Abstract
Objectives
Excessive blood loss during liver surgery contributes to postoperative morbidity and mortality and the minimizing of blood loss improves outcomes. This study examines pre- and intraoperative factors contributing to blood loss and identifies areas for improvement.
Methods
All patients who underwent elective hepatic resection between June 2007 and June 2009 were identified. Detailed information on the pre- and perioperative clinical course was analysed. Univariate and multivariate analyses were used to identify factors associated with intraoperative blood loss.
Results
A total of 175 patients were studied, of whom 95 (54%) underwent resection of three or more segments. Median blood loss was 782 ml. Greater blood loss occurred during major resections and prolonged surgery and was associated with an increase in postoperative complications (P = 0.026). Peak central venous pressure (CVP) of >10 cm H2O was associated with increased blood loss (P = 0.01). Although no differences in case mix were identified, blood loss varied significantly among anaesthetists, as did intraoperative volumes of i.v. fluids and transfusion practices.
Conclusions
This study confirms a relationship between CVP and blood loss in hepatic resection. Intraoperative CVP values were higher than those described in other studies. There was variation in the intraoperative management of patients. Collaboration between surgical and anaesthesia teams is required to minimize blood loss and the standardization of intraoperative anaesthesia practice may improve outcomes following liver surgery.
Keywords: liver, resection, hepatectomy, colorectal liver metastases, hepatocellular carcinoma, blood loss, haemorrhage, transfusion, complication, perioperative outcome
Introduction
Liver resection offers the only possibility of cure for patients with colorectal metastases1 and is an important treatment modality for patients with hepatocellular carcinoma.2 Initial experiences in hepatic resection were associated with significant morbidity and mortality; however, increased understanding of hepatic segmental anatomy and improvements in surgical equipment, associated with the accumulation of surgical experience, have led to continued improvements in perioperative outcomes and most major series now report mortality rates of <5%.3 As a result, liver resection is now seen as a first-line treatment in suitable patients.
Intraoperative blood loss is a predictor of perioperative outcome following liver resection3,4 and may have an effect on longterm disease-free survival.5,6 In addition to surgical techniques such as total vascular exclusion, modifications of intraoperative patient management procedures, such as low central venous pressure (CVP) anaesthesia and acute haemodilution, have been shown to reduce blood loss and perioperative morbidity.7,8
Many previous reports of liver resection series describe procedures in which expertise was still being accumulated or centre on small numbers of surgeons and anaesthetists, or refer to contexts in which less advanced techniques were utilized.3,7 In the current era of low CVP management and contemporary techniques, this study sought to examine the factors in perioperative care that are associated with blood loss and postoperative morbidity in an established tertiary referral centre for hepatic surgery.
Materials and methods
Data were collected from the Lothian Surgical Audit database and by retrospective case note review for patients who underwent liver resection at the Royal Infirmary of Edinburgh between June 2007 and June 2009. A standardized data collection form was used. Variables recorded included patient characteristics (age, gender, diagnosis, comorbidities), surgical, anaesthetic and intraoperative details, postoperative analgesia and i.v. fluids received, transfusion on the day of surgery (including intraoperative transfusion and transfusion administered in the anaesthesia recovery room), complications and overall length of hospital stay. Major resections were classified as those involving three or more segments. Complications were recorded using the Dindo–Clavien classification system.9 Intraoperative stability and volume status were assessed by collecting the highest and lowest recorded CVPs during the procedure, and the lowest recorded mean arterial pressure (MAP). Operative blood loss was recorded at the end of the operation by operating department staff. The names of surgeons and anaesthetists were coded during data entry and the analyses were performed in a blinded manner.
All data were entered into a database using Microsoft® Access 97. Data were analysed using spss Version 15.0 (SPSS, Inc., Chicago, IL, USA). The Mann–Whitney U-test was used for continuous variables and the chi-squared test for categorical variables. Data are presented as medians, with ranges in parentheses, except when stated otherwise. Spearman rank correlation analysis was performed to assess the relationship between peak and minimum CVP and operative blood loss. All factors identified on univariate analysis as predictive of operative blood loss of ≥1000 ml in major resections with a P-value of <0.1 were entered into a backwards stepwise multiple logistic regression model.
