Abstract
Objectives
Accurate prediction of safe remnant liver volume to minimize complications following liver resection remains challenging. The aim of this study was to assess whether quantification of steatosis improved the predictive value of preoperative volumetric analysis.
Methods
Thirty patients undergoing planned right or extended right hemi-hepatectomy for colorectal metastases were recruited prospectively. Magnetic resonance imaging was used to assess the level of hepatic steatosis and future remnant liver volume. These data were correlated with data on postoperative hepatic insufficiency, complications and hospital stay. Correlations of remnant percentage, remnant mass to patient mass and remnant mass to body surface area with and without steatosis measurements were assessed.
Results
In 10 of the 30 patients the planned liver resection was altered. Moderate–severe postoperative hepatic dysfunction was seen in 17 patients. Complications arose in 14 patients. The median level of steatosis was 3.8% (range: 1.2–17.6%), but was higher in patients (n= 10) who received preoperative chemotherapy (P= 0.124), in whom the median level was 4.8% (range: 1.5–17.6%). The strongest correlation was that of remnant liver mass to patient mass (r= 0.77, P < 0.001). However, the addition of steatosis quantification did not improve this correlation (r= 0.76, P < 0.001).
Conclusions
This is the first study to combine volumetric with steatosis quantifications. No significant benefit was seen in this small pilot. However, these techniques may be useful in operative planning, particularly in patients receiving preoperative chemotherapy.
Keywords: fatty liver, morbidity, volumetry, liver resection, steatosis, complications
Introduction
Liver resection, if possible, is the favoured management option for patients with colorectal liver metastases, offering 5-year survival rates of 35–58%1,2 and the only chance of cure. The only contraindications to resection are patient fitness, presence of unresectable extrahepatic disease and insufficient future liver remnant (FLR) volume.3 Despite some previous studies,4–8 data on the precise definition of insufficient FLR volume are unavailable and figures of 20–30% have often been arbitrarily cited. Some groups have hypothesized that the proportion of fat within the liver may be an important factor in predicting which patients are at higher risk for post-resection hepatic insufficiency,5,9 but the presence of fat within the liver has not been assessed in any previous volumetric studies. This study aimed to assess whether the quantification of hepatic steatosis improves the ability of volumetric analysis of FLR to predict hepatic insufficiency.
Materials and methods
Patient selection
Ethics committee approval was obtained in line with national guidelines (ref no. 06/Q1205/168; Leeds (West) Research Ethics Committee). Thirty patients who had previously undergone bowel resection and in whom right hemi-hepatectomy or extended right hemi-hepatectomy as a first liver resection for colorectal metastases was planned in 2007 and 2008 were included in the study. The decision on the procedure was made after the evaluation of tumour distribution using magnetic resonance imaging (MRI) of the liver, computed tomography (CT) of the chest, abdomen and pelvis, and positron emission tomography (PET), followed by discussion at a multidisciplinary team meeting. This unit's standard protocol of utilizing 2-mm slices with and without contrast was applied; this has been described in detail elsewhere.10 This group of patients was selected to ensure both uniformity of disease and a high risk for hepatic insufficiency as all of these patients would be expected to have at least 50% of their liver resected. All were deemed to have sufficient liver function to undergo a major hepatic resection.
Demographic information was recorded. This included preoperative height and weight so that the body surface area (BSA) could be calculated. Body mass index (BMI) was calculated as weight in kilograms divided by height in metres squared (kg/m2) and BSA was calculated as weight in kilograms to the power of 0.5 multiplied by height in centimetres to the power of 0.5 divided by 60 (m2).
All patients underwent daily routine blood tests to assess liver function on days 1–5 and at least every other day thereafter until hospital discharge. Arterial blood gas analysis was carried out routinely while patients were in high-dependency care. Encephalopathy was also assessed.11 The primary outcome measures were hepatic insufficiency and patient morbidity. Hepatic insufficiency was quantified using the definitions described previously (Table 1).5 Morbidity was collected and quantified according to the Clavien grade.12 In addition, postoperative length of stay was included as a secondary outcome measure.
