Abstract
β-Adrenoceptor antagonists differ in their degree of partial agonism. In vitro assays have provided information on ligand affinity, selectivity, and intrinsic efficacy. However, the extent to which these properties are manifest in vivo is less clear. Conscious freely moving rats, instrumented for measurement of heart rate (β1; HR) and hindquarters vascular conductance (β2; HVC) were used to measure receptor selectivity and ligand efficacy in vivo. CGP 20712A caused a dose-dependent decrease in basal HR (P<0.05, ANOVA) at 5 doses between 6.7 and 670 μg/kg (i.v.) and shifted the dose-response curve for isoprenaline to higher agonist concentrations without altering HVC responses. In contrast, at doses of 67 μg/kg (i.v.) and above, ICI 118551 substantially reduced the HVC response to isoprenaline without affecting HR responses. ZD 7114, xamoterol, and bucindolol significantly increased basal HR (ΔHR: +122±12, +129±11, and +59±11 beats/min, respectively; n=6), whereas other β-blockers caused significant reductions (all at 2 mg/kg i.v.). The agonist effects of xamoterol and ZD 7114 were equivalent to that of the highest dose of isoprenaline. Bucindolol, however, significantly antagonized the response to the highest doses isoprenaline. An excellent correlation was obtained between in vivo and in vitro measures of β1-adrenoceptor efficacy (R2=0.93; P<0.0001).—Baker, J. G., Kemp, P., March, J., Fretwell, L., Hill, S. J., Gardiner, S. M. Predicting in vivo cardiovascular properties of β-blockers from cellular assays: a quantitative comparison of cellular and cardiovascular pharmacological responses.
Keywords: β-adrenoceptor, partial agonism, intrinsic sympathomimetic activity, heart rate, vascular conductance
Following their initial discovery in the 1960s (1), β-adrenoceptor antagonists (β-blockers) have become one of the most widely prescribed classes of drugs in clinical practice (2, 3). They prevent endogenous catecholamines from binding to β-adrenoceptors, which, in the heart, reduces the rate and force of contraction, thereby reducing myocardial oxygen demand. In addition to their cardiac effects, beneficial effects on renin release and, in some cases, sympathetic nerve activity have meant that β-blockers have been extremely valuable, and, indeed, in some conditions life-saving, in the management of many cardiovascular disorders, including angina, myocardial infarction, arrhythmias (including perioperative arrhythmias), and hypertension (2, 4–9). Over the past decade, β-blockers have been proven to reduce mortality in patients with heart failure by mechanisms that reduce catecholamine-driven myocardial apoptosis, fibrosis, and remodeling (2, 5, 10). In addition, β-blockers are also used in the management of glaucoma, portal hypertension, anxiety, migraine, tremor, and hyperthyroidism (8, 11–16).
However, in clinical cardiovascular studies, β-blockers do not seem to behave as a single class of drugs (2, 9, 17–23). For example, following the original reports of the efficacy of β-blockers in heart failure (24, 25), 6 β-blockers have undergone trials in the management of heart failure, 4 of which have been found to be helpful and prolong life: bisoprolol (26), carvedilol (27), metoprolol (28), and nebivolol (29); one was found to be of no benefit, bucindolol (30), and one (xamoterol) actually increased mortality (31). In the only head-to-head trial, carvedilol was found to be superior to metoprolol in prolonging life, for the same degree of β-blockade (32). β-Blockers differ in their receptor selectivity (between β1 and β2-adrenoceptors; refs. 33–36) and in the extent of intrinsic sympathomimetic activity (ISA; refs. 2, 10, 17, 20, 21). Interestingly, β-blockers with ISA (e.g., pindolol, xamoterol, bucindolol) appear to be less effective in the treatment of cardiovascular disease (10, 37, 38) and migraine (14).
In pharmacological terms, β-blockers with ISA are partial agonists; thus, they block the stimulatory effects of higher-efficacy agonists, such as adrenaline (e.g., adrenaline-induced increases in heart rate), but on their own, they actually stimulate a low-level response (2, 39). Several studies have confirmed the partial agonist effects of certain β-blockers at the cellular level in model cell systems (34, 39, 40–44). Furthermore, some of these stimulatory actions of β-blockers have been observed in cardiac tissues (37, 38, 41, 45). The correlation between ISA in neonatal rat cardiomyocytes and cardiovascular responses in pithed rats was high, although that between adult cardiac tissues and the whole animal was poor (17). However, what is needed to reduce number, time, and cost on animal experimentation is to know the extent to which partial agonist effects of β-blockers in a model cell system are predictive of ISA in vivo in intact animals.
In this study, we have compared the pharmacological properties of a range of β-blockers, including those prescribed for heart failure, in an in vitro Chinese hamster ovary (CHO) cell model to cardiovascular responses in vivo in conscious rats. Thus, the correlation between the extent of partial agonism at the β1-adenoceptor and selectivity between β1 and β2-adrenoceptors in vitro, and their effects on resting and β agonist-induced heart rate (HR; β1 response) and hindquarters vascular conductance (HVC; β2 response) in parasympathetically blocked conscious rats has been examined. This has allowed us to test the predictive power of the in vitro assays for in vivo ISA and receptor selectivity in conscious animals.
MATERIALS AND METHODS
Materials
FCS was obtained from PAA Laboratories (Teddington, UK). Microscint 20 scintillation fluid was obtained from PerkinElmer (Shelton, CT, USA). 3H-CGP 12177, 3H-adenine, and 14C-cAMP were obtained from Amersham International (Little Chalfont, UK). Bisoprolol, bucindolol, carvedilol, CGP 20712A, xamoterol and ZD7114 were from Tocris Life Sciences (Avonmouth, UK). Fentanyl citrate was from Janssen-Cilag (High-Wycombe, UK); medetomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were from Pfizer (Sandwich, UK); buprenorphine (Vetergesic) was from Alstoe Animal Health (York, UK). Nebivolol hydrochloride was from Sequoia Research Products (Pangbourne, UK). All other reagents were from Sigma Chemicals (Poole, UK).
Constructs, cell lines, and cell culture
CHO K1 cells stably expressing either the human β1-adrenoceptor (at 1146 fmol/mg protein) or the human β2-adrenoceptor (at 466 fmol/mg protein) were used (33, 34). All cell lines were grown in Dulbecco's modified Eagle's medium nutrient mix F12 (DMEM/F12) containing 10% FCS and 2 mM l-glutamine in a 37°C humidified 5% CO2:95% air atmosphere.
3H-CGP 12177 whole-cell binding
Cells were grown to confluence in white-sided tissue culture-treated 96-well view plates. 3H-CGP 12177 whole-cell competition binding was performed as described previously (33) using 10 μM propranolol to define nonspecific binding and 3H-CGP 12177 in the range of 0.80–1.23 nM (total volume 200 μl/well). The KD value for 3H-CGP 12177 at the human β1 and β2 adrenoceptors is 0.42 and 0.17 nM, respectively (33). KD values for the competing ligands were calculated from the IC50 values, as described below.
3H-cAMP accumulation
Cells were grown to confluence in clear plastic tissue culture-treated 24-well plates. Following 3H-adenine prelabeling, 3H-cAMP accumulation in the wells was measured as described previously (34). Ligands were incubated for 5 h. 3H-cAMP was separated from other 3H-nucleotides by sequential Dowex and alumina column chromatography, as described previously (46).
Animals and surgery
Adult, male, Sprague-Dawley rats (Charles River, Margate, UK), weighing 300–350 g, were housed in groups in a temperature-controlled (21–23°C) environment with a 12-h light-dark cycle (lights on at 6 AM) and free access to food (18% Protein Rodent Diet; Teklad Global, Bicester, UK) and water for ≥7 d after arrival from the supplier before any surgical intervention.
Surgery was performed in 2 stages under general anesthesia (fentanyl and medetomidine, 300 μg/kg i.p. of each, supplemented as required), with reversal of anesthesia and postoperative analgesia provided by atipamezole (1 mg/kg s.c.) and buprenorphine (0.02 mg/kg s.c.). At the first surgical stage, a miniature pulsed Doppler flow probe was sutured around the distal abdominal aorta to monitor hindquarters hemodynamics. The wires from the probe were taped and sutured at the nape of the neck, and the animals were returned to the holding room. At the second surgical stage, which took place at least 10 d after the surgery for probe implantation, and following a satisfactory inspection from the named veterinary surgeon, catheters were implanted in the distal abdominal aorta via the caudal artery [for arterial blood pressure (BP) monitoring and the derivation of HR], and in the right jugular vein (for drug administration). Three separate intravenous catheters were placed in the jugular vein to enable concurrent administration of different substances. At this stage, the wires from the probe were soldered into a miniature plug (Microtech, Boothwyn, PA, USA), which was mounted onto a custom-designed harness worn by the rat. The catheters emerged from the same point as the probe wires and were fed through a protective spring secured to the harness and attached to a counter-balanced pivot system. The arterial catheter was connected to a fluid-filled swivel for overnight infusion of heparinized (15 U/ml) saline to maintain patency.
Experiments began 24 h after surgery for catheter implantation, with animals fully conscious and unrestrained in home cages, with free access to food and water. All procedures were carried out with approval of the University of Nottingham Local Ethical Review Committee, under Home Office Project and Personal License Authority.
Cardiovascular recordings
Cardiovascular variables were recorded using a customized, computer-based system [Instrument Development Engineering Evaluation (IDEEQ), Maastrich Instruments Bv, Maastrich, The Netherlands] connected to a transducer amplifier (Gould, Eastlake, Ohio, USA) model 13–4615-50) and a Doppler flowmeter (Crystal Biotech, Holliston, MA, USA) VF-1 mainframe (pulse repetition frequency 125 kHz) was fitted with high-velocity (HVPD-20) modules). Raw data were sampled by IDEEQ every 2 ms, averaged and stored to disc every cardiac cycle. HVC changes were calculated from the changes in BP and Doppler shift.
Experimental protocol
In all experiments, atropine methyl nitrate (1 mg/kg/h; 0.4 ml/h) was infused continuously to remove any parasympathetic influence on the control of HR. Starting 2 h after the onset of the atropine infusion, rats were given 3-min infusions (0.15 ml/min) of isoprenaline (4, 12, 40, and 120 ng/kg/min) in ascending order separated by ≥20 min. At least 45 min after the last infusion of isoprenaline, a β-adrenoceptor antagonist or vehicle was given as an i.v. bolus (0.1 ml) maintained by continuous infusion (0.4 ml/h), and the isoprenaline infusions were repeated, starting 30 min thereafter. Experiments were run in 4 series, and within each series, there was a contemporaneous control, and the antagonist doses (series 1 and 2) or different antagonists (series 3 and 4) were given in a randomized order.
Series 1
Rats (n=6/group) were given isoprenaline before and after administration of saline (0.1 ml bolus, 0.4 ml/h infusion) or CGP 20712A at doses of 2, 6.7, 20, 67, 200, and 670 μg/kg bolus with 1, 3.3, 10, 33, 100, and 333 μg/kg/h infusions, respectively.
Series 2
The above experiment was repeated in groups of rats (n=6/group) given saline or ICI 118551 (volumes and doses as above).
Series 3
Groups of rats (n=6) were given isoprenaline before and after administration of vehicle (saline, as above) or ZD 7114, labetolol, xamoterol, pronethalol, bisoprolol, or acebutolol, all at a dose of 2 mg/kg bolus, 1 mg/kg/h infusion. An additional 2 groups of rats (n=6/group) were given lower doses of bisoprolol (20 μg/kg bolus, 10 μg/kg/h infusion and 200 μg/kg bolus, 100 μg/kg/h infusion).
Series 4
Groups of rats (n=6/group) were given isoprenaline before and after administration of vehicle (5% propylene glycol, 2% Tween 80 in sterile saline; 0.1 ml bolus, 0.4 ml/h infusion) or nebivolol, carvedilol, or bucindolol, all at a dose of 2 mg/kg bolus, 1 mg/kg/h infusion.
In vitro data analysis
Whole-cell binding
For competition binding, all data points on each binding curve were performed in triplicate, and each 96-well plate also contained 6 determinations of total and nonspecific binding. In all cases in which a KD value is stated, the competing ligand completely inhibited the specific binding of 3H-CGP 12177.
A 1-site sigmoidal response curve was then fitted to the data using GraphPad Prism 2.01 (GraphPad, San Diego, CA, USA) and the IC50 was then determined as the concentration required to inhibit 50% of the specific binding:
where [A] is the concentration of the competing ligand, IC50 is the concentration at which half of the specific binding of 3H-CGP 12177 has been inhibited, and NS is the nonspecific binding.
From the IC50 value and the known concentration of 3H-CGP 12177, a KD value (concentration at which half the receptors are bound by the competing ligand) was calculated using the equation:
Functional assays: 3H-cAMP accumulation
Most agonist responses were best described by a 1-site sigmoidal concentration response curve:
where Emax is the maximum response, [A] is the agonist concentration, and EC50 is the concentration of agonist that produces 50% of the maximal response.
The responses to bucindolol and carvedilol were, however, best fitted to a 2-site concentration response:
where N is the percentage of site 1, [A] is the concentration of agonist, and EC150 and EC250 are the respective EC50 values for the two agonist sites.
A 10 μM (maximal) isoprenaline concentration was included in 2–3 wells in each plate for each separate experiment for 3H-cAMP accumulation to allow agonist responses to be expressed as a percentage of the isoprenaline maximum for each experiment. All data are presented as means ± se of triplicate determinations, and n in the text refers to the number of separate experiments.
In vivo data analysis
Data were analyzed offline using IDEEQ software. Responses to isoprenaline were measured as the difference between steady-state values immediately before the isoprenaline infusion and during the third minute of infusion. Changes in baseline were measured as the difference between the control values for the last dose of isoprenaline before, and the first dose of isoprenaline after, β-adrenoceptor antagonist administration. Data were analyzed by t test or ANOVA with Bonferroni correction as appropriate; P < 0.05 was taken as significant (GraphPad Prism 5.02).
RESULTS
Ligand affinity and selectivity: 3H-CGP 12177 whole-cell binding
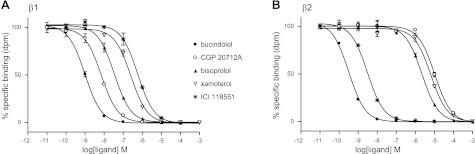
All ligands completely inhibited specific 3H-CGP 12177 binding to both the human β1- and human β2-adrenoceptors to yield KD values as shown in Table 1 and Fig. 1. As expected, CGP 20712A was the most selective β1-adrenoceptor antagonist, and ICI 118551 was the most selective β2-antagonist. Bisoprolol (36-fold), nebivolol (23-fold) and xamoterol (20-fold) had some selectivity for the β1-adrenoceptor, while pronethalol (4-fold), bucindolol (5-fold), and carvedilol (6-fold) had marginal preference for the β2-adrenoceptor (Table 1).
Table 1.
Log KD values obtained from 3H-CGP 12177 whole-cell binding studies in CHO cells stably expressing either the human β1-adrenoceptor or the human β2-adrenoceptor
| Ligand | β1 |
β2 |
Selectivity |
|||
|---|---|---|---|---|---|---|
| Log KD | n | Log KD | n | β1 | β2 | |
| CGP 20712A | −8.63 ± 0.09 | 8 | −5.65 ± 0.05 | 8 | 955 | |
| Bisoprolol | −7.98 ± 0.04 | 8 | −6.43 ± 0.04 | 8 | 35.5 | |
| Nebivolol | −8.79 ± 0.09 | 8 | −7.42 ± 0.04 | 8 | 23.4 | |
| Xamoterol | −7.08 ± 0.04 | 8 | −5.79 ± 0.06 | 8 | 19.5 | |
| Acebutolol | −6.57 ± 0.02 | 8 | −5.70 ± 0.03 | 8 | 7.4 | |
| ZD 7114 | −7.61 ± 0.02 | 8 | −7.14 ± 0.04 | 8 | 3.0 | |
| Labetolol | −7.99 ± 0.03 | 8 | −8.25 ± 0.08 | 8 | 1.8 | |
| Pronethalol | −6.67 ± 0.03 | 8 | −7.27 ± 0.02 | 8 | 4.0 | |
| Bucindolol | −9.47 ± 0.04 | 8 | −10.19 ± 0.07 | 8 | 5.3 | |
| Carvedilol | −9.26 ± 0.05 | 8 | −10.06 ± 0.06 | 8 | 6.3 | |
| ICI 118551 | −6.74 ± 0.03 | 8 | −9.27 ± 0.05 | 8 | 338 | |
Values represent means ± se for n separate experiments. Ligands are arranged in order of β1/β2 selectivity.
Figure 1.
Inhibition of 3H-CGP 12177-specific binding in whole CHO cells expressing human β1-adrenoceptor (A) and human β2-adrenoceptor (B). Nonspecific binding was determined by 10 μM propranolol. Concentration of 3H-CGP 12177 present was 0.97 nM (A) and 1.11 nM (B). Data points are means ± se of triplicate determinations. Results of single experiments shown are representative of 8 separate experiments in each case.
Agonist efficacy: 3H-cAMP accumulation at the human β1-adrenoceptor
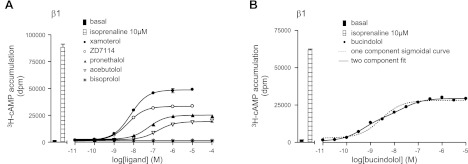
The degree of partial agonism for each of the ligands was then established by comparing the maximum 3H-cAMP accumulation response obtained with each ligand with that for the isoprenaline maximum response stimulated in each experiment (Table 2 and Fig. 2). Xamoterol, ZD 7114, and bucindolol were the most efficacious partial agonists producing maximal responses that were 59, 49, and 49% respectively, of that produced by isoprenaline. Most ligands stimulated a concentration response that was best described by a single component sigmoidal response curve. The responses to bucindolol and carvedilol, however, were best described by a 2-component dose-response curve. In both cases, the first component seen in 3H-cAMP accumulation yielded a log EC50 value (−9.23 or −9.22) that was very similar to the log KD derived from ligand binding studies (Tables 1 and 2) suggestive of partial agonist activation of the high-affinity catecholamine site. The higher concentration of ligands required for the second component (log EC50 values of −7.29 and −7.37 for bucindolol and carvedilol, respectively) suggest secondary (low affinity) site activation.
Table 2.
Log EC50 values and percentage isoprenaline maximal responses obtained from 3H-cAMP accumulation assays in cells expressing the human β1-adrenoceptor
| Ligand | Log EC50 cAMP, β1 | Isomax (%) | n |
|---|---|---|---|
| Xamoterol | −8.06 ± 0.10 | 59.3 ± 2.3 | 5 |
| ZD 7114 | −8.37 ± 0.05 | 49.1 ± 4.1 | 6 |
| Bucindolol | 49.0 ± 2.8 | 6 | |
| Site 1 | −9.23 ± 0.04, 64.7 ± 2.5% | ||
| Site 2 | −7.29 ± 0.20 | ||
| Pronethalol | −7.34 ± 0.04 | 28.6 ± 2.8 | 6 |
| Acebutolol | −7.00 ± 0.05 | 20.0 ± 2.2 | 7 |
| Labetolol | −6.96 ± 0.07 | 17.5 ± 2.1 | 6 |
| Carvedilol | 14.8 ± 1.0 | 11 | |
| Site 1 | −9.22 ± 0.06, 42.6 ± 1.1% | ||
| Site 2 | −7.37 ± 0.05 | ||
| Nebivolol | −8.80 ± 0.04 | 2.28 ± 0.28 | 7 |
| Bisoprolol | No response | 0 | 3 |
| ICI 118551 | No response | 0 | 3 |
| CGP 20712A | No response | 0 | 3 |
Values are presented as means ± se of n separate determinations. Values provided for isoprenaline maximum (Isomax) response for bucindolol and carvedilol are for response at the maximum obtained, i.e., site 1 + site 2. Ligands are ranked in order of partial agonism.
Figure 2.
3H-cAMP accumulation in CHO cells expressing the human β1-adrenoceptor in response to xamoterol, ZD 7114, pronethalol, acebutolol and bisoprolol (A) and bucindolol (B). Bars represent basal 3H-cAMP accumulation and that in response to 10 μM isoprenaline. Data points are means ± se of triplicate determinations. Results are representative of 3 (A) and 6 (B) separate experiments.
Effects of CGP 20712A and ICI 118551 on baseline cardiovascular responses and responses to isoprenaline in conscious rats (series 1 and 2)
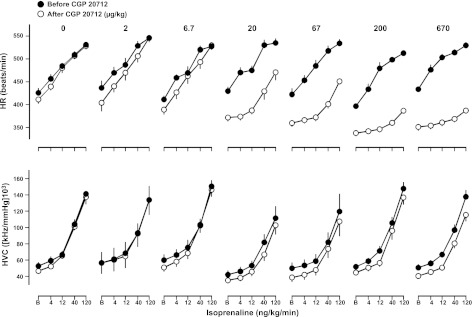
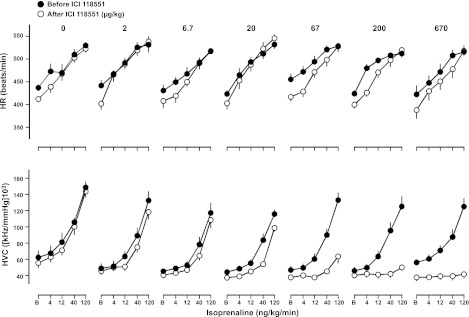
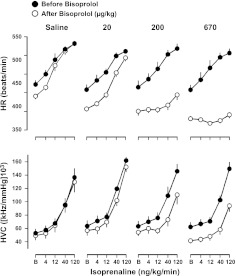
CGP 20712A caused a significant decrease in basal HR at doses of 20 μg/kg bolus and 10 μg/kg/h infusion and above (Fig. 3, top panel, and Table 3). No change in basal HR was seen following administration of ICI 118551 (Fig. 4, top panel, and Table 3). CGP 20712A did not cause any change in basal HVC (Fig. 3, bottom panel, and Table 3); however, a reduction in HVC was seen at the highest dose of ICI 118551 (Fig. 4, bottom panel, and Table 3).
Figure 3.
Absolute values for HR (top panel) and HVC (bottom panel) in conscious, atropine-treated, freely moving rats in response to isoprenaline (at 0, 4, 12, 40, and 120 ng/kg/min) before and after administration of CGP 20712A at doses of 0–670 μg/kg bolus, 0–333 μg/kg/h infusion. Values (mean±se; n=6) were measured at baseline (B; i.e., in the absence of isoprenaline) and at the end of 3-min infusions of isoprenaline.
Table 3.
Changes in baseline cardiovascular variables following administration of CGP 20712A, ICI 118551, and bisoprolol
| Antagonist and variable | Antagonist concentration (μg/kg) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 6.7 | 20 | 67 | 200 | 670 | 2000 | |
| CGP 20712A | ||||||||
| ΔHR (beats/min) | −1 ± 3 | −22 ± 10 | −12 ± 7 | −42 ± 7* | −56 ± 6* | −48 ± 3* | −69 ± 4* | |
| ΔMAP (mmHg) | +3 ± 2 | +0 ± 2 | +2 ± 2 | +3 ± 1 | +2 ± 3 | +3 ± 4 | +5 ± 3 | |
| ΔHVC (%) | −8 ± 6 | +3 ± 4 | −8 ± 3 | −15 ± 5 | −16 ± 5 | −12 ± 5 | −19 ± 3 | |
| ICI 118551 | ||||||||
| ΔHR (beats/min) | −17 ± 6 | −27 ± 5 | −15 ± 5 | −14 ± 11 | −24 ± 11 | −27 ± 6 | −32 ± 6 | |
| ΔMAP (mmHg) | +3 ± 1 | +1 ± 2 | +4 ± 3 | −0 ± 2 | +5 ± 2 | +5 ± 6 | +13 ± 4 | |
| ΔHVC (%) | −4 ± 6 | −8 ± 3 | −12 ± 5 | −8 ± 3 | −19 ± 3 | −17 ± 6 | −30 ± 6* | |
| Bisoprolol | ||||||||
| ΔHR (beats/min) | +14 ± 9 | −45 ± 5* | −64 ± 9* | −70 ± 17* | ||||
| ΔMAP (mmHg) | −2 ± 3 | −2 ± 2 | −2 ± 3 | −5 ± 2 | ||||
| ΔHVC (%) | −3 ± 2 | −11 ± 4 | −14 ± 6 | −23 ± 5* | ||||
Values are calculated as the difference (mean±se) between the values immediately before the last dose of isoprenaline prior to antagonist administration, and the values immediately before the first dose of isoprenaline after antagonist administration; n = 6/group. HR, heart rate, MAP, mean arterial pressure, HVC, hindquarters vascular conductance.
P < 0.05 vs. 0 dose; ANOVA.
Figure 4.
Absolute values for HR (top panel) and HVC (bottom panel) in conscious, atropine-treated, freely moving rats in response to isoprenaline (at 0, 4, 12, 40, and 120 ng/kg/min) before and after administration of ICI 118551 at doses of 0–670 μg/kg bolus and 0–333 μg/kg/h infusion. Values (means±se; n=6) were measured at baseline (B; i.e., in the absence of isoprenaline) and at the end of 3-min infusions of isoprenaline.
Isoprenaline infusions caused dose-dependent increases in HR and HVC that were unaffected by saline administration (Figs. 3–5). Administration of CGP 20712A caused a significant reduction in the HR response to isoprenaline at doses of 20 μg/kg bolus and 10 μg/kg/h infusion and above, but had no significant effect on the HVC responses to isoprenaline at any dose tested (Fig. 3 and Table 3). In contrast, at doses of 67 μg/kg bolus and 33 μg/kg/h infusion and above, ICI 118551 substantially reduced the HVC response to isoprenaline without affecting the HR responses (Fig. 4 and Table 3). This confirms that the HR response observed is predominantly occurring via the β1-adrenoceptor, whereas the HVC response is predominantly via the β2-adrenoceptor.
Figure 5.
Absolute values for HR (top panel) and HVC (bottom panel) in conscious, atropine-treated, freely moving rats in response to isoprenaline (at 0, 4, 12, 40, 120 ng/kg/min) following administration of bisoprolol at doses of 0–2000 μg/kg bolus, 0–1000 μg/kg/h infusion. Values (means±se; n=6) were measured at baseline (B; i.e., in the absence of isoprenaline) and at the end of 3-min infusions of isoprenaline.
For comparison, we also evaluated the cardiovascular responses to bisoprolol, which demonstrated the best selectivity between β1 and β2 adrenoceptors among the clinically used β-blockers in cellular systems (Table 1). Given the lower affinity of bisoprolol for the human β1-adrenoceptor (as compared to CGP 20712A), we extended the concentration of bisoprolol to 2000 μg/kg bolus and 1000 μg/kg/h infusion. Bisoprolol produced a concentration-dependent decrease in both basal HR (Table 3) and isoprenaline-induced tachycardia (Fig. 5). However, at a dose of 2000 μg/kg bolus and 1000 μg/kg/h infusion, there was a significant basal vasoconstriction (Table 3) and an inhibition of isoprenaline-stimulated HVC responses (Fig. 5), indicative of β2-adrenoceptor antagonism.
Selectivity and efficacy of β-blockers in conscious rats (series 3 and 4)
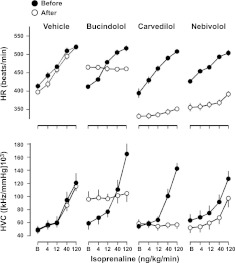
The cardiovascular studies were then extended to ligands that exhibited partial agonist effects in measurements of cAMP in transfected cells. Given the poor solubility of carvedilol, nebivolol, and bucindolol, these ligands were dissolved in 5% propylene glycol and 2% Tween 80 in sterile saline, rather than saline, and this was used as the vehicle control in series 4 (see Fig. 7). Neither saline (series 3) nor vehicle (series 4) had any significant effect on baseline cardiovascular variables.
Figure 7.
Absolute values for HR (top panel) and HVC (bottom panel) in conscious, atropine-treated, freely moving rats in response to isoprenaline (at 0, 4, 12, 40, and 120 ng/kg/min) following administration of vehicle (5% propylene glycol, 2% Tween 80 in saline), bucindolol, carvedilol, and nebivolol (2 mg/kg bolus, 1 mg/kg/h infusion). Values (means±se; n=6) were measured at baseline (B; i.e., in the absence of isoprenaline) and at the end of 3-min infusions of isoprenaline.
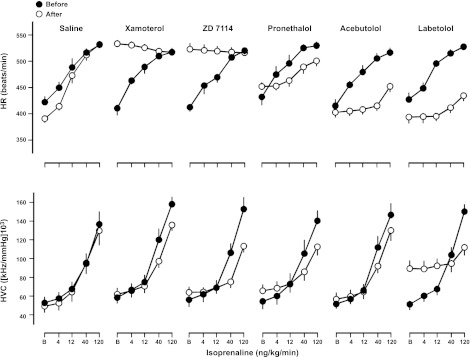
Changes in baseline cardiovascular variables following administration of the chosen β-adrenoceptor antagonists at a dose of 2 mg/kg bolus and 1 mg/kg/h infusion are shown in Table 4. ZD 7114, xamoterol, and bucindolol caused significant increases in basal HR, whereas labetolol, bisoprolol, carvedilol, and nebivolol all caused significant reductions in basal HR (Table 4 and Fig. 6). In the case of both xamoterol and ZD 7114, the agonist effect was equivalent to that of the highest dose of isoprenaline, and as a consequence, no antagonism of isoprenaline-stimulated increases in HR was apparent (Fig. 6). Bucindolol (Fig. 7) exhibited a lower agonist effect than xamoterol and ZD 7114, and it was able to significantly antagonise the response to the highest doses isoprenaline in a manner expected for a partial agonist (Fig. 7). Significant antagonism of the HR responses to all four doses of isoprenaline were obtained with acebutolol pronethalol, labetolol, carvedilol, and nebivolol (Figs. 6 and 7).
Table 4.
Changes in baseline cardiovascular variables following administration of saline, vehicle, and different β-blockers (series 3 and 4)
| Ligand | ΔHR, β1 (beats/min) | ΔBP (mmHg) | ΔHVC, β2 (%) |
|---|---|---|---|
| Saline | −14 ± 9 | −2 ± 3 | +3 ± 2 |
| Xamoterol | +129 ± 11* | −1 ± 3 | +12 ± 8 |
| ZD 7114 | +122 ± 12* | −5 ± 3 | +20 ± 6* |
| Pronethalol | +23 ± 10 | −1 ± 2 | +33 ± 12* |
| Acebutolol | +5 ± 6 | −1 ± 2 | +9 ± 5 |
| Labetolol | −26 ± 9* | −19 ± 2* | +72 ± 12* |
| Bisoprolol | −70 ± 17* | +5 ± 2 | −23 ± 5* |
| Vehicle | −10 ± 8 | −1 ± 2 | +3 ± 11 |
| Bucindolol | +59 ± 11* | −19 ± 2* | +51 ± 10* |
| Carvedilol | −54 ± 10* | −17 ± 3* | +13 ± 5 |
| Nebivolol | −55 ± 13* | −4 ± 2 | +3 ± 7 |
Values are calculated as the difference (mean±se) between the values immediately before the last dose of isoprenaline prior to antagonist administration, and the values immediately before the first dose of isoprenaline after antagonist administration; n = 6 group. β-Adrenoceptor antagonists were all given at a dose of 2 mg/kg bolus followed by 1 mg/kg/h infusion.
P < 0.05; paired t test.
Figure 6.
Absolute values for HR (top panel) and HVC (bottom panel) in conscious, atropine-treated, freely moving rats in response to isoprenaline (at 0, 4, 12, 40, and 120 ng/kg/min) following administration of saline and several different β-blockers (2 mg/kg bolus, 1 mg/kg/h infusion). Values (means±se; n=6) were measured at baseline (B; i.e., in the absence of isoprenaline) and at the end of 3-min infusions of isoprenaline.
Administration of labetolol, pronethalol, and bucindolol caused increases in HVC which, in the case of labetolol and bucindolol, were accompanied by falls in blood pressure. Interestingly, carvedilol caused a reduction in blood pressure, which was not accompanied by an increase in HVC, possibly indicative of vasodilatation in other vascular beds.
All drugs tested inhibited the HVC responses to the 2 highest doses of isoprenaline, with the exception of nebivolol (which only inhibited the highest dose of isoprenaline) and bucindolol (Figs. 6 and 7). In the case of bucindolol, there was a marked partial agonist effect on HVC, and this compound was also only able to attenuate the response to the highest dose of isoprenaline (Figs. 6 and 7).
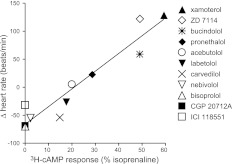
A comparison of the in vitro efficacy of the different β-blockers (i.e., the size of the response as compared to the isoprenaline maximum responses, taken from Table 1) and their ISA activity as judged by basal HR effects in vivo is shown in Fig. 8. The excellent correlation between the two (R2=0.93; P<0.0001) is striking. Clearly, the partial agonism seen on cAMP accumulation in transfected CHO cells will depend on the level of receptor expression, and it is notable that the expression level used in this study is relatively high (1146 fmol/mg protein). However, it is also clear from the in vivo studies that it is only ligands exhibiting >20% of the maximal isoprenaline response in this cell line that have detectable ISA at the level of HR responses in the conscious rat (Fig. 8).
Figure 8.
Correlation plot of the efficacy of β-blockers for the in vitro vs. in vivo data. In vitro efficacy is the maximum 3H-cAMP accumulation response (given as percentage of the maximum response to isoprenaline) in cells expressing the human β1-adrenoceptor. The in vivo efficacy is the change in HR (beats/min) in response to the administration of the β-blocker alone in conscious freely moving rats (ISA). Ligands are at 2 mg/kg bolus, 1 mg/kg/h infusion except for CGP 20712A and ICI 118551, which were at 670 μg/kg bolus, 333 μg/kg/h infusion. For ligands with a 2-component 3H-cAMP accumulation response (bucindolol and carvedilol), efficacy is given as the maximum efficacy obtained for that ligand (i.e., includes response at site 1 plus that at site 2). Line is that of best fit through all the ligands.
DISCUSSION
β-Blockers have been used in the management of cardiovascular disorders with great success for several decades. However the results from more recent heart failure trials have suggested that not all β-blockers are the same, with some being helpful and prolonging life, while others are detrimental. Recent evidence from cell studies has suggested that many β-blockers actually stimulate agonist responses but that the degree of stimulation (efficacy) varies among β-blockers. The primary aim of this study was, therefore, to examine the correlation between agonist actions of β-blockers observed in a model cell system with any ISA seen in a whole animal.
In keeping with previous studies (33, 35, 47, 48), CGP 20712A was a very selective β1-adrenoceptor antagonist in the CHO cell system and had high affinity and high selectivity for the human β1-adrenoceptor (955 fold β1-selective), and ICI 118551 was confirmed to be a high-affinity highly β2-selective ligand (388 fold β2-selective). These compounds were, therefore, used to determine the contribution of β1- and β2-adrenoceptors to the basal and isoprenaline-induced changes in rat HR and HVC. Alone, CGP 20712A lowered HR below basal. It also inhibited the isoprenaline-induced tachycardia but had no effect on either resting HVC or isoprenaline-induced hindquarters vasodilatation. In contrast, ICI 118551 had no effect on HR (either basal or isoprenaline-induced) but did reduce HVC (basal and isoprenaline-stimulated). From these studies, it is possible to conclude that in the conscious rat model used here, the changes in HR were predominantly mediated by β1-adrenoceptors and the changes in HVC via the β2-adrenoceptor.
To provide a comparison for the in vivo experiments, the degree of partial agonism was determined for each ligand in the CHO cell line expressing the human β1-adrenoceptor. From this, it can be seen that the ligands with the highest degree of partial agonism (biggest cAMP stimulation) were xamoterol and bucindolol, with several of the ligands having no basal cAMP stimulatory effect (Table 2), most notably bisoprolol. When examined in vivo, several ligands stimulated substantial increases in basal HR (Table 4 and Fig. 8), and the rank order of the compounds was exactly the same as that obtained from measurements of basal cAMP accumulation (Fig. 8). Interestingly, this is in keeping with previous studies in human myocardium, both normal and failing human heart (37, 38, 45) and rats (17). It has been previously proposed (38, 39) that the cellular basis for ISA observed in animals occurs from cAMP-driven partial agonism. Clinically, the drugs with highest ISA, xamoterol (31) and bucindolol (30), were the ones found to be nonbeneficial/detrimental in clinical heart failure studies, whereas the ligands with little agonist efficacy were life-prolonging: carvedilol (27), nebivolol (29), and bisoprolol (26).
Several β-blockers (especially propranolol) have been shown to have biased signaling at the human β2-adrenoceptor (i.e., they are inverse agonists on one pathway, e.g., cAMP, while stimulating agonist actions on another pathway, e.g., MAP kinase; refs. 43, 49). This has also been demonstrated for propranolol at the β1-adrenoceptor (50, 51). Interestingly, at the β1-adrenoceptor, carvedilol and bucindolol have been shown to activate both cAMP and MAP kinase pathways (51). However, as carvedilol was found to be beneficial in heart failure (27), whereas bucindolol was not (30), it is difficult to know what role MAP kinase signaling has in a clinical context. It will be interesting to examine how the effects of MAP kinase activity correlate with longer-term physiological cardiovascular responses and potential pathological changes, e.g., remodeling and cell proliferation in heart failure.
The β1-adrenoceptor has been reported to exist in at least two active agonist conformations or sites: the catecholamine site, at which isoprenaline and many agonists act and where many β-blockers bind with high affinity, and a secondary site, where certain β-blockers can elicit agonist responses at higher concentration (52–55). In cell-based assays, xamoterol, ZD 7114, labetolol, and acebutolol have previously been shown to have their agonist action via the catecholamine site, while the agonist action of carvedilol was via the secondary site (42, 44). Bucindolol has been shown to be a partial agonist at the human β1-adrenoceptor in human myocardium (37, 45, 56), and this partial agonist action has been reported to occur via the secondary site of the human β1-adrenoceptor (45).
In this study, bucindolol stimulated an increase in 3H-cAMP that was best described by a 2-component concentration-response curve. The most likely explanation for this is that the first major (high-affinity) component is occurring via the catecholamine site and the second (low-affinity) component via the secondary site on the receptor. This is similar to other previously reported ligands, e.g., pindolol, where a 2-component response is seen in cell-based assays (34, 42, 57), and the ISA response in human myocardium has been shown to be via the secondary site (41). In the present study, the 3H-cAMP response to carvedilol was also best described by a 2-component response, although here the secondary site appeared to be the more abundant. Taken together, these data suggest that ISA can occur from partial agonism at either the catecholamine site (xamoterol, ZD 7114) or via the secondary site (bucindolol). Furthermore, the clinical effectiveness of the drugs used in heart failure also appears to be independent of the site activated by the ligand. Thus, bucindolol (not helpful clinically in heart failure; ref. 30) and carvedilol (beneficial in heart failure; ref. 27) can both access both sites of the β1-adrenoceptor. The agonist action of xamoterol (catecholamine site) is detrimental in humans (31), as is that of bucindolol [secondary site (45)]. Therefore, it appears that it is the overall amount of agonism/ISA that is detrimental in humans, not the site on the β1-adrenoceptor at which it occurs.
The selectivity of β-blockers was also examined in both the cellular model and in the rats. Unlike CGP 20712A, bisoprolol, nebivolol, and xamoterol were relatively poor β1-adrenoceptor-selective ligands in the CHO cell studies, in keeping with previous selectivity studies (33, 35, 36, 47, 48). CGP 20712A and ICI 118551 clearly demonstrated marked selectivity in the rat studies. Interestingly, bisoprolol (an inverse agonist at the human β2-adrenoceptor; ref. 43), one of the most β1-selective drugs that are clinically available, was also shown to have relatively poor selectivity in the rat cardiovascular studies, in as much as bisoprolol caused a reduction in isoprenaline-stimulated HR (β1) but also a reduction in HVC (β2) at the highest dose tested.
When this was extended to the other β-blockers, it can be seen that the other β-blockers had effects on the both the isoprenaline-stimulated HR response (β1) and the hindquarters β2-response and that this was most marked for the least selective (or even slightly β2-selective) ligands (e.g., bucindolol, carvedilol). The lack of selectivity among clinically used β-blockers, seen in the CHO cell model system, is, therefore, also seen in the conscious rat model. Given that it is possible for a β1-selective compound to be devoid of β2 effects (i.e., CGP 20712A), there is clearly a need for the development of β-blockers with improved selectivity for clinical use.
CONCLUSIONS
The degree of partial agonism seen in a cell-based cAMP assay correlated very well with the observed degree of ISA in vivo, as judged by changes in basal HR in conscious rats. The cell-based assay is, therefore, highly predictive for the level of clinically relevant ISA seen in animals. ISA can occur from stimulation of either the catecholamine site or the secondary site of the receptor or both; however, the site of partial agonist action does not matter for clinical effectiveness in heart failure studies—the ligands with the least overall stimulatory activity are those most useful in heart failure. Many clinically used β-blockers have been previously shown to have poor selectivity between the β1- and β2-adrenoceptors in cell-based assays. The in vivo assay used here (i.e., parasympathetically blocked conscious rats instrumented for measuring isoprenaline-induced tachycardia and hindquarters vasodilatation) can also clearly demonstrate β1- and β2-selectivity and identify compounds with good (e.g., CGP 20712A and ICI 118551) and poor selectivity (bisoprolol). Thus, in vitro 3H-CGP 12177 whole-cell binding and 3H-cAMP accumulations assays are good predictors of the ISA (partial agonism) and ligand selectivity as seen in whole animals.
Acknowledgments
J.G.B. thanks the Wellcome Trust for her Clinician Scientist Fellowship (073377/Z/03/Z). The authors thank June McCulloch and Marleen Groenen technical assistance in running the cAMP chromatography columns. The authors declare no conflicts of interest.
REFERENCES
- 1. Black J. W., Duncan W. A. M., Shanks R. G. (1965) Comparison of some properties of pronethalol and propranolol. Br. J. Pharmacol. 25, 577–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hollenberg N. K. (2005)The role of β-blockers as a cornerstone of cardiovascular therapy. Am. J. Hypertens. 18, 165S–168S [DOI] [PubMed] [Google Scholar]
- 3. Baker J. G., Hill S. J., Summers R. J. (2011)Evolution of β-blockers—from anti-anginal drugs to ligand-directed signalling Trends Pharmacol Sci. 32, 227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Auerbach A. D., Goldman L. (2002) Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 287, 1435–1444 [DOI] [PubMed] [Google Scholar]
- 5. Ellison K. E., Gandhi G. (2005) Optimising the use of beta-adrenoceptor antagonists in coronary artery disease. Drugs 65, 787–797 [DOI] [PubMed] [Google Scholar]
- 6. Bangalore S., Messerli F. H., Kostis J. B., Pepine C. J. (2007) Cardiovascular protection using beta-blockers: a critical review of the evidence. J. Am. Coll. Cardiol. 50, 563–572 [DOI] [PubMed] [Google Scholar]
- 7. Echahidi N., Pibarot P., O'Hara G., Mathieu P. (2008) Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J. Am. Coll. Cardiol. 51, 793–801 [DOI] [PubMed] [Google Scholar]
- 8. Aronow W. S. (2010) Current role of beta-blockers in the treatment of hypertension. Expert Opin. Pharmacother. 11, 2599–2607 [DOI] [PubMed] [Google Scholar]
- 9. Cruickshank J. M. Beta-blockers and hypertension. (2010) J. Am. Coll. Cardiol. 53, 2105–2106 [DOI] [PubMed] [Google Scholar]