Results
Population characteristics
Over the 2-year period studied, 190 patients were submitted to liver resection. Comprehensive data were available for 175 (92%) of these patients. Demographic data are presented in Table 1.
Table 1.
Patient demographics and comorbidities (n = 175)
| Variable | |
|---|---|
| Age, years, median (range) | 61 (19–84) |
| Gender, male, n (%) | 92 (52.6%) |
| ASA grade, n (%) | |
| I | 23 (13.1%) |
| II | 115 (65.7%) |
| III | 28 (16.0%) |
| IV | 1 (0.6%) |
| Not stated | 8 (4.6%) |
| Smoker, n (%) | 35 (20.0%) |
| Diagnosis, n (%) | |
| Metastatic colorectal cancer | 106 (60.6%) |
| Cholangiocarcinoma | 10 (5.7%) |
| Hepatocellular carcinoma | 22 (12.6%) |
| Benign lesion | 16 (9.1%) |
| Other malignant lesion | 21 (12.0%) |
| Comorbidity, n (%) | |
| Diabetes mellitus | 17 (9.7%) |
| Hypertension | 50 (28.6%) |
| Ischaemic heart disease | 17 (9.7%) |
| Cerebrovascular disease | 6 (3.4%) |
| Chronic obstructive pulmonary disease | 2 (1.1%) |
ASA, American Society of Anesthesiologists.
Operative details and blood loss
Complete blood loss data were available for 166 (95%) patients. Intraoperative events and postoperative details are shown in Table 2. Operative details and complication rates for all resections stratified by blood loss are shown in Table 3. Underlying hepatic fibrosis or steatosis had no significant bearing on blood loss.
Table 2.
Intraoperative events and postoperative course
| Operating time, min, median (range) | 220 (50–735) |
| Estimated blood loss, ml, median (range) | 800 (25–15 000) |
| Extent of resection, n (%) | |
| Minor (<3 segments) | 80 (45.7%) |
| Major (≥3 segments) | 95 (54.3%) |
| Intraoperative fluids, ml, median (range) | 2000 (500–14 400) |
| Peak CVP, cm H2O, median (range) | 10 (2–17) |
| Minimum CVP, cm H2O, median (range) | 5 (−3–13) |
| Transfusion on day of surgery, n (%) | 45 (25.7%) |
| Length of ICU stay, days, median (range) | 0 (0–23) |
| Length of HDU stay, days, median (range) | 3 (0–8) |
| Length of hospital stay, days, median (range) | 6 (2–58) |
| Complications, n (%) | 60 (34.3%) |
| Dindo–Clavien grade of complication, n (%) | |
| I | 19 (10.9%) |
| II | 23 (13.1%) |
| IIIa | 4 (2.3%) |
| IIIb | 5 (2.9%) |
| IVa | 1 (0.6%) |
| IVb | 2 (1.1%) |
| V | 6 (3.4%) |
CVP, central venous pressure; ICU, intensive care unit; HDU, high dependency unit.
Table 3.
Operative details and complication rates for all resections stratified by blood loss
| Blood loss, ml | ||||
|---|---|---|---|---|
| 0–500 (n = 54) | 501–999 (n = 42) | ≥1000 (n = 79) | P-valuea | |
| Major resection, n (%) | 10 (18.5%) | 24 (57.1%) | 61 (77.2%) | <0.001 |
| Operative time, min, median (range) | 150 (50–270) | 235 (105–500) | 285 (140–735) | <0.001 |
| Intraoperative crystalloid, ml, median (range) | 1000 (200–3000) | 1250 (260–3000) | 2000 (900–7000) | 0.001 |
| Intraoperative colloid, ml, median (range) | 0 (0–2000) | 875 (0–2000) | 1500 (0–7000) | <0.001 |
| Complications, n (%) | 11 (20.4%) | 14 (33.3%) | 34 (43.0%) | 0.026 |
| In-hospital stay, days, median (range) | 6 (2–18) | 6 (3–58) | 7 (4–54) | <0.001 |
Chi-squared test.
