Abstract
Objectives
Traditionally, a gallbladder removed for presumed benign disease has been sent for histopathological examination (HPE), but this practice has been the subject of controversy. This study was undertaken to compare patients in whom gallbladder cancer (GBC) was diagnosed after cholecystectomy on HPE with GBC patients in whom the gallbladder was not sent for HPE and who therefore presented late with symptoms.
Methods
A retrospective analysis of prospectively collected data for 170 GBC patients diagnosed after cholecystectomy was conducted. All patients presented to one centre during 2000–2011. These patients were divided into two groups based on the availability of histopathology reports: Group A included patients who presented early with HPE reports (n = 93), and Group B comprised patients who presented late with symptoms and without HPE reports (n = 77).
Results
The median time to presentation in Group A was significantly lower than in Group B (29 days vs. 152 days; P < 0.001). Signs or symptoms suggestive of recurrence (pain, jaundice or gastric outlet obstruction) were present in four (4.3%) patients in Group A and all (100%) patients in Group B (P < 0.001). Patients deemed operable on preoperative evaluation included all (100%) patients in Group A and 38 (49.4%) patients in Group B (P < 0.0001). The overall resectability rate (69.9% vs. 7.8%) and median survival (54 months vs. 10 months) were significantly higher in Group A compared with Group B (P < 0.0001).
Conclusions
Patients in whom a cholecystectomy specimen was sent for HPE presented early, had a better R0 resection rate and longer overall survival. Hence, routine HPE of all cholecystectomy specimens should be performed.
Keywords: resection < gallbladder, surgery < cholelithiasis, outcomes < gallbladder
Introduction
Gallbladder cancer (GBC) is the most common cancer of the biliary tract worldwide.1 Surgical resection of the tumour and its loco-regional spread remains the only hope for longterm cure and survival. Incidental GBC (IGBC) refers to GBC that is not suspected before or at operation and even on gross examination of the opened gallbladder specimen by the surgeon, but is detected for the first time on histopathological examination (HPE) of a gallbladder removed for presumed (clinical, ultrasound, operative) diagnosis of gallstone disease (GSD). In practical terms, all GBCs not detected preoperatively and diagnosed during or following surgery are considered as IGBC. The incidence of IGBC has been reported to range from 0.3–1.5% in various series.2 Although the overall prognosis of GBC is poor, IGBC is associated with better outcomes.3,4 The literature-based support for a better prognosis in IGBC is based on the fact that tumours detected on HPE usually represent early-stage disease and patients are referred promptly by their treating physicians for definitive surgery. Completion radical cholecystectomy is the standard treatment for IGBC of stage Ib and beyond.3,5,6
The early-stage tumours for which surgical resection provides the greatest benefit are difficult to diagnose preoperatively and are often missed even after intraoperative examination of the cholecystectomy specimen.7–9 Hence, it has been standard practice to submit all gallbladders removed for presumed GSD to routine HPE to exclude gallbladder malignancy.10 In recent years, however, the role of routine HPE of cholecystectomy specimens has been questioned.11–15 In India, some centres do not send all cholecystectomy specimens for HPE and this centre often manages post-cholecystectomy GBC patients who present late in the course of disease without HPE data and with symptoms of recurrence. This provided an opportunity to study the impact of avoiding routine histopathology of all cholecystectomy specimens.
This study was conducted to analyse the clinical presentation, resectability rate and survival in patients with GBC diagnosed after cholecystectomy based on HPE of the gallbladder and to compare these data with equivalent data for GBC patients in whom the gallbladder was not sent for HPE and who therefore presented late with symptoms suggestive of recurrence.
Materials and methods
This study was a retrospective analysis of prospectively collected GBC data for 170 patients with IGBC diagnosed after cholecystectomy, who were referred to this centre during the period 2000–2011. When HPE of the resected gallbladder had not been carried out, the presence of a mass in the region of the gallbladder fossa seen either on preoperative imaging or on exploratory laparotomy was taken as corroborative evidence of GBC. Ultrasonography of the abdomen was performed in all patients. Patients without evidence of metastatic disease on ultrasound underwent contrast-enhanced computed tomography (CT) of the abdomen to assess resectability. Magnetic resonance imaging with cholangiography and endoscopic retrograde cholangiography were carried out selectively in patients with jaundice.
Patients who were considered unresectable on the basis of preoperative imaging were offered palliative non-surgical treatment; a few patients, particularly in the earlier part of the study period, underwent palliative surgery. Patients were treated based on their tumour (T) stage: patients with T-stage Ib or higher and without evidence of metastatic disease underwent a completion radical cholecystectomy. If required, the bile duct, colon and any other contiguous organs were resected en bloc to achieve a negative margin (R0) resection. Patients with T-stage Ia disease underwent regular follow-up.
Patients were divided into two groups: Group A comprised patients who presented early with HPE reports, and Group B consisted of patients who presented late with symptoms and without HPE reports. Statistical analysis was performed using GraphPad instat Version 4 (GraphPad Software, Inc., La Jolla, CA, USA). Continuous variables were compared using the Mann–Whitney test. Categorical variables were compared using Fisher's exact test and the chi-squared test. Survival probabilities were estimated using the Kaplan–Meier method and compared using the log-rank test. P-values of < 0.05 were considered to indicate statistical significance.
Results
Of the 170 patients with GBC diagnosed after cholecystectomy, 93 were in Group A and 77 in Group B. Table 1 depicts the demographics and clinical features of patients in the two groups. Time to presentation in Group A (median = 29 days, range: 1–70 days) was significantly less than in Group B (median = 152 days, range: 38–1080 days) (P < 0.001). There were no significant differences in age distribution or gender ratio between the two groups. Abdominal pain as the presenting symptom was reported for 25 patients in Group B and four patients in Group A (P < 0.001). None of the patients in Group A had jaundice or gastric outlet obstruction (GOO), whereas 53 patients in Group B had jaundice (n = 45) or GOO (n = 8) (P < 0.0001). Abdominal distension secondary to malignant ascites or intestinal (colonic) obstruction was present in seven patients in Group B and no patients in Group A (P = 0.003).
Table 1.
Patient demographics and clinical features in gallbladder cancer patients presenting early with biopsy reports (Group A) or late without biopsy reports (Group B)
| Clinical parameter | Group A (n = 93) | Group B (n = 77) | P-value |
|---|---|---|---|
| Time to presentation, days | 29 (1–70) | 152 (38–1080) | < 0.001 |
| Age, years, median (range) | 51 (25–75) | 53 (32–67) | 0.983 |
| Sex, male : female | 1:4.5 | 1:3.5 | 0.571 |
| Jaundice, n | 0 | 45 | <0.001 |
| Gastric outlet obstruction, n | 0 | 8 | 0.008 |
| Abdominal pain, n | 4 | 25 | <0.001 |
| Ascites/intestinal obstruction, n | 0 | 7 | 0.003 |
Table 2 depicts resectability and survival rates in the two groups. On preoperative evaluation, all patients in Group A and 38 (49.4%) patients in Group B were found to be operable (P < 0.0001). Of the 93 patients in Group A in whom surgery with curative intent was performed, 65 underwent curative resection (resectability rate: 69.9%). Of the 77 patients in Group B, 54 were referred for surgery and 27 (35.1%) underwent surgery with curative intent because palliative segment III bypass was performed frequently in patients with jaundice during the early part of the study period. Only six of the 27 patients in Group B referred for surgery with curative intent underwent curative resection (resectability rate: 22.2%). Overall resectability was significantly higher in Group A (69.9%, 65/93) than in Group B (7.8%, 6/77) (P < 0.0001). Adjacent organ resection was performed in 13 patients in Group A and two patients in Group B (P = 0.591). The median duration of follow-up was 36 months (range: 6–72 months) in Group A and 8 months (range: 3–22 months) in Group B. Median survival was significantly higher in Group A (54 months) than in Group B (10 months) (P < 0.001) (Fig. 1, Table 3).
Table 2.
