Abstract
Previous Western studies showed a consistent and marked reduction in health-related quality of life (HRQOL) in patients chronically infected with HCV. However, these studies were conducted on patients whose knowledge of their serological status may have affected their HRQOL. This HRQOL survey conducted in the Egyptian rural population provides a unique opportunity to clarify this issue among a population whose serological status is unknown. HRQOL was assessed by an Arabic translation of the Short-Form 12, and a visual analogue scale (VAS) of the relative severity of one’s health status. HCV chronic infection was defined by positive tests for anti-HCV antibody and HCV-RNA. HRQOL was compared according to HCV chronic infection status in linear mixed models adjusted for potential confounding factors, i.e. age, sex, education, and health care-related risk factors, and adjusted for interviewer as a random effect. One hundred and forty-six Egyptians chronically infected with HCV had similar Short-Form 12 and VAS scores compared to 1140 uninfected controls from the same rural community. In individuals chronically infected with HCV, serum aminotransferase levels did not correlate with HRQOL. In conclusion, this study did not find a significant reduction of HRQOL in patients chronically infected with HCV compared to uninfected, contemporaneous controls. This may in part be explained by a lower morbidity amongst patients chronically infected with HCV in rural Egypt and a higher morbidity amongst uninfected controls as compared to those of Western studies as well as a lack of awareness of hepatitis C serological status.
Keywords: Adult; Egypt; epidemiology; Female; Hepatitis C, Chronic; epidemiology; psychology; Humans; Male; Middle Aged; Multivariate Analysis; Prevalence; Quality of Life; Rural Population; Transaminases; blood
Keywords: HCV, SF-12, Visual analogue scale, disease labelling effect, Developing Countries.
Introduction
About 15% of 59 million Egyptians in 1996 were estimated to have positive test for anti-HCV antibody, and based on 60% viremia, more than five million Egyptians are chronically infected with hepatitis C virus (HCV) (1). The treatment of chronic hepatitis C (CHC) patients is currently considered to be a public health priority in Egypt to reduce both the burden of liver disease and the transmission of HCV. However, dramatic health care budget constraints limit access to the costly treatment recommended in Western countries (2–4). Decision to treat will depend on the expected benefits from treatment of CHC patients, who are mostly infected with the genotype 4, living in the Nile Delta rural areas, and generally unaware of their HCV serological status in the absence of systematic screening (1).
Previous Western studies have reported a consistent and marked reduction in health-related quality of life (HRQOL) among CHC patients as compared to nationally representative samples of adults, particularly in physical health-related domains (5–16). The HRQOL of CHC patients declines even more during the 6 to 12 months of treatment, but it returns to the pre-treatment level during the 6 months after treatment due to significant improvements on HRQOL of sustained virological responders (7, 10, 11, 15, 17). However, these studies were conducted on patients whose knowledge of their serological status may have affected their HRQOL instead of the disease itself (12, 18).
Egyptian rural population with the highest prevalence of hepatitis C in the world provides a unique opportunity to clarify this issue. Our primary objective was to compare HRQOL in individuals chronically infected with HCV and unaware of their serological status to that of uninfected controls from the same Egyptian rural community. Our secondary objective was to compare HRQOL within individuals chronically infected with HCV across serum aminotransferase levels.
Material and methods
Study design
All adults and children from a village in the lower Nile Delta region (Zwayat-Razin, Meynoufeya Governorate), Egypt, were invited to participate in a cohort study on HCV risk factors between May and December 2002 (n=5130; response rate of 78%). The village of Zwayat-Razin was selected because it is an endemic area for Schistosomiasis mansoni and, as such, best represents the epidemic of hepatitis C in rural Egypt (1). The HRQOL sub-study was conducted among all adults of five out of six districts of the village included in the cohort study due to budget constraints. The HRQOL interview occurred at a median time of three weeks (extremes: same day to 6 months) following the risk factors interview. Three interviewers blinded to HCV serological status of participants followed an initial two-month training period with joint interviews, and were then purposely matched to the interviewee’s gender. Participants were not yet aware of their HCV serological status at the time of the HRQOL survey. There was no difference in chronic HCV infection prevalence between participants included in the HRQOL sub-study (11.4%) and others from the risk factors study (11.9%). The study was approved by the institutional review board at the University of Ain Shams, Cairo, and informed consent was obtained from each participant.