- 10. Cruickshank J. M. (2007) Are we misunderstanding beta-blockers. Int. J. Cardiol. 20, 10–27 [DOI] [PubMed] [Google Scholar]
- 11. Peet M., Yates R. A. (1981) Beta-blockers in the treatment of neurological and psychiatric disorders. J. Clin. Hosp. Pharm. 6, 155–171 [DOI] [PubMed] [Google Scholar]
- 12. Feely J., Peden N. (1984) Use of beta-adrenoceptor blocking drugs in hyperthyroidism. Drugs 27, 425–446 [DOI] [PubMed] [Google Scholar]
- 13. Uitti R. J. (1998) Medical treatment of essential tremor and Parkinson's disease. Geriatrics 53, 46–48 and 53–57 [PubMed] [Google Scholar]
- 14. Gray R. N., Goslin R. E., McCrory D. C., Eberlein K., Tulsky J., Hasselblad V. (1999) Drug Treatments for the Prevention of Migraine Headache. AHRQ Technical Reviews and Summaries, U.S. Agency for Health Care Policy and Research, Rockville, MD, USA: [PubMed] [Google Scholar]
- 15. Limmroth V., Michel M. C. (2001) The prevention of migraine: a critical review with special emphasis of β-adrenergic blockers. Br. J. Clin. Pharmacol. 52, 237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stamper R. L., Wiggington S. A., Higgingbotham E. J. (2002) Primary drug treatment for glaucoma: beta-blocker versus other medications. Surv. Ophthalmol. 47, 63–67 [DOI] [PubMed] [Google Scholar]
- 17. Willette R. N., Aiyar N., Yue T. L., Mitchell M. P., Disa J., Storer B. L., Naselsky D. P., Stadel J. M., Ohlstein E. H., Ruffolo R.R., Jr. (1999) In vitro and in vivo characterization of intrinsic sympathomimetic activity in normal and heart failure rats. J. Pharmacol. Exp. Ther. 289, 48–53 [PubMed] [Google Scholar]
- 18. Wollert K. C., Drexler H. (2002) Carvedilol prospective randomized cumulative survival (COPERNICUS) trial carvedilol as the sun and center of the β-blocker world? Circulation 106, 2164–2166 [DOI] [PubMed] [Google Scholar]
- 19. Metra M., Cas L. D., di Lenarda A., Poole-Wilson P. (2004) Beta-blockers in heart failure: are pharmacological differences clinically important? Heart Fail. Rev. 9, 123–130 [DOI] [PubMed] [Google Scholar]
- 20. Karter Y. (2007) Nebivolol: more than a highly selective beta blocker. Recent Pat. Cardiovasc. Drug Discov. 2, 152–155 [DOI] [PubMed] [Google Scholar]
- 21. Kountz D. S. (2009) Are tolerability concerns a class effect of beta-blockers in treating patients with hypertension? Postgrad. Med. 121, 14–24 [DOI] [PubMed] [Google Scholar]
- 22. Basile J. N. (2010) One size does not fit all: the role of vasodilating beta-blockers in controlling hypertension as a means of reducing cardiovascular and stroke risk. Am. J. Med. 123, S9–S15 [DOI] [PubMed] [Google Scholar]
- 23. Lipsic E., Van Veldhuisen D. J. (2010) Nebivolol in chronic heart failure: current evidence and future perspectives. Expert Opin. Pharmacother. 11, 983–992 [DOI] [PubMed] [Google Scholar]
- 24. Waagstein F., Hjalmarson A., Varnauskas E., Wallentin I. (1975) Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br. Heart J. 37, 1022–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swedberg K., Hjalmarson A., Waagstein F., Wallentin I. (1979) Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet 1, 1374–1376 [DOI] [PubMed] [Google Scholar]
- 26. CIBIS-II Investigators and Committees: The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. (1999) Lancet 353, 9–13 [PubMed] [Google Scholar]
- 27. Packer M., Fowler M. B., Roecker E. B., Coats A. J. S., Katus H. A., Krum H., Mohacsi P., Rouleau J. L., Tendera M., Staiger C., Holcslaw T. L., Amann-Zalan I., DeMets D. L., for the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group (2002) Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 106, 2194–2199 [DOI] [PubMed] [Google Scholar]
- 28. MERIT-HF Study Group: Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). (1999) Lancet 353, 2001–2007 [PubMed] [Google Scholar]
- 29. Flather M. D., Shibata M. C., Coats A. J., Van Veldhuisen D. J., Parkhomenko A., Borbola J., Cohen-Solal A., Dumitrascu D., Ferrari R., Lechat P., Soler-Soler J., Tavazzi L., Spinarova L., Toman J., Böhm M., Anker S. D., Thompson S. G., Poole-Wilson P. A. (2005) SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur. Heart J. 26, 215–225 [DOI] [PubMed] [Google Scholar]
- 30. Beta-blocker Evaluation of Survival Trial Investigators (BEST) (2001) A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N. Engl. J. Med. 344, 1659–1667 [DOI] [PubMed] [Google Scholar]
- 31. Nicholas G., Oakley C., Pouleur H., Rousseau M. F., Ryden L. E., Wellens H. (1990) Xamoterol in severe heart failure. The Xamoterol in Severe Heart Failure Study Group. Lancet 336, 1–6 1694945 [Google Scholar]
- 32. Poole-Wilson P. A., Swedberg K., Cleland J. G., Di, Lenarda. A., Hanrath P., Komajda M., Lubsen J., Lutiger B., Metra M., Remme W. J., Torp-Pedersen C., Scherhag A., Skene A. (2003) Carvedilol or metoprolol European trial investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the carvedilol or metoprolol European trial (COMET): randomised controlled trial. Lancet 362, 7–13 [DOI] [PubMed] [Google Scholar]
- 33. Baker J. G. (2005) The selectivity of β-adrenoceptor antagonists at the β1, β2 and β3 adrenoceptors. Br. J. Pharmacol. 144, 317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baker J. G. (2010) The selectivity of β-adrenoceptor agonists at the human β1, β2 and β3 adrenoceptors. Br. J. Pharmacol. 160, 1048–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoffmann C., Leitz M. R., Oberdorf-Maass S., Lohse M. J., Klotz K. N. (2004) Comparative pharmacology of human β-adrenergic receptor subtypes—characterization of stably transfected receptor in CHO cells. Naunyn-Schmiedebergs Arch. Pharmacol. 369, 151–159 [DOI] [PubMed] [Google Scholar]
- 36. Smith C., Teitler M. (1999) Beta-blocker selectivity at cloned human beta1- and beta2-adrenoceptors. Cardiovasc. Drugs Ther. 13, 123–126 [DOI] [PubMed] [Google Scholar]
- 37. Maack C., Cremers B., Flesch M., Höper A., Südkamp M., Böhm M. (2000) Different intrinsic activities of bucindolol, carvedilol and metoprolol in human failing myocardium. Br. J. