Abstract
Objectives
For patients with unresectable pancreatic cancer (PC), the efficacy and safety of molecular targeted agents (MTAs) in combination with gemcitabine are still unclear. Published randomized controlled trials (RCTs) have reported conflicting results. This study aimed to conduct a systematic review of the literature and to perform a meta-analysis if appropriate.
Methods
Seven electronic databases were searched using a standard technique to November 2011 without restriction on publication status or language. The primary aim was to assess overall survival (OS). Secondary aims were to assess progression-free survival (PFS), overall response rates (ORRs) and grade 3, 4 and 5 toxicities. A random-effects model was used for the meta-analysis.
Results
Seven Phase III RCTs were identified; 1981 patients were treated with MTAs and gemcitabine, and 1992 patients received gemcitabine with or without placebo. No statistically significant difference in OS was found between the two groups [hazard ratio (HR) = 0.93, 95% confidence interval (CI) 0.85–1.02; P = 0.13]. The addition of MTAs improved PFS (HR = 0.86, 95% CI 0.79–0.93; P = 0.000) and ORR (odds ratio 1.35, 95% CI 1.05–1.74; P = 0.01). However, these benefits were accompanied by significantly higher toxicity (P = 0.001).
Conclusions
The findings of this study suggest that the palliation of PC with gemcitabine and MTAs does not provide a significant survival benefit and is associated with increased grade 3 and 4 toxicities.
Keywords: pancreatic cancer, palliation, gemcitabine, molecular targeted agents, meta analysis, overall survival
Introduction
Pancreatic adenocarcinoma is a common gastrointestinal malignancy. In the USA it is the fourth largest cause of cancer-related mortality and accounts for approximately 42 500 new cases and 35 000 deaths each year.1 Its prognosis is poor and surgical resection is the only potential cure,2 but feasible in only a minority of patients because of metastatic or locally advanced disease.3–5 Palliative chemotherapy with gemcitabine offers better overall survival (OS) and quality of life6 in comparison with fluorouracil or best supportive care,7,8 but its benefits are modest.
In recent years, the use of biological agents in combination with standard chemotherapy has improved the outcomes of patients with advanced colorectal cancer and other gastrointestinal tumours.9 Consequently, several Phase III trials10–14 testing the role of gemcitabine in combination with new biological agents have been carried out in patients with unresectable pancreatic cancer (PC). These studies have produced contradictory results with only one trial10 showed a positive effect on OS. In view of this a systematic review of the literature was performed, with the appraisal and analysis of all published Phase III randomized controlled trials (RCTs) that used molecular targeted agents (MTAs) in addition to standard chemotherapy for patients with unresectable PC.
Materials and methods
Data sources and study selection
PubMed, Excerpta Medical Database, Scopus, Web of Science, the Cochrane Central Register of Controlled Trials, the hepatobiliary group in the Cochrane Library, EMBASE and CINAHL were searched for Phase III RCTs comparing standard palliative chemotherapy with gemcitabine alone or in combination with placebo vs. gemcitabine with the addition of MTAs. The search applied to papers published to November 2011 without restriction on publication status or language. To identify all potential papers, medical subject headings reported in Table 1 were used with a Boolean search strategy.15 Articles cited in the reference lists of all potential studies were further reviewed and a comprehensive database to catalogue the medical literature on this topic was developed.
Table 1.
