Abstract
Adrenergic receptors and their ligands are important regulators of synaptic plasticity and metaplasticity, but the exact mechanisms underlying their action are still poorly understood. Octopamine, the invertebrate homolog of mammalian adrenaline or noradrenaline, plays important roles in modulating behavior and synaptic functions. We previously uncovered an octopaminergic positive-feedback mechanism to regulate structural synaptic plasticity during development and in response to starvation. Under this mechanism, activation of Octß2R autoreceptors by octopamine at octopaminergic neurons initiated a cAMP-dependent cascade that stimulated the development of new synaptic boutons at the Drosophila larval neuromuscular junction (NMJ). However, the regulatory mechanisms that served to brake such positive feedback were not known. Here, we report the presence of an alternative octopamine autoreceptor, Octß1R, with antagonistic functions on synaptic growth. Mutations in octß1r result in the overgrowth of both glutamatergic and octopaminergic NMJs, suggesting that Octß1R is a negative regulator of synaptic expansion. As Octß2R, Octß1R functioned in a cell-autonomous manner at presynaptic motorneurons. However, unlike Octß2R, which activated a cAMP pathway, Octß1R likely inhibited cAMP production through inhibitory Goα. Despite its inhibitory role, Octß1R was required for acute changes in synaptic structure in response to octopamine and for starvation-induced increase in locomotor speed. These results demonstrate the dual action of octopamine on synaptic growth and behavioral plasticity, and highlight the important role of inhibitory influences for normal responses to physiological stimuli.
Introduction
Adrenaline/noradrenaline and their receptors have emerged as important modulators of synaptic plasticity, metaplasticity, and behavior in the mammalian brain (Murchison et al., 2004; Hu et al., 2007; Kuzmiski et al., 2009). However, the mechanisms underlying this regulation of synaptic structure are not known.
In insects, adrenergic signaling is accomplished through octopamine and octopamine receptors (Balfanz et al., 2005) and is a powerful modulator of behaviors such as appetitive behavior (Long and Murdock, 1983; Suo et al., 2006) and aggression (Hoyer et al., 2008; Zhou et al., 2008). It also regulates synaptic function (Breen and Atwood, 1983) and synaptic structure (Koon et al., 2011).
We have previously demonstrated that at the Drosophila larval neuromuscular junction (NMJ) octopamine regulates the expansion of both modulatory and excitatory nerve terminals (Koon et al., 2011). Larval NMJs are innervated by glutamatergic, octopaminergic, and peptidergic motorneurons (Prokop, 2006). Of these, glutamatergic nerve terminals provide classical excitatory transmission (Jan and Jan, 1976), while octopaminergic nerve endings support global modulation of excitability and synaptic growth (Monastirioti et al., 1995; Koon et al., 2011). Larval NMJs are continuously expanding to compensate for muscle cell growth (Schuster et al., 1996) and respond to acute changes in activity by extending new synaptic boutons (Ataman et al., 2008; Koon et al., 2011). We previously demonstrated that, by binding to the octopamine autoreceptor Octß2R, octopamine activated a cAMP second messenger pathway that led to CREB activation and transcription, which in turn promoted the extension of new octopaminergic nerve endings (Koon et al., 2011). This positive-feedback mechanism was required for an increase in locomotor activity in response to starvation. In addition, this mechanism positively regulated the growth of glutamatergic nerve endings through Octß2R receptors present in glutamatergic neurons. An important question regards the mechanisms that serve to brake such positive feedback.
Here, we demonstrate the presence of a second octopamine receptor, Octß1R, which serves as such a brake. We show that Octß1R is also an autoreceptor in octopaminergic neurons that serves to inhibit synaptic growth. This inhibitory influence is excerpted through the activation of the inhibitory G-protein subunit, Goα, and thus by limiting cAMP production. Like Octß2R receptors, Octß1R receptors are also present at excitatory glutamatergic endings. Thus, octopamine release induces a dual excitatory (through Octß2R) and inhibitory (through Octß1R) function on the growth of both octopaminergic and glutamatergic endings. The presence of both the excitatory and inhibitory receptors is required for normal structural plasticity at octopaminergic terminals and for normal responses to starvation, as obliterating Octß1R (this study) or Octß2R (Koon et al., 2011) prevents the acute growth of octopaminergic ending in response to octopamine and the increase in locomotor speed in response to starvation. Thus, this study highlights the requirement of both excitatory and inhibitory influences for normal synaptic and behavioral plasticity.
Materials and Methods
Fly strains.
Flies were reared in standard Drosophila medium at 25°C except where indicated. Both males and female larvae were used in these studies. Animals used in RNAi experiments were reared at 29°C to increase knockdown efficiency but were incubated at 25°C for 1 h before experiments. The following stocks were used: the wild-type strain Canton-S (CS), Tdc2-Gal4 (Bloomington Stock Center, Bloomington, IN), C380-Gal4 (Budnik et al., 1996), BG439-Gal4 (Koon et al., 2011), 19H07-Gal4, 20C11-Gal4, 20E11-Gal4, 21E03-Gal4 (Pfeiffer et al., 2008), UAS-mCD8-GFP (Bloomington), UAS-PTX (Ferris et al., 2006), y dncM14 cv v f (Bloomington), UAS-Dnc (remobilized to the second chromosome) (Cheung et al., 1999), rut2080 (Bloomington), goα007 (Frémion et al., 1999), UAS-Dcr2 (Bloomington), PBac{WH}oa2[f02819] (Bloomington), PBac{WH}w[f06195] (Harvard Exelixis, Cambridge, MA), PBac{WH}Octß2R[f05679] (Bloomington), w[1118]; Df(3R)Exel6191, P{w[+mC]=XP-U}Exel6191 (Bloomington), UAS-Octß1R-RNAi (110537; Vienna Drosophila RNAi Center, Vienna, Austria), UAS-Goα-RNAi (110552 and 19124; Vienna Drosophila RNAi Center) (110552 is Goα-RNAi1 and 19124 is Goα-RNAi2), UAS-Giα-RNAi (28510; Vienna Drosophila RNAi Center; JF01608; Transgenic RNAi Project, Harvard Medical School, Cambridge, MA) (28510 is Giα-RNAi1 and JF01608 is Giα-RNAi2).
Generation and analysis of octβ1r mutants.
octβ1r mutants were generated using PBac{WH}oa2[f02819] and PBac{WH}w[f06195], which contain piggyback-based transposons in the same orientation on the third chromosome. A heat shock Flipase was crossed into the first chromosome to induce recombination between the two transposons and to excise the flanked DNA. The heat shock processes were performed twice during development at 37°C for 30 min each (once during first-instar and once during second-instar larval stages, respectively). To obtain a stable mutant, the X-chromosome containing the heat shock Flipase was then crossed out and substituted with an X-chromosome in the w- background. Genomic PCR was performed to verify the desired deletion. Primers used to verify the deletion were GTCATGCGGCACCGGAAATTG (5′ genomic primer) paired with CCTCGATATACAGACCGATAAAAC (WH3′) (to verify the presence of the 3′ end of PBac[f02819]) and CTAAAGTGCATTGCACCTGG (3′ genomic primer) paired with TCCAAGCGGCGACTGAGATG (WH5′) (to verify the 5′ end of PBac[f06195]). Negative control primers against deleted Octβ1R genomic sequence were ACAGGAGCGTCTGGTGTAC paired with CGGAGTGATGCAACTATCGC, TGTCAAGCGCACAGAACTC paired with GCGTTGGTTGGTTCCAAGG, and AGTGCTGTACAGTAGCGAGC paired with CCTGACTCCATGACACCTAAATATG. Primers used to verify the presence of PBac{WH}Octß2R[f05679] in octβ1r, octβ2r double mutant were CGCAGGTCATGGAGAGTGTG and WH5′. RT-PCR was performed by extracting total mRNA from dissected larval body wall muscles and larval brains using a combination of Trizol (Invitrogen) and the RNeasy kit (QIAGEN). Synthesis of cDNA for +RT reactions was performed using the Superscript III kit (Invitrogen), where −RT reactions lacked reverse transcriptase. The +RT and −RT reactions were then used for PCR using forward primer CCGCCTGGCAACGAGTAAC and reverse primer CTCGTCGATGAGCCCGTC. These primers were specifically designed to recognize all known splice variants of Octß1R, and across an exon–intron junction to avoid false signal from any contaminating genomic DNA.
Immunocytochemistry.
Larval body wall muscles were dissected and fixed for 15 min in 4% paraformaldehyde. For tyramine-β-hydroxylase (TBH) immunocytochemistry, samples were fixed in Bouin's fixative. Antibodies and their concentrations were as follows: anti-TBH, 1:400 (Koon et al., 2011); anti-HRP-Dylite594, 1:500 (Jackson ImmunoResearch). Secondary antibodies conjugated to either FITC or Dylite594 (Jackson ImmunoResearch) were used at a concentration of 1:200. Imaging of fixed preparations was described previously (Ataman et al., 2008).
Animal rearing conditions for synaptopod analysis.
All animals used in synaptopod analysis carried a copy of Tdc2-Gal4 and a copy of UAS-mCD8-GFP to visualize the type II terminals. Egg collection was done in standard 25-mm-diameter cornmeal/agar/molasses food vials at 25°C with ∼60% humidity. Larvae were grown at low density. Wandering late third-instar larvae were used for experiments.
Animal rearing conditions for RNAi experiments.
All animals used in RNAi experiments carried a copy of UAS-Dcr2. Egg collection was done at 29°C instead of 25°C to increase RNAi efficiency. For behavioral experiments, food vials rearing animals at 29°C were incubated at 25°C for 1 h before crawling assay or starvation assay.
Stimulation procedures and live imaging of dissected preparations.
Synaptopods were imaged from live preparations as described by Koon et al. (2011). For octopamine stimulation, larvae were dissected in HL3 saline (Stewart et al., 1994) containing 0.1 mm Ca2+ and preparations gently glued onto a custom-made glass imaging chamber using surgical glue. Then, identified NMJs were imaged on an Improvision spinning disc confocal microscope (PerkinElmer) with a C9100-13 Hamamatsu cooled EM-CCD camera and using a 40×, 1.2 NA objective, with a 2.4× optical zoom. After imaging for <30 min, animals were partially unglued to allow muscles to contract freely, and 10 μm octopamine in HL3 containing 1.5 mm Ca2+ was then applied for 15 min followed by five washes for 15 min each with 0.1 mm Ca2+ HL3 saline before imaging again. For experiments involving pertussis toxin (PTX) application, dissected living larval prep was preincubated in HL3 containing 0.1 mm Ca2+, 0.03% DMSO, 30 μm ATP, and 1.5 μg/ml PTX (purchased from Sigma-Aldrich) for 2 h. Then, HL3 containing 1.5 mm Ca2+, 10 μm octopamine, 0.03% DMSO, 30 μm ATP, and 1.5 μg/ml PTX was applied for 15 min, followed by five washes for 15 min each with the preincubation HL3. Control animals were preincubated, stimulated, and washed in the same way but without PTX.
Crawling assay and starvation assay.
Details of crawling and starvation assays were described by Koon et al. (2011). Synchronized mid-third-instar larvae were washed with water, and individually loaded onto a 3% agar plate. Animals were allowed a pre-run of 25 s on the agar before manual recordings were made for 1 min. Experiments were performed in a 25°C, 60% humidity behavioral room under red light. For starvation assays, larvae were maintained in food or food-free moisturized 35 mm Petri dishes for 2 h, and then subjected to the crawling assay. For behavioral assays, N represents number of animals.
Quantification of boutons and synaptopod number.
Type I boutons number was obtained at muscles 6 and 7 of abdominal segment A3, while type II bouton number was quantified at muscle 12 in A3. For muscle area measurements, the muscle length and width were measured using an ocular scale bar. Measurements of synaptopod number were from muscles 12 of segment A4 in dissected preparations. Number of synaptopods in the histograms represents the total number of synaptopods per 100 μm of each arbor. Synaptopods were defined as such if they measured at least 0.5 μm in length and at most 0.5 μm in width. For morphometric analysis of NMJs, N represents the number of NMJs analyzed (at most two per animal).
Statistical analysis.
For comparisons between more than two sample groups, an ANOVA with Tukey's post hoc test was performed. For pairwise comparisons, Student's t test was used. The numbers in histograms represent mean ± SEM. ***p < = 0.0001; **p < = 0.001; *p < 0.05. Unless otherwise noted, sample number (N) represents the number of NMJs for anatomical measurements, or the number of animals for behavioral analyses. Statistical analysis for animals reared at 25 or 29°C was done separately.
Genotype abbreviations.
Type I motorneuron driver control is BG439/+ and BG439>Dcr2 for RNAi experiments. Type II motorneuron driver control is Tdc2/+ and Tdc2>Dcr2 for RNAi experiments. Type I+II motorneuron driver control is C380/+ and C380>Dcr2 for RNAi experiments. [Transgene]-typeI is BG439>[Transgene] and BG439>[Transgene],Dcr2 for RNAi experiments. [Transgene]-typeII is Tdc2>[Transgene] and Tdc2>[Transgene],Dcr2 for RNAi experiments. [Transgene]-typeI+II is C380>[Transgene] and C380>[Transgene],Dcr2 for RNAi experiments, unless otherwise indicated.
Results
Octß1R receptors are present in motorneurons
We have recently demonstrated that octopamine-containing synaptic terminals (type II terminals) undergo structural changes in response to behavioral states that induce an increase in locomotion (Koon et al., 2011). Underlying this structural change is the activation of a positive-feedback mechanism, in which octopamine release, presumably by type II octopaminergic synaptic boutons, activates Octß2R autoreceptors (Koon et al., 2011). In turn, Octß2R turns on a cAMP- and CREB-dependent signaling cascade at octopaminergic neurons, which induces synaptic expansion. This positive control mechanism not only promotes the proliferation of type II synaptic boutons but also functions in a paracrine fashion to stimulate the growth of type I boutons (Koon et al., 2011), primary mediators of excitatory transmission at the NMJ.
Analysis of the Gal4 transcriptional reporters, 19H07-Gal4, 21E03-Gal4, 20C11-Gal4, and 20E11-Gal4, generated by fusing Gal4 to four different intronic regions of the Octß1R (also known as OA2) octopamine receptor in Drosophila (Pfeiffer et al., 2008) (Fig. 1I) revealed that reporter (mCD8-GFP) signal could be observed in all bouton types, including type I and type II boutons, but not in postsynaptic muscles (Fig. 1A–D). The intensity of the reporter signal varied among the different strains or was present in only a subset of bouton types. For example, 19H07-Gal4 displayed mCD8-GFP signal primarily in type Ib nerve endings, very weak signal in type II terminals, and no detectable signal in type Is and type III terminals (Fig. 1A). 21E03-Gal4 showed strong signal in type Is and type II boutons, but the signal was not detected in type Ib and type III endings (Fig. 1B). 20C11-Gal4 had strong mCD8-GFP expression in type III endings, but no signal was observed in type I or type II terminals (Fig. 1C). Finally, 20E11-Gal4 had weak reporter signal in type Ib boutons, barely detectable signal in type Is boutons, and undetectable signal in type II and type III boutons. Most importantly, these results suggest that, like Octß2R, Octß1R is also expressed in motorneurons.
Figure 1.
Expression of Octß1R-Gal4 transcriptional reporters at the NMJ. A–D, NMJs at muscle 12 of third-instar larvae expressing mCD8-GFP using four different Octß1R-Gal4 strains generated from different intronic regions of the octß1r locus (Pfeiffer et al., 2008). NMJs were double labeled with anti-GFP (green) and anti-HRP (red). The panels represent confocal Z-stack projections. As shown by the white arrowheads, reporter expression is found in the following synaptic endings: 19H07-Gal4: type Ib and type II (A1–3); 21E03-Gal4: type Is and type II (B1–3); 20C11-Gal4: type III (C1–3); 20E11-Gal4: type Ib and type Is (D1–3). E, F, Reporter gene expression in the CNS and imaginal discs of 19H07-Gal4, showing reporter expression in the CNS and proximal band of retinal cells in the optic disc (E); 21E03-Gal4, showing signal in the CNS and edge of imaginal discs (F); 20C11-Gal4: CNS, showing GFP signal in differentiated retinal cells at the optic disc, one to two cells in other imaginal discs, and neurons innervating the pharyngeal muscles (inset) (G); 20E11-Gal4, showing Gal4 expression in the CNS (H). Scale bar: A–D, 20 μm; E–H, 240 μm; G, inset, 70 μm. I, Schematic representation of the octß1r genomic region, showing predicted alternatively spliced isoforms A–C, and the approximate locations (brackets) of different intronic regions used to generate the Gal4 transcriptional reporters (Pfeiffer et al., 2008). The approximate location of the two PBac insertions used to generate the octß1r mutant is shown with blue arrowheads. Orange boxes, Coding region within exons. Gray boxes, Noncoding regions within exons (UTRs). Black lines, Introns. J, RT-PCR from wild-type and octß1r mutant RNA. Virtually no expression of the remaining fragment in octß1r mutants was observed, indicating that the mutant is likely a null mutant. +RT, Reverse transcription reactions with reverse transcriptase added; −RT, reverse transcriptase absent.
Apart from motorneurons, the above Gal4 lines also displayed reporter gene expression in the CNS and imaginal discs. 19H07-Gal4 had weak reporter signal in the brain lobes, strong signal in a few neurons per segment in the ventral ganglion, and signal in a proximal band of retinal cells in the optic disc (Fig. 1E). 21E03-Gal4 demonstrated GFP reporter expression in several cells in the brain, as well as in octopaminergic motorneurons in the ventral ganglion (Fig. 1F). In addition, a strong GFP band at one edge of each imaginal disc was observed (Fig. 1F). 20C11-Gal4 displayed GFP reporter expression in many neurons of the brain and ventral ganglion (Fig. 1G). In addition, strong label was observed in differentiated retinal cells in the optic disc, in one to two cells per imaginal disc (Fig. 1G), and in neurons innervating the pharyngeal muscles (Fig. 1G, inset). Finally, 20E11-Gal4 had broad reporter signal in many cells of the larval brain and ventral ganglion (Fig. 1H). Although we attempted to verify the above pattern of reporter gene expression by raising anti-Octß1R antibodies, these efforts were unsuccessful. Nevertheless, a recent study reported the pattern of Octß1R expression using in situ hybridization, and reported the distribution of receptor transcript in the CNS, imaginal discs, and salivary glands (Ohhara et al., 2012).
To determine whether Octß2R and Octß1R served redundant roles at the NMJ, we generated an octß1r mutant by FRT-mediated recombination of two P-element insertions (PBac[WH]oa2[f02819] and PBac[WH]w[f06195]) (Fig. 1I). The recombination was verified by genomic PCR. Analysis of the mutant strain demonstrated that most of the octß1r coding region had been removed, excluding a fragment encompassing the 5′-UTR and the first exon of Octß1R, which is predicted to encode the N-terminal extracellular domain, the first transmembrane domain, and 4 aa of the first intracellular loop of the receptor. However, RT-PCR demonstrated the virtual absence of the predicted transcript fragment (Fig. 1J), suggesting that octß1r is likely a null mutant. No other known genes were disrupted by the excision.
Octß1R receptors are negative regulators of synaptic growth
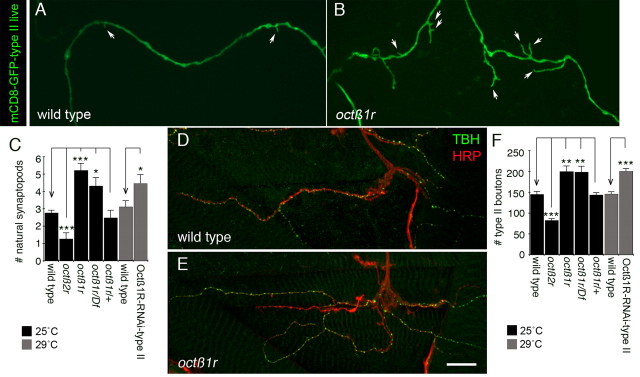
We previously demonstrated that octopaminergic type II NMJs expand by extending “natural synaptopods,” motile filopodia-like extensions observed during the expansion of type II terminals through larval development (Koon et al., 2011). Furthermore, we found that the number of natural synaptopods is reduced in octß2r mutants due to an autonomous function of Octß2R in octopaminergic neurons (Koon et al., 2011). To determine whether Octß1R receptors had a redundant role in type II boutons, we examined natural synaptopods in octß1r mutants by expressing mCD8-GFP in octopaminergic neurons using the Tdc2-Gal4 driver, which drives Gal4 expression in these neurons (Cole et al., 2005). Notably, the number of natural synaptopods was substantially increased in this mutant (Fig. 2A–C), in complete opposition to the phenotype found in octß2r mutants. A similar phenotype was found when Octß1R receptor was downregulated in octopaminergic neurons alone by expressing Octß1R-RNAi in these neurons (Fig. 2C), suggesting a cell-autonomous function of the receptor. Thus, Octß1R receptors appear to negatively regulate the formation of synaptopods at type II terminals. Consistent with this role, the number of type II boutons was also increased both in octß1r mutants and in larvae expressing Octß1R-RNAi in octopaminergic neurons (Fig. 2D–F).
Figure 2.
octß1r mutants display an overgrowth of octopaminergic endings at the NMJ. A, B, Confocal Z-stack projections of type II arbors at muscle 12 in larvae expressing mCD8-GFP in octopaminergic neurons of control (A) and octß1r mutant (B), showing a marked increase in the number of natural synaptopods (white arrows). C, Quantification of the number of natural synaptopods per 100 μm of type II arbor in octß2r mutants, octß1r mutants, and animals expressing Octß1R-RNAi in type II motorneurons, showing increased natural synaptopods in octß1r mutants and Octß1R-RNAi animals [N (left to right) = 175, 13, 21, 10, 11, 25, 17 NMJs]. D, E, Third-instar larval NMJs at muscles 12 of wild-type (D) and octß1r mutant (E), showing a marked increase in the number of type II boutons (shown by TBH labeling). NMJs were double labeled with anti-TBH (green) and anti-HRP (red). The panels represent confocal Z-stack projections. F, Quantification of the number of type II boutons at muscle 12 in octß2r mutants, octß1r mutants, and animals expressing Octß1R-RNAi in type II motorneurons, showing increased type II boutons in octß1r mutants and Octß1R-RNAi animals [N (left to right) = 22, 17, 15, 11, 16, 20, 11 NMJs]. Animals used in RNAi experiments were reared at 29°C to increase knockdown efficiency. Scale bar: A, B, 8 μm; D, E, 20 μm. Error bars indicate SEM. ***p < = 0.0001; **p < = 0.001; *p < 0.05.