HRQOL data collection
HRQOL was evaluated by two instruments: Short-Form 12 (SF-12) and a visual analogue scale (VAS). The SF-12 is a widely-used generic HRQOL questionnaire (19), developed by the Medical Outcome Trust to reduce the time of administering the SF-36 (20, 21). The health concepts underlying the SF-12 and the SF-36 range from those reflecting predominantly physical wellness, including items on physical functioning, the ability to perform expected physical roles, the degree of bodily pain, and the overall sense of general health, to those reflecting predominantly social and emotional well-being, including items on the ability to perform expected emotional and social roles, overall sense of vitality, and overall sense of mental health. The twelve items of the SF-12 were selected from the SF-36 to best reflect its physical health summary score (PCS) and its mental health summary score (MCS) (21). We performed a translation and cross-cultural adaptation of the SF-12 by a forward translation from US English followed by an independent backward translation into English, and adaptations were made to the context of rural Egypt, e.g. description of activities of a typical day. The Arabic SF-12 version showed satisfactory psychometric properties (i.e. reliability, construct validity, convergent validity) in our sample, except for the item Mental Health 2 about ‘Have you felt downhearted and blue?’ which had a low sensitivity. We decided, however, to include all component items in the PCS and the MCS using the Likert method of summated items (22). Presented results did not change after the removal of Mental Health 2 from the MCS (data not shown). We also developed a visual analogue scale (VAS) to assess the relative severity of one’s health state that was shown to be feasible in developing countries (23), with a high sensitivity to change in CHC patients (24) and other chronic diseases of mild to moderate severity (25). The VAS was designed as a 100-point scale between death and the best imaginable health state, and participants were asked to rate their health state at different points in time, i.e. during the preceding month, one year ago, and then five years ago. The SF-12 PCS, SF-12 MCS, and VAS ranged from 0 to 100, with higher scores reflecting better HRQOL.
Data analyses
HCV chronic infection was defined by positive tests for anti-HCV antibody with two enzyme-linked immunosorbent assays (INNOTEST™ HCV Ab IV, 4th generation test - Innogenetics - Ghent, Belgium; and ABBOTT HCV EIA 3.0, 3rd generation test - Abbott Laboratories - Wiesbaden, Delknheim, Germany) and a detectable HCV viremia (reverse transcriptase polymerase chain reaction - in house assay using 5′UTR primers with modification) (26) with or without elevated liver enzymes. All univariate and multivariate analyses of HRQOL were adjusted for interviewer as a random effect in linear mixed models. Mean HRQOL score in individuals chronically infected with HCV was compared to that of uninfected controls adjusting for potential confounding factors, i.e. risk factors for HCV chronic infection that decreased HRQOL. In individuals chronically infected with HCV, the association of HRQOL and serum aminotransferase (ALT, AST) level was tested by using Pearson correlation. Significance was attributed at the 5% level, and data were analyzed with SAS software (SAS 8.02, Cary, NC).
Results
Overall 1286 adult Egyptians from the village of Zwayat Razin were interviewed to collect HRQOL data following the survey on HCV infection risk factors. Participants were 58.9% female and had a mean (SD) age of 33.1 (12.0) years, 60.3% were illiterate and 73.6% were farmers. The prevalence of self-declared chronic diseases was 19.0% (12.4% high blood pressure, 6.7% rheumatic diseases, 1.9% diabetes, 0.5% liver diseases). About 54.6% reported a previous admission to hospital or clinic, 6.1% had been transfused, and 6.5% had received parenteral antischistosomal therapy. None of the 146 (11.4%) individuals chronically infected with HCV were aware of their serological status.
We looked for potential confounding factors between HCV chronic infection and HRQOL scores. On the one hand, sex, age, education, self-declared chronic disease, previous admission to hospital or clinic, past history of transfusion, and past parenteral antischistosomal therapy were significant risk factors for HCV chronic infection (Table 1). On the other hand, these socio-demographic or disease-related variables, except past parenteral antischistosomal therapy, were significant predictors of HRQOL scores in individuals chronically infected with HCV as well as uninfected controls (Table 2). Accordingly, further multivariate analyses on HRQOL included those six potential confounding factors, i.e. sex, age, education, self-declared chronic disease, previous admission to hospital or clinic, and past history of transfusion.
Table 1.
Baseline characteristics of subjects (n=1286) according to HC V chronic infection. Except where stated otherwise, values are expressed in percentage of subjects.
| Infected by HC V (n=146) | Uninfected controls (n=1140) | p value* | |
|---|---|---|---|
| Socio-dem ographic variables | |||
| Fem ale sex | 43.1 | 60.9 | <0.0001 |
| Mean (SD) age, y | 39.6 (12.3) | 32.2 (11.8) | <0.0001 |
| Marital status: | |||
| Married/partnered | 86.3 | 76.8 | ns |
| Single/never married | 8.9 | 18.1 | |
| Divorced/separated | 1.4 | 1.5 | |
| Widowed | 3.4 | 3.6 | |
| Number of pregnancies (in women): | |||
| 0 | 3.5 | 5.1 | ns |
| 1–2 | 22.8 | 29.8 | |
| 3–4 | 26.3 | 29.0 | |
| >4 | 47.4 | 36.1 | |
| Education: | |||
| Illiterate | 60.3 | 60.3 | |
| Read and write | 24.6 | 15.3 | <0.01 |
| Any education | 15.1 | 24.4 | |
| Farmer job | 74.7 | 73.4 | ns |
| Lived whole life here | 87.7 | 89.2 | ns |
| Disease-related variables | |||
| Self-declared chronic disease: | 28.8 | 17.7 | <0.01 |
| High blood pressure | 17.8 | 11.7 | <0.05 |
| Rheumatism | 11.0 | 6.1 | <0.05 |
| Diabetes mellitus | 3.4 | 1.7 | ns |
| Liver disease | 0 | 0.5 | ns |
| Previous admission to hospital or clinic | 66.4 | 53.1 | <0.01 |
| Past history of transfusion | 12.3 | 5.4 | <0.001 |
| Parenteral antischistosomal therapy | 19.2 | 4.8 | <0.0001 |
Chi-square test or exact Fisher test for qualitative variables; Student t-test for quantitative variable.
ns: non significant.