Summary of the terms used singly or in combination for evidence acquisition
| Primary MeSH terms | Secondary MeSH terms | Keywords |
|---|---|---|
| Pancreas | Antineoplastic protocol(s) | Gemcitabine |
| Pancreatic duct(s) | Drug therapy, combination | Pemetrexed |
| Pancreatic disease(s) | Antineoplastic combined chemotherapy protocols | Marimastat |
| Pancreatic neoplasm(s) | Randomized controlled trial | Tipifarnib |
| Adenocarcinoma(s) | Clinical trials, Phase III | Bevacizumab |
| Neoplasm metastasis | Controlled clinical trial(s) | Cetuximab |
| Palliative care | Double-blind method | Erlotinib |
| Drug therapy | Research design | Axitinib |
| Treatment outcome | Epidemiologic research design | |
| Outcome assessment | Adult | |
| Clinical trial(s) | Deoxycitidine | |
| Random allocation | Early termination of clinical trials | |
| Humans | Treatment failure | |
| Cytidine | Angiogenesis inhibitor(s) | |
| Deoxyribonucleosides | Antimetabolite(s) | |
| Antineoplastic agent(s) | Anticarcinogenic agent(s) | |
| Molecular targeted therapy | Receptor protein-tyrosine kinase(s) | |
| Antibodies, monoclonal | Receptor, epidermal growth factor | |
| Angiogenesis modulating agent(s) | Angiogenesis inhibitor(s) | |
| Growth inhibitor(s) | Receptor, vascular endothelial growth factor | |
| Metalloendopeptidases | Platelet-derived growth factor | |
| Folic acid antagonists | ||
Two reviewers (KME and MM) independently assessed the eligibility of all potential abstracts and titles. In cases of disagreement or in the presence of insufficient information, the full text of the study was reviewed for eligibility. The decision to include articles in this study was reached by consensus. Principal investigators of potential trials were asked for missing data and updates by electronic mail whenever the study data were insufficiently described. Phase I and II RCTs, non-controlled clinical trials, studies on animals and review articles were excluded.
Definition of molecular target agents
Molecular targeted agents were defined as all the biological molecules able to alter at least one of the following neoplastic cellular pathways: intracellular or intercellular signalling; metabolic activities, and mytosis.16 The MTAs included for this study were required to be approved for clinical trials on human subjects, alone or in combination with other chemotherapy agents, for the scope of improving tumour response, survival and symptoms in patients affected by PC.
Outcomes
The primary outcome of this study was OS in patients receiving at least one cycle of chemotherapy. Secondary outcomes were: progression-free survival (PFS); overall response rate (ORR), and incidences of grade 3, 4 and 5 toxicities according to the National Cancer Institute Common Toxicity Criteria.17 Overall survival was defined as the time interval between the date of enrolment in the protocol and death. Progression-free survival was defined as the time interval during which the size of the tumour remained stable and ORR was defined as the sum of complete and partial tumour responses by RECIST (response evaluation criteria in solid tumours) criteria,18 divided by the number of included patients.
Data extraction
Two reviewers (KME and MM) independently extracted the data of interest in each study. The following variables were collected: the name of the primary author; year of publication; country in which the study was performed; number of patients randomized in each arm; dosage of anti-cancer therapy; allocation sequence generation; allocation concealment; power calculation; study design; methods used to deal with missing data, and appropriate description of attrition and drop-outs. For each study, the following clinical variables of interest were extracted: OS; PFS; ORR, and grade 3, 4 and 5 drug-induced toxicities.
Assessment of study quality
The Cochrane Collaboration risk assessment tool was used to score the quality of the studies.19 The randomization methods were classified as the primary means to control bias and the randomization process was evaluated by the methods used to generate and conceal the allocation sequence. Adequate randomization methods were based on a table of random numbers, computer-generated allocation or equivalent techniques. Allocation concealment was considered adequate if it was obtained by a central randomization system, if coded drugs that appeared identical were used, if serially numbered opaque sealed envelopes were employed or if other equivalent methods were used. Blinding was extracted and appraised for caregivers, patients and assessors. Risk for attrition bias was assessed by the number of and reason for drop-outs and withdrawals and whether all patients were accounted for and analysed. The quality of studies was assessed according to sample size calculations and whether or not the sample size had been achieved, whether the study included clear definitions of primary outcomes, and whether or not a crossover design had been used.
Statistical analysis
Statistical analysis was performed using Comprehensive Meta-Analysis Version 2 software.20 Meta-analysis was performed using a random-effects model21 in response to the expected clinical heterogeneity among the trials. The results were reported as hazard ratios (HRs) with 95% confidence intervals (CIs) for OS and PFS analyses. Pooled odds ratios (ORs) with 95% CIs were applicable for response rates and drug toxicities. For the primary outcome, sensitivity analysis was performed to assess the impact of studies with higher risk for bias. The main modality for presenting numerical data in visual form was the forest plot.
