (Puil et al., 1994, Tennigkeit et al., 1997).
Since we have not performed any lesion or specific inactivation studies, the generators of the EFRs and ABRs for each channel can only be estimated based on latencies of ABRs, sensitivities to isoflurane in ABRs and EFRs, and modulation frequency preferences. Lesion studies and studies using electrode recordings from various auditory nuclei of humans and animals suggests that primary generators of the ABR waves are the distal portion of the VIII nerve for waves I and II, pontine sources for waves III and IV and the midbrain for wave V (Lev and Sohmer, 1972, Buchwald and Huang, 1975, Hashimoto et al., 1981). Based on previously published ABR waveform morphologies of lesion studies, the generators of our Channel 1 ABR seem to include primarily the caudal auditory brainstem structures. The generator of the most prominent wave III seems to be the cochlear nucleus, with the superior olivary complex and the lateral lemniscus making up the small waves and the negativity after wave III (Chen and Chen, 1991). The generators for the most prominent peaks of our Channel 2 ABRs seem to be primarily made up of, after the auditory nerve, the lateral lemniscus, lateroventral inferior colliculus and IC outputs (Funai and Funasaka, 1983, Kaga et al., 1997). This is also similar to studies in humans recording ABRs from various auditory using depth electrodes, and confirmed by studies in patients with lesions at various nuclei (Stockard and Rossiter, 1977), that the primary generators of waves III and IV are below the level of the mid-brain, and are potentially the cochlear nucleus and the superior olivary complex (Buchwald and Huang, 1975), while wave V is primarily generated from the inferior colliculus (Hashimoto et al., 1981). Hence it would be beneficial to see if these electrode configurations can be reproduced in human recordings and if similar selectivity to different auditory nuclei can be observed.
It should be noted that the generators for FFRs and EFRs are not similar to the generators of the specific waves of the ABRs, with a large component of the EFRs coming from the IC as well as the auditory forebrain (Kiren et al., 1994, Kuwada et al., 2002). The absence of any effect of isoflurane on modulation frequencies higher than 362 Hz in Channel 1, with responses in 90–362Hz being most affected suggests that the major generators for Channel 1 include the auditory nuclei including and below the IC since 362 Hz is close to the upper limit of phase locking observed in the rodent IC (Rees and Moller, 1987, Zhang and Kelly, 2003) whereas cochlear nucleus and other auditory brainstem nuclei can phase-lock up to much higher modulation frequencies (Joris et al. 2004). In Channel 2, the selective degradation of lower modulation frequencies up to 90Hz with isoflurane suggests that the main EFR generators include the IC as well as potentially more rostral auditory nuclei as the upper limit of phase locking for neurons in the primary auditory cortex and MGB in un-anesthetized rodents are typically around 100 Hz (Creutzfeldt et al., 1980). Hence the two channels seem to emphasize the caudal or the rostral auditory nuclei with the separation being near the cochlear nucleus/superior olivary complex, though the exact nature of this can only be determined by future lesion or inactivation studies. Age related changes in our study begin to emerge in Channel 1, for the faster modulation frequencies, and continue in Channel 2, also for the faster modulation frequencies (above 90 Hz AM). This seems to suggest that age related neural changes in temporal processing begin to emerge early on in the auditory pathway, in the caudal brainstem structures like the cochlear nucleus, which has also been shown in single unit studies (Schatteman et al., 2008). This selective degradation of responses at faster modulation frequencies could be due to a reduction in inhibitory neurotransmitters glycine and GABA with age, which has been observed in various anatomical and single unit studies (reviewed in (Caspary et al., 2008). Whether changes in any other neurotransmitters or metabolic factors like oxidative stress also play a role in this degradation of responses at faster AM frequencies remains to be studied.
Potential analogous configurations for the two electrode configurations in humans based on anatomical landmarks could be from Fz to mastoid for Channel 1 and from either Cz or more caudal sources like Pz to mastoid for Channel 2. Studies in humans have shown a larger wave V when recording from the vertex to the mastoid, as compared to recording from the forehead to the mastoid (Beattie et al., 1986, Beattie and Taggart, 1989). However, the precise configurations require careful study in multichannel human recording configurations to determine whether similar sensitivities to complementary modulation frequency ranges and changes with age can be observed, as the generators of the human FFR are also known to be similar to the animal FFR, comprising the cochlear nucleus, inferior colliculus as well as the auditory forebrain (Sohmer et al., 1977, Hoormann et al., 1992).