Table 2.
Univariate analysis of explanatory variables of HRQOL scores† according to HCV chronic infection in 1286 rural Egyptians. Results are expressed in least-squares mean (CI95%) scores with adjustment for interviewer as a random effect in linear mixed models.
| Explanatory variable of HRQOL scores | Infected by HCV (n=146)
|
Uninfected controls (n=1140)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n= | Physical health summary score | p value | Mental health summary score | p value | VAS during the last month | p value | n= | Physical health summary score | p value | Mental health summary score | p value | VAS during the last month | p value | |
| Socio-demographic variables | ||||||||||||||
| Sex: | ||||||||||||||
| Female | 63 | 32.5 (24.9–40.1) | * | 45.3 (39.8–50.9) | ns | 62.2 (58.4–66.0) | ns | 694 | 45.8 (43.5–48.2) | ** | 48.6 (46.9–50.3) | * | 64.9 (63.8–66.2) | * |
| Male | 83 | 55.3 (48.4–62.3) | 54.9 (49.8–60.1) | 63.5 (60.0–67.1) | 446 | 65.2 (62.2–68.2) | 58.6 (56.4–60.8) | 71.5 (70.0–73.1) | ||||||
| Age: | ||||||||||||||
| >38 years old | 89 | 39.7 (33.4–46.0) | * | 48.1 (43.5–52.7) | * | 58.4 (55.6–61.2) | ** | 368 | 31.2 (28.4–34.0) | *** | 42.8 (40.5–44.9) | *** | 56.9 (55.5–58.2) | *** |
| 26 to 38 years old | 33 | 43.4 (30.7–56.1) | 50.3 (41.0–59.6) | 68.3 (62.8–73.8) | 397 | 56.3 (53.5–59.1) | 54.5 (52.3–56.7) | 69.4 (68.0–70.8) | ||||||
| <26 years old | 24 | 60.7 (47.0–74.3) | 63.6 (53.7–73.6) | 73.1 (67.1–79.0) | 375 | 70.6 (67.8–73.4) | 60.3 (58.1–62.5) | 76.1 (74.7–77.5) | ||||||
| Education: | ||||||||||||||
| Illiterate | 88 | 38.0 (31.9–44.2) | *** | 48.2 (43.7–52.8) | * | 59.4 (56.6–62.2) | ** | 687 | 44.3 (42.1–46.6) | *** | 48.9 (47.2–50.5) | ** | 64.1 (62.9–65.2) | *** |
| Read and write | 36 | 53.5 (41.0–66.0) | 54.8 (45.5–64.0) | 64.7 (58.9–70.3) | 174 | 59.9 (55.3–64.6) | 57.7 (54.3–61.2) | 69.0 (66.7–71.4) | ||||||
| Any education | 22 | 68.3 (52.8–83.8) | 61.7 (50.3–73.2) | 76.5 (69.5–83.6) | 279 | 71.0 (67.3–74.7) | 59.1 (56.4–61.8) | 75.5 (73.6–77.3) | ||||||
| Disease-related variables | ||||||||||||||
| Self-declared chronic disease: | ||||||||||||||
| Yes | 42 | 23.4 (14.9–31.9) | ** | 37.4 (31.4–43.5) | ns | 54.3 (50.1–58.5) | * | 202 | 21.9 (17.6–26.1) | *** | 36.6 (33.4–39.8) | ** | 54.3 (52.1–56.5) | ** |
| No | 104 | 56.1 (50.1–62.1) | 55.7 (51.5–59.9) | 66.6 (63.6–69.5) | 938 | 60.3 (58.3–62.2) | 56.4 (54.9–57.8) | 70.3 (69.3–71.3) | ||||||
| Admission to hospital or clinic: | ||||||||||||||
| Yes | 97 | 42.5 (36.2–48.8) | * | 51.3 (46.8–55.8) | ns | 62.0 (58.9–65.0) | ns | 605 | 47.8 (45.3–50.4) | ** | 48.8 (47.0–50.6) | *** | 64.3 (63.1–65.5) | * |
| No | 49 | 52.9 (43.1–63.7) | 51.1 (43.3–58.9) | 67.1 (61.9–72.3) | 535 | 59.0 (56.3–61.8) | 57.3 (55.3–59.2) | 71.3 (69.9–72.6) | ||||||
| Past history of transfusion: | ||||||||||||||
| Yes | 18 | 18.9 (3.8–33.9) | *** | 30.0 (19.4–40.6) | *** | 52.8 (45.5–60.1) | *** | 61 | 38.5 (30.5–46.5) | * | 42.9 (37.3–48.6) | * | 59.9 (56.0–63.9) | * |
| No | 128 | 48.9 (43.5–54.3) | 54.1 (50.3–57.9) | 64.5 (61.9–67.1) | 1079 | 53.8 (51.9–55.8) | 53.3 (51.9–54.7) | 68.0 (67.0–68.9) | ||||||
| Parenteral antischistosomal therapy: | ||||||||||||||
| Yes | 28 | 42.1 (28.5–55.7) | ns | 52.3 (42.5–62.0) | ns | 61.6 (55.1–68.1) | ns | 55 | 44.4 (36.0–52.7) | ns | 52.1 (46.1–58.0) | ns | 62.4 (58.2–66.5) | ns |
| No | 118 | 44.9 (38.8–51.0) | 51.8 (47.4–56.2) | 63.8 (60.9–66.8) | 1085 | 53.5 (51.6–55.5) | 52.8 (51.4–54.2) | 67.9 (66.9–68.8) | ||||||
SF-12 Physical and Mental health summary scores and VAS score range from 0 to 100, with higher scores reflecting better HRQOL.