Reporting
The PRISMA Statement was used for reporting the strategy used to identify Phase III RCTs, inclusion and exclusion criteria, assessment of bias and the results of this meta-analysis.22
Results
Ascertainment of the studies
The search strategy used to identify Phase III trials on the topic of interest detected a total of 163 publications. Initial screening reduced this number to a total of 61 potentially relevant trials. Reading of the content of abstracts and appraisals of eligibility further reduced this number to seven Phase III RCTs that satisfied the inclusion criteria of the present study (Fig. 1).
Figure 1.
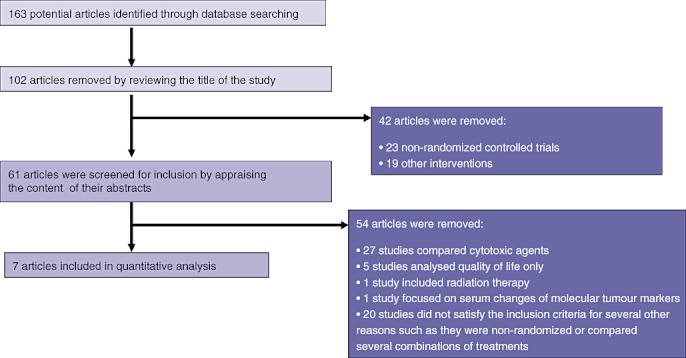
Flow of information through the different phases of the systematic review
Study characteristics
Table 2 summarizes the characteristics of all included studies. All trials were multicentric; the total number of patients per study ranged from 239 to 745, giving a combined total of 3973 participants. Each of the RCTs that satisfied the inclusion criteria involved an experimental arm in which patients received gemcitabine chemotherapy combined with one of the following MTAs: bevacizumab; cetuximab; erlotinib; marimastat; axitinib; pemetrexed, and tipifarnib. In five trials10,11,13,14,23 the control groups received gemcitabine and placebo, and in two trials12,24 the control groups received gemcitabine only.
Table 2.
Characteristics of the included trials
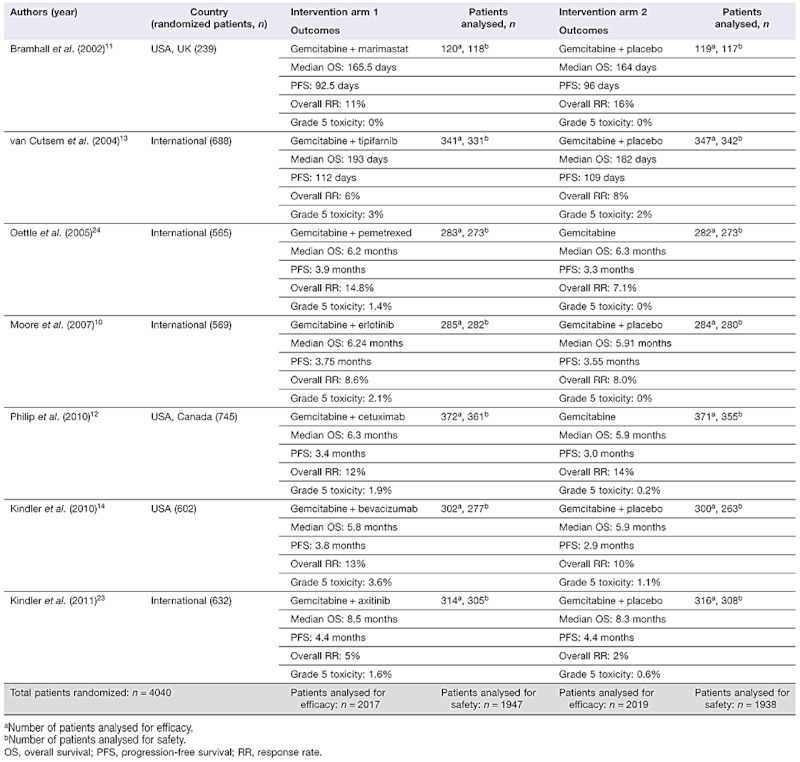 |
In total, 1981 patients received gemcitabine and MTAs, and 1992 subjects underwent conventional chemotherapy with a single cytotoxic agent.
Risk for bias
The allocation system was described in all seven trials; however, none reported enough details on the strategy used for allocation concealment. The majority of the trials were blinded to both patients and observers. Methods of handling missing data were not adequately described in any of the included studies (Table 3).
Table 3.
Risk for bias in the published controlled trials
| Authors (year) | Allocation system described | Allocation concealment | Blinding: patient | Blinding: personnel | Blinding: assessor | Handling of missing data | Power calculation for number of patients to be treated |
|---|---|---|---|---|---|---|---|
| Kindler et al. (2010)14 | Yes | NA | Yes | Yes | NA | Unclear | Yes |
| Philip et al. (2010)12 | Yes | NA | NA | NA | NA | Unclear | Yes |
| Moore et al. (2007)10 | Yes | NA | Yes | Yes | NA | Unclear | Yes |
| Bramhall et al. (2002)11 | Yes | NA | Yes | Yes | NA | Unclear | Yes |
| van Cutsem et al. (2004)13 | Yes | NA | Yes | Yes | NA | Unclear | Yes |
| Kindler et al. (2011)23 | Yes | Yes | Yes | Yes | NA | Unclear | Yes |
| Oettle et al. (2005)24 | Yes | NA | Yes | NA | NA | Unclear | Yes |
NA, not available.
All the RCTs had similar designs and compared the effects of gemcitabine vs. gemcitabine in combination with a single MTA for palliation of unresectable PC without the addition of radiation therapy. Consequently, both authors agreed to perform a meta-analysis for the primary and secondary outcomes.
Primary outcome
Overall survival
Although the overall trend favoured the use of MTAs, the pooled analysis did not demonstrate any significant difference between the two groups (HR = 0.94, 95% CI 0.87–1.01; P = 0.09) (Fig. 2). A sensitivity analysis was performed by excluding the 2010 study by Kindler et al.14 as it was the only trial to show a relatively better survival benefit in the placebo group and was therefore considered as an outlier. The meta-analysis of the six remaining studies confirmed no difference in OS between the two arms.
Figure 2.
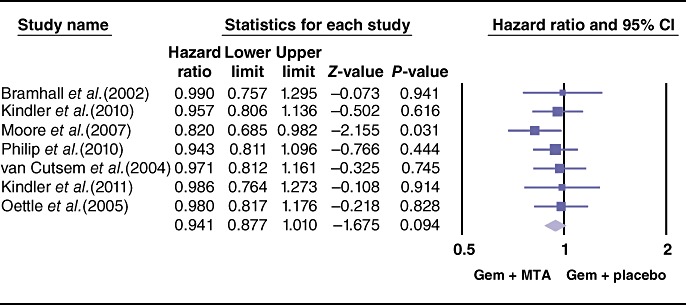
Forest plot representing the pooled results for overall survival in patients treated with gemcitabine (Gem) and molecular targeted agents (MTAs) vs. Gem alone or in combination with placebo. 95% CI, 95% confidence interval
Secondary outcomes
The pooled analysis for PFS showed a statistically significant improvement in the study arm treated with MTAs (HR = 0.86, 95% CI 0.79–0.93; P = 0.000) (Fig. 3). Similarly, the ORR differed the two therapeutic arms, favouring the use of MTAs (OR = 1.35, 95% CI 1.05–1.74; P = 0.01) (Fig. 4). By contrast, effects of grade 3 and 4 toxicities (e.g. anaemia, neutropenia, thrombocytopenia, cerebrovascular accidents, proteinuria, venous thrombosis, skin rash, nausea/vomiting, anorexia) were more common in the group treated with MTAs (OR = 1.79, 95% CI 1.50–2.13; P = 0.00) (Fig. 5). A higher risk for treatment-related mortalities (grade 5 toxicities) was also seen in patients receiving a combination of gemcitabine and MTAs in all studies except one11 (pooled OR = 2.19, 95% CI 1.19–4.0; P = 0.01) (Fig. 6).
Figure 3.
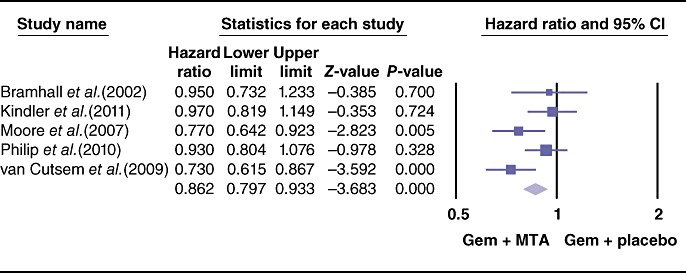
Forest plot representing the pooled results for progression-free survival in patients treated with gemcitabine (Gem) and molecular targeted agents (MTAs) vs. Gem alone or in combination with placebo. Progression-free survival was reported in median months by Oettle et al.24 and by Kindler et al.14 and therefore these were not included in the meta-analysis for this outcome. 95% CI, 95% confidence interval
Figure 4.
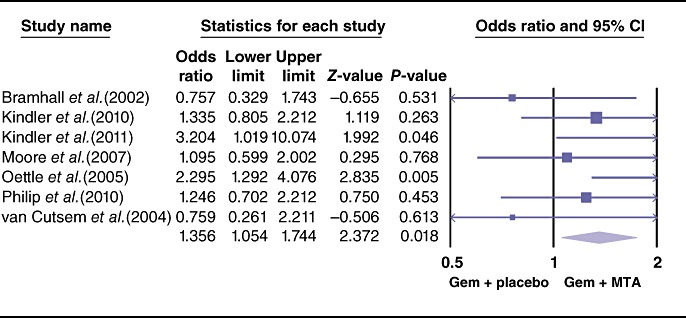
Forest plot representing the pooled results for objective response rate in patients treated with gemcitabine (Gem) and molecular targeted agents (MTAs) vs. Gem alone or in combination with placebo. 95% CI, 95% confidence interval
Figure 5.
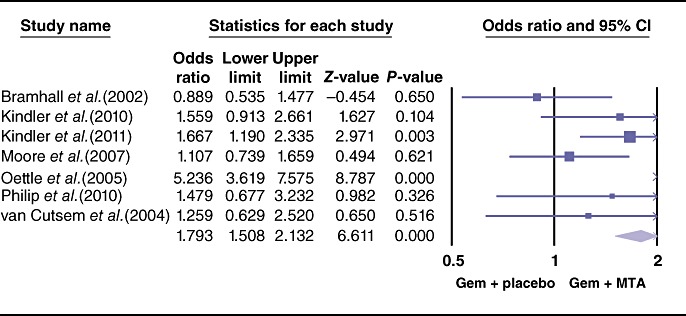
Forest plot representing the pooled results for grade 3 and 4 toxicities in patients treated with gemcitabine (Gem) and molecular targeted agents (MTAs) vs. Gem alone or in combination with placebo. 95% CI, 95% confidence interval
Figure 6.
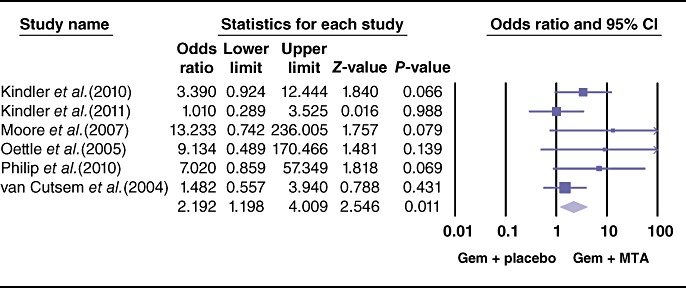
Forest plot representing the pooled results for grade 5 toxicities (treatment-related deaths) in patients treated with gemcitabine (Gem) and molecular targeted agents (MTAs) vs. Gem alone or in combination with placebo. Bramhall et al.11 reported no grade 5 toxicities in either treatment arm. 95% CI, 95% confidence interval
Sensitivity analysis and publication bias
Sensitivity analysis did not show any difference in results for either primary or secondary outcomes. Assessment for publication bias performed using a funnel plot of the standard errors suggested that the risk for publication bias was minimal as a result of the symmetrical distribution of the relationship between treatment effect and study size25 (Fig. 7).
Figure 7.
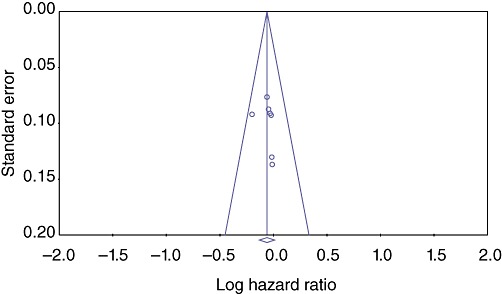
Assessment for publication bias by funnel plot of the standard errors. The symmetrical distribution of the relationship between treatment effect and study size25 suggests a minimal risk for publication bias
Discussion
Since 1997, gemcitabine-based chemotherapy has become the reference treatment for patients with unresectable PC.7 During the subsequent years, many combinations of chemotherapy regimens have been tried for unresectable PC without considerable benefits. Only one Phase III trial showed a synergistic effect of gemcitabine and erlotinib, with a median survival of 6.24 months compared with 5.91 months in the gemcitabine-only arm.10
Among many negative chemotherapy trials, a recent European multicentre study shown a significant improvement in OS in patients treated with the combination of oxaliplatin, irinotecan, leucovorin and fluorouracil (FOLFIRINOX) in comparison with gemcitabine alone.26 This trial generated some optimism for chemotherapy protocols that combine several agents to minimize side-effects and take advantage of the synergistic effects of compounds that act at different cellular levels.
This trial fits well with the recent identification of several molecular abnormalities occurring in solid tumours, and the development of new chemotherapy agents designed to act on specific cellular targets.27
Data from laboratory research have shown that MTAs are able to alter the expected course of neoplastic cells28,29 with precision and without altering the cycle of normal cells.30 For some solid and haematological tumours, these effects have been proven in clinical trials, with significant extension of OS and, in some cases, complete response.30 The success of MTAs for the treatment of some other gastrointestinal tumours9 has generated the hypothesis that MTAs might also be beneficial in PC.30 Similarly to other solid tumours, the development of PC involves multiple genetic and epigenetic alterations, chromosomal aberrations, gene mutations and several changes of molecular pathways31 that may be amenable to pharmacologic interference by MTAs.
Because PC cells are resistant to conventional chemotherapy agents, and there is no single very effective cytotoxic agent, several Phase III RCTs have been carried out to investigate the effects of MTAs in combination with gemcitabine. All these trials were very well designed, adequately powered and reported proper follow-up and survival. However, despite optimistic expectations, only one study10 was able to show a positive statistical difference in OS between the treatment arms.
Single experiments very rarely provide definitive answers to research questions; therefore a systematic review of the literature was performed. After an extensive search of several databases, all the potential Phase III RCTs that satisfied the inclusion criteria were selected. The available studies had very similar designs and objectives, and agreement was reached that the criteria for a quantitative synthesis were satisfied and a meta-analysis was subsequently performed.
The results of this study confirmed that the addition of an MTA did not translate into significant OS benefits, although PFS and ORR were statistically better than in the control arms. By contrast, the addition of MTAs resulted in a significant increase of grade 3 and 4 and, more importantly, grade 5 toxicities.
One of the limitations of this study was the fact that the MTAs tested in the trials were heterogeneous in terms of their molecular structure and specific targets. Some MTAs were selective inhibitors of trans-membrane receptors and others were inhibitors of intracellular enzymes responsible for cellular replication, angiogenesis and promotion of metastases. However, although each MTA acted on a unique target, their final effects could be unified as one of the following: inhibition of angiogenesis and cell growth, or prevention of the degradation of basement membranes and migration of endothelial cells needed to form blood vessels. All the trials were based on the concept that MTAs are complementary drugs to the main cytotoxic agent gemcitabine and tested the hypothesis that MTAs might be synergistic.
Another limitation was the relatively small number of trials, although the total number of patients included in the meta-analysis was conspicuous. In fact, the final number of subjects pooled in this meta-analysis was sufficient to power an RCT able to detect a minimum difference in HR of 0.1.
Along with these limitations, this study has several strengths. The first of these was the high quality and the homogeneity of the design and interventions of all the included studies and the fact that an extensive literature search was performed to establish the most up-to-date information on the effects of novel MTAs in PC. Secondly, a relatively large number of patients (3973) was included, and two reviewers were involved in assessing potential bias and in extracting data in a manner that has been shown to improve the quality of meta-analysis. As a final point, this study assessed not only potential survival benefits, but also other important clinical outcomes such as response rates, grade 3 and 4 toxicities, and treatment-related mortality.