Table 1.
Definition of postoperative hepatic dysfunction based on results from blood tests and clinical observation
| Severity of hepatic dysfunctiona | |||
|---|---|---|---|
| 0 | 1 | 2 | |
| Total serum bilirubin, µmol/l | 20 | 21–60 | >60 |
| Prothrombin time, s above normal | <4 | 4–6 | >6 |
| Serum lactate, mmol | <1.5 | 1.6–3.5 | >3.5 |
| Encephalopathy grade | No | 1 and 2 | 3 and 4 |
Severity of hepatic dysfunction: 0, none; 1–2, mild; 3–4, moderate; >4, severe.
MRI assessment
Examination by MRI for volumetry and steatosis assessment was carried out with an additional scan on the day before surgery. All MRI was performed at a field strength of 1.5 T using a Symphony system (Siemens AG, Erlangen, Germany) and a body phased array coil. Imaging comprised steady-state precession (TrueFISP) sequences acquired in coronal and axial planes with a slice thickness of 7 mm and standard spoiled gradient echo (GRE) T1-weighted in-phase and opposed-phase sequences. In addition, several breath-hold sequences were acquired to quantitatively assess hepatic fat. The details of these sequences are provided in the section on steatosis quantification.
All images were acquired during breath-holding with an acquisition time of approximately 20 s per image, resulting in a total examination time of 15–20 min.
Volumetry assessment
Slices of 7 mm in thickness were used to ensure that the entire liver was imaged. On each slice, regions of interest were drawn to measure the total liver volume (TLV), tumour volume (TV) and remnant volume (RV), thus enabling the volume of each in the whole liver to be assessed in each patient.
Steatosis quantification
In- and out-of-phase two-dimensional (2-D) spoiled GRE images of the liver were obtained with TR = 112 ms, TE = 2.3 ms (opposed phase) and TE = 4.6 ms (in-phase) with a flip angle of 10 °. Additional TE = 9.2 ms images were acquired to correct for T2* decay.
The hepatic fat fraction (FF) was calculated according to the equation:
where Sip is the signal from the 10° in-phase images and Sop is the signal intensity from the 10° opposed-phase images.
The fat fraction was calculated for three regions of interest selected from the background normal liver parenchyma in the predicted liver remnant and a mean was derived. These three regions were selected randomly (by ALY and JW) to avoid regions close to any tumour or major blood vessels. These data were used to calculate the functional remnant liver volume (FRLV) defined as the remnant liver volume (RLV)*(1 − FF). This methodology has been validated at other centres13,14 and locally where the R2 value was 0.88.15
Magnetic resonance spectra were acquired from a 20 × 20 × 10-mm voxel in the liver using a single-voxel PRESS sequence with a TR of 5000 ms. Spectra at TEs of 30, 40, 50, 60 and 70 ms enabled the calculation of T2 for both fat and water. The fat fraction was calculated by measuring the areas under the T2 corrected fat and water peaks.
Predicting hepatic dysfunction
In addition to the Edinburgh model5 for predicting hepatic dysfunction (Table 1), secondary outcome measures were infective complications, Clavien grade and postoperative stay. These outcome measures were compared with five different assessments using the volumetric and steatosis analyses:
remnant percentage: RLV/TLV × 100;
remnant liver volume relative to body mass: RLV/body mass × 100;
functional remnant liver volume relative to body mass: FRLV/body mass × 100;
total remnant liver volume relative to body surface area: RLV/BSA × 100, and
functional remnant liver volume relative to body surface area: FRLV/BSA × 100.
Validation
The resection specimen was used to calculate the displacement volume of normal saline and was weighed using electronic scales.
Statistics
Non-parametric data are presented as medians (ranges); categorical data are presented as frequencies. Correlation analyses were carried out using Spearman's correlation coefficient. Continuous variables were assessed using the Mann–Whitney U-test. All statistical tests were carried out using spss Version 14.0 (SPSS, Inc., Chicago, IL, USA). Statistical significance was taken at 5%. For correlation analyses, an r-value of >0.7 indicated a strong correlation.