p<0.05;
p<0.01;
p<0.001;
ns: non significant;
CI95%: confidence interval at 95%.
Multivariate analyses showed that HCV chronic infection was not associated to the SF-12 PCS, SF-12 MCS, and VAS score, whereas sex, age, education, self-declared chronic disease, previous admission to hospital or clinic remained independent predictors of HRQOL scores (Table 3). Further comparison of adjusted mean scores of the eight subscales of the SF-12 between individuals chronically infected with HCV and uninfected controls did not show any significant difference (Figure 1). Using a VAS, participants assessed retrospectively the relative severity of their health status at three different points in time from the preceding month to five years ago. Adjusted mean VAS scores did not differ significantly at any points in time between the two groups.
Table 3.
Multivariate analysis of explanatory variables of HRQOL scores† in 1286 rural Egyptians. Results are expressed in least-squares mean (CI95%) scores of fixed effects with adjustment for interviewer as a random effect in linear mixed models.
| Explanatory variable of HR Q O L scores | n= | Physical health summary score‡ | p value | Mental health summary score‡ | p value | V AS during the last month‡ | p value |
|---|---|---|---|---|---|---|---|
| HC V chronic infection: | |||||||
| Yes | 146 | 50.7 (45.5–55.8) | ns | 51.9 (47.6–56.2) | ns | 66.9 (64.4–69.6) | ns |
| No | 1140 | 50.1 (46.7–53.4) | 49.2 (46.4–51.9) | 66.5 (64.8–68.2) | |||
| Socio-dem ographic variables | |||||||
| Sex: | |||||||
| Female | 757 | 43.2 (39.3–47.1) | * | 46.7 (43.5–50.0) | * | 64.7 (62.7–66.6) | ns |
| Male | 529 | 57.5 (53.4–61.6) | 54.3 (50.9–57.8) | 68.8 (66.7–70.8) | |||
| Age: | |||||||
| >38 years old | 457 | 36.6 (32.7–40.5) | *** | 44.3 (41.0–47.5) | ** | 58.9 (56.9–60.8) | *** |
| 26 to 38 years old | 430 | 51.1 (46.8–55.4) | 51.1 (47.6–54.7) | 67.9 (65.7–70.1) | |||
| <26 years old | 399 | 63.5 (58.9–68.0) | 56.2 (52.4–60.0) | 73.4 (71.1–75.7) | |||
| Education: | |||||||
| Illiterate | 775 | 44.6 (40.8–48.4) | ** | 48.5 (45.4–51.7) | ns | 64.2 (62.3–66.1) | * |
| Read and write | 210 | 51.1 (46.2–56.0) | 52.1 (47.9–56.2) | 66.6 (64.1–69.1) | |||
| Any education | 301 | 55.4 (50.9–59.9) | 51.0 (47.3–54.7) | 69.4 (67.1–71.6) | |||
| Disease-related variables | |||||||
| Self-declared chronic disease: | |||||||
| Yes | 244 | 40.2 (35.5–44.9) | *** | 44.6 (40.7–48.5) | * | 63.2 (60.8–65.6) | * |
| No | 1042 | 60.6 (56.9–64.2) | 56.4 (53.4–59.5) | 70.3 (68.4–72.1) | |||
| Admission to hospital or clinic: | |||||||
| Yes | 702 | 47.2 (43.6–50.9) | * | 48.1 (45.1–51.1) | * | 64.7 (62.8–66.5) | ns |
| No | 584 | 53.5 (49.3–57.8) | 53.0 (49.4–56.5) | 68.8 (66.6–70.9) | |||
| Past history of transfusion: | |||||||
| Yes | 79 | 49.7 (43.7–55.7) | ns | 49.2 (44.1–54.2) | ns | 66.5 (63.5–69.6) | ns |
| No | 1207 | 51.1 (48.2–53.9) | 51.9 (49.5–54.3) | 66.9 (65.5–68.4) | |||
SF-12 Physical and Mental health summary scores and VAS score range from 0 to 100, with higher scores reflecting better HRQOL.