Following the identification of an increased complication rate in patients with greater blood loss, analysis was undertaken to determine the pre- and intraoperative factors that might predict greater blood loss in major resections. Greater losses were seen in patients with hepatocellular carcinoma and cholangiocarcinoma. Neither pre-existing comorbidity nor operating surgeon affected blood loss, but the amount of blood loss was associated with the anaesthetist involved. Assessment of the intraoperative volume status of patients undergoing major resection revealed that higher peak CVP levels were associated with greater rates of blood loss of ≥1000 ml (P = 0.010) and peak CVP was weakly correlated with operative blood loss (Fig. 1). Analysis of preoperative laboratory results did not show any relationship between prothrombin time or platelets and bleeding.
Figure 1.
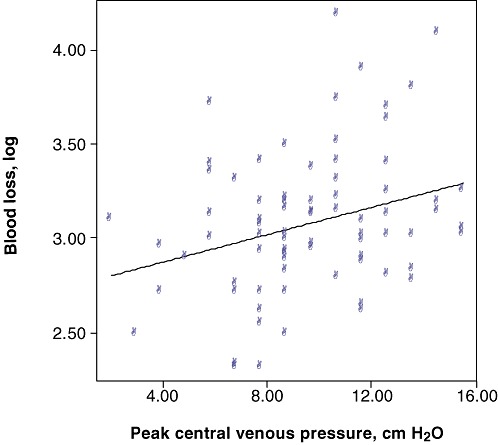
Correlation between blood loss and peak central venous pressure (CVP) showing a weak association between peak CVP and increased blood loss (P = 0.023, Spearman's rank coefficient R = 0.253)
Logistic regression was performed to establish which indices were independent predictors of blood loss of ≥1000 ml. Two factors remained independently significant: CVP of >10 cm H2O, and the responsible anaesthetist (P = 0.02) (Table 4). The regression model showed no lack of fit to the model (Hosmer–Lemeshow test, P = 0.963), but limited predictive value (Nagelkerke R2= 0.354).
Table 4.
Univariate and multivariate analyses of pre- and intraoperative factors and associations with blood loss of ≥1000 ml in major resections (≥3 segments)
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Univariate analysis | |||
| ≥1 comorbidity | 1.017 | 0.408–2.535 | 0.971 |
| Peak CVP >10 cm H2O | 3.339 | 1.340–8.310 | 0.010 |
| Minimum CVP <5 cm H2O | 1.314 | 0.556–3.101 | 0.534 |
| Age >70 years | 0.636 | 0.219–1.848 | 0.406 |
| Albumin <35 | 0.385 | 0.041–3.635 | 0.404 |
| Bilirubin >16 | 2.50 | 0.62–10.10 | 0.198 |
| Platelets <150 | 1.870 | 0.349–10.000 | 0.465 |
| Prothrombin time >12 s | 1.793 | 0.552–5.250 | 0.331 |
| Multivariate analysis | |||
| CVP >10 cm H2O | 5.52 | 1.70–17.20 | 0.003 |
| Anaesthetist | 0.020 | ||
| 1 | 1 | – | – |
| 2 | 0.036 | 0.002–0.670 | 0.026 |
| 3 | 0.01 | 0.00–0.26 | 0.006 |
| 4 | 0.10 | 0.10–1.04 | 0.054 |
| 5 | 0.111 | 0.004–2.780 | 0.181 |
| 6 | 0.278 | 0.025–3.162 | 0.302 |
| 7 | 0.600 | 0.049–7.407 | 0.691 |
OR, odds ratio; 95% CI, 95% confidence interval; CVP, central venous pressure.
Blood transfusion
Transfusion practices varied among anaesthetists. A total of 45 patients (26%) received blood on the day of surgery. Five anaesthetists administered blood only to patients in whom the total blood loss was >1500 ml. However, two anaesthetists took a more liberal approach to blood administration and transfused patients with lesser blood loss (minimum blood losses resulting in transfusions of 205 ml and 885 ml, respectively). Fifteen of the 62 patients under the care of these two anaesthetists received blood transfusions despite having lost <1500 ml of blood; these accounted for 15 of the 16 patients in whom blood loss was below this threshold and who received transfusions (Table 5).
Table 5.
Transfusion practices of different anaesthetists. Analysis was performed only in 159 patients for whom complete data were available and who were cared for by the ‘usual’ anaesthetists. Case mix data include those patients for whom blood loss data were unavailable
| Anaesthetist | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total | P-valuea | |
| Patients, n | 17 | 11 | 12 | 49 | 8 | 38 | 24 | 159 | |
| Patients transfused, n | 8 | 1 | 0 | 14 | 2 | 7 | 10 | 42 | 0.036 |
| Minimum blood loss resulting in transfusion, ml | 1630 | 2000 | N/A | 205 | 1500 | 2300 | 885 | N/A | N/A |
| Patients transfused with blood loss ≤1500 ml, n | 0/9 | 0/10 | 0/11 | 9/43 | 1/7 | 0/28 | 6/19 | 16/127 | N/A |
| Patients transfused with blood loss >1500 ml, n | 8/8 | 1/1 | 0/1 | 5/6 | 1/1 | 7/10 | 4/5 | 26/32 | N/A |
| Median blood loss, ml | 1200 | 400 | 568 | 705 | 750 | 813 | 1173 | 800 (25–15 000)b | 0.028 |
| Intraoperative crystalloid, ml | 1000 | 1000 | 1000 | 1000 | 2000 | 1500 | 2000 | 1500 (200–7000)b | <0.001 |
| Intraoperative colloid, ml | 1000 | 0 | 1000 | 1000 | 500 | 750 | 1000 | 1000 (0–7000)b | 0.285 |
| Total intraoperative fluids, ml | 2000 | 1500 | 2000 | 2000 | 3000 | 2000 | 3500 | 2000 (500–12 000)b | 0.011 |
| Case mix, n | |||||||||
| Major resections | 9 | 6 | 6 | 28 | 3 | 22 | 16 | 90 | |
| Minor resections | 9 | 5 | 7 | 23 | 5 | 17 | 9 | 75 | |
Kruskal–Wallis test.
Median (range).
N/A, not available.
Patients who received a blood transfusion on the day of surgery were more likely to suffer a postoperative complication (22 of 45 vs. 38 of 130; P = 0.001) and to have a prolonged hospital inpatient stay (median stay 6 days vs. 9 days; P < 0.001), and had a greater likelihood of in-hospital mortality (four of 45 vs. two of 130; P = 0.039). There was no difference in baseline clotting characteristics between patients who received transfusions and those who did not.
Discussion
This study demonstrates a relationship between CVP and blood loss in hepatic resection and suggests that the standardization of intraoperative management may improve outcomes associated with liver surgery.
The link between higher CVP values and greater intraoperative blood loss during parenchymal transection would appear to be intuitive. However, this has not been consistently demonstrated in previous studies because only approximately half of the published series have shown higher CVP to have an effect.10–12 This is one of the largest studies to show that CVP correlates with blood loss. Several recent studies on living donor liver transplantation (LDLT) have found that CVP had no effect on blood loss13,14 and some have advocated that CVP monitoring is not required in this type of surgery.15 However, this may relate to absolute CVP level. The findings of older studies correlating high CVP and blood loss7 have been adopted by these LDLT centres, which actively strive to maintain low target CVPs.13–15
The positioning of the pressure transducer is an important consideration in the interpretation of CVP because the relationship between the CVP transducer and the operative site will change depending on the segmental location of the resection and the physical dimensions of the liver.16
The data recorded in the present study represent maximum and minimum CVP levels, collected retrospectively from anaesthesia charts. The optimal index measurement is CVP during parenchymal transection, taking into account other factors such as positive end-expiratory pressure and transducer position. However, in this study the highest recorded CVP was predictive of blood loss. The strength of the association between CVP and blood loss might have been improved if transection CVP had been used; this measurement should be used in any future prospective study.
Variations in anaesthesia practice within and among institutions have been demonstrated previously in liver transplantation17,18 and in open heart surgery.19 This study found that practices in intraoperative fluid administration and blood transfusion differed within a group of anaesthetists experienced in the care of patients undergoing hepatic resection.
Intraoperative fluid administration varied among anaesthetists. There is increasing evidence that volume restriction may influence outcomes in critical care and major surgery.20 A recent randomized controlled clinical trial in cardiac surgery demonstrated that restrictive intraoperative fluid regimens are associated with reduced operative blood loss.21 In liver surgery, many centres have embraced a policy of restricted fluid administration.8,13,22 From this retrospective study, it is not possible to ascertain whether the relationship between intraoperative fluid administration and blood loss reflects one of cause or of effect, but the differences seen among different anaesthetists may suggest that it reflects inter-individual practice. Restricted fluid administration does not impair renal function in hepatic surgery22 and the current evidence would appear to favour a restrictive regimen in liver resection.