Resectability rates and survival in gallbladder cancer patients presenting early with biopsy reports (Group A) or late without biopsy reports (Group B)
| Parameter | Group A (n = 93) | Group B (n = 77) | P-value |
|---|---|---|---|
| Deemed unresectable on preoperative workup, n (%) | 0 | 39 (50.7%) | <0.001 |
| Referred for surgery (curative intent), n (%) | 93 (100%) | 27 (35.1%) | <0.001 |
| Resectability in patients referred for surgery with curative intent, n (%) | 65 (69.9%) | 6 (22.2%) | <0.001 |
| Overall resectability, n (%) | 65 (69.9%) | 6 (7.8%) | <0.001 |
| Adjacent organ resection, n (%) | 13 (14.0%) | 2 (2.6%) | 0.591 |
| Median survival, months | 54 | 10 | <0.001 |
Figure 1.
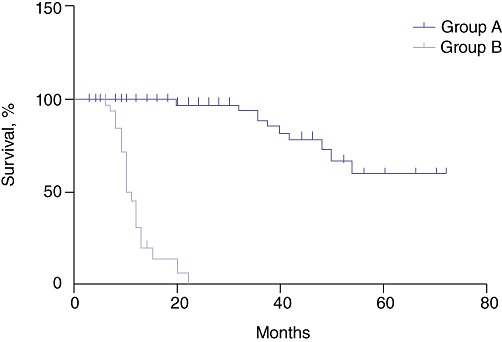
Survival rates in Groups A and B
Table 3.
Survival in gallbladder cancer patients presenting early with biopsy reports (Group A) or late without biopsy reports (Group B)
| Patients at risk, n | Time in months | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 6 | 9 | 20 | 24 | 48 | 72 | |
| Group A | 93 | 93 | 83 | 68 | 64 | 23 | 2 |
| Group B | 77 | 64 | 34 | 3 | 0 | 0 | 0 |
Discussion
Gallbladder cancer is the most common malignancy of the extrahepatic biliary tree.1 It is usually detected at an advanced stage and is associated with a dismal prognosis.3 Although early-stage tumours are associated with good prognosis, the preoperative detection of tumours that are more likely to benefit from surgical resection is difficult.7 The clinical presentation of early GBC is non-specific and symptoms are similar to those of acute or chronic cholecystitis. Although an expert radiologist can detect the presence of early lesions in the form of focal gallbladder wall thickening or lesions of small mass, not all cases of early GBC present with an obvious lesion on abdomen ultrasonography.7 Some of the small lesions missed on preoperative ultrasound can be picked up on gross examination of the cholecystectomy specimen. Histopathological examination of the cholecystectomy specimen facilitates the detection of tumours that are not apparent even on gross examination of the specimen.8 Hence, it has been traditional practice to send all cholecystectomy specimens for HPE.
Recently, however, various reports in the literature have questioned the role of routine HPE in all cholecystectomy specimens.11–15 The reasons given are that the incidence of IGBC is too low to justify routine HPE, that routine HPE of all cholecystectomy specimens overburdens pathology and hospital resources, that almost all cases of IGBC are associated with positive findings on gross examination of the gallbladder when it is cut open and examined in the operating theatre, and that simple cholecystectomy is adequate for early-stage tumours (carcinomas in situ and T-stage Ia tumours).
The suggestion that routine HPE should be avoided is based on the belief that examination of the cholecystectomy specimen in the operating room reveals some suspicious findings in all cases of IGBC. Some reports support this claim. Darmas et al. reported IGBC in only four of 1452 (0.3%) patients for whom cholecystectomy specimens were examined over a period of 5 years, all four of whom demonstrated mass on gross examination of the cholecystectomy specimen and two of whom showed preoperative suspicions of malignancy.15 Bazoua et al. reported an analysis of HPE reports for 2890 cholecystectomy specimens which showed malignancy in 10 cases, all of which had demonstrated thick-walled gallbladders on gross examination and in two of which suspicious mass had been apparent.13 Recently, a centre in the south of India reported its experience with 1312 cholecystectomy cases over a 10-year period.14 Of these, 610 (46.5%) cholecystectomy specimens showed macroscopic abnormalities in the form of thickening, mucosal ulcerations or polypoidal lesions. Malignancy was found in 13 of these 610 cholecystectomy specimens with macroscopic abnormalities. None of the cholecystectomy specimens without macroscopic abnormalities were found to have GBC.14 Based on these findings, all these reports recommend the selective HPE of cholecystectomy specimens with either preoperative or intraoperative suspicious findings.
Most of the GBC cases reported in these series showed suspicions of malignancy on either preoperative evaluation or intraoperative examination of the cholecystectomy specimen. Incidental GBC is, by definition, detected for the first time on HPE of gallbladders removed for presumed (clinical, imaging or operative including the examination of the cut surface of the cholecystectomy specimen) diagnosis of GSD. Several authors have reported that preoperative imaging findings and intraoperative gross examination may not be reliable in identifying malignancy.8,9 Roa et al., in a report from Chile, observed that 37% of primary tumours were macroscopically inapparent.8 Lohsiriwat et al. reported the absence of pre- or intraoperative suspicion in all 24 cases of IGBC diagnosed on HPE of 4317 cholecystectomy specimens examined over a period of 8 years.16 Recently, a report from Nepal found that pre- and intraoperative examination was accurate in identifying only 55% of all IGBC cases.9 All these reports underscore the importance of routine HPE of all cholecystectomy specimens.
In the present study, a retrospective analysis of 503 cholecystectomy specimens removed from patients with presumed diagnoses of GSD over a period of 2 years showed that 33 patients had shown intraoperative suspicion of malignancy and the gallbladder had been sent for frozen-section examination. Of these, only five patients had evidence of malignancy. Of the remaining 470 specimens in which there was no suspicion of malignancy on macroscopic examination of the cut-open cholecystectomy specimen, GBC was diagnosed in four cases. Of these, one patient had stage Ia disease, one had stage Ib disease and two had stage II disease. Thus, it is evident that, despite careful macroscopic examination, GBC can be missed and that implementing a policy of selective HPE of cholecystectomy specimens will fail to identify tumours in patients who might otherwise have a good prognosis.
Darmas et al. suggested that adopting a more selective approach would reduce costs, resulting in an overall saving of GBP10 875 in their series.15 They also suggested the histopathology workload would be reduced by 3.5–4.0% per year if only selected cholecystectomy specimens were examined. However, these benefits should be weighed against the risk that early gallbladder malignancies and potential chances to provide curative treatment in this dismal disease might be lost. The projected cost-saving does not look justifiable.
In patients with GBC, a radical R0 resection is the only hope for cure. Patients with early-stage GBC benefit most from radical resection. Although a proportion of IGBC patients with T-stage Ia disease do not require further treatment (three patients in the present series), a significant number of IGBC patients have stage Ib or II disease and will benefit from radical resection.6 In the present series, patients who were referred early by their treating physicians (based on biopsy reports for gallbladders removed at index surgery) had a 69.9% overall resectability rate. By contrast, patients who presented late with symptoms (because HPE reports were either not available or neglected) had a poor overall resectability rate (7.8%). Time to presentation, which determined resectability, was affected by the availability of an HPE report. Thus, patients in whom evidence of malignancy was found on HPE of the cholecystectomy specimen were referred early by their treating physicians for completion radical cholecystectomy and had a better rate of resectability.
In India, it is not uncommon for the removed gallbladder not to be sent for HPE, especially in peripheral centres. As a result, many patients present late at a time when their disease is usually advanced or metastatic. The present series included a few patients (nine patients in Group B) in whom a biopsy specimen had been sent for HPE at the time of surgery, but the HPE report had not been collected on follow-up by either the patient or the surgeon until the patient had developed a recurrence. These patients also presented late with signs and symptoms of advanced disease; their outcomes were dismal and only on retrospective review of all details was it found that the gallbladder specimen had been sent for HPE. Based on this analysis, the present authors recommend that all cholecystectomy specimens should be opened and examined. Any patient in whom findings on intraoperative examination are suspicious should benefit from frozen-section examination of the cholecystectomy specimen. In all other patients, the cholecystectomy specimen should be sent for routine HPE so that the detection of early GBC is not missed in a subgroup of patients who might derive maximum benefit from radical resection.
Conclusions
Patients in whom a cholecystectomy specimen was sent for HPE presented early and achieved a better R0 resection rate and better overall survival, whereas those in whom the gallbladder was not sent for HPE presented late with symptoms of recurrence, and had a poor resectability rate and poor longterm survival. Hence, all cholecystectomy specimens should be sent for histopathology.
Conflicts of interest
None declared.