R-square=0.499, 0.319, and 0.436, when PCS, M CS, and VAS is the dependent variable, respectively.
p<0.05;
p<0.01;
p<0.001;
ns: non significant;
CI95%: confidence interval at 95%.
Figure 1.
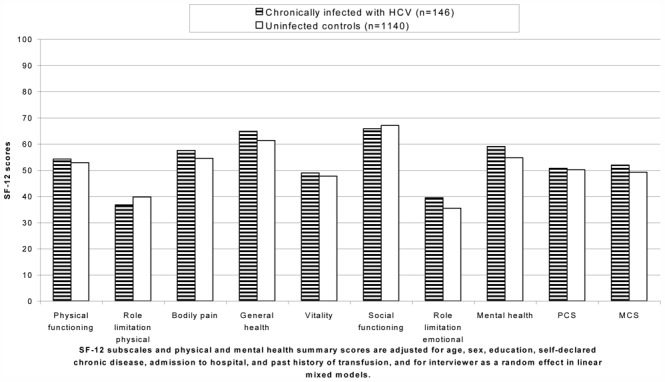
Health-related quality of life according to HCV chronic infection in rural Egypt.
One-hundred and fifteen out of 146 (79%) individuals chronically infected with HCV came to the local hepatology clinic for the results of their HCV serological status at a median time of seven weeks following the HRQOL interview (interquartile range: 4–10 weeks). ALT and AST levels were above normal values (40 IU/ml) in 32% and 23% of individuals, respectively. ALT and AST levels did not correlate significantly with PCS, MCS, and VAS during the preceding month.
Discussion
In this study, 1286 Egyptians unaware of their HCV serological status were prospectively interviewed on their health-related quality of life (HRQOL) by interviewers blinded to HCV serological status of the participants. An Arabic version of the SF-12 of satisfactory psychometric properties did not differ between the 146 individuals chronically infected with HCV and uninfected controls. The assessment of the relative severity of one’s health status by a visual analogue scale (VAS) was also similar between the two groups at different points in time from the preceding month to five years ago. In individuals chronically infected with HCV, serum aminotransferase levels did not correlate with HRQOL measures.
Contrary to previous Western studies, this study did not find a significant reduction of HRQOL in individuals chronically infected with HCV compared to uninfected, contemporaneous controls (5–16). This may be explained by a lower morbidity amongst patients chronically infected with HCV in rural Egypt and a higher morbidity amongst uninfected controls as compared to those of Western studies; a more precise handling of confounding factors in the comparison with population controls as well as a lack of awareness of hepatitis C serological status.
The lack of reduction of HRQOL in individuals chronically infected with HCV could be related to better outcomes of HCV infection in Egypt. The epidemic of hepatitis C is largely attributed to the parenteral antischistosomal therapy program which exposed children and young adults of the Nile Delta areas to contaminated needles from 1961 to 1986 (1). First, other studies have shown consistently that subjects infected at younger ages have better outcomes (27–31). Second, HCV-related morbidity was apparently low in the cohort, as shown by the small proportion (32%) of chronically infected subjects with any elevation of ALT. However, it compares to that of the cohort of Irish women among whom 55% had elevated liver enzymes 17 years after contamination by anti-D immune globulin (28), and it may be attributed in part to the low alcohol consumption expected in the Egyptian community. Conversely, Western HRQOL studies included CHC patients from clinical trials (5–8, 10, 11, 14, 15) or tertiary care centres (9, 12, 13, 16), and they may be subject to referral bias, since these centres attract primarily individuals with already established chronic liver disease. In addition, eligibility criteria excluded individuals with normal ALT levels. However, none of the Western studies showed an association between HRQOL and disease severity markers, as evidenced again in this study in case of ALT levels.
In this study, rural Egyptians appeared to have significantly lower HRQOL scores than comparable Western populations. If the SF-12 PCS and MCS scales had been scored using U.S. norm-based methods – where the mean (SD) is 50 (10) in the 1998 general U.S. population (page 227, (32)) – then the SF-12 PCS and MCS of the uninfected rural Egyptians would have been significantly lower than in the U.S. (44.1 (11.9) and 44.7 (9.4), respectively; p<0.0001). On the other hand, the SF-12 PCS and MCS of the HCV chronically infected Egyptians would not differ significantly from the U.S. norm for liver disease including chronic hepatitis and cirrhosis (PCS: 41.6 (11.8) vs. 39.9 (11.9); MCS: 45.6 (9.4) vs. 45.4 (10.7), respectively; page 120, (32)). Accordingly, one might postulate that chronic hepatitis C infection might have little impact compared to the numerous other factors impacting HRQOL in this remote area of Egypt.