The overall transfusion rate in this series was 26%. The published literature describes highly variable rates of transfusion, which vary according to centre and the years for which data are analysed, and typically range from 17% to 50%.3,4,23 Although the overall transfusion rates in this study compare well with those of other studies, it is interesting to note that there were differences in transfusion triggers among anaesthetists. Variable transfusion practices have been noted both within and among institutions in liver transplantation.17,18 A relationship between increased transfusion rates and poorer postoperative outcomes has been demonstrated repeatedly in liver surgery. However, it remains unclear whether or not this simply reflects more difficult surgery. Although this study did not assess the transfusion trigger on a case-by-case basis, the implementation of a unit-wide agreement on transfusion triggers might reduce transfusion heterogeneity in this patient group.
There are a number of interventions to change transfusion practices which have been used previously in different settings, albeit with mixed levels of success.24 An international definition and grading of the severity of post-hepatectomy haemorrhage has been proposed to facilitate the comparison of results from different studies.25 Similarly, standardized grading of intraoperative blood loss could be applied to operative blood loss and replacement to facilitate and standardize the reporting of complications in future trials.
The anaesthetist has previously been recognized as a risk factor in cardiac surgery.26,27 No effect of anaesthetists on mortality or morbidity emerged in this study, but it is insufficiently powered to make such an assessment. There were no differences among anaesthetists in overall case mix and the relationship between anaesthetist and blood loss may reflect variations in intraoperative management and may be attributable to the amount of crystalloid administered intraoperatively or to CVP management.
This study has a number of limitations. These include its retrospective nature, potential unaccounted bias and the way in which CVP was recorded (peak CVP). Furthermore, the true triggers for blood ordering and administration in individual patients cannot be established. It will be essential to test the findings of this study in a prospective controlled study. In addition to improving the detail of CVP measurement (mean, peak and transectional CVP), such a study should seek to definitively identify the factors that trigger blood transfusion, including the assessment of the speed of blood loss, intraoperative haemoglobin levels and the time and point of blood ordering.
In conclusion, this study identified variations in the intraoperative management of patients undergoing liver surgery which were associated with increased operative blood loss. Collaboration between surgical and anaesthesia teams and the development of standardized protocols may contribute to the minimizing of blood loss and improve the outcomes associated with liver surgery.
Conflicts of interest
None declared.
References
- 1.Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55(Suppl. 3):iii1–iii8. doi: 10.1136/gut.2006.098053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarnagin W, Chapman WC, Curley S, D'Angelica M, Rosen C, Dixon E, et al. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB. 2010;12:302–310. doi: 10.1111/j.1477-2574.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephenson KR, Steinberg SM, Hughes KS, Vetto JT, Sugarbaker PH, Chang AE. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Ann Surg. 1988;208:679–687. doi: 10.1097/00000658-198812000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asahara T, Katayama K, Itamoto T, Yano M, Hino H, Okamoto Y, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999;23:676–680. doi: 10.1007/pl00012367. [DOI] [PubMed] [Google Scholar]
