Abstract
Background
Non-functional endocrine pancreatic tumours (NPT) of more than 2 cm have an increased risk of malignancy. The aim of the present study was: (i) to define the guidelines for laparoscopic enucleation (LapEn) in patients with a non-functional NPT ≤3 cm in diameter; (ii) to evaluate pancreas-related complications; and (iii) to present the long-term outcome.
Methods
Between April 1998 and September 2010, 30 consecutive patients underwent laparoscopic surgery for a non-functional NPT (median age 56.5 years, range 44–83). Only 13 patients with tumours ≤3 cm in size underwent LapEn. Local lymph node dissection to exclude lymph node involvement was performed in all patients.
Results
The median tumour size, operative time and blood loss were 2.8 cm (range 2.8–3), 130 min (range 90–280) and 220 ml (range 120–300), respectively. A pancreatic fistula occurred in five patients: International Study Group of Pancreatic Fistula (ISGPF) A in two patients and ISGPF B in three patients. The median follow-up was 48 months (12–144). Three patients with well-differentiated carcinoma are free of disease 2, 3 and 4 years after LapEn and a regional lymphadenectomy. One patient, 5 years after a LapEn, presented with lymph node and liver metastases.
Conclusions
The present study confirms the technical feasibility and acceptable morbidity associated with LapEn. Intra-operative lymph node sampling and frozen-section examination should be performed at the time of LapEn; when a malignancy is confirmed, oncologically appropriate lymph node dissection should be performed.
Keywords: non-functional neuroendocrine pancreatic tumours, laparoscopy, enucleation, regional lymphadenectomy, pancreatic fistula, laparoscopic resection
Introduction
Neuroendocrine pancreatic tumours (NPT) are a rare group of neoplasms with complex patterns of behaviour requiring detailed specialist management. Surgery remains the only curative modality currently available for resectable NPT. Complete surgical resection may be possible in those tumours that are localized at presentation. The surgical management varies according to tumour type, location and size. Functional and non-functional NPT ≤ 3 cm in diameter may be treated with enucleation.1–3
In 1996, Gagner et al.1 reported an early experience with laparoscopic resection (LPR) of islet cell tumours. Since then, the majority of reports on LPR are based on limited experience with a short follow-up.2–14 In a recent systematic review of laparoscopic enucleation of pancreatic tumours by Briggs et al.,4 the conversion rate ranged from 10.5% to 44.4%, and the morbidity associated with the procedure ranged from 22% to 66.6%.
Presently, pancreatic enucleation is considered the procedure of choice to treat insulinomas.5–15 Non-functional tumours are a poorly understood group of lesions that may behave more aggressively.16 Only non-functional NPT with a low likelihood of malignancy (<3 cm in size, no enlarged locoregional lymph nodes or no distant metastasis) can be treated with enucleation. In the context of NPT, the semantics of benign vs. malignant have to be viewed with caution. A small non-functional NPT that has been completely enucleated may be found years later to have lymph nodes and liver metastases.16,17 Based on this behaviour, in patients with non-functional NPT, nodal sampling should be routinely performed during enucleation to rule out metastatic disease and to increase the diagnostic effectiveness.
We have previously reported5 the outcome of 16 patients with non-functional pancreatic tumours. In the present study, a further 14 patients have been added.
The aim of the present study was: (i) to define the guidelines for laparoscopic enucleation (LapEn) in patients with non-functional NPT; (ii) to evaluate pancreas-related complications after a LapEn; and (iii) to present the long-term outcome.
Methods
Laparoscopic pancreatic resections were carried out at the Hospital Clínic de Barcelona.
The diagnosis and location of a non-functional NPT was established with a combination of endoscopic ultrasonography and computed tomography. Magnetic resonance imaging was performed to define the anatomic relation between the tumour and the main pancreatic duct.
The technical details of LapEn have been previously published.18 The use of intra-operative laparoscopic ultrasonography (LapUS) was an integral part of the procedure. All patients were managed with local lymph node dissection to exclude metastatic lymph node involvement. Local lymphadenectomy varied depending on the localization of the tumour. For tumours located in the pancreatic head, lymph node sampling was performed from lymph node stations 8 (lymph nodes around the common hepatic artery), 14 (lymph nodes around the superior mesenteric artery) and 17 (anterior pancreaticoduodenal lymph nodes) as defined by the Japanese Pancreas Society.19 For tumours located in the body-tail of the pancreas, lymph node sampling was performed from lymph node stations 8, 9 (lymph nodes around the celiac trunk), 11 (lymph nodes around the splenic artery) and 18 (lymph nodes along the inferior border of the body and tail of the pancreas), as defined by the Japanese Pancreas Society19 (Figs 1,2). When frozen section examination revealed malignancy, a complete lymphadenectomy of the involved lymph node station was performed.