Overall, in this study we have demonstrated simultaneous two channel recordings from young and aged un-anesthetized animals to obtain ABRs and EFRs. We have shown that this simple recording configuration provides responses complementary to each other and when put together can be used to effectively and sensitively test across a range of modulation frequencies more effectively than a single channel. Two-channel ABR and EFR recordings can be useful to better understand temporal processing difficulties with aging, as it can be seen that age related loss of AM depth processing does not occur at lower modulation frequencies but only at higher modulation frequencies, which would be missed by only recording from Channel 2. This dual channel configuration can be applied to a wide range of animal models to pinpoint more accurately the auditory regions and frequency ranges affected by a given manipulation.
Highlights.
ABRs & EFRs were obtained from two electrode configurations in young and aged rats
The two channels emphasize complementary AM frequencies
Aging deficits due to sound level only in preferred AM ranges for either channel
Aging deficits in AM depth processing evident for faster AM frequencies only
Anesthesia effects suggest two channels may emphasize different auditory nuclei
Acknowledgements
This work for supported by a grant from the American federation for aging research (AFAR) to EB. The authors would like to thank Christopher Evenson for his help with data collection, and Dr. Ravi Krishnan for his helpful comments and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hearing Research. 2008;245:35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Young ED. Isoflurane/N2O anesthesia suppresses narrowband but not wideband inhibition in dorsal cochlear nucleus. Hearing Research. 2004;188:29–41. doi: 10.1016/S0378-5955(03)00348-4. [DOI] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, Yi HG, Kraus N. A Neural Basis of Speech-in-Noise Perception in Older Adults. Ear and Hearing. 2011;32:750–757. doi: 10.1097/AUD.0b013e31822229d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argence M, Saez I, Sassu R, Vassias I, Vidal PP, De Waele C. Modulation in inhibitory and excitory synaptic transmission in rat inferior colliculus after unilateral cochleectomy: An in situ and immunofluorescence study. Neuroscience. 2006;141:1193–1207. doi: 10.1016/j.neuroscience.2006.04.058. [DOI] [PubMed] [Google Scholar]
- Arnold S, Burkard R. Inner hair cell loss and steady-state potentials from the inferior colliculus and auditory cortex of the chinchilla; Meeting of the Association-for-Research-in-Otolaryngology; St Petersburg, Florida. 2001. pp. 590–599. [DOI] [PubMed] [Google Scholar]
- Backoff PM, Caspary DM. AGE-RELATED-CHANGES IN AUDITORY BRAIN-STEM RESPONSES IN FISCHER-344 RATS - EFFECTS OF RATE AND INTENSITY. Hearing Research. 1994;73:163–172. doi: 10.1016/0378-5955(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Dual actions of volatile anesthetics on GABA(A) IPSCs - Dissociation of blocking and prolonging effects. Anesthesiology. 1999;90:120–134. doi: 10.1097/00000542-199901000-00018. [DOI] [PubMed] [Google Scholar]
- Basu M, Krishnan A, Weber-Fox C. Brainstem correlates of temporal auditory processing in children with specific language impairment. Developmental Science. 2010;13:77–91. doi: 10.1111/j.1467-7687.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- Beattie RC, Beguwala FE, Mills DM, Boyd RL. LATENCY AND AMPLITUDE EFFECTS OF ELECTRODE PLACEMENT ON THE EARLY AUDITORY EVOKED-RESPONSE. Journal of Speech and Hearing Disorders. 1986;51:63–70. doi: 10.1044/jshd.5101.63. [DOI] [PubMed] [Google Scholar]
- Beattie RC, Taggart LA. ELECTRODE PLACEMENT AND MODE OF RECORDING (DIFFERENTIAL VS SINGLE-ENDED) EFFECTS ON THE EARLY AUDITORY-EVOKED RESPONSE. Audiology. 1989;28:1–18. doi: 10.3109/00206098909081607. [DOI] [PubMed] [Google Scholar]
