Abstract
Objectives
This paper presents an innovative technique to address complex multiple hepatic vein (HV) reconstruction in right lobe graft living donor liver transplantation (RL-LDLT).
Methods
A patient with hepatitis C virus-related cirrhosis underwent RL-LDLT. The graft had seven HVs, including: the right HV (17 mm); one segment VII HV (11 mm); two segment VI HVs (6 mm and 16 mm), and three segment V HVs. The graft weighed 663 g (53% of standard liver volume; ratio of graft weight to recipient body weight: 0.96). Each HV had significant drainage territory requiring reconstruction. A cryopreserved iliac vein graft was used to create a sleeve patch to incorporate the HV openings. The holes were anastomosed to their corresponding HV tributaries using continuous 6–0 polydioxanone (PDS) sutures. Two of the three segment V HVs were combined using a smaller iliac vein patch, which was anastomosed in an end-to-side fashion to a previously harvested recipient umbilical vein interposition graft. The other end of the umbilical vein graft was anastomosed to the larger iliac vein sleeve patch.
Results
Overall, six HV openings were incorporated in one sleeve patch to allow a single wide anastomosis with the recipient inferior vena cava. Doppler ultrasound after reconstruction showed adequate flow patterns in all the HVs.
Conclusions
All-in-one sleeve patch graft venoplasty simplifies the reconstruction of multiple HVs and reduces warm ischaemia time in RL-LDLT with excellent outcomes.
Keywords: right lobe graft, living donor liver transplantation, venoplasty, multiple hepatic veins, outflow reconstruction
Introduction
The success of adult living donor liver transplantation (LDLT) utilizing a right lobe graft (RLG) relies on the provision of ample volume, adequate arterial and portal inflow, biliary drainage and the establishment of an outflow conduit that will allow sufficient drainage into the inferior vena cava (IVC). The obstruction of hepatic vein (HV) outflow could potentially result in liver congestion, graft failure, sepsis and death.1 However, by contrast with the whole organ and its left lobe counterpart, the anatomy of the right lobe, particularly of its HVs, is highly variable and remarkably intricate.
The RLG may contain multiple HV openings, each of which may drain significant portions of the graft and require reconstruction. Reconstructing the individual openings is tedious, challenging, time-consuming, and associated with prolonged warm ischaemia and cross-clamping time. This report describes a technique of sleeve patch graft venoplasty that overcomes the difficulty of multiple HV outflow reconstruction in an RLG.
Materials and methods
A 60-year-old woman (height: 160 cm; weight: 69 kg; body surface area: 1.77 m2) with hepatitis C virus-related end-stage liver cirrhosis was referred for liver transplantation. Computed tomography (CT) angiography studies showed partial thrombus formation in the right and main portal veins (PVs). The hepatic artery, HV and IVC appeared normal. Collateral vessels, including a re-canalized umbilical vein (9.9 mm in diameter), were also noted. Her standard liver volume (SLV) was estimated to be 1251 ml.
The live liver donor was the patient's 35-year-old daughter (height: 172 cm; weight: 63 kg). The CT volumetric estimation of the donor's liver was 1197 ml (right lobe: 827 ml; left lobe: 370 ml) with mild (5%) fatty infiltration, which was confirmed by liver biopsy. The right and left lobe volumes of the donor's liver corresponded to 66% and 30% of the recipient's SLV, respectively. After correcting for the degree of fatty infiltration, the donor's right and left lobe corrected volumes represented 62% and 28%, respectively, of the recipient's SLV. The right lobe was selected to provide an adequate volume (>40% of SLV). The remnant left lobe, which represented 31% of the donor's total liver volume, was considered adequate. Preoperative CT angiography showed that the donor's right lobe was supplied by a single right PV and a single right hepatic artery. In total, the RLG was drained by a single right HV, a segment VII HV, two segment VI HVs and three segment V HVs.