This study showed that several confounding factors could explain the association of HRQOL and hepatitis C. Socio-demographic factors related to past parenteral antischistosomal therapy such as older age, male sex and illiteracy, and ongoing iatrogenic factors such as self-declared chronic disease, previous admission to hospital or clinic, and past history of transfusion, were significant risk factors for HCV. In addition, these factors were all significantly associated to a reduction in HRQOL, hence confounding the association between HCV infection and HRQOL. This contrasts with previous Western HRQOL studies that compared CHC patients to national population norms with adjustments made for age, sex, and sometimes co-morbidity. One may argue that adjustment was performed on a too limited number of HRQOL explanatory factors when matching CHC patients to population norms. For instance, low education may have acted as a confounding factor as it was associated with hepatitis C infection, (33, 34) and it decreased consistently HRQOL (35–37).
As compared with previous Western HRQOL studies, none of the 146 Egyptians chronically infected with HCV were aware of their serological status. The lack of a reduction of HRQOL in these patients underlines the HCV labeling effect on HRQOL suggested in previous studies (12, 18). This labeling effect of HCV may be related to the significant reduction of HRQOL associated to the perceived negative impact of HCV on health (38), emotional distress (38), and stigmatization experienced by CHC patients (39). In rural Egypt, further studies should evaluate the effect of disease labeling on HRQOL. However, we may expect that a low education level, the limited access to health care, and the current large burden of disease as compared to the potential distressing long-term outcomes related to HCV infection would limit the impact of HCV labeling on HRQOL.
These study results may be limited by the instruments used to assess HRQOL. There is no current HRQOL instrument that has been validated in the Arabic language. Our choice of the SF-12 questionnaire was guided by its reduced time for administration as compared to the SF-36 or the WHOQOL-BREF (26 items); its cross-cultural validation in many languages around the world, including developing countries (40); its ability to predict SF-36 physical and mental health summary scores widely used in the field of hepatitis C (6–16, 18, 38, 41). Our Arabic translation and cross-cultural adaptation of the SF-12 showed satisfactory but poorer reliability than the original US SF-12. However, it showed a good convergent validity against VAS (Pearson correlation with PCS and MCS of 0.65 and 0.49, respectively) and a good construct validation in extreme groups. Another limitation may be related to a lower sensitivity of SF-12 to changes in HRQOL as compared to the SF-36 or the SF-36 expanded with specific questions related to hepatitis infection (11), although findings were similar with VAS of proven sensitivity in hepatitis C (24). Finally, we had to rely on interviews in an illiterate community instead of the self-assessment of HRQOL used in most previous Western studies. Possible interaction with interviewer was taken into account in all statistical analyses by adjusting for interviewer as a random effect in linear mixed models. However, two Western studies showed consistently that SF-36 scores collected by self completion were lower than by interview administration, the largest differences being in the health concepts reflecting social and emotional well-being (42, 43). To the extent that the Egyptians surveyed as well as the interviewers were unaware of the HCV serological status of the interviewee, the difference in the mode of administration of HRQOL questionnaires should not undermine the presented results, but it may limit the comparability with previous Western studies.
The generalizability of the study results to Western settings is limited by the many differences observed in rural Egypt with regard to socio-demographic variables (e.g., 60% were illiterate, 78% were married or partnered, and 89% lived their whole life in the village), the higher morbidity, the risk factors for HCV infection (intravenous drug use is very rare in rural Egypt, and the attributable fraction of HCV infections related to past injections, including parenteral antischistosomal therapy, was estimated around 50% (M.K. Mohammed, personal communication)), the viral genotype 4, and the unawareness of HCV serological status. Accordingly, it would be of interest to replicate the study results in Western settings by adjusting HRQOL on larger sets of explanatory variables when matching to a population norm, or alternatively by using a common source population.
In conclusion, this study shows that HRQOL was not significantly reduced in adults chronically infected with HCV and unaware of their HCV status in the long phase before clinical manifestations. As Egypt is currently considering options to treat HCV, this study shows that public health policies should concentrate on their health benefits in the long-term, i.e. reduction in mortality (2, 44, 45) and HCV transmission, and their opportunity costs compared to other options, e.g., safer procedures in the health care system.
Acknowledgments
Funding agencies:
Agence Nationale de Recherche sur le SIDA – France (ANRS 12.79), and European Community (INCOMED Programme: Viral Hepatitis Surveillance in Mediterranean Countries, Contract ICA3-CT-2000-30011).