The inhibitory function of Octß1R is likely mediated by Goα
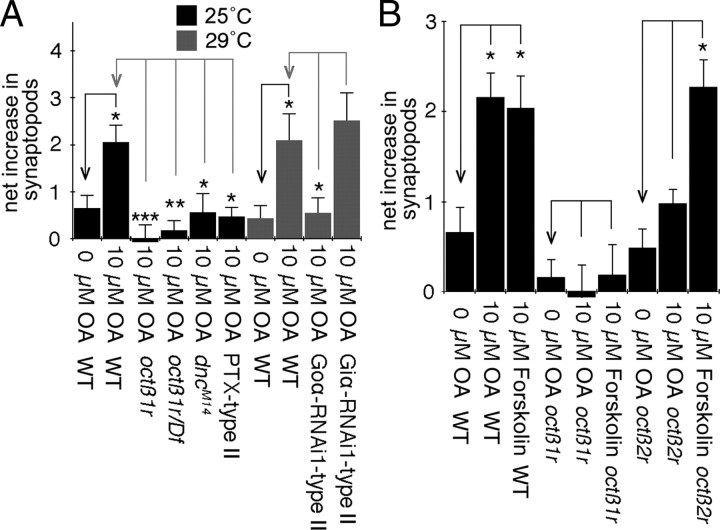
Synaptopod formation is downstream of elevated cAMP levels mediated by octopamine-dependent activation of Octß2Rs (Koon et al., 2011), a G-protein-coupled receptor. Octß1R is also predicted to function as a G-protein-coupled receptor (Balfanz et al., 2005; Evans and Maqueira, 2005). Therefore we wondered whether the negative regulation of type II synaptic growth by Octß1R could be mediated through activation of G-protein inhibitory subunits, such as Goα or Giα (El-Armouche et al., 2003; Johnston and Watts, 2003). This hypothesis was first examined by bath applying PTX, which in Drosophila specifically inhibits Goα (Thambi et al., 1989), and determining its consequences on octopamine-dependent synaptopod formation. As previously reported, application of 10 μm octopamine to wild-type control NMJs resulted in a significant increase in the number of synaptopods (Fig. 3A,C). Application of 10 μm octopamine in conjunction with 1.5 μg/ml PTX significantly enhanced this effect (Fig. 3B,C). This is consistent with the idea that activation of Goα partially inhibits octopamine-dependent synaptopod formation. In support of this interpretation, the number of natural synaptopods was substantially increased in larvae expressing UAS-PTX in octopamine neurons throughout larval development (Fig. 3D,E,G).
Figure 3.
Disruption of Goα phenocopies synaptic overgrowth of octß1r. A, B, Confocal Z-stack projections of type II arbors at muscle 12 in larvae expressing mCD8-GFP in octopaminergic neurons before and after bath application of octopamine. A, Bath application of octopamine increases the number of synaptopods on type II arbors (white arrowheads). B, Bath application of octopamine in the presence of PTX results in significantly larger increase of synaptopods than octopamine alone (white arrowheads). C, Quantification of the net increase of synaptopods in A and B per 100 μm of type II arbor, showing that octopamine induces a larger increase in synaptopods in larval preparations with 2 h PTX incubation than preparations without PTX treatment [N (left to right) = 18, 13, 16 NMJs]. D–F, Confocal Z-stack projections of type II arbors at muscle 12 in larvae expressing mCD8-GFP in octopaminergic neurons of control (D), animals expressing PTX in type II (E), and animals expressing Goα-RNAi1 in type II (F), showing a marked increase in the number of natural synaptopods (white arrowheads). G, Quantification of natural synaptopods per 100 μm of type II arbor in animals expressing PTX, Goα-RNAi, or Giα-RNAi in type II, showing that that the disruption of Goα function but not Giα results in increased number of natural synaptopods [N (left to right) = 175, 12, 25, 19, 10, 11, 10 NMJs]. Animals used in RNAi experiments were reared at 29°C to increase knockdown efficiency. H, Quantification of type II boutons at muscle 12 in animals expressing PTX, Goα-RNAi, or Giα-RNAi in type II motorneurons alone or type I+II motorneurons, showing that that the disruption of Goα function but not Giα results in increased number of type II boutons. [N (left to right) = 22, 16, 12, 20, 24, 16, 16, 10, 16 NMJs]. I, Quantification of natural synaptopods per 100 μm of type II arbor in octß1r mutants, octß1r/+ heterozygotes, goα007/+ heterozygotes, and goα007/+; octß1r/+ transheterozygotes, showing that goα007/+; octß1r/+ transheterozygotes have increased number of natural synaptopods, indicating a genetic interaction [N (left to right) = 175, 21, 11, 10, 11 NMJs]. J, Quantification of type II boutons at muscle 12 in animals of the same genotypes in I, showing that octß1r/+; goα007/+ transheterozygotes have increased number of type II boutons, indicating a genetic interaction [N (left to right) = 22, 15, 16, 12, 16 NMJs]. Scale bar, 12 μm. Error bars indicate SEM. ***p < = 0.0001; **p < = 0.001; *p < 0.05.
To corroborate an involvement of Goα in inhibiting synaptopod formation, we expressed two different Goα-RNAi constructs in octopaminergic neurons, Goα-RNAi1 and Goα-RNAi2, and examined the number of natural synaptopods at type II arbors in third-instar larvae. In agreement with our model, downregulating Goα by either RNAi resulted in significant increase in the number of natural synaptopods (Fig. 3F,G). In contrast, downregulating Giα by using two different Giα-RNAi constructs was without effect (Fig. 3G).
Since synaptopods are precursors of type II boutons (Koon et al., 2011), we also expected that inhibiting or downregulating Goα function should result in increased type II bouton growth. Indeed, expressing PTX or the two Goα-RNAi constructs in octopaminergic neurons resulted in significant increase in the number of type II boutons (Fig. 3H). To further test the hypothesis that Octß1R mediates inhibition via Goα, we also looked for evidence of genetic interactions between octß1r and goα genes. Heterozygotes goα007/+ (homozygous lethal) or octß1r/+ showed no differences in the number of natural synaptopods or type II boutons (Fig. 3I,J). However, in transheterozygotes (goα007/+; octß1r/+), both the number of natural synaptopods and type II boutons were significantly increased (Fig. 3I,J). This nonadditive effect is a strong indication that both genes act in the same pathway (Anholt and Mackay, 2004; Greenspan, 2004). Together, these results suggest that Octß1R receptors inhibit the growth of type II endings via inhibitory G-protein Goα.
Octß1R functions upstream of cAMP production and is partially dependent on Octß2R function
Goα functions by inhibiting cAMP production (Johnston and Watts, 2003). Thus, we predicted that decreasing cAMP levels by an independent approach should suppress the synaptic overgrowth phenotype in octß1r mutants. This hypothesis was tested by examining synaptopod formation in animals also lacking the adenylate cyclase, Rutabaga (Rut), or by overexpressing the phosphodiesterase, Dunce (Dnc), in the octß1r mutant background. Consistent with this hypothesis, both conditions prevented the increase in synaptopods elicited by mutations in octß1r (Fig. 4A). Similarly, they also prevented the increase in the number of type II boutons (Fig. 4B). Since the resulting phenotypes are no different from the phenotypes observed in the rut mutant alone or upon overexpressing Dnc alone, it is likely that Rut and Dnc are downstream components of the Octß1R pathway.
Figure 4.

Suppression of the overgrowth phenotype of octß1r by decreasing cAMP. A, B, Quantification of natural synaptopods per 100 μm of type II arbor (A) and type II boutons at muscle 12 (B) in octß1r mutants, rut mutants, animals overexpressing Dnc in type II and combinations of these genetic manipulations, showing that rut and overexpressing Dnc fully suppress the synaptic overgrowth phenotype of octß1r, suggesting that Rut and Dnc are downstream components of the Octß1R signaling pathway [N (left to right) = 175, 21, 24, 13, 14, 13 NMJs in A; N (left to right) = 22, 15, 18, 18, 12, 12 NMJs in B]. C, D, Quantification of natural synaptopods per 100 μm of type II arbor (C) and type II boutons at muscle 12 (D) in octß2r mutants, octß1r mutants, and octß1r, octß2r double mutants, showing that Octß1R and Octß2R function partially independently [N (left to right) = 175, 13, 21, 12 NMJs in C; N (left to right) = 22, 17, 15, 16 NMJs in D]. Error bars indicate SEM. ***p < = 0.0001; **p < = 0.001; *p < 0.05.