The techniques used for both donor2 and recipient3 procedures in LDLT have been described in previous reports. The hepatic parenchymal transection in the donor was carried out to the right of the middle HV. Following procurement, the graft was perfused with cold (4 °C) histidine-tryptophan-ketoglutarate (HTK) solution. The graft's actual weight was 663 g (53% of SLV; graft weight : recipient body weight ratio: 0.96). Overall, the graft was drained by seven HV tributaries (Fig. 1): one right HV (17 mm); one segment VII HV (11 mm); two segment VI HVs (6 mm and 16 mm), and three segment V HVs (each measuring 5 mm). The estimated volume drained by the HVs was assessed by the extent of hepatic congestion induced by temporary clamping of the right hepatic artery following division of the HVs. Reconstruction of all but one HV was deemed necessary based on the measured diameter of the HVs, extent of congestion and effluent flow during perfusion. One of the segment V HVs was closed by suture because insignificant effluent was noted at the back table.
Figure 1.
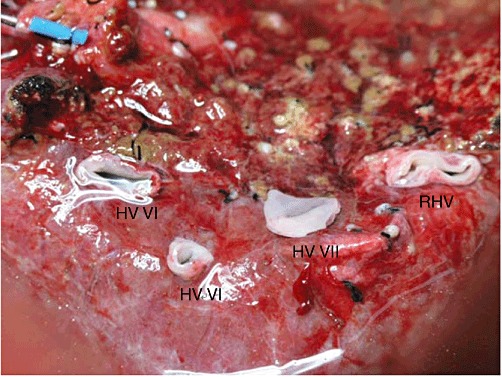
Right lobe graft with multiple hepatic veins (HVs). Right hepatic vein (RHV): 17 mm; segment VII HV: 11 mm; segment VI HVs: 6 mm and 16 mm
All-in-one sleeve patch graft venoplasty
In view of the number of HVs that required reconstruction, it was decided to reconstruct the HV tributaries collectively using a long sleeve cryopreserved iliac vein graft (IVG) from a deceased donor. The IVG had a flat diameter of 8 mm and a length of 120 mm. The IVG was cut along its entire length to create a rectangular venous patch with a width of 16 mm. Four oval holes in the IVG patch were created corresponding to the locations of the right HV, the segment VII HV and the segment VI HVs. A fifth hole was created to accommodate the umbilical vein graft. Each hole was fashioned slightly larger and anastomosed to its respective HV using a double-arm 6–0 polydioxanone (PDS) continuous suturing technique. All knots were tied extraluminally without growth factor.
The two segment V HVs were incorporated to a smaller patch graft taken beforehand from a portion of the cryopreserved IVG (Fig. 2). The combined segment V HV patch graft was anastomosed end-to-side to the recipient's umbilical vein interposition graft, which, in turn, was anastomosed to the large IVG sleeve patch (Fig. 3). Figure 4 shows the completed all-in-one sleeve patch graft venoplasty ready for anastomosis with the recipient's IVC.
Figure 2.
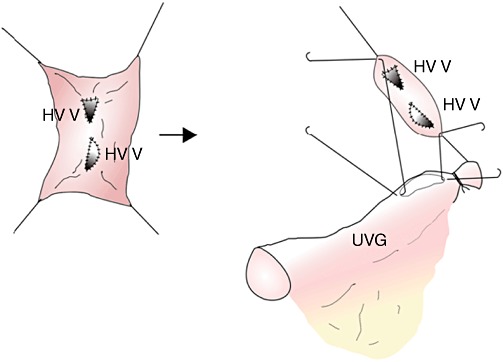
Two segment V hepatic veins were incorporated to a small patch taken from a portion of the cryopreserved iliac vein graft. The segment V patch was subsequently anastomosed to the procured recipient re-canalized umbilical vein graft (UVG)
Figure 3.
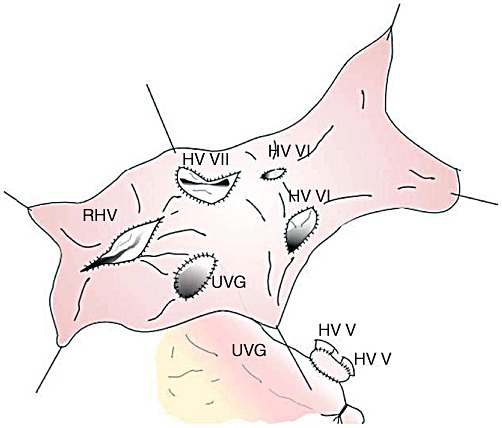
Four oval holes were created in the iliac vein graft patch to accommodate the hepatic vein (HV) openings for one right HV (RHV), one segment VII HV and two segment VI HVs. A fifth hole was created to accommodate the umbilical vein graft (UVG) previously anastomosed to the segment V HV patch graft
Figure 4.