List of Abbreviations
- ALT
serum alanine aminotransferase
- AST
serum aspartate aminotransferase
- CHC
chronic hepatitis C
- HCV
Hepatitis C virus
- HRQOL
Health-related quality of life
- MCS
mental health summary score
- PCS
physical health summary score
- SD
Standard deviation
- SF-12
Short-Form 12
- SF-36
Short-Form 36
- VAS
Visual analogue scale
Footnotes
Conflict of interest: none
References
- 1.Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 2.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90:1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth BM, Manns MP, et al. Cost effectiveness of peginterferon alpha-2b plus ribavirin versus interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52:425–432. doi: 10.1136/gut.52.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290:228–237. doi: 10.1001/jama.290.2.228. [DOI] [PubMed] [Google Scholar]
- 5.Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC, Jr, Perrillo RP, Carey W, et al. Assessing health-related quality of life in chronic hepatitis C using the Sickness Impact Profile. Clin Ther. 1994;16:334–343. discussion 271–332. [PubMed] [Google Scholar]
- 6.Carithers RL, Jr, Sugano D, Bayliss M. Health assessment for chronic HCV infection: results of quality of life. Dig Dis Sci. 1996;41:75S–80S. doi: 10.1007/BF02087879. [DOI] [PubMed] [Google Scholar]
- 7.Hunt CM, Dominitz JA, Bute BP, Waters B, Blasi U, Williams DM. Effect of interferon-alpha treatment of chronic hepatitis C on health-related quality of life. Dig Dis Sci. 1997;42:2482–2486. doi: 10.1023/a:1018852309885. [DOI] [PubMed] [Google Scholar]
- 8.Bayliss MS, Gandek B, Bungay KM, Sugano D, Hsu MA, Ware JE., Jr A questionnaire to assess the generic and disease-specific health outcomes of patients with chronic hepatitis C. Qual Life Res. 1998;7:39–55. doi: 10.1023/a:1008884805251. [DOI] [PubMed] [Google Scholar]
- 9.Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27:209–212. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- 10.Bonkovsky HL, Woolley JM. Reduction of health-related quality of life in chronic hepatitis C and improvement with interferon therapy. The Consensus Interferon Study Group. Hepatology. 1999;29:264–270. doi: 10.1002/hep.510290124. [DOI] [PubMed] [Google Scholar]
- 11.Ware JE, Jr, Bayliss MS, Mannocchia M, Davis GL. Health-related quality of life in chronic hepatitis C: impact of disease and treatment response. The Interventional Therapy Group. Hepatology. 1999;30:550–555. doi: 10.1002/hep.510300203. [DOI] [PubMed] [Google Scholar]
- 12.Rodger AJ, Jolley D, Thompson SC, Lanigan A, Crofts N. The impact of diagnosis of hepatitis C virus on quality of life. Hepatology. 1999;30:1299–1301. doi: 10.1002/hep.510300504. [DOI] [PubMed] [Google Scholar]
- 13.Hussain KB, Fontana RJ, Moyer CA, Su GL, Sneed-Pee N, Lok AS. Comorbid illness is an important determinant of health-related quality of life in patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:2737–2744. doi: 10.1111/j.1572-0241.2001.04133.x. [DOI] [PubMed] [Google Scholar]
- 14.Fontana RJ, Moyer CA, Sonnad S, Lok ASF, Sneed-Pee N, Walsh J, Klein S, et al. Comorbidities and quality of life in patients with interferon-refractory chronic hepatitis C. Am J Gastroenterol. 2001;96:170–178. doi: 10.1111/j.1572-0241.2001.03473.x. [DOI] [PubMed] [Google Scholar]
- 15.McHutchison JG, Ware JE, Jr, Bayliss MS, Pianko S, Albrecht JK, Cort S, Yang I, et al. The effects of interferon alpha-2b in combination with ribavirin on health related quality of life and work productivity. J Hepatol. 2001;34:140–147. doi: 10.1016/s0168-8278(00)00026-x. [DOI] [PubMed] [Google Scholar]
- 16.Younossi ZM, Boparai N, Price LL, Kiwi ML, McCormick M, Guyatt G. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol. 2001;96:2199–2205. doi: 10.1111/j.1572-0241.2001.03956.x. [DOI] [PubMed] [Google Scholar]
- 17.Rasenack J, Zeuzem S, Feinman SV, Heathcote EJ, Manns M, Yoshida EM, Swain MG, et al. Peginterferon alpha-2a (40kD) [Pegasys] improves HR-QOL outcomes compared with unmodified interferon alpha-2a [Roferon-A]: in patients with chronic hepatitis C. Pharmacoeconomics. 2003;21:341–349. doi: 10.2165/00019053-200321050-00005. [DOI] [PubMed] [Google Scholar]
- 18.Cordoba J, Reyes J, Esteban JI, Hernandez JM. Labeling may be an important cause of reduced quality of life in chronic hepatitis C. Am J Gastroenterol. 2003;98:226–227. doi: 10.1111/j.1572-0241.2003.07195.x. [DOI] [PubMed] [Google Scholar]
- 19.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 20.Tarlov AR, Ware JE, Jr, Greenfield S, Nelson EC, Perrin E, Zubkoff M. The Medical Outcomes Study. An application of methods for monitoring the results of medical care. JAMA. 1989;262:925–930. doi: 10.1001/jama.262.7.925. [DOI] [PubMed] [Google Scholar]
- 21.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Nunnally JC, Bernstein IH. Psychometric theory. 3. NY: Mc Graw-Hill, Inc; 1994. [Google Scholar]