We previously demonstrated that Octß2R receptors promote the formation of synaptopods and the expansion of type II terminals by increasing cAMP production (Koon et al., 2011). However, other G-protein-coupled receptors, in addition to Octß2R receptors, may also regulate cAMP production at octopaminergic neurons. To determine whether Octß1R receptors function by antagonizing the action of Octß2R receptors alone, other G-protein-coupled receptors, or a combination of both, we generated octß1r, octß2r double mutants by recombining the mutations on the third chromosome. The presence of the double mutation was verified by genomic PCR. If Octß1R functions by exclusively inhibiting Octß2R, then the octß1r, octß2r double mutant should not be different from octß2r mutants alone. However, we found that, in the octß1r, octß2r double mutant, the number of natural synaptopods was significantly higher than that in the octß2r mutant and similar to wild-type controls (Fig. 4C). Similar results were obtained when counting the number of type II boutons, which were significantly larger than octß2r mutants but not significantly different from wild type (Fig. 4D). These observations suggest that Octß1R receptors function at least partially through Octß2R receptors, but that they additionally inhibit an alternative G-protein-coupled receptor during synaptopod formation and type II terminal extension.
That the octß1r, octß2r double mutant was no different from control suggests that the two receptors are not absolutely required for normal extension of synaptopods or proliferation of type II boutons, but that, rather, they exert a modulatory role, which may be relevant for physiological responses to stimuli. Indeed, as described below, although the double mutant has a normal number of synaptopods and type II boutons, it has impaired locomotion and abnormal response to starvation.
Acute octopamine- and cAMP-induced synaptic growth is occluded in octß1r mutants
We also examined the effect of octopamine application on synaptopod formation at type II endings in octß1r mutants and in larvae expressing Goα-RNAi, Giα-RNAi, or PTX in octopaminergic neurons. No response to octopamine was observed at type II terminals in octß1r mutants, when Goα was downregulated, or when PTX was expressed throughout larval development in octopaminergic neurons (Fig. 5A). In contrast, the response to octopamine when Giα was downregulated was normal (Fig. 5A). A likely explanation for the lack of response to octopamine in octß1r mutants or when expressing either Goα-RNAi or PTX, is our observation that, in these conditions, the number of natural synaptopods is substantially increased (Figs. 2A–C, 3D–G). It is possible that synaptopod formation has reached saturation in these animals, which would occlude a further increase in the number of synaptopods by octopamine application. This interpretation was supported by studies of dnc mutants. dnc encodes a cAMP-specific phosphodiesterase, and thus when mutated it results in significant increase in cAMP levels and consequently a drastic increase in the number of natural synaptopods, likely to saturation (Koon et al., 2011). As in the above strains, dnc mutants did not show any increase in the number of synaptopods in response to octopamine (Fig. 5A).
Figure 5.
octß1r mutation or disruption of Goα function likely results in saturating levels of cAMP. A, Quantification of the net increase of synaptopods per 100 μm of type II arbor in response to exogenous octopamine application in octß1r mutants, dnc mutants, and animals expressing PTX, Goα-RNAi, or Giα-RNAi in type II, showing that bath application of octopamine increases synaptopods in control and Giα-RNAi animals, but not in animals with disrupted Octß1R or Goα pathway, which have increased natural synaptopods [N (left to right) = 14, 13, 12, 10, 10, 11, 12, 11, 19, 10 NMJs]. Animals used in RNAi experiments were reared at 29°C to increase knockdown efficiency. B, Quantification of the net increase of synaptopods per 100 μm of type II arbor in response to exogenous octopamine or forskolin application in octß1r mutants and octß2r mutants, showing that bath application of octopamine fails to increase synaptopods in both octß1r and octß2r, whereas bath application of forskolin increases synaptopods in octß2r but not in octß1r. This indicates that the lack of response to octopamine in octß1r is likely due to saturating levels of cAMP [N (left to right) = 14, 13, 11, 10, 12, 13, 11, 13, 10 NMJs]. Error bars indicate SEM. ***p < = 0.0001; **p < = 0.001; *p < 0.05.
To test whether the lack of response to octopamine in octß1r mutants was due to occlusion resulting from saturating cAMP levels, we induced a maximal increase in cAMP levels with forskolin, which activates adenylate cyclases and increases intracellular cAMP, thus bypassing the activation of GPCRs by ligand binding (Seamon et al., 1981). If cAMP levels are saturated, this manipulation is expected not to further increase the number of synaptopods in octß1r mutants. Forskolin at 10 μm was bath applied to control and octß1r mutant body wall muscle preparations. In control animals, forskolin resulted in an increase of synaptopods similar to that produced by octopamine (Fig. 5B). In contrast, no increase in synaptopod formation was observed in octß1r mutants (Fig. 5B).
Our previous studies demonstrated that octß2r mutants also fail to respond to octopamine by forming new synaptopods, which is similar to the findings above with octß1r mutants (Koon et al., 2011). However, in contrast to octß1r mutants, octß2r mutants responded normally to forskolin (Fig. 5B) and had an NMJ undergrowth phenotype (Fig. 2C,F). Together, the above results support the notion that the response to octopamine in octß1r mutants is occluded due to saturating cAMP levels.
Octß1R is required for the increase in locomotion in response to starvation
In our previous study, we demonstrated that Octß2R receptors are necessary for octopamine-induced cAMP increase in type II motorneurons and starvation-induced larval locomotor increase (Koon et al., 2011). Since type II terminals of octß1r mutants also do not respond to octopamine, we wondered whether the increase in locomotor activity upon starvation would also be blocked in octß1r mutants similar to octß2r mutants. As we expected, octß1r failed to respond to starvation by increasing locomotor speed (Fig. 6A), possibly due to high levels of cAMP hindering normal Octß2R-dependent cAMP increase and as observed when dnc levels are reduced (Koon et al., 2011). The same defect was also observed in animals expressing UAS-PTX or Goα-RNAi1 in octopaminergic neurons, but not Giα-RNAi1 (Fig. 6A). This result indicates that both inhibitory Octß1Rs and excitatory Octß2Rs are required for normal starvation-induced behavior in larva. It also confirms our previous findings that octopaminergic and cAMP-dependent signaling within octopaminergic neurons is necessary for this type of behavioral plasticity. Interestingly, octß1r, octß2r double mutants were still defective in the starvation response (Fig. 6A), even though they have wild-type levels of natural synaptopods and boutons (Fig. 4C,D). These results suggest that both Octß1R and Octß2R are required for proper locomotor increase during starvation. Moreover, it is apparently not the endogenous amount of octopaminergic innervation that determines the animals' ability to increase locomotion during starvation. Instead, it seems likely that it is the capability of increasing octopaminergic innervation and cAMP levels in response to octopamine during starvation that regulates this type of behavioral change. However, since our manipulations involved the entire complement of octopaminergic neurons, and not octopaminergic motorneurons alone, whether all effects are directly due to type II innervation of the NMJ, or to a more central octopamine function in the brain, remains to be established.
Figure 6.
Octß1R is required for starvation-induced locomotor increase. A, Quantification of the percentage increase of larval crawling speed in response to 2 h starvation in octß1r mutants, octß2r mutants, octß1r, octß2r double mutants, and animals expressing PTX, Goα-RNAi, or Giα-RNAi in octopaminergic neurons, showing that the disruption of the Octß1R pathway results in defects in the increase of locomotor activity in response to starvation [N (left to right) = 26, 16, 16, 15, 16, 16, 16, 16, 17, 13, 19, 16, 16, 17 animals]. B, Quantification of the basal crawling speed of the same genotypes in A, showing that the defect starvation response of PTX-type II and Goα-RNAi1-type II in A is not due to a defect in basal locomotor speed [N (left to right) = 31, 16, 18, 29, 16, 18, 20, 14, 17, 15, 20, 17, 16, 20 animals]. Animals used in RNAi experiments were reared at 29°C to increase knockdown efficiency. Error bars indicate SEM. ***p < = 0.0001; **p < = 0.001; *p < 0.05.