- Benkwitz C, Banks MI, Pearce RA. GABAa receptor gamma2 subunit influences desensitization and block by isoflurane. Society for Neuroscience Abstract Viewer and Itinerary Planner 2003:Abstract No. 571.573. 2003 [Google Scholar]
- Boettcher FA, Madhotra D, Poth EA, Mills JH. The frequency-modulation following response in young and aged human subjects. Hearing Research. 2002;165 doi: 10.1016/s0378-5955(01)00398-7. PII S0378/5955, 0301 00398-00397. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Mills JH, Norton BL. AGE-RELATED-CHANGES IN AUDITORY-EVOKED POTENTIALS OF GERBILS .1. RESPONSE AMPLITUDES. Hearing Research. 1993;71:137–145. doi: 10.1016/0378-5955(93)90029-z. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Poth EA, Mills JH, Dubno JR. The amplitude-modulation following response in young and aged human subjects. Hearing Research. 2001;153:32–42. doi: 10.1016/s0378-5955(00)00255-0. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Huang CM. FAR-FIELD ACOUSTIC RESPONSE - ORIGINS IN CAT. Science. 1975;189:382–384. doi: 10.1126/science.1145206. [DOI] [PubMed] [Google Scholar]
- Buckiova D, Popelar J, Syka J. Aging cochleas in the F344 rat: Morphological and functional changes. Experimental Gerontology. 2007;42:629–638. doi: 10.1016/j.exger.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Burton MJ, Cohen LT, Rickards FW, McNally KI, Clark GM. STEADY-STATE EVOKED-POTENTIALS TO AMPLITUDE MODULATED TONES IN THE MONKEY. Acta Oto-Laryngologica. 1992;112:745–751. doi: 10.3109/00016489209137469. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. Journal of Experimental Biology. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Raza A, Armour BAL, Pippin J, Arneric SP. IMMUNOCYTOCHEMICAL AND NEUROCHEMICAL EVIDENCE FOR AGE-RELATED LOSS OF GABA IN THE INFERIOR COLLICULUS - IMPLICATIONS FOR NEURAL PRESBYCUSIS. Journal of Neuroscience. 1990;10:2363–2372. doi: 10.1523/JNEUROSCI.10-07-02363.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Li MN, Tanaka C, Bielefeld EC, Hu BH, Kermany MH, Salvi R, Henderson D. Aging outer hair cells (OHCs) in the Fischer 344 rat cochlea: Function and morphology. Hearing Research. 2009;248:39–47. doi: 10.1016/j.heares.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Chen TJ, Chen SS. GENERATOR STUDY OF BRAIN-STEM AUDITORY EVOKED-POTENTIALS BY A RADIOFREQUENCY LESION METHOD IN RATS. Experimental Brain Research. 1991;85:537–542. doi: 10.1007/BF00231737. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Nagarajan SS, Bedenbaugh PH, Schreiner CE, Wang XQ, Wong A. Auditory cortical neuron response differences under isoflurane versus pentobarbital anesthesia. Hearing Research. 2001;156:115–127. doi: 10.1016/s0378-5955(01)00272-6. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: evidence from human frequency-following response recordings. Hear Res. 2010;264:48–55. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P, Benna P, Bianco C, Ferrero P, Bergamasco B. AGING EFFECTS ON BRAINSTEM AUDITORY EVOKED POTENTIALS. Electromyography and Clinical Neurophysiology. 1990;30:495–500. [PubMed] [Google Scholar]
- Creutzfeldt O, Hellweg FC, Schreiner C. THALAMOCORTICAL TRANSFORMATION OF RESPONSES TO COMPLEX AUDITORY-STIMULI. Experimental Brain Research. 1980;39:87–104. doi: 10.1007/BF00237072. [DOI] [PubMed] [Google Scholar]
- Dolphin WF, Mountain DC. THE ENVELOPE FOLLOWING RESPONSE - SCALP POTENTIALS ELICITED IN THE MONGOLIAN GERBIL USING SINUSOIDALLY AM ACOUSTIC-SIGNALS. Hearing Research. 1992;58:70–78. doi: 10.1016/0378-5955(92)90010-k. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Dirks DD, Morgan DE. EFFECTS OF AGE AND MILD HEARING-LOSS ON SPEECH RECOGNITION IN NOISE. Journal of the Acoustical Society of America. 1984;76:87–96. doi: 10.1121/1.391011. [DOI] [PubMed] [Google Scholar]
- Finneran JJ, London HR, Houser DS. Modulation rate transfer functions in bottlenose dolphins (Tursiops truncatus) with normal hearing and high-frequency hearing loss. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2007;193:835–843. doi: 10.1007/s00359-007-0238-6. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Auditory temporal processing in elderly listeners. J Am Acad Audiol. 1996;7:183–189. [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: Relations to possible neural mechanisms. Hearing Research. 1997;106:95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Funai H, Funasaka S. EXPERIMENTAL-STUDY ON THE EFFECT OF INFERIOR COLLICULUS LESIONS UPON AUDITORY BRAIN-STEM RESPONSE. Audiology. 1983;22:9–19. doi: 10.3109/00206098309072766. [DOI] [PubMed] [Google Scholar]
- Galbraith G, Waschek J, Armstrong B, Edmond J, Lopez I, Liu WX, Kurtz I. Murine auditory brainstem evoked response: Putative two-channel differentiation of peripheral and central neural pathways. Journal of Neuroscience Methods. 2006;153:214–220. doi: 10.1016/j.jneumeth.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Galbraith GC. 2-CHANNEL BRAIN-STEM FREQUENCY-FOLLOWING RESPONSES TO PURE-TONE AND MISSING FUNDAMENTAL STIMULI. Electroencephalography and Clinical Neurophysiology. 1994;92:321–330. doi: 10.1016/0168-5597(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Galbraith GC, Bagasan B, Sulahian J. Brainstem frequency-following response recorded from one vertical and three horizontal electrode derivations. Perceptual and Motor Skills. 2001;92:99–106. doi: 10.2466/pms.2001.92.1.99. [DOI] [PubMed] [Google Scholar]
- Galbraith GC, Threadgill MR, Hemsley J, Salour K, Songdej N, Ton J, Cheung L. Putative measure of peripheral and brainstem frequency-following in humans. Neuroscience Letters. 2000;292:123–127. doi: 10.1016/s0304-3940(00)01436-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto I, Ishiyama Y, Yoshimoto T, Nemoto S. BRAIN-STEM AUDITORY-EVOKED POTENTIALS RECORDED DIRECTLY FROM HUMAN BRAIN-STEM AND THALAMUS. Brain. 1981;104:841–859. doi: 10.1093/brain/104.4.841. [DOI] [PubMed] [Google Scholar]
- He NJ, Mills JH, Ahlstrom JB, Dubno JR. Age-related differences in the temporal modulation transfer function with pure-tone carriers. Journal of the Acoustical Society of America. 2008;124:3841–3849. doi: 10.1121/1.2998779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez O, Espinosa N, Perez-Gonzalez D, Malmierca MS. The inferior colliculus of the rat: A quantitative analysis of monaural frequency response areas. Neuroscience. 2005;132:203–217. doi: 10.1016/j.neuroscience.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Hoormann J, Falkenstein M, Hohnsbein J, Blanke L. THE HUMAN FREQUENCY-FOLLOWING RESPONSE (FFR) - NORMAL VARIABILITY AND RELATION TO THE CLICK-EVOKED BRAIN-STEM RESPONSE. Hearing Research. 1992;59:179–188. doi: 10.1016/0378-5955(92)90114-3. [DOI] [PubMed] [Google Scholar]
- Izquierdo MA, Gutierrez-Conde PM, Merchan MA, Malmierca MS. Non-plastic reorganization of frequency coding in the inferior colliculus of the rat following noise-induced hearing loss. Neuroscience. 2008;154:355–369. doi: 10.1016/j.neuroscience.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Kaga K, Shinoda Y, Suzuki JI. Origin of auditory brainstem responses in cats: Whole brainstem mapping, and a lesion and HRP study of the inferior colliculus. Acta Oto-Laryngologica. 1997;117:197–201. doi: 10.3109/00016489709117768. [DOI] [PubMed] [Google Scholar]
- Kiren T, Aoyagi M, Furuse H, Koike Y. AN EXPERIMENTAL-STUDY ON THE GENERATOR OF AMPLITUDE-MODULATION FOLLOWING RESPONSE. Acta Oto-Laryngologica. 1994:28–33. [PubMed] [Google Scholar]