References
- 1.Diehl AK. Epidemiology of gallbladder cancer: a synthesis of recent data. J Natl Cancer Inst. 1980;65:1209–1214. [PubMed] [Google Scholar]
- 2.Targarona EM, Pons MJ, Viella P, Trias M. Unexpected carcinoma of the gallbladder, a laparoscopic dilemma. Surg Endosc. 1994;8:211–213. doi: 10.1007/BF00591833. [DOI] [PubMed] [Google Scholar]
- 3.Lai CH, Lau WY. Gallbladder cancer – a comprehensive review. Surgeon. 2008;6:101–110. doi: 10.1016/s1479-666x(08)80073-x. [DOI] [PubMed] [Google Scholar]
- 4.Kaushik SP. Current perspectives in gallbladder carcinoma. J Gastroenterol Hepatol. 2001;16:848–854. doi: 10.1046/j.1440-1746.2001.02520.x. [DOI] [PubMed] [Google Scholar]
- 5.You DD, Lee HG, Paik KY, Heo JS, Choi SH, Choi DW. What is an adequate extent of resection for T1 gallbladder cancers? Ann Surg. 2008;247:835–838. doi: 10.1097/SLA.0b013e3181675842. [DOI] [PubMed] [Google Scholar]
- 6.Wakai T, Shirai Y, Hatakeyama K. Radical second resection provides survival benefit for patients with T2 gallbladder carcinoma first discovered after laparoscopic cholecystectomy. World J Surg. 2002;26:867–871. doi: 10.1007/s00268-002-6274-z. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor VK, Pradeep R, Haribhakti SP, Sikora SS, Kaushik SP. Early carcinoma of the gallbladder: an elusive disease. J Surg Oncol. 1996;62:284–287. doi: 10.1002/(SICI)1096-9098(199608)62:4<284::AID-JSO12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Roa I, Araya JC, Villaseca M, Roa J, de Aretxabala X, Ibacache G. Gallbladder cancer in a high risk area: morphological features and spread patterns. Hepatogastroenterology. 1999;46:1540–1546. [PubMed] [Google Scholar]
- 9.Shrestha R, Tiwari M, Ranabhat SK, Aryal G, Rauniyar SK, Shrestha HG. Incidental gallbladder carcinoma: value of routine histological examination of cholecystectomy specimens. Nepal Med Coll J. 2010;12:90–94. [PubMed] [Google Scholar]
- 10.Royal College of Pathologists. Histopathology and Cytopathology of Limited or No Clinical Value. Report of Working Group of the Royal College of Pathologists. 2nd. London: Royal College of Pathologists; 2005. [Google Scholar]
- 11.Dix FP, Bruce IA, Krypcyzk A, Ravi S. A selective approach to histopathology of the gallbladder is justifiable. Surgeon. 2003;1:233–235. doi: 10.1016/s1479-666x(03)80023-9. [DOI] [PubMed] [Google Scholar]
- 12.Oommen CM, Prakash A, Cooper JC. Routine histology of cholecystectomy specimens is unnecessary. Ann R Coll Surg Engl. 2007;89:738. doi: 10.1308/003588407X209473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bazoua G, Hamza N, Lazim T. Do we need histology for a normal-looking gallbladder? J Hepatobiliary Pancreat Surg. 2007;14:564–568. doi: 10.1007/s00534-007-1225-6. [DOI] [PubMed] [Google Scholar]
- 14.Mittal R, Jesudason MR, Nayak S. Selective histopathology in cholecystectomy for gallstone disease. Indian J Gastroenterol. 2010;29:32–36. doi: 10.1007/s12664-010-0005-4. [DOI] [PubMed] [Google Scholar]
- 15.Darmas B, Mahmud S, Abbas A, Baker AL. Is there any justification for the routine histological examination of straightforward cholecystectomy specimens? Ann R Coll Surg Engl. 2007;89:238–241. doi: 10.1308/003588407X168361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohsiriwat V, Vongjirad A, Lohsiriwat D. Value of routine histopathologic examination of three common surgical specimens: appendix, gallbladder, and haemorrhoid. World J Surg. 2009;33:2189–2193. doi: 10.1007/s00268-009-0164-6. [DOI] [PubMed] [Google Scholar]