It is also possible that the lack of behavioral response to starvation is due to defects in basal levels of locomotion in the above genotypes. Thus, we compared the basal larval locomotor speed in these animals. We found that octß1r mutants indeed had a decreased basal locomotor activity (Fig. 6B). Nevertheless, this defect was not observed in animals expressing PTX or Goα-RNAi1 in octopamine neurons, indicating that, at least in these genotypes, their starvation response defect is unlikely a secondary effect of a basal alteration in locomotion (Fig. 6B).
Octß1R receptors inhibit type I synaptic growth in a cell-autonomous manner
We previously demonstrated that blocking octopamine synthesis by a mutation in tyramine-ß-hydroxylase (tbh), the gene encoding the octopamine biosynthetic enzyme, or octopamine release by ablating type II endings, results in a decrease in the growth of type I boutons, implicating type II endings in regulating the plasticity of type I terminals (Koon et al., 2011). This effect was mediated by the function of Octß2R receptors in type I motorneurons. Thus, we wondered whether Octß1R receptors, in addition to antagonizing the effects of Octß2R receptors in octopaminergic neurons, could also antagonize the growth of type I boutons in a cell-autonomous fashion. Indeed, as predicted, mutations in octß1r led to a significant increase in the number of type I boutons (Fig. 7A,B,E). Similarly, an increase in the number of type I boutons was also observed in goα007/+; octß1r/+ transheterozygotes (Fig. 7E) again, supporting a genetic interaction between octß1r and goα. Furthermore, expressing PTX, downregulating Octß1R or Goα in type I motorneurons alone, using BG439-Gal4, or simultaneously in type I and type II motorneurons, using C380-Gal4, led to a significant increase in the number of type I boutons (Fig. 7C,D,F,G). In contrast, the same genetic manipulations in octopamine neurons alone did not result in any change in the expansion of type I boutons (Fig. 7H), and downregulation of Giα in type I and/or type II also had no effect (Fig. 7F–H). These results suggest that Octß1R receptors and Goα regulate the growth of type I terminals in a cell-autonomous fashion, similar to that in type II.
Figure 7.
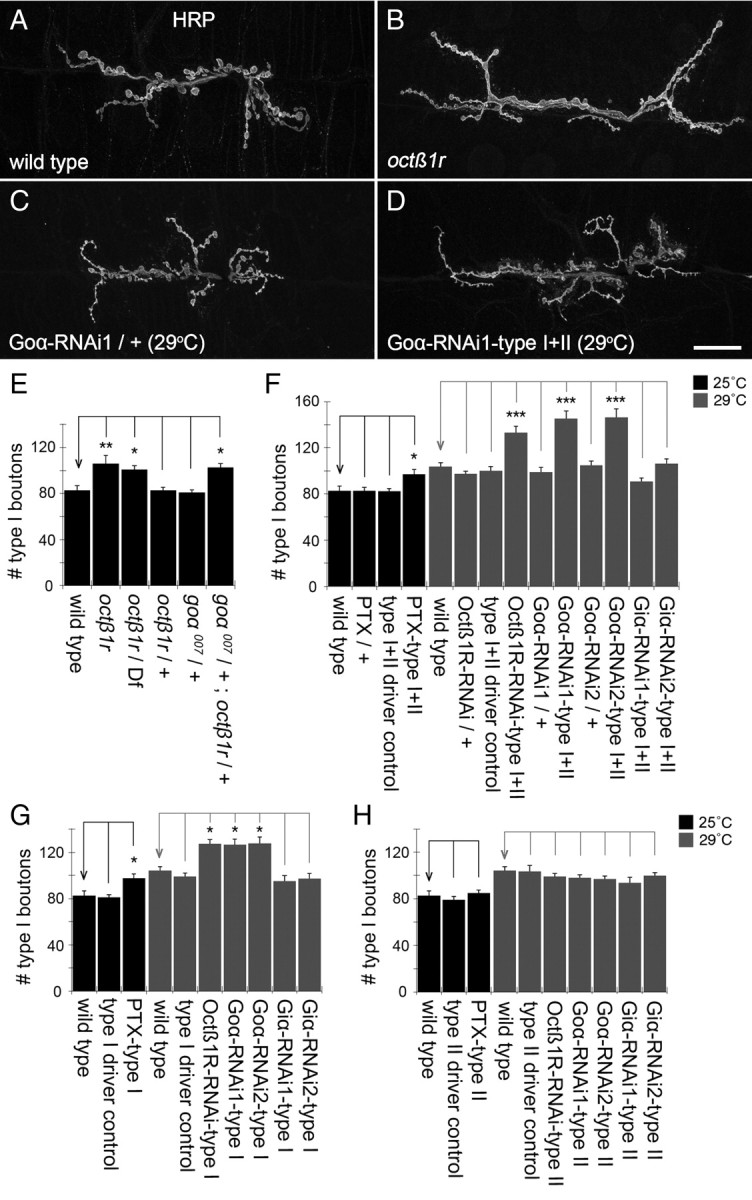
Octß1R negatively regulates type I synaptic growth in a cell-autonomous manner. A–D, Third-instar larval NMJs at muscles 6/7 of wild type (A), octß1r mutant (B), Goα-RNAi1/+ control (reared at 29°C) (C), and Goα-RNAi1-type I+II (reared at 29°C) (D), showing a marked increase in the number of type I boutons. NMJs were labeled with anti-HRP. The panels represent confocal Z-stack projections. E, Quantification of type I boutons at muscle 6/7 in octß1r mutants, octß1r/+ heterozygotes, goα007/+ heterozygotes, and goα007/+; octß1r/+ transheterozygotes, showing that octß1r mutants and goα007/+; octß1r/+ transheterozygotes have increased number of type I boutons [N (left to right) = 18, 15, 16, 16, 12, 18 NMJs]. F–H, Quantification of type I boutons at muscle 6/7 in animals expressing PTX, Octß1R-RNAi, Goα-RNAi, or Giα-RNAi in type I and type II (F), type I (G), and type II (H), showing that the disruption Octß1R or Goα in type I and type II simultaneously or type I alone increase type I boutons [N (left to right) = 18, 14, 14, 16, 15, 12, 14, 12, 12, 16, 12, 16, 14, 16 NMJs in F; N (left to right) = 18, 12, 16, 15, 13, 18, 12, 10, 12, 13 NMJs in G; N (left to right) = 18, 12, 14, 15, 11, 12, 16, 16, 10, 16 NMJs]. Animals used in RNAi experiments were reared at 29°C to increase knockdown efficiency. Scale bar: A–D, 10 μm. Error bars indicate SEM. ***p < = 0.0001; **p < = 0.001; *p < 0.05.
Discussion
We previously demonstrated that octopamine regulates synaptic and behavioral plasticity through an autoregulatory positive-feedback mechanism involving Octß2R, which promotes both type I and type II outgrowth (Koon et al., 2011). We have now identified an octopamine receptor, Octß1R, which antagonizes the function of Octß2R. We propose that Octß1R may serve as a brake for the positive feedback induced by Octß2R. We demonstrated that Octß1R receptors inhibit the cAMP pathway via the inhibitory G-protein Goα, as loss of Octß1R or Goα function results in synaptic overgrowth of type I and type II endings in an octopamine cell-autonomous manner, and as octß1r and goα interact genetically. Notably, defective Octß1R signaling appears to saturate cAMP levels, occluding the function of Octß2R. Thus, the loss of Octß1R function results in insensitivity to octopamine stimulation. In turn, this abolishes starvation-induced behavioral changes that require Octß2R signaling. While in this study we centered primarily on Octß1R function at octopaminergic NMJ terminals, it is important to emphasize that octopamine neurons are also present in the larval brain. Thus, with current tools we cannot discern whether the defects are exclusively due to the function of octopaminergic motorneurons, or whether other central octopaminergic neurons contribute to these effects. While the phenotypes on NMJ development are most parsimoniously explained by a local function at NMJ terminals, it is likely that the behavioral effects are more complex, also involving important contribution from brain octopaminergic neurons.