- 23.Baltussen RM, Sanon M, Sommerfeld J, Wurthwein R. Obtaining disability weights in rural Burkina Faso using a culturally adapted visual analogue scale. Health Econ. 2002;11:155–163. doi: 10.1002/hec.658. [DOI] [PubMed] [Google Scholar]
- 24.Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, Krahn M. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–638. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 25.Hollingworth W, Deyo RA, Sullivan SD, Emerson SS, Gray DT, Jarvik JG. The practicality and validity of directly elicited and SF-36 derived health state preferences in patients with low back pain. Health Econ. 2002;11:71–85. doi: 10.1002/hec.650. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Hamid M, Edelman DC, Highsmith WE, Constantine NT. Optimization, assessment, and proposed use of a direct nested reverse transcription-polymerase chain reaction protocol for the detection of hepatitis C virus. J Hum Virol. 1997;1:58–65. [PubMed] [Google Scholar]
- 27.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 28.Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 29.Vogt M, Lang T, Frosner G, Klingler C, Sendl AF, Zeller A, Wiebecke B, et al. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med. 1999;341:866–870. doi: 10.1056/NEJM199909163411202. [DOI] [PubMed] [Google Scholar]
- 30.Deuffic S, Buffat L, Poynard T, Valleron AJ. Modeling the hepatitis C virus epidemic in France. Hepatology. 1999;29:1596–1601. doi: 10.1002/hep.510290528. [DOI] [PubMed] [Google Scholar]
- 31.Minola E, Prati D, Suter F, Maggiolo F, Caprioli F, Sonzogni A, Fraquelli M, et al. Age at infection affects the long-term outcome of transfusion-associated chronic hepatitis C. Blood. 2002;99:4588–4591. doi: 10.1182/blood-2001-12-0192. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Jr, Kosinski M, RTurner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12 Health Survey (With a Supplement Documenting Version 1) Lincoln, RI: QualityMetric Incorporated; 2002. [Google Scholar]
- 33.Alter HJ, Conry-Cantilena C, Melpolder J, Tan D, Van Raden M, Herion D, Lau D, et al. Hepatitis C in asymptomatic blood donors. Hepatology. 1997;26:29S–33S. doi: 10.1002/hep.510260705. [DOI] [PubMed] [Google Scholar]
- 34.Stuver SO, Boschi-Pinto C, Trichopoulos D. Infection with hepatitis B and C viruses, social class and cancer. IARC Sci Publ. 1997:319–324. [PubMed] [Google Scholar]
- 35.Johnson JA, Coons SJ. Comparison of the EQ-5D and SF-12 in an adult US sample. Qual Life Res. 1998;7:155–166. doi: 10.1023/a:1008809610703. [DOI] [PubMed] [Google Scholar]
- 36.Lim LL, Fisher JD. Use of the 12-item short-form (SF-12) Health Survey in an Australian heart and stroke population. Qual Life Res. 1999;8:1–8. doi: 10.1023/a:1026409226544. [DOI] [PubMed] [Google Scholar]
- 37.Sprangers MA, de Regt EB, Andries F, van Agt HM, Bijl RV, de Boer JB, Foets M, et al. Which chronic conditions are associated with better or poorer quality of life? J Clin Epidemiol. 2000;53:895–907. doi: 10.1016/s0895-4356(00)00204-3. [DOI] [PubMed] [Google Scholar]
- 38.Fontana RJ, Hussain KB, Schwartz SM, Moyer CA, Su GL, Lok AS. Emotional distress in chronic hepatitis C patients not receiving antiviral therapy. J Hepatol. 2002;36:401–407. doi: 10.1016/s0168-8278(01)00280-x. [DOI] [PubMed] [Google Scholar]
- 39.Zickmund S, Ho EY, Masuda M, Ippolito L, LaBrecque DR. “They treated me like a leper”. Stigmatization and the quality of life of patients with hepatitis C. J Gen Intern Med. 2003;18:835–844. doi: 10.1046/j.1525-1497.2003.20826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ware JE, Jr, Gandek B, Kosinski M, Aaronson NK, Apolone G, Brazier J, Bullinger M, et al. The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1167–1170. doi: 10.1016/s0895-4356(98)00108-5. [DOI] [PubMed] [Google Scholar]
- 41.Unal G, de Boer JB, Borsboom GJ, Brouwer JT, Essink-Bot M, de Man RA. A psychometric comparison of health-related quality of life measures in chronic liver disease. J Clin Epidemiol. 2001;54:587–596. doi: 10.1016/s0895-4356(00)00372-3. [DOI] [PubMed] [Google Scholar]
- 42.Weinberger M, Oddone EZ, Samsa GP, Landsman PB. Are health-related quality-of-life measures affected by the mode of administration? J Clin Epidemiol. 1996;49:135–140. doi: 10.1016/0895-4356(95)00556-0. [DOI] [PubMed] [Google Scholar]
- 43.Lyons RA, Wareham K, Lucas M, Price D, Williams J, Hutchings HA. SF-36 scores vary by method of administration: implications for study design. J Public Health Med. 1999;21:41–45. doi: 10.1093/pubmed/21.1.41. [DOI] [PubMed] [Google Scholar]
- 44.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002;156:761–773. doi: 10.1093/aje/kwf100. [DOI] [PubMed] [Google Scholar]
- 45.Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095–2100. doi: 10.1016/s0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]