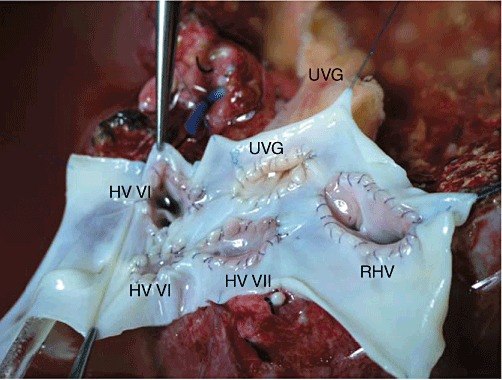
Completed all-in-one sleeve patch graft venoplasty ready for anastomosis with the recipient vena cava. HV, hepatic vein; UVG, umbilical vein graft; RHV, right hepatic vein
In the recipient, the middle and left HVs were closed by sutures. The right HV orifice was incised caudally along the IVC to 7 cm to approximate the length of the all-in-one sleeve patch graft. The all-in-one sleeve patch graft was anastomosed to the IVC using a double-arm 5–0 PDS continuous suturing technique (Fig. 5). The overall cold and warm ischaemia times were 159 min and 47 min, respectively. When outflow reconstruction had been completed, end-to-end PV and hepatic artery reconstructions were performed. The latter was carried out using a microsurgical technique. Doppler ultrasonography performed after graft revascularization showed adequate flow in all reconstructed HV tributaries.
Figure 5.
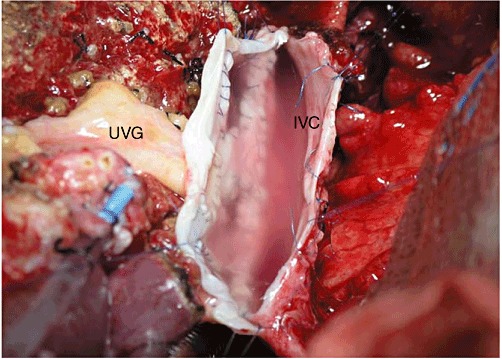
All-in-one sleeve patch anastomosed to the inferior vena cava (IVC) of the recipient. UVG, umbilical vein graft
The recipient's and the donor's postoperative courses were uneventful. The reconstructed HV remained patent with adequate flow velocities at 26 months of follow-up.
Discussion
Because of the anatomical complexities of HV drainage of the RLG, the extent of venous outflow reconstruction in adult LDLT remains widely debated. It is clear that the right HV requires reconstruction. However, the best approach to managing the multiple HV tributaries is unclear.
Lo et al.4,5 advocated the use of an RLG together with the middle HV trunk. This approach has the advantage of providing a larger graft with better outflow drainage. However, others believe this technique may pose a theoretical risk to the donor.6,7
Although some authors have advocated that the reconstruction of the middle HV tributaries may be omitted in patients in whom pre-existing venous channels between the middle and right HVs are apparent,8–10 these channels are difficult to demonstrate in the majority of cases.11 The occlusion of the middle HV tributaries may lead to congestion, with subsequent atrophy of the segments drained by these HVs, or graft failure if the congested portion is large.1
Sano et al. proposed to estimate the volume drained by a particular middle HV tributary by temporarily clamping both the hepatic artery and middle HV tributary to induce congestion of the involved portions of the graft and recommended reconstruction of the middle HV tributary if congestion was large and the volume of the unaffected portion of the graft was <40% of the recipient's SLV.12 Other investigators have reconstructed the middle HV tributaries using an interposition graft anastomosed to the recipient's middle and left HVs.13,14 At the present institution, it is routine practice to estimate the extent of temporary congestion, and cryopreserved vascular grafts have been used extensively to reconstruct the divided middle HV or its tributaries since a vascular bank was set up in 2005.
In some situations, the RLG is drained by several HVs. Reconstructing these vessels individually is tedious and prolongs the warm ischaemia time, as well as the anhepatic phase. To overcome this problem, various technical innovations have been proposed to reconstruct multiple venous openings. Hwang et al. described quilt venoplasty using the recipient's autologous greater saphenous vein15 and composite cluster reconstructions using homologous iliac or autologous paraumbilical veins.16 Similarly, Soejima et al.17 utilized a left internal jugular patch and a PV graft to reconstruct multiple HV openings into a single orifice.