At the Drosophila larval NMJ, three type II motorneurons innervate most of the body wall muscles in each segment (Koon et al., 2011). This layout suggests that octopamine is likely to globally regulate plasticity, by tuning the excitability levels of multiple excitatory synapses on the body wall muscles. Together, the observations in our previous study (Koon et al., 2011) and this investigation identify the presence of excitatory and inhibitory octopamine receptors that are coexpressed in the same cells. This suggests that global regulation of synapses and behavior by octopamine can be tipped toward excitation or inhibition depending on receptor expression levels, affinity of the receptors for octopamine, and availability of these receptors for binding octopamine on the target cells. This dual mode of controlling excitability likely provides enhanced flexibility, allowing a broader level of control over synaptic functions.
An important question is how can Octß1R and Octß2R regulate development of innervation and behavior given that they are activated by the same ligand, are localized in the same cells, and their functions are antagonistic. Several alternatives can be proposed. Octß1R and Octß2R might have different affinities for octopamine binding. Thus, different levels of octopamine release could differentially activate the receptors. For instance, if Octß1R receptors have higher affinity for octopamine, and octopamine is normally released at low levels, a stable degree of innervation could be maintained by continuous inhibition of synaptic growth-promoting signals. High levels of octopamine release, as would occur during starvation (Davenport and Evans, 1981), would then activate the lower affinity Octß2R, eliciting synaptic growth. Precedence for this type of regulation has been obtained in honeybees and olive fruit flies, where low concentrations of octopamine are inhibitory while high concentrations are excitatory to cardiac contraction (Papaefthimiou and Theophilidis, 2011).
An alternative possibility is based on the well known internalization of GPCRs upon ligand binding (Calebiro et al., 2010). It is possible that such a mechanism would maintain an appropriate ratio of Octß1R and Octß2R at the cell surface, actively keeping or removing octopamine receptor-mediated excitation or inhibition, depending on physiological states. A third alternative is that receptors could be posttranslationally modified upon ligand binding, which might also affect their downstream functions. For example, dimerization of ß2-adrenergic receptors can inhibit its adenylate cyclase-activating activity (Hebert et al., 1996) and phosphorylation of ß1-adrenergic receptor by PKA reduces its affinity for Gsα and increases its affinity for Giα/oα (Martin et al., 2004). Last, Octß1R and Octß2R receptors could be spatially separated in neurons, with one receptor being closer and the other distant to sites of octopamine release. In this scenario, the receptors would likely be exposed to different octopamine concentration.
Simultaneous expression of excitatory and inhibitory GPCRs in the same neuron has been reported previously. For instance, mammalian dopamine receptors can couple to both stimulatory and inhibitory G-proteins, with the D1 receptor-like family being coupled to Gsα and the D2-like family being coupled to Giα/oα (Beaulieu and Gainetdinov, 2011).
Previous studies have investigated the effect of octopamine on synaptic transmission at the Drosophila first-instar (Nishikawa and Kidokoro, 1999) and third-instar (Kutsukake et al., 2000; Nagaya et al., 2002; Koon et al., 2011) larval NMJ. While the studies at the third-instar larval NMJ demonstrated an excitatory effect of octopamine in neurotransmission (Kutsukake et al., 2000; Nagaya et al., 2002; Koon et al., 2011), the study on the first-instar larval stage substantiated an inhibitory effect (Nishikawa and Kidokoro, 1999). A recent study now provides a potential explanation for such discrepancy between the responses to octopamine at the two larval stages (Ohhara et al., 2012). In particular, it was found that that Octß1R is expressed at high levels in first instar and at low levels in third instar. In contrast, Octß2R is expressed at low levels in first instar and at high levels in third instar (Ohhara et al., 2012). Our studies demonstrating an inhibitory role for Octß1R (this study) and an excitatory role for Octß2R (Koon et al., 2011) are in agreement with the idea that octopamine may play an inhibitory role during first instar, but an excitatory role during third instar.
Octopamine receptors have been shown to elicit intracellular Ca2+ and/or cAMP increase (Han et al., 1998; Balfanz et al., 2005). OAMB, the only α-adrenergic-like receptor in Drosophila, has been implicated to function via Ca2+ signaling in the Drosophila oviduct (Lee et al., 2009). However, OAMB is expressed in the oviduct epithelium, and not in the oviduct muscle cells (Lee et al., 2009). Given that octopamine induces relaxation of oviduct muscles, the presence of an alternative, inhibitory octopamine receptor in oviduct muscles was proposed (Lee et al., 2009). Our identification of Octß1R receptor as an inhibitory receptor raises the possibility that this is the inhibitory receptor in the oviduct.
In apparent contradiction to our findings, a previous study has shown that Octß1R (also known as OA2) is capable of increasing cAMP (Balfanz et al., 2005). In this study, HEK293 cells transfected with Octß1R were exposed to different octopamine concentrations, which resulted in an increase in cAMP levels (Balfanz et al., 2005). A potential explanation for the disparate results is that GPCR overexpression might alter its coupling to downstream pathways. For instance, mammalian ß2-adrenergic receptors are known to couple to both Gsα and Giα/oα proteins (Xiao, 2001). However, overexpression of ß2-adrenergic receptors constitutively couples the receptor to Gsα and not to Giα or Goα (Milano et al., 1994; Bond et al., 1995). Furthermore, analysis of its binding specificity through immunoprecipitation shows that, when the receptor was overexpressed in transgenic mice, it coprecipitated with Gsα but not with Giα/oα in the absence of agonist (Gurdal et al., 1997). An additional explanation is that human embryonic kidney HEK293 cells are unlikely to express the same transduction pathways as endogenous Drosophila cells. Indeed, a recent study showed that HEK293 cells express virtually no Goα (Atwood et al., 2011), which could also explain the lack of inhibitory response of overexpressed Octß1R in this cell line.
Goα is expressed in the nervous system of Drosophila and shows a marked increase in levels during the development of axonal tracts (Guillén et al., 1991). Goα levels are altered in memory mutants including dunce and rutabaga (Guillén et al., 1990), and Goα is necessary for associative learning (Ferris et al., 2006). PTX overexpression in mushroom bodies of adult Drosophila severely disrupts memory (Ferris et al., 2006), suggesting a role of Goα in synaptic plasticity. However, homozygous goα mutants are lethal due to defective development of the heart (Frémion et al., 1999) preventing the use of null mutants in studies of the NMJ or the adult brain. Moreover, overexpression of inhibitory G-proteins is known to sequester available Gß and Gγ subunits, resulting in unspecific downregulation of other G-protein signaling (Katanayeva et al., 2010). Thus, there are significant problems associated with the use of an overexpression approach to study Goα function. Fortunately, the availability of PTX and multiple Goα-RNAi strains allowed us to downregulate Goα function in a cell-specific manner to examine synaptic development at the NMJ, which was found to phenocopy defects observed at the NMJ of octß1r mutants. The presence of genetic interactions between the octß1r and goα genes further support the notion that the two proteins act in the same signaling pathway to inhibit synaptic growth. These results provide strong evidence for the involvement of Goα in synaptic plasticity at the NMJ.
In summary, our studies reveal that octopamine acts both as an inhibitory and excitatory transmitter to regulate synaptic growth and behavior. Thus, the inhibitory function of octopamine in global synaptic growth is as crucial as its excitatory function in maintaining plasticity in a dynamic range.
Footnotes
This work was supported by NIH Grant R01 MH070000 (V.B.). We thank Dr. James Ashley for thoughtful comments on this manuscript, and Ceren Korkut for her help on some studies. We also thank Dr. Bulent Ataman and Dr. Comert Kural for their assistance in acquiring the reagents for generating the octß1r mutant, and Dr. Andrew Tomlinson for sending us the goα007 mutant.
The authors declare no competing financial interests.