This is the first meta-analysis on the results of MTAs in the treatment of unresectable PC. For reasons that are still not very well understood, PC appears to be very resistant, not only to well-known cytotoxic agents, but also to the MTAs currently available.
The findings of this study do not support the use of available MTAs in combination with gemcitabine for the palliation of patients affected by advanced PC. Although the enthusiasm for MTAs in PC has declined because of their modest effects and high costs, further RCTs combining MTAs with FOLFIRNOX should be encouraged. In further trials, cost-effective analyses should also be included among the outcomes of interest as the costs of adding MTAs to other cytotoxic agents will remain a significant constraint on the wide use of these agents in the treatment of patients with PC.
Conflicts of interest
None declared.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Sharma C, Eltawil KM, Renfrew PD, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990–2010. World J Gastroenterol. 2011;17:867–897. doi: 10.3748/wjg.v17.i7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaib YH, Davila JA, El-Serag HB. The epidemiology of pancreatic cancer in the United States: changes below the surface. Aliment Pharmacol Ther. 2006;24:87–94. doi: 10.1111/j.1365-2036.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- 4.Espat NJ, Brennan MF, Conlon KC. Patients with laparoscopically staged unresectable pancreatic adenocarcinoma do not require subsequent surgical biliary or gastric bypass. J Am Coll Surg. 1999;188:649–655. doi: 10.1016/s1072-7515(99)00050-2. discussion 655–657. [DOI] [PubMed] [Google Scholar]
- 5.Di Marco M, Di Cicilia R, Macchini M, Nobili E, Vecchiarelli S, Brandi G, et al. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? (Review) Oncol Rep. 2010;23:1183–1192. doi: 10.3892/or_00000749. [DOI] [PubMed] [Google Scholar]
- 6.Strimpakos AS, Syrigos KN, Saif MW. The molecular targets for the diagnosis and treatment of pancreatic cancer. Gut Liver. 2010;4:433–449. doi: 10.5009/gnl.2010.4.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 8.van Cutsem E, Haustermans K, van Steenbergen W. New treatment possibilities for pancreatic and biliary tumours. Ann Oncol. 2000;11(Suppl. 3):165–169. [PubMed] [Google Scholar]
- 9.Sobrero A, Ackland S, Clarke S, Perez-Carrión R, Chiara S, Gapski J, et al. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77:113–119. doi: 10.1159/000229787. [DOI] [PubMed] [Google Scholar]
- 10.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 11.Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomized study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161–167. doi: 10.1038/sj.bjc.6600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philip PA, Benedetti J, Corless CL, Wong R, O'Reilly EM, Flynn PJ, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group – directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–1438. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 14.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karimi S, Pohl S, Scholer F, Cavedon L, Zobel J. Boolean versus ranked querying for biomedical systematic reviews. BMC Med Inform Decis Mak. 2010;10:58. doi: 10.1186/1472-6947-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997;57:2140–2143. [PubMed] [Google Scholar]
- 17.Cancer Therapy Evaluation Program. 1998. Common Toxicity Criteria, Version 2.0. DCTD, NCI, NIH, DHHS. March.
- 18.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.2. [Updated September 2009.] http://www.cochrane-handbook.org. [Accessed May 2011]
- 20.Biostat, Inc. Englewood, NJ: Biostat, Inc; 2006. Comprehensive Meta-Analysis, Version 2. [Google Scholar]
- 21.DerSimonian RLN. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomized phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 24.Oettle H, Richards D, Ramanathan RK, van Laethem JL, Peeters M, Fuchs M, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639–1645. doi: 10.1093/annonc/mdi309. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 27.Almhanna K, Philip PA. Defining new paradigms for the treatment of pancreatic cancer. Curr Treat Options Oncol. 2011;12:111–125. doi: 10.1007/s11864-011-0150-8. [DOI] [PubMed] [Google Scholar]
- 28.Huettner CS, Zhang P, van Etten RA, Tenen DG. Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nat Genet. 2000;24:57–60. doi: 10.1038/71691. [DOI] [PubMed] [Google Scholar]
- 29.Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein IB, Joe AK. Mechanisms of disease: oncogene addiction – a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol. 2006;3:448–457. doi: 10.1038/ncponc0558. [DOI] [PubMed] [Google Scholar]
- 31.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signalling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]