In the present patient, the use of a cryopreserved IVG together with the autologous re-canalized umbilical vein obviated the need for additional surgery to procure a vascular graft from either recipient or donor. This technique is suitable in grafts with multiple accessory right HVs and tributaries from the middle HV.
The previous use of cryopreserved veins in the late 1990s and early 2000s resulted in dismal outcomes related to aneurysm formation, thrombosis and stenosis.18,19 However, the technology and techniques of vascular cryopreservation and its use in liver transplantation, particularly in the reconstruction of PVs rather than HVs, were evolving at that time. In 2006, the use of cryopreserved veins in PV reconstruction in adult LDLT was highlighted by late PV stenosis and thrombosis.20 However, the PV haemodynamic differs from the HV haemodynamic. Portal vein flow in end-stage liver disease and cirrhosis is hyperdynamic as a result of portal hypertension, whereas flow in the HV is similar to central venous pressure even after graft placement. Further, the possible adverse effect of cryopreserved vessel graft shrinkage is avoided by utilizing a large patch.
At the present institution, 1-month and 3-month patency rates in outflow reconstruction of segment V and VIII HVs using cryopreserved vessels have been reported as 82% and 41%, respectively.21 The 1-year patency rate was only 9%, but the rate of overall graft survival was 96%. This implies that the graft has already regenerated by the time the cryopreserved vessels begin to obstruct as a result of late stenosis and thrombosis, and recruitment of HV collaterals has developed. The cold and warm ischaemia times in this series were 74 min and 69 min, respectively. The cold and warm ischaemia times in the all-in-one sleeve patch graft venoplasty technique were 159 min and 47 min, respectively. Thus, warm ischaemia time, which is more important, was reduced by 22 min.
In conclusion, all-in-one sleeve patch graft venoplasty is a feasible technique to simplify the reconstruction of multiple HVs in the RLG. This approach utilizes back-table reconstruction to reduce warm ischaemia time and does not require additional surgical procedures for procurement of vein grafts. The single large anastomosis is technically easier to perform and provides excellent outflow, preventing congestion in the graft.
Conflicts of interest
None declared.
References
- 1.Lee SG, Park KM, Hwang S, Lee YJ, Choi DN, Kim KH, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001;71:812–814. doi: 10.1097/00007890-200103270-00021. [DOI] [PubMed] [Google Scholar]
- 2.Chen CL, Chen YS, De Villa V, Wang CC, Lin CL, Goto S, et al. Minimal blood loss living donor hepatectomy. Transplantation. 2000;69:2580–2586. doi: 10.1097/00007890-200006270-00018. [DOI] [PubMed] [Google Scholar]
- 3.De Villa V, Chen CL, Chen YS, Wang CC, Wang SH, Chiang YC, et al. Outflow reconstruction in living donor liver transplantation. Transplantation. 2000;70:1604–1608. doi: 10.1097/00007890-200012150-00011. [DOI] [PubMed] [Google Scholar]
- 4.Lo CM, Fan ST, Liu CL, Lo RJW, Lau GK, Wei WI, et al. Extending the limit on the size of adult recipient in living donor liver transplantation using extended right lobe graft. Transplantation. 1997;63:1524–1528. doi: 10.1097/00007890-199705270-00027. [DOI] [PubMed] [Google Scholar]
- 5.Lo CM, Fan ST, Liu CL, Wong J. Hepatic venoplasty in living donor liver transplantation using right lobe graft with middle hepatic vein. Transplantation. 2003;75:358–360. doi: 10.1097/01.TP.0000046527.19422.3E. [DOI] [PubMed] [Google Scholar]
- 6.Inomata Y, Uemoto S, Asonuma K, Egawa H, Kiuchi T, Fujita S, et al. Right lobe graft in living donor liver transplantation. Transplantation. 2000;69:258–264. doi: 10.1097/00007890-200001270-00011. [DOI] [PubMed] [Google Scholar]