Figure 1.
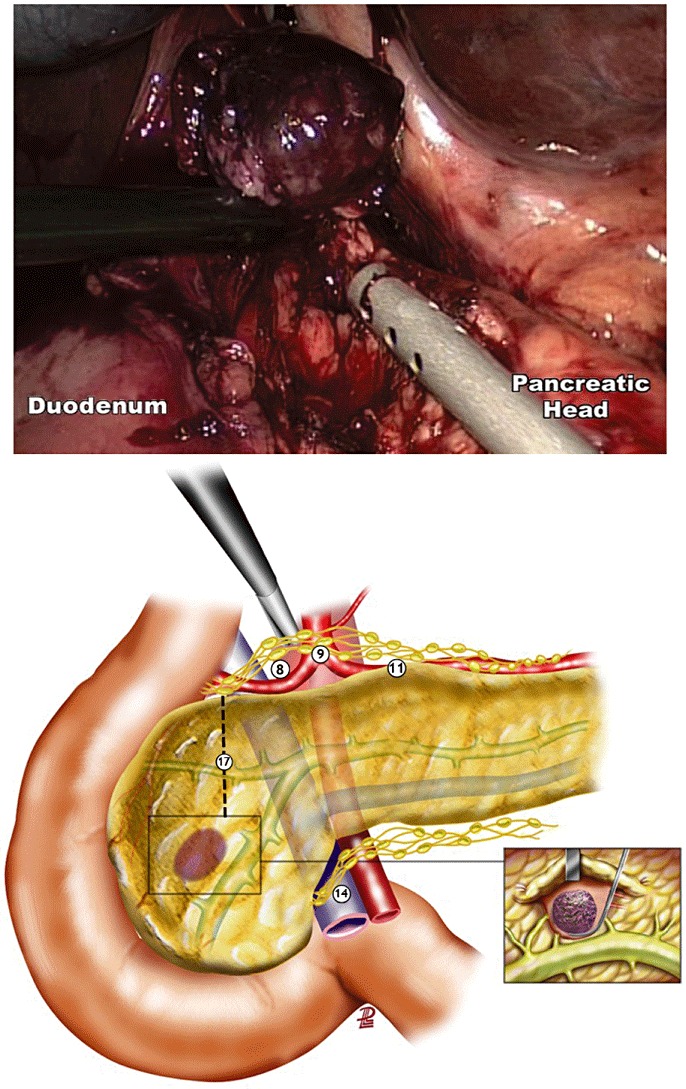
Complete laparoscopic enucleation of a tumour located in the pancreatic head. Caution should be taken to avoid injury of the Wirsung duct. Lymph node sampling of the areas around the hepatic artery (8), anterior pancreatoduodenal (17) and superior mesenteric vein (14); numbers correspond to areas according to the Japanese Pancreas Society
Figure 2.
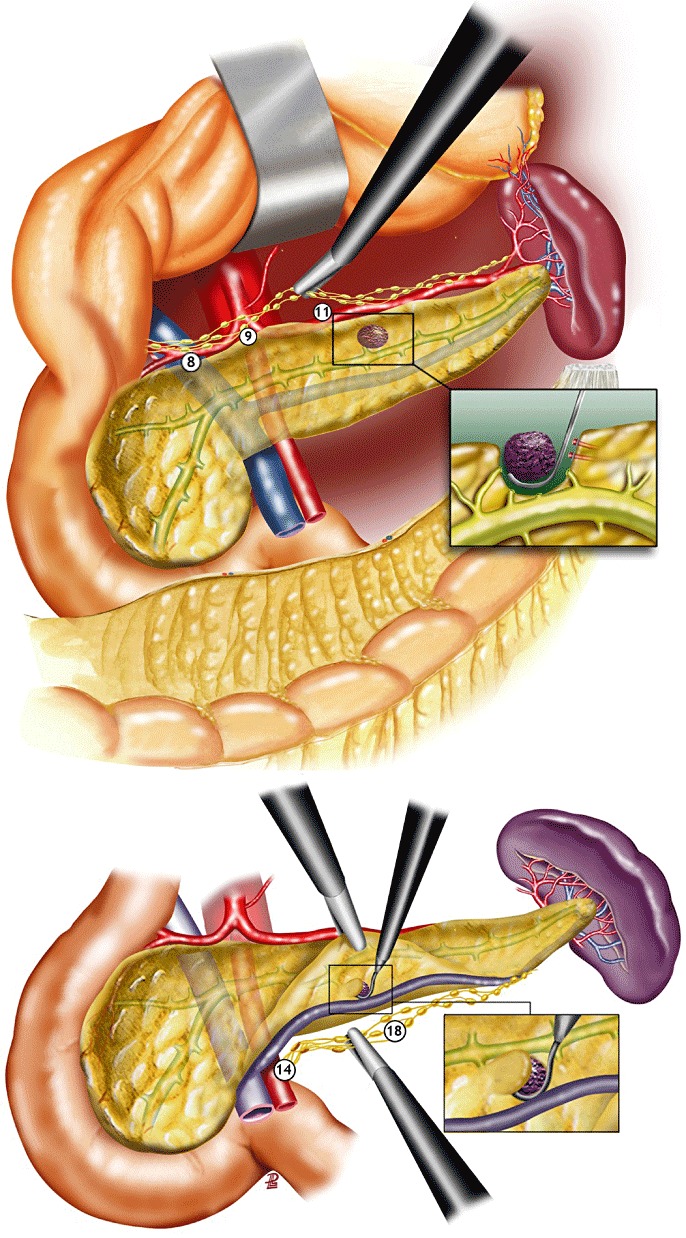
Laparoscopic enucleation of tumours located in the body of the pancreas. Depending on the location of the tumour, lymph node sampling along the splenic artery (11) and at the inferior border of the pancreas (18); numbers correspond to areas according to the Japanese Pancreas Society
Data were collected prospectively. Patient demographics and intra-operative parameters, including total operative time, blood loss and blood transfusions, were recorded. Post-operative events were recorded using the following definitions.
Pancreatic fistula (PF), according to the International Study Group on Pancreatic Fistula (ISGPF).20
Splenic complications: splenic infarct, focal or massive, detected by colour Doppler ultrasonography (CD-US).
Abscess: culture-positive purulent drainage from an intra-abdominal fluid collection obtained percutaneously or intra-operatively, and/or radiographically-confirmed fluid collection, with systemic or localized signs of infection.
Pneumonia: presence of a new infiltrate on chest radiograph, with the following: temperature >38°C; abnormal elevation of white blood count; or positive symptoms Gram stain or culture, and requiring intravenous antibiotic treatment.
Length of hospital stay (LHS): days from the initial operation to hospital discharge.
Conversion: need for an abdominal incision to deal with any intra-operative complication; the need to make an abdominal incision when a safe dissection is not possible on oncological grounds.