- 7.Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058–1060. doi: 10.1046/j.1365-2168.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- 8.Balci ST, Pirat A, Torgay A, Cinar O, Sevmis S, Arslan G. Effect of restrictive fluid management and acute normovolemic intraoperative haemodilutionon transfusion requirements during living donor hepatectomy. Transplant Proc. 2008;40:224–227. doi: 10.1016/j.transproceed.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12:935–939. doi: 10.3748/wjg.v12.i6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Merchant NB, Didolkar MS. Hepatic resection using intermittent vascular inflow occlusion and low central venous pressure anaesthesia improves morbidity and mortality. J Gastrointest Surg. 2000;4:162–167. doi: 10.1016/s1091-255x(00)80052-9. [DOI] [PubMed] [Google Scholar]
- 12.Johnson M, Mannar R, Wu AV. Correlation between blood loss and inferior venacaval pressure during liver resection. Br J Surg. 1998;85:188–190. doi: 10.1046/j.1365-2168.1998.00570.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim YK, Chin JH, Kang SJ, Jun IG, Song JG, Jeong SM, et al. Association between central venous pressure and blood loss during hepatic resection in 984 living donors. Acta Anaesthesiol Scand. 2009;53:601–606. doi: 10.1111/j.1399-6576.2009.01920.x. [DOI] [PubMed] [Google Scholar]
- 14.Chhibber A, Dziak J, Kolano J, Norton JR, Lustik S. Anaesthesia care for adult live donor hepatectomy: our experiences with 100 cases. Liver Transpl. 2007;13:537–542. doi: 10.1002/lt.21074. [DOI] [PubMed] [Google Scholar]
- 15.Niemann CU, Feiner J, Behrends M, Eilers H, Ascher NL, Roberts JP. Central venous pressure monitoring during living right donor hepatectomy. Liver Transpl. 2007;13:266–271. doi: 10.1002/lt.21051. [DOI] [PubMed] [Google Scholar]
- 16.Giordano C, Deitte LA, Gravenstein N, Rice MJ. What is the preferred central venous pressure zero reference for hepatic resection? Anesth Analg. 2010;111:660–664. doi: 10.1213/ANE.0b013e3181c76d3e. [DOI] [PubMed] [Google Scholar]
- 17.Massicotte L, Sassine MP, Lenis S, Roy A. Transfusion predictors in liver transplant. Anesth Analg. 2004;98:1245–1251. doi: 10.1213/01.ane.0000111184.21278.07. [DOI] [PubMed] [Google Scholar]
- 18.Ozier Y, Pessione F, Samain E, Courtois F French Study Group on Blood Transfusion in Liver Transplantation. Institutional variability in transfusion practice for liver transplantation. Anesth Analg. 2003;97:671–679. doi: 10.1213/01.ANE.0000073354.38695.7C. [DOI] [PubMed] [Google Scholar]
- 19.Karkouti K, Wijeysundera DN, Beattie WS, Callum JL, Cheng D, Dupuis JY, et al. Reducing Bleeding in Cardiac Surgery (RBC) Research Group. Variability and predictability of large-volume red blood cell transfusion in cardiac surgery: a multicentre study. Transfusion. 2007;47:2081–2088. doi: 10.1111/j.1537-2995.2007.01432.x. [DOI] [PubMed] [Google Scholar]
- 20.Adesanya A, Rosero E, Timaran C, Clagett P, Johnston WE. Intraoperative fluid restriction predicts improved outcomes in major vascular surgery. Vasc Endovascular Surg. 2008;42:531–536. doi: 10.1177/1538574408318474. [DOI] [PubMed] [Google Scholar]
- 21.Vretzakis G, Kleitsaki A, Stamoulis K, Bareka M, Georgopoulou S, Karanikolas M, et al. Intraoperative intravenous fluid restriction reduces perioperative red blood cell transfusion in elective cardiac surgery, especially in transfusion-prone patients: a prospective, randomized controlled trial. J Cardiothorac Surg. 2010;5:7. doi: 10.1186/1749-8090-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anaesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 23.Cockbain AJ, Masudi T, Lodge JP, Toogood GJ, Prasad KR. Predictors of blood transfusion requirement in elective liver resection. HPB. 2010;12:50–55. doi: 10.1111/j.1477-2574.2009.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tinmouth A. Reducing the amount of blood transfused by changing clinicians' transfusion practices. Transfusion. 2007;47(Suppl. 2):132–136. doi: 10.1111/j.1537-2995.2007.01369.x. [DOI] [PubMed] [Google Scholar]
- 25.Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS) HPB. 2011;13:528–535. doi: 10.1111/j.1477-2574.2011.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slogoff S, Keats AS. Does perioperative myocardial ischaemia lead to postoperative myocardial infarction? Anesthesiology. 1985;62:107–114. doi: 10.1097/00000542-198502000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Merry AF, Ramage MC, Whitlock RM, Laycock GJ, Smith W, Stenhouse D, et al. First-time coronary artery bypass grafting: the anaesthetist as a risk factor. Br J Anaesth. 1992;68:6–12. doi: 10.1093/bja/68.1.6. [DOI] [PubMed] [Google Scholar]