- Konrad-Martin D, Dille MF, McMillan G, Griest S, McDermott D, Fausti SA, Austin DF. Age-Related Changes in the Auditory Brainstem Response. Journal of the American Academy of Audiology. 2012;23:18–35. doi: 10.3766/jaaa.23.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A. Human frequency-following responses to two-tone approximations of steady-state vowels. Audiology and Neuro-Otology. 1999;4:95–103. doi: 10.1159/000013826. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Bidelman GM, Gandour JT. Neural representation of pitch salience in the human brainstem revealed by psychophysical and electrophysiological indices. Hearing Research. 2010;268:60–66. doi: 10.1016/j.heares.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada S, Anderson JS, Batra R, Fitzpatrick DC, Teissier N, D'Angelo WR. Sources of the scalp-recorded amplitude-modulation following response. J Am Acad Audiol. 2002;13:188–204. [PubMed] [Google Scholar]
- Lev A, Sohmer H. SOURCES OF AVERAGED NEURAL RESPONSES RECORDED IN ANIMAL AND HUMAN SUBJECTS DURING COCHLEAR AUDIOMETRY (ELECTRO-COCHLEOGRAM) Archiv Fur Klinische Und Experimentelle Ohren-Nasen-Und Kehlkopfheilkunde. 1972;201:79. doi: 10.1007/BF00341066. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Freeberg TM, Long GR, Krishnan A. Seasonal variation in avian auditory evoked responses to tones: a comparative analysis of Carolina chickadees, tufted titmice, and white-breasted nuthatches. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2007;193:201–215. doi: 10.1007/s00359-006-0180-z. [DOI] [PubMed] [Google Scholar]
- Mazelova J, Popelar J, Syka J. 6th International Symposium on the Neurobiology and Neuroendocrinology of Aging. Bregenz, Austria: Pergamon-Elsevier Science Ltd; 2002. Auditory function in presbycusis: peripheral vs. central changes; pp. 87–94. [Google Scholar]
- Mendelson JR, Lui B. The effects of aging in the medial geniculate nucleus: a comparison with the inferior colliculus and auditory cortex. Hearing Research. 2004;191:21–33. doi: 10.1016/j.heares.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Backoff PM, Caspary DM. Responses of young and aged rat inferior colliculus neurons to sinusoidally amplitude modulated stimuli. Hearing Research. 2001;153:174–180. doi: 10.1016/s0378-5955(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A, Strait DL, Kraus N. Context-dependent encoding in the auditory brainstem subserves enhanced speech-in-noise perception in musicians. Neuropsychologia. 2011;49:3338–3345. doi: 10.1016/j.neuropsychologia.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL. AGE-RELATED AUDITORY DEFICITS IN TEMPORAL PROCESSING IN F-344 RATS. Neuroscience. 2011;192:619–630. doi: 10.1016/j.neuroscience.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Cunningham PA, Bartlett EL. Age-related differences in auditory processing as assessed by amplitude-modulation following responses in quiet and in noise. Frontiers in aging neuroscience. 2010;2:152. doi: 10.3389/fnagi.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping JL, Li NX, Du Y, Wu XH, Li L, Galbraith G. Auditory evoked responses in the rat: Transverse mastoid needle electrodes register before cochlear nucleus and do not reflect later inferior colliculus activity. Journal of Neuroscience Methods. 2007;161:11–16. doi: 10.1016/j.jneumeth.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Puil E, Hutcheon B, Reiner PB. ISOFLURANE INHIBITS CALCIUM CURRENTS IN NEOCORTICAL NEURONS. Neuroscience Letters. 1994;176:63–66. doi: 10.1016/0304-3940(94)90872-9. [DOI] [PubMed] [Google Scholar]
- Rees A, Moller AR. STIMULUS PROPERTIES INFLUENCING THE RESPONSES OF INFERIOR COLLICULUS NEURONS TO AMPLITUDE-MODULATED SOUNDS. Hearing Research. 1987;27:129–143. doi: 10.1016/0378-5955(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen S, Haapalinna A, Riekkinen PJ, Sirvio J. Dexmedetomidine reduces response tendency, but not accuracy of rats in attention and short-term memory tasks. Pharmacology Biochemistry and Behavior. 1997;56:31–40. doi: 10.1016/S0091-3057(96)00151-7. [DOI] [PubMed] [Google Scholar]