References
- Anholt RR, Mackay TF. Quantitative genetic analyses of complex behaviours in Drosophila. Nat Rev Genet. 2004;5:838–849. doi: 10.1038/nrg1472. [DOI] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics. 2011;12:14. doi: 10.1186/1471-2164-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfanz S, Strünker T, Frings S, Baumann A. A family of octopamine [corrected] receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J Neurochem. 2005;93:440–451. doi: 10.1111/j.1471-4159.2005.03034.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bond RA, Leff P, Johnson TD, Milano CA, Rockman HA, McMinn TR, Apparsundaram S, Hyek MF, Kenakin TP, Allen LF, Lefkowitz RJ. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the beta 2-adrenoceptor. Nature. 1995;374:272–276. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- Breen CA, Atwood HL. Octopamine—a neurohormone with presynaptic activity-dependent effects at crayfish neuromuscular junctions. Nature. 1983;303:716–718. doi: 10.1038/303716a0. [DOI] [PubMed] [Google Scholar]
- Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Persani L, Lohse MJ. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci. 2010;31:221–228. doi: 10.1016/j.tips.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Cheung US, Shayan AJ, Boulianne GL, Atwood HL. Drosophila larval neuromuscular junction's responses to reduction of cAMP in the nervous system. J Neurobiol. 1999;40:1–13. doi: 10.1002/(sici)1097-4695(199907)40:1<1::aid-neu1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Evans PD. Changes in haemolymph octopamine levels associated with food deprivation in the locust, Schistocerca gregaria. Physiol Entomol. 1981;9:269–274. [Google Scholar]
- El-Armouche A, Zolk O, Rau T, Eschenhagen T. Inhibitory G-proteins and their role in desensitization of the adenylyl cyclase pathway in heart failure. Cardiovasc Res. 2003;60:478–487. doi: 10.1016/j.cardiores.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Evans PD, Maqueira B. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci. 2005;5:111–118. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Ferris J, Ge H, Liu L, Roman G. Go signaling is required for Drosophila associative learning. Nat Neurosci. 2006;9:1036–1040. doi: 10.1038/nn1738. [DOI] [PubMed] [Google Scholar]
- Frémion F, Astier M, Zaffran S, Guillèn A, Homburger V, Sémériva M. The heterotrimeric protein Go is required for the formation of heart epithelium in Drosophila. J Cell Biol. 1999;145:1063–1076. doi: 10.1083/jcb.145.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan RJ. Fly pushing: the theory and practice of Drosophila genetics, Chap 5. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2004. Analysis of mutations. [Google Scholar]
- Guillén A, Jallon JM, Fehrentz JA, Pantaloni C, Bockaert J, Homburger V. A Go-like protein in Drosophila melanogaster and its expression in memory mutants. EMBO J. 1990;9:1449–1455. doi: 10.1002/j.1460-2075.1990.tb08261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén A, Sémériva M, Bockaert J, Homburger V. The transduction signalling protein Go during embryonic development of Drosophila melanogaster. Cell Signal. 1991;3:341–352. doi: 10.1016/0898-6568(91)90063-z. [DOI] [PubMed] [Google Scholar]
- Gurdal H, Seasholtz TM, Wang HY, Brown RD, Johnson MD, Friedman E. Role of Gαq or Gαo proteins in α1-adrenoceptor subtype-mediated responses in Fischer 344 rat aorta. Mol Pharmacol. 1997;52:1064–1070. doi: 10.1124/mol.52.6.1064. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci. 1998;18:3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, Bouvier M. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem. 1996;271:16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, Heisenberg M. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976;262:215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CA, Watts VJ. Sensitization of adenylate cyclase: a general mechanism of neuroadaptation to persistent activation of Gαi/o-coupled receptors? Life Sci. 2003;73:2913–2925. doi: 10.1016/s0024-3205(03)00703-3. [DOI] [PubMed] [Google Scholar]
- Katanayeva N, Kopein D, Portmann R, Hess D, Katanaev VL. Competing activities of heterotrimeric G proteins in Drosophila wing maturation. PLoS One. 2010;5:e12331. doi: 10.1371/journal.pone.0012331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koon AC, Ashley J, Barria R, DasGupta S, Brain R, Waddell S, Alkema MJ, Budnik V. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat Neurosci. 2011;14:190–199. doi: 10.1038/nn.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake M, Komatsu A, Yamamoto D, Ishiwa-Chigusa S. A tyramine receptor gene mutation causes a defective olfactory behavior in Drosophila melanogaster. Gene. 2000;245:31–42. doi: 10.1016/s0378-1119(99)00569-7. [DOI] [PubMed] [Google Scholar]
- Kuzmiski JB, Pittman QJ, Bains JS. Metaplasticity of hypothalamic synapses following in vivo challenge. Neuron. 2009;62:839–849. doi: 10.1016/j.neuron.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Rohila S, Han KA. The octopamine receptor OAMB mediates ovulation via Ca2+/calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium. PLoS One. 2009;4:e4716. doi: 10.1371/journal.pone.0004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long TF, Murdock LL. Stimulation of blowfly feeding. Proc Natl Acad Sci U S A. 1983;80:4159–4163. doi: 10.1073/pnas.80.13.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NP, Whalen EJ, Zamah MA, Pierce KL, Lefkowitz RJ. PKA-mediated phosphorylation of the beta1-adrenergic receptor promotes Gs/Gi switching. Cell Signal. 2004;16:1397–1403. doi: 10.1016/j.cellsig.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Milano CA, Dolber PC, Rockman HA, Bond RA, Venable ME, Allen LF, Lefkowitz RJ. Myocardial expression of a constitutively active alpha 1B-adrenergic receptor in transgenic mice induces cardiac hypertrophy. Proc Natl Acad Sci U S A. 1994;91:10109–10113. doi: 10.1073/pnas.91.21.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, Budnik V. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol. 1995;356:275–287. doi: 10.1002/cne.903560210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Nagaya Y, Kutsukake M, Chigusa SI, Komatsu A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosci Lett. 2002;329:324–328. doi: 10.1016/s0304-3940(02)00596-7. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Kidokoro Y. Octopamine inhibits synaptic transmission at the larval neuromuscular junction in Drosophila melanogaster. Brain Res. 1999;837:67–74. doi: 10.1016/s0006-8993(99)01676-5. [DOI] [PubMed] [Google Scholar]
- Ohhara Y, Kayashima Y, Hayashi Y, Kobayashi S, Yamakawa-Kobayashi K. Expression of beta-adrenergic-like octopamine receptors during Drosophila development. Zoolog Sci. 2012;29:83–89. doi: 10.2108/zsj.29.83. [DOI] [PubMed] [Google Scholar]
- Papaefthimiou C, Theophilidis G. Octopamine—a single modulator with double action on the heart of two insect species (Apis mellifera macedonica and Bactrocera oleae): acceleration vs. inhibition. J Insect Physiol. 2011;57:316–325. doi: 10.1016/j.jinsphys.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, Mungall C, Svirskas R, Kadonaga JT, Doe CQ, Eisen MB, Celniker SE, Rubin GM. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A. Organization of the efferent system and structure of neuromuscular junctions in Drosophila. Int Rev Neurobiol. 2006;75:71–90. doi: 10.1016/S0074-7742(06)75004-8. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron. 1996;17:655–667. doi: 10.1016/s0896-6273(00)80198-1. [DOI] [PubMed] [Google Scholar]
- Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Suo S, Kimura Y, Van Tol HH. Starvation induces cAMP response element-binding protein-dependent gene expression through octopamine-Gq signaling in Caenorhabditis elegans. J Neurosci. 2006;26:10082–10090. doi: 10.1523/JNEUROSCI.0819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambi NC, Quan F, Wolfgang WJ, Spiegel A, Forte M. Immunological and molecular characterization of Go alpha-like proteins in the Drosophila central nervous system. J Biol Chem. 1989;264:18552–18560. [PubMed] [Google Scholar]
- Xiao RP. Beta-adrenergic signaling in the heart: dual coupling of the beta2-adrenergic receptor to Gs and Gi proteins. Sci STKE. 2001;2001:re15. doi: 10.1126/stke.2001.104.re15. [DOI] [PubMed] [Google Scholar]
- Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]