All tumours were classified according to the World Health Organization (WHO) classification,21Table 1.
Table 1.
Clinicopathological features of pancreatic neuroendocrine neoplasms
| Classification | Classes | Criteria |
|---|---|---|
| WHO | 1 Well-differentiated tumour | |
| 1.1 Benign | <2 cm contained in the pancreas, no angioinvasion/perineural invasion, ≤2 mitoses/10 HPF, <2% Ki-67 | |
| 1.2 Uncertain | ≥2 cm contained in the pancreas, angioinvasion/perineural invasion, >2–10 mitoses/10 HPF, >2% Ki-67 | |
| 2 Well-differentiated carcinoma | Low-grade malignant, gross local invasion and/or metastases | |
| 3 Poorly-differentiated carcinoma | High-grade malignant >10/10 HPF, >16% Ki-67 | |
Results
A total of 30 consecutive patients with a non-functional NPT between April 1998 and September 2010 underwent laparoscopic pancreatic surgery (median age 56.5 years, range 44 to 83).
Only 13 patients with tumours ≤3 cm in size (median 2.8 cm and range 2.8–3 cm) underwent LapEn. Lymph node sampling of the areas in close proximity to the LapEn was performed in all patients, and these samples were sent for frozen-section examination. In three patients with tumours localized in the head (n = 1) and the body (n = 2) of the pancreas, the pathological report was positive for lymph node metastases. Lymph node dissection was performed in one patient along the hepatic artery (area 8), superior mesenteric vein (area 14) and anterior head of the pancreas (area 17); ten lymph nodes were retrieved, and 2 from station 8 were malignant. In two other patients, a regional lymphadenectomy was performed, including area 8 (hepatic artery), 9 (celiac trunk), 11 (splenic artery) and 18 (inferior border of the pancreas); eight and 9 lymph nodes, respectively, were retrieved and malignancy was found in one (1/5) node from station 11 in one patient, and in 2 (2/4) nodes from station 18 in the other patient.
The median operating time was 130 min (range 90–280) and the median blood loss was 220 ml (120–300).
A pancreatic fistula occurred in five patients: International Study Group on Pancreatic Fistula (ISGPF) A in two patients and ISGPF B in three patients.
According to the WHO classification,22 10 patients were classified as uncertain behaviour and 3 patients had tumours with evidence of malignancy (well differentiated carcinoma).
There were no deaths in the series. The median LHS was 6 days (range 6–7).
The median follow-up and range was 48 (12–144) months, respectively. All three patients with lymph node metastases at the time of LapEn and regional lymphadenectomy are free of disease 2, 3 and 4 years after surgery. In the course of the study, one patient 5 years after LapEn for non-functional uncertain behaviour tumour presented with lymph node and liver metastases (segments II and III) which were successfully resected using a laparoscopic approach.
Discussion
The operative approach to NPT is still a matter of debate. Enucleation has become an alternative procedure in patients with benign-appearing tumours. The potential advantages of this less invasive procedure are less blood loss and better preservation of pancreatic function. Therefore, enucleation has become an accepted procedure for the treatment of insulinoma.5–14 The question of laparoscopic enucleation in non-functional NPT is still not settled.
Enucleation for NPT is not a simple procedure, whether performed open or laparoscopically. In addition, pancreas-related complications may occur. In an international review of benign insulinomas treated by open surgery, Rothmund et al.22 reported a complication rate of 32%. Hellman et al.23 evaluated the outcome of 65 patients operated on for organic hiperinsulinism using the open approach. A pancreatic fistula occurred after enucleation from the head in 50%, from the body-tail of the pancreas in 24% and after distal pancreatectomy in 26%. Park et al.24 reported that experience with open surgical treatment in patients with islet cell tumours arising in the head of the pancreas (70% sporadic insulinoma). In 22 patients with simple enucleation, the mean operative time was 4 h, the mean blood loss 360 ml, the median time for return to regular diet 7 days and the median time to drain removal 22 days. In that previous series, 67% of patients had no complications; however, a PF, requiring intravenous alimentation and prolonged hospital stay, occurred in 15% of patients. We interpret this as a grade C pancreatic fistula according to ISGPF. Furthermore, 12 patients (44%) had some amount of amylase-rich fluid drainage (biochemical grade A fistula). Other complications observed were pancreatitis (4%), an intra-abdominal abscess (4%), post-operative bleeding (4%) and delayed gastric emptying (4%). Crippa et al.25 reported 61 consecutive patients who underwent pancreatic enucleation, among whom 38 had neuroendocrine tumours (22 insulinomas and 16 non-functional tumors). Open enucleation was performed in 93% of patients and LapEn in 7%. Overall morbidity was 43%. Pancreatic fistula was also reported in 38% of patients (ISGPF type A 39%, type B 43%, and type C 18%). Reoperation was performed in 8% of patients. The mean LHS was 9 days.
Recently, Pitt et al.26 in a multi-institutional retrospective review compared the outcomes of enucleation and resection in 87 patients with a small NPT. The pancreatic fistula rate was 38% after enucleation (ISGPF type A 57%, type B 43%) and 15% after resection (ISGPF type A 23%, type B 62%, and type C 15%). Length of hospital stay after enucleation and resection were 9 and 10 days, respectively. The authors concluded that, although enucleated patients had a higher incidence of a PF formation compared with the resection group, the fistulas that formed after resection were more clinically significant (grades B and C).
Recently, Dedieu et al.27 summarized the series of LapEn for cystic tumours and NPT. Mortality was 4%, morbidity 7%–60%, pancreatic fistula rate 13%–50%, re-operation rate 0%–8% and LHS 9–19.5 days.
Compared with small, benign insulinomas that are readily curable by surgical resection,5–14 non-functional NPT have a much less-favourable prognosis.16,17 Approximately 50% to 80% of these neoplasms will have a recurrence or metastasize, and up to one-third of the patients already have metastases at initial presentation. The present study has demonstrated the feasibility and safety of LapEn for a non-functional NPT ≤3 cm. However, one of the concerns in the management of non-functional NPT is the question of malignancy. At the time of LapEn the distinction between benign and malignant is difficult. Although macroscopically most tumours appear well demarcated, they often fail to exhibit a well-defined capsule and are usually white-grey to pinkish-brown and of firm consistency. The clearest evidence of malignancy is provided by local or capsular invasion or the presence of lymph node metastases. In the present study, three patients were found with lymph node metastases at the time of LapEn. Recently, Edil et al.28 reported the outcome of the surgical treatment of 218 sporadic, non-functional NPT. In all, 140 patients (44%) had positive lymph nodes. Tumour size was associated with lymph node metastasis: <1 cm: 14%; 1–1.9 cm: 9%; 2–2.9 cm: 37%; 3–3.9 cm: 56%; 4–4.9 cm: 72%; and ≥5 cm: 56%. The previous authors concluded that size of NPT correlates with lymph node metastases, and even small, <1 cm NPT metastasize to lymph nodes. Lymph node metastases, furthermore, correlate with survival. We believe lymph node sampling should be mandatory to rule out malignancy. Lymph node dissection should be performed after LapEn as an oncologically sound operation. So far, there is no evidence that a more extended pancreatic resection will result in less recurrence and better patient survival.
Conclusion
Surgical resection is the treatment of choice for patients with NPT. For small tumours ≤3 cm, NPT enucleation preserves healthy parenchyma and pancreatic function. In addition, the laparoscopic approach offers clear advantages to the patients in terms of reduced parietal damage in the abdomen. It seems that the risk of PF is high after LapEn for tumours in the pancreatic head vs. those located in the body-tail. The present study provides evidence for the surgical strategy in patients with non-functional NPT. Intra-operative lymph node sampling and frozen-section examination should be part of the evaluation in patients with non-functional NPT. When malignancy is confirmed the surgeon may continue with LapEn provided an oncologically appropriate lymph node dissection is performed.
Conflicts of interest
Laureano Fernández-Cruz, Víctor Molina, Rodrigo Vallejos, Enrique Jiménez Chavarria, Miguel-Angel López-Boado and Joana Ferrer have no conflicts of interest or financial ties to disclose.
References
- 1.Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resection of islet cell tumors. Surgery. 1996;120:1051–1054. doi: 10.1016/s0039-6060(96)80054-7. [DOI] [PubMed] [Google Scholar]
- 2.Assalia A, Gagner M. Laparoscopic pancreatic surgery for islet cell tumors of the pancreas. World J Surg. 2004;28:1239–1247. doi: 10.1007/s00268-004-7617-8. [DOI] [PubMed] [Google Scholar]
- 3.Mabrut JY, Fernández-Cruz L, Azagra JS, Bassi C, Delvaux G, Weerts J, et al. Laparoscopic pancreatic resection: Results of a multicenter European study of 127 patients. Surgery. 2005;137:597–605. doi: 10.1016/j.surg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Briggs CD, Mann CD, Irving GRB, Neal C, Peterson M, Cameron IC, et al. Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg. 2009;13:1129–1137. doi: 10.1007/s11605-008-0797-z. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Cruz L, Blanco L, Cosa R, Rendon H. Is laparoscopic resection adequate in patients with neuroendocrine pancreatic tumors? World J Surg. 2008;32:904–917. doi: 10.1007/s00268-008-9467-2. [DOI] [PubMed] [Google Scholar]
- 6.Berends FJ, Cuesta MA, Kazemier G, van Eijck CH, de Herder WW, van Muiswinkel JM, et al. Laparoscopic detection and resection of insulinomas. Surgery. 2000;128:386–390. doi: 10.1067/msy.2000.107413. [DOI] [PubMed] [Google Scholar]
- 7.Gramatica L, Herrera MF, Mercado-Luna A, Sierra M, Verasay G, Brunner N. Videolaparoscopic resection of insulinomas. World J Surg. 2002;26:1297–1300. doi: 10.1007/s00268-002-6711-z. [DOI] [PubMed] [Google Scholar]
- 8.Ihiara M, Obara T. Minimally invasive endocrine surgery: Laparoscopic resection of insulinomas. Biomed Pharmacother. 2002;56:227–230. doi: 10.1016/s0753-3322(02)00238-x. [DOI] [PubMed] [Google Scholar]
- 9.Ayav A, Bresler L, Brunand L, Boissel P. Laparoscopic approach for insulinoma: a multicenter study. Langenbecks Arch Surg. 2005;390:134–140. doi: 10.1007/s00423-004-0526-3. [DOI] [PubMed] [Google Scholar]
- 10.Toniato A, Meduri F, Foletto M, Avogaro A, Pelizzo M. Laparoscopic treatment of benign insulinomas localized in the body and tail of the pancreas. World J Surg. 2006;30:1916–1919. doi: 10.1007/s00268-005-0645-1. [DOI] [PubMed] [Google Scholar]
- 11.Jaroszewski D, Schlinkert RT, Thompson GB, Schlinker DK. Laparoscopic localization and resection of insulinomas. Arch Surg. 2004;139:270–274. doi: 10.1001/archsurg.139.3.270. [DOI] [PubMed] [Google Scholar]
- 12.Sa Cunha A, Beau C, Rault A, Catargi B, Collet D, Masson B. Laparoscopic versus open approach for solitary insulinoma. Surg Endosc. 2007;21:103–108. doi: 10.1007/s00464-006-0021-8. [DOI] [PubMed] [Google Scholar]
- 13.Sweet MP, Izumisato Y, Way LW, Clerk OH, Masharani U, Duh AY. Laparoscopic enucleation of insulinomas. Arch Surg. 2007;142:1202–1204. doi: 10.1001/archsurg.142.12.1202. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Peng C, Zhang S, Wu Y, Fang H, Sheng H, et al. Strategy for the surgical management of insulinomas: analysis of 52 cases. Dig Surg. 2007;24:463–470. doi: 10.1159/000111822. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Fredland A. The Diagnosis and Treatment of Insulinomas. Philadephia, PA: W.B. Saunders; 1983. pp. 60–70. [Google Scholar]
- 16.Kloppel G, Heitz PU. Pancreatic endocrine tumors. Pathol Res Pract. 1988;183:155–168. doi: 10.1016/S0344-0338(88)80043-8. [DOI] [PubMed] [Google Scholar]
- 17.Heitz PU, Kasper M, Polak JM, et al. Pancreatic endocrine tumors. Hum Pathol. 1982;13:263–271. doi: 10.1016/s0046-8177(82)80183-4. [DOI] [PubMed] [Google Scholar]
- 18.Fernández-Cruz L, César-Borges G. Laparoscopic strategies for resection of insulinoma. J Gastrointest Surg. 2006;10:752–760. doi: 10.1016/j.gassur.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Japan Pancreas Society. General Rules for Surgery and Pathological Studies on Cancer of the Pancreas. 3rd. Tokyo: Kanehara; 1986. pp. 2–5. [Google Scholar]
- 20.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Heitz PU, Komminoth P, Perren A. WHO histological classification of tumors of the endocrine pancreas. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. Pathology and Genetic Tumors of Endocrine Organs. 1st. Lyon, France: IARC Press; 2004. pp. 177–182. [Google Scholar]
- 22.Rothmund M, Angelini L, Brunt LM, Farndon JR, Geelhoed G, Grama D, et al. Surgery for benign insulinoma: an international review. World J Surg. 1990;14:393–398. doi: 10.1007/BF01658536. [DOI] [PubMed] [Google Scholar]
- 23.Hellman P, Goretzki P, Simon D, Dotzenrath C, Röher HD. Therapeutic experience of 65 cases with organic hyperinsulinism. Langenbecks Arch Surg. 2000;385:329–336. doi: 10.1007/s004230000148. [DOI] [PubMed] [Google Scholar]
- 24.Park BJ, Alexander HR, Libatti SK, Huang J, Royalty D, Skarulis MC, et al. Operative management of islet cell tumor arising in the head of the pancreas. Surgery. 1998;124:1056–1061. doi: 10.1067/msy.1998.92171. [DOI] [PubMed] [Google Scholar]
- 25.Crippa S, Bassi C, Salvia R, Falconi M, Butturini G, Pederzoli P. Enucleation of pancreatic neoplasms. Br J Surg. 2007;94:1254–1259. doi: 10.1002/bjs.5833. [DOI] [PubMed] [Google Scholar]
- 26.Pitt S, Pitt HA, Baker MS, Christians K, Tonzios JG, Kiely JM, et al. Small pancreatic and periampullary neuroendocrine tumors: resect or enucleat. J Gastrointest Surg. 2009;13:1692–1698. doi: 10.1007/s11605-009-0946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dedieu A, Rault A, Collet D, Masson B, Sa Cunha A. Laparoscopic enucleation of pancreatic neoplasm. Surg Endosc. 2011;25:572–576. doi: 10.1007/s00464-010-1223-7. [DOI] [PubMed] [Google Scholar]
- 28.Edil BH, Ellison JL, Cameron R, Venkat R, Pawlik M, Choti M, et al. Even small pancreatic endocrine neoplasm have lymph node metastasis. Abstract. Pancreas Club 2011 program page 55.