- Santarelli R, Carraro L, Conti G, Capello M, Plourde G, Arslan E. Effects of isoflurane on auditory middle latency (MLRs) and steady-state (SSRs) responses recorded from the temporal cortex of the rat. Brain Research. 2003;973:240–251. doi: 10.1016/s0006-8993(03)02520-4. [DOI] [PubMed] [Google Scholar]
- Schatteman TA, Hughes LF, Caspary DM. Aged-related loss of temporal processing: Altered responses to amplitude modulated tones in rat dorsal cochlear nucleus. Neuroscience. 2008;154:329–337. doi: 10.1016/j.neuroscience.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BA, Pichorafuller MK, Kowalchuk D, Lamb M. GAP DETECTION AND THE PRECEDENCE EFFECT IN YOUNG AND OLD ADULTS. Journal of the Acoustical Society of America. 1994;95:980–991. doi: 10.1121/1.408403. [DOI] [PubMed] [Google Scholar]
- Snell KB, Ison JR, Frisina DR. THE EFFECTS OF SIGNAL FREQUENCY AND ABSOLUTE BANDWIDTH ON GAP DETECTION IN NOISE. Journal of the Acoustical Society of America. 1994;96:1458–1464. doi: 10.1121/1.410288. [DOI] [PubMed] [Google Scholar]
- Sohmer H, Pratt H, Kinarti R. SOURCES OF FREQUENCY FOLLOWING RESPONSES (FFR) IN MAN. Electroencephalography and Clinical Neurophysiology. 1977;42:656–664. doi: 10.1016/0013-4694(77)90282-6. [DOI] [PubMed] [Google Scholar]
- Stockard JJ, Rossiter VS. CLINICAL AND PATHOLOGIC CORRELATES OF BRAIN-STEM AUDITORY RESPONSE ABNORMALITIES. Neurology. 1977;27:316–325. doi: 10.1212/wnl.27.4.316. [DOI] [PubMed] [Google Scholar]
- Szalda K, Burkard R. The effects of nembutal anesthesia on the auditory steady-state response (ASSR) from the inferior colliculus and auditory cortex of the chinchilla. Hearing Research. 2005;203:32–44. doi: 10.1016/j.heares.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Tadros SF, D'Souza M, Zettel ML, Zhu XX, Waxmonsky NC, Frisina RD. Glutamate-related gene expression changes with age in the mouse auditory midbrain. Brain Research. 2007;1127:1–9. doi: 10.1016/j.brainres.2006.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennigkeit F, Ries CR, Schwarz DWF, Puil E. Isoflurane attenuates resonant responses of auditory thalamic neurons. Journal of Neurophysiology. 1997;78:591–596. doi: 10.1152/jn.1997.78.2.591. [DOI] [PubMed] [Google Scholar]
- Ter-Mikaelian M, Sanes DH, Semple MN. Transformation of temporal properties between auditory midbrain and cortex in the awake Mongolian gerbil. Journal of Neuroscience. 2007;27:6091–6102. doi: 10.1523/JNEUROSCI.4848-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Billings C, Rohila N. Speech evoked cortical potentials: effects of age and stimulus presentation rate. Journal of the American Academy of Audiology. 2004;15:226–264. doi: 10.3766/jaaa.15.3.5. [DOI] [PubMed] [Google Scholar]
- Vander Werff KR, Brown CJ. Effect of audiometric configuration on threshold and suprathreshold auditory steady-state responses; 26th Midwinter Meeting of the Association-for-Research-in-Otolaryngology; Daytona Beach, FL: Lippincott Williams & Wilkins; 2003. pp. 310–326. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O'Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. Journal of Neuroscience. 1998;18:2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier CM, Abrams DA, Nicol TG, Kraus N. Inferior colliculus contributions to phase encoding of stop consonants in an animal model. Hearing research. 2011;282:108–118. doi: 10.1016/j.heares.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Kelly JB. Glutamatergic and GABAergic regulation of neural responses in inferior colliculus to amplitude-modulated sounds. Journal of Neurophysiology. 2003;90:477–490. doi: 10.1152/jn.01084.2002. [DOI] [PubMed] [Google Scholar]