Results
Patient population
The 30 patients included 21 male and nine female patients with a median age of 65 years (range: 45–82 years) (Table 2). Their median height was 1.70 m (range: 1.54–1.94 m), median mass was 75 kg (range: 60–113 kg) and median BMI was 26.3 kg/mm2 (range: 21.3–34.8 kg/mm2). Their median body surface area was 1.91 m2 (range: 1.09–2.47 m2). Ten patients had received preoperative chemotherapy, predominantly with oxaliplatin and 5-fluorouracil (5-FU).
Table 2.
Patient characteristics (n= 30)
| Sex, n | |
| Male | 21 |
| Female | 9 |
| Age, years, median (range) | 65 (45–82) |
| Preoperative chemotherapy, n | 10 |
| Height, m, median (range) | 1.70 (1.54–1.94) |
| Mass, kg, median (range) | 75 (60–113) |
| Body mass index, kg/m2, median (range) | 26.3 (21.3–34.8) |
| Body surface area, m2, median (range) | 1.91 (1.65–2.47) |
| Total liver volume, cm3, median (range) | 1614 (1256–2889) |
| Total tumour volume, cm3, median (range) | 96.5 (5–865) |
| Remnant volume, cm3, median (range) | 562.9 (238–1784) |
| Remnant liver, %, median (range) | 35.36 (15–100) |
| Liver steatosis, %, median (range) | 3.76 (1.18–17.57) |
| Extent of resections, n | |
| Right trisectionectomy | 2 |
| Extended right hemi-hepatectomy | 3 |
| Right hemi-hepatectomy + caudate | 1 |
| Right hemi-hepatectomy + left-sided metastasectomies | 7 |
| Right hemi-hepatectomy | 7 |
| Central hepatectomy | 1 |
| Right anterior sectionectomy + left-sided metastasectomies | 1 |
| Non-anatomical resections | 8 |
Assessment of volume and steatosis
The median TLV was 1614 cm3 (range: 1256–2889 cm3) and median TV was 96.5 cm3 (range: 5–865 cm3). The median level of steatosis was 3.8% (range: 1.2–17.6%) (Fig. 1). The level of steatosis was higher in patients who received preoperative chemotherapy (median 4.8%; range: 1.5–17.6%) relative to those who did not (median 3.0%; range: 1.2–15.4%), although this difference did not reach statistical significance (P= 0.124, Mann–Whitney U-test).
Figure 1.
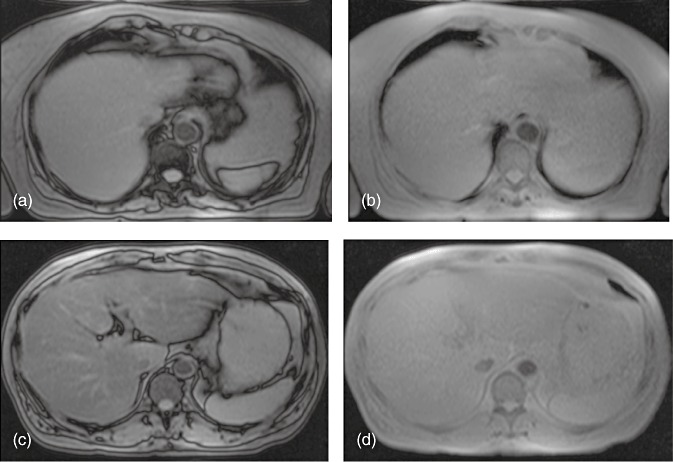
Magnetic resonance imaging showing (a, b) mild steatosis (3.4%) in (a) opposed-phase and (b) in-phase scans and (c, d) severe steatosis (17.6%) in (c) opposed-phase and (d) in-phase scans
Validation
The volume (saline displacement) and mass of the resected specimen showed an excellent correlation with one another (r= 0.967, P < 0.001) (Fig. 2). Both the volume and mass of the resected specimen correlated well with the predicted resection volume (volume: r= 0.897, P < 0.001; mass: r= 0.901, P < 0.001) (Fig. 2).
Figure 2.
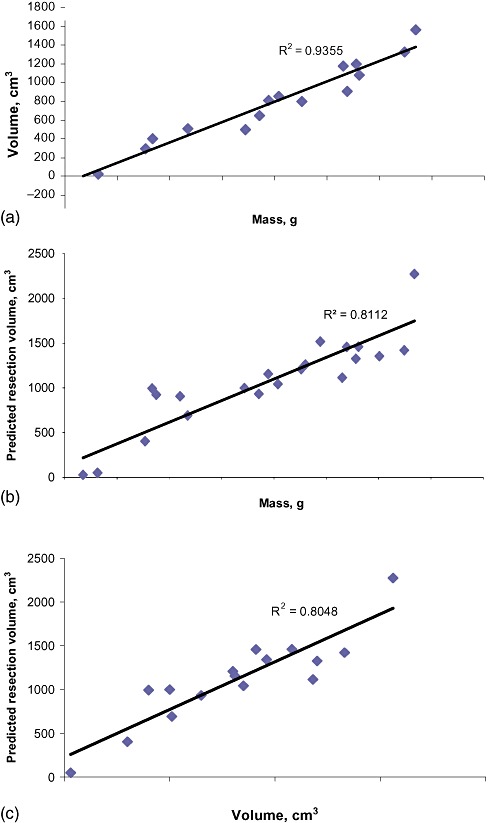
Graphs showing strong correlations between (a) the mass and volume of the resected specimen, (b) the mass of the resected specimen and predicted resection volume, and (c) the volume of the resected specimen and predicted resection volume
Resections
Although right or extended right hemi-hepatectomy (resection of segments V, VI, VII and VIII with or without segments I and IV) was planned in all patients, intraoperative surgical assessment changed the extent of resection in 10 patients (Table 2). This decision reflected changes in the disease extent or concern that the condition of the liver parenchyma put the patient at high risk for postoperative hepatic insufficiency. Eight patients had solitary tumours and the rest had multiple metastases. The median size of the largest tumour was 40 mm (range: 11–162 mm). A total of 27 patients underwent a Pringle manoeuvre applied for a median of 30 min (range: 12–75 min). The median length of surgery was 150 min (range: 60–330 min). No patients required perioperative blood transfusion.
Complications
There were no deaths. Overall, 14 patients suffered 19 complications: three patients experienced Clavien Grade IV complications; six experienced Clavien Grade III complications; three experienced Clavien Grade II complications, and two experienced Clavien Grade I complications. Eight patients had infective complications (five urinary tract infections, two postoperative collections and one wound infection). Other complications included two bile leaks, one gastrointestinal bleed, one atrial fibrillation, one pulmonary oedema, one case of transient postoperative jaundice, one case of transient encephalopathy with four other complications. The median postoperative stay was 10 days (range: 4–79 days). Two patients had no hepatic dysfunction, 11 had mild, 14 had moderate and three had severe dysfunction (Table 3).
Table 3.
Assessment of outcomes in 30 patients
| Hepatic dysfunction, n | |
| None | 2 |
| Mild | 11 |
| Moderate | 14 |
| Severe | 3 |
| Infective complications, n | 8 |
| Clavien score complications, n | 14 |
| Postoperative stay, days, median (range) | 10 (4–79) |
Correlations for predicting hepatic dysfunction
The five predictive assessments were evaluated to determine which most accurately predicted hepatic dysfunction (Fig. 3). This showed that remnant percentage had marginally the weakest predictive value and that total RLV to BMI, and FRLV to BMI both showed slightly stronger correlations (Table 4). Correlations using BSA rather than BMI were slightly less accurate in predicting hepatic dysfunction (Table 4).
Figure 3.
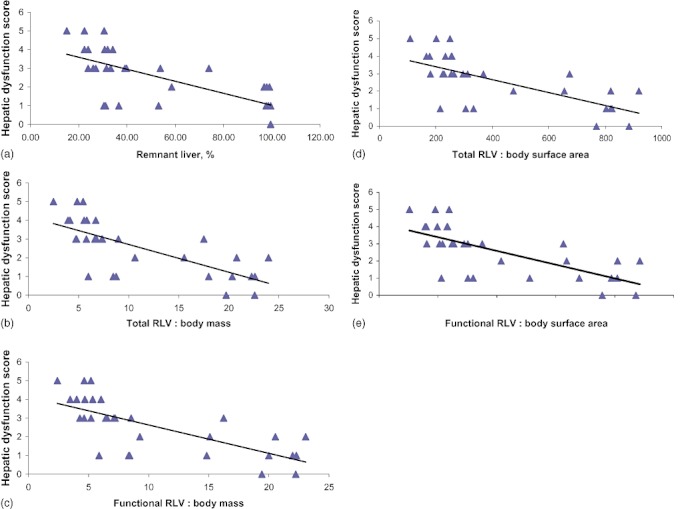
Correlations between hepatic dysfunction as assessed by the Edinburgh score5 and (a) percentage of remnant liver volume (RLV), (b) RLV and body mass, (c) functional RLV and body mass, (d) RLV and body surface area and (e) functional RLV and body surface area
Table 4.
Correlations between outcome measures and predictive indices
| RLV, % | TRLV : body mass | FRLV : body mass | TRLV : BSA | FRLV : BSA | |
|---|---|---|---|---|---|
| Hepatic dysfunction | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| r=−0.710 | r=−0.770 | r=−0.763 | r=−0.715 | r=−0.741 | |
| Infectious complications | P= 0.071 | P= 0.031 | P= 0.049 | P= 0.015 | P= 0.028 |
| Clavien score | P= 0.025 | P= 0.018 | P= 0.024 | P= 0.008 | P= 0.011 |
| r=−0.403 | r=−0.430 | r=−0.411 | r=−0.476 | r=−0.458 | |
| Postoperative stay | P= 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| r=−0.509 | r=−0.631 | r=−0.621 | r=−0.613 | r=−0.630 | |
RLV, remnant liver volume; TRLV, total remnant liver volume; FRLV, functional remnant liver volume; BSA, body surface area.
Predicting morbidity and postoperative stay
The five ways of potentially predicting the postoperative course from the volumetric and steatosis analyses were used to assess their value in predicting infectious complications, complications classified by Clavien score and postoperative stay (Table 4).
All five methods of volumetric ± steatosis analyses were useful in predicting infectious complications and complications according to Clavien score, but all were more able to predict postoperative stay. None of the five methods of assessment were clearly superior at predicting infectious complications, all complications (by Clavien grade) or postoperative stay.
Discussion
In hepatic surgery, the accurate preoperative prediction of a safe future liver remnant in terms of volume remains challenging, whether the liver parenchyma is normal or diseased. The increasing incidence of obesity and the steatotic changes induced by chemotherapy make this assessment more difficult. This study aimed to take these factors into account and to examine the impact of steatosis on volumetric assessment in terms of predicting postoperative hepatic dysfunction and the occurrence of postoperative complications. If this method of prediction proved successful, this information could be used to help quantify perioperative risk in patients undergoing liver resection. It might also be helpful in determining which patients might benefit from further preoperative optimization by using techniques such as portal vein embolization.16
The assessment of postoperative hepatic dysfunction remains controversial and various systems for doing this have been suggested.5,6,12,17 The 50-50 criterion appears to be the most valid at this point,17 but none of the patients in this pilot study met this criterion. The system used in the current study appeared to allow a dispersion of dysfunction which has proved to be useful in other similar studies.5 Agreement on the most useful and valid method of assessing postoperative hepatic dysfunction would assist in the interpretation of future studies.
This pilot study is likely to be biased because patients in whom predicted functional liver volume was borderline (as assessed by combining the subjective assessments of volume and steatosis during surgery) were switched by the surgeon from resection to an oncologically potentially more risky procedure, but one that might decrease the risks for perioperative mortality and morbidity. This stance is supported by data showing that perioperative morbidity leads to oncologically poorer outcomes.18 It is unlikely that this bias can be reduced in future studies.
Assessment of immediate outcome after liver resection often relies upon perioperative morbidity, but this is problematic for several reasons. For example, five of 30 patients in the current series developed culture-proven urinary tract infections. All patients required urinary catheterization for fluid monitoring. Whether these infections reflected impairments to the immune system caused by hepatic dysfunction or urinary tract comorbidity cannot be ascertained. The use of the recently published Clavien system of classification represented an attempt to correct for the severity of complications.12 Postoperative hospital stay was thought to represent a potentially useful outcome measure. However, this also has the potential to skew the data. One patient underwent a right hemi-hepatectomy and achieved a dysfunction score of only 1, but remained in hospital with cardiac complications for 79 days. This patient developed atrial fibrillation and heart block and eventually required pacing and a prolonged period of monitoring under the joint care of surgeons and cardiologists.
Previous studies that have carried out volumetric analyses have made several assumptions about uniform hepatic function.5–8 There is no evidence that these assumptions are accurate and, in addition to variations among different segments, such as the caudate lobe, it is likely that there are variations in peritumoral blood flow and levels of cholestasis. Clearly, patient age, which affects regenerative capacity,19 in addition to the fat content of the liver,20 will affect the risk for postoperative morbidity, but age was not factored into the current model. In addition, steatosis is known to correlate with postoperative morbidity, but the direct effects of marked steatosis, such as that of making the liver more friable and therefore resection more technically demanding, must also be considered when attempting to quantitate perioperative risk.9,21 Correlating with a more quantitative measure of liver function, such as indocyanide green clearance, might have helped to address these issues to some extent.
The radiological assessment of hepatic steatosis using MRI has been previously shown to be accurate.15,22,23 As chemotherapy becomes increasingly common in patients prior to liver resection,24 with associated damage to the liver,25 accurate non-invasive assessments of hepatic steatosis are likely to improve preoperative risk stratification. This is the first study to quantify steatosis to assist in volumetric analysis in predicting liver dysfunction following major hepatic resection. However, this study does not show a clear predictive benefit of the addition of steatosis quantification. This may be related to the size of the current pilot study or to the inherent bias caused by the alterations in operative technique described above, or may indicate that the steatosis observed in this patient population was not severe enough to impact the results. However, further investigation may illuminate areas in which these techniques may be useful, such as in the assessment of patients for live donor liver transplantation and patients at high risk for post-resection complications, such as those with borderline FLR volume, obese patients, patients with metabolic syndrome and patients who have received large amounts of potentially hepatotoxic chemotherapy.
In summary, this pilot study has shown that a measure of hepatic steatosis is practical in volumetric analysis prior to liver resection. However, because of the limitations described above, its role in determining clinical decisions at this time is limited. A significantly larger study population would be required to show any potential benefit, at which point receiver operator characteristic curves could be plotted to produce a more accurate cut-off figure. There are a number of variables of importance which must be accounted for before any volumetric score can be used to accurately predict postoperative hepatic dysfunction.
Acknowledgments
This study was funded by a grant from Yorkshire Cancer Research.
Conflicts of interest
None declared.
References
- 1.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in longterm survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vauthey JN, Choti MA, Helton WS. AHPBA/SSO/SSAT Consensus Conference on hepatic colorectal metastases: rationale and overview of the conference. January 25, 2006. Ann Surg Oncol. 2006;13:1259–1260. doi: 10.1245/s10434-006-9017-9. [DOI] [PubMed] [Google Scholar]
- 4.van der Vorst JR, van Dam RM, van Stiphout RS, van den Broek MA, Hollander IH, Kessels AG, et al. Virtual liver resection and volumetric analysis of the future liver remnant using open source image processing software. World J Surg. 2010;34:2426–2433. doi: 10.1007/s00268-010-0663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 7.Chun YS, Ribero D, Abdalla EK, Madoff DC, Mortenson MM, Wei SH, et al. Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg. 2008;12:123–128. doi: 10.1007/s11605-007-0323-8. [DOI] [PubMed] [Google Scholar]
- 8.Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O, et al. Remnant liver volume to body weight ratio > or = 0.5%: a new cut-off to estimate postoperative risks after extended resection in non-cirrhotic liver. J Am Coll Surg. 2007;204:22–33. doi: 10.1016/j.jamcollsurg.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Gomez D, Malik HZ, Bonney GK, Wong V, Toogood GJ, Lodge JP, et al. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395–1402. doi: 10.1002/bjs.5820. [DOI] [PubMed] [Google Scholar]
- 10.Ward J, Robinson PJ, Guthrie JA, Downing S, Wilson D, Lodge JP, et al. Liver metastases in candidates for hepatic resection: comparison of helical CT and gadolinium- and SPIO-enhanced MR imaging. Radiology. 2005;237:170–180. doi: 10.1148/radiol.2371041444. [DOI] [PubMed] [Google Scholar]
- 11.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy – definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahl M, Qayyum A, Westphalen AC, Noworolski SM, Chu PW, Ferrell L, et al. Liver steatosis: investigation of opposed-phase T1-weighted liver MR signal intensity loss and visceral fat measurement as biomarkers. Radiology. 2008;249:160–166. doi: 10.1148/radiol.2491071375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McPherson S, Jonsson JR, Cowin GJ, O'Rourke P, Clouston AD, Volp A. Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J Hepatol. 2009;51:389–397. doi: 10.1016/j.jhep.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Biglands JD, Wilson D, Ward J, Treanor D, Gurthry A, Nijhawan A, et al. Comparison of MRI and histopathologic methods of quantifying hepatic fat fraction. Proc Intl Soc Magn Reson Med. 2006;14:2703. [Google Scholar]
- 16.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50-50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. discussion 828–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farid SG, Aldouri A, Morris-Stiff G, Khan AZ, Toogood GJ, Lodge JP, et al. Correlation between postoperative infective complications and longterm outcomes after hepatic resection for colorectal liver metastasis. Ann Surg. 2010;251:91–100. doi: 10.1097/SLA.0b013e3181bfda3c. [DOI] [PubMed] [Google Scholar]
- 19.Furrer K, Rickenbacher A, Tian Y, Jochum W, Bittermann AG, Käch A, et al. Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway. Proc Natl Acad Sci U S A. 2011;108:2945–2950. doi: 10.1073/pnas.1012531108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veteläinen R, van Vliet AK, van Gulik TM. Severe steatosis increases hepatocellular injury and impairs liver regeneration in a rat model of partial hepatectomy. Ann Surg. 2007;245:44–50. doi: 10.1097/01.sla.0000225253.84501.0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Mennesson N, Dumortier J, Hervieu V, Milot L, Guillaud O, Scoazec JY, et al. Liver steatosis quantification using magnetic resonance imaging: a prospective comparative study with liver biopsy. J Comput Assist Tomogr. 2009;33:672–677. doi: 10.1097/RCT.0b013e318199d883. [DOI] [PubMed] [Google Scholar]
- 23.Cesbron-Métivier E, Roullier V, Boursier J, Cavaro-Ménard C, Lebigot J, Michalak S, et al. Non-invasive liver steatosis quantification using MRI techniques combined with blood markers. Eur J Gastroenterol Hepatol. 2010;22:973–982. doi: 10.1097/meg.0b013e32833775fb. [DOI] [PubMed] [Google Scholar]
- 24.Nordlinger B, van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P, et al. European Colorectal Metastases Treatment Group; Sixth International Colorectal Liver Metastases Workshop. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985–992. doi: 10.1093/annonc/mdn735. [DOI] [PubMed] [Google Scholar]
- 25.Fong Y, Bentrem DJ. CASH (chemotherapy-associated steatohepatitis) costs. Ann Surg. 2006;243:8–9. doi: 10.1097/01.sla.0000193599.57858.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]