- 7.Marcos A, Fisher RA, Ham JH, Shiffman ML, Sanyal AJ, Luketic VA, et al. Right lobe living donor liver transplantation. Transplantation. 1999;68:798–803. doi: 10.1097/00007890-199909270-00012. [DOI] [PubMed] [Google Scholar]
- 8.Satou S, Sugawara Y, Kokudo N, Makuuchi M. Preoperative detection of hepatic venous collaterals in right liver graft. Liver Transpl. 2005;11:708–709. doi: 10.1002/lt.20412. [DOI] [PubMed] [Google Scholar]
- 9.Cescon M, Sugawara Y, Sano K, Ohkubo T, Kaneko J, Makuuchi M. Right liver graft without middle hepatic vein reconstruction from a living donor. Transplantation. 2002;73:1164–1166. doi: 10.1097/00007890-200204150-00028. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko T, Kaneko K, Sugimoto H, Inque S, Hatsuno Y, Sawada K, et al. Intrahepatic anastomosis formation between the hepatic veins in the graft liver of the living related liver transplantation: observation by Doppler ultrasonography. Transplantation. 2000;70:982–985. doi: 10.1097/00007890-200009270-00018. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura S, Tsuzuki T. Surgical anatomy of the hepatic veins and the inferior vena cava. Surg Gynecol Obstet. 1981;152:43–50. [PubMed] [Google Scholar]
- 12.Sano K, Makuuchi M, Miki K, Maema A, Sugawara Y, Imamura H, et al. Evaluation of hepatic venous congestion: proposed indication criteria for hepatic vein reconstruction. Ann Surg. 2002;236:241–247. doi: 10.1097/00000658-200208000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang S, Lee SG, Ahn CS, Park KM, Kim KH, Moon DB, et al. Cryopreserved iliac artery is indispensible interposition graft material for middle hepatic vein reconstruction of right liver grafts. Liver Transpl. 2005;11:644–649. doi: 10.1002/lt.20430. [DOI] [PubMed] [Google Scholar]
- 14.Sugawara Y, Makuuchi M, Sano K, Imamura H, Kaneko J, Ohkubo T, et al. Vein reconstruction in modified right liver graft for living donor liver transplantation. Ann Surg. 2003;237:180–185. doi: 10.1097/01.SLA.0000048444.40498.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang S, Lee SG, Park KM, Kim KH, Ahn CS, Moon DB, et al. Quilt venoplasty using recipient saphenous vein graft for reconstruction of multiple short hepatic veins in right liver grafts. Liver Transpl. 2005;11:104–107. doi: 10.1002/lt.20314. [DOI] [PubMed] [Google Scholar]
- 16.Hwang S, Lee SG, Ahn CS, Park KM, Kim KH, Song GW, et al. Composite cluster reconstruction of multiple middle hepatic vein branches in right lobe graft. Liver Transpl. 2005;11:1144–1146. doi: 10.1002/lt.20531. [DOI] [PubMed] [Google Scholar]
- 17.Soejima Y, Ueda N, Fukuhara T, Yoshizumi T, Ikegami T, Yamashita Y, et al. One-step venous reconstruction for a right lobe graft with multiple venous orifices in living donor liver transplantation. Liver Transpl. 2008;14:706–708. doi: 10.1002/lt.21401. [DOI] [PubMed] [Google Scholar]
- 18.Kuang AA, Renz JF, Ferrel LD, Ring EJ, Rosenthal P, et al. Failure patterns of cryopreserved grafts in liver transplantation. Transplantation. 1996;62:742–747. doi: 10.1097/00007890-199609270-00007. [DOI] [PubMed] [Google Scholar]
- 19.Millis MJ, Cronin D, Brady LM, Newell KA, Woodle ES, Bruce DS, et al. Primary living-donor liver transplantation at the University of Chicago. Ann Surg. 2000;232:104–111. doi: 10.1097/00000658-200007000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugawara Y, Makuuchi M, Tamura S, Matsui Y, Kaneko J, Hasegawa K, et al. Portal vein reconstruction in adult living donor liver transplantation using cryopreserved vein grafts. Liver Transpl. 2006;12:1233–1236. doi: 10.1002/lt.20786. [DOI] [PubMed] [Google Scholar]
- 21.Jordan A, Chen CL, Yong CC, Wang CC, Wang SH, Liu YW, et al. Cryopreserved vascular conduits in living donor liver transplantation. Liver Transpl. 2008;14(Suppl. 1):232. [Google Scholar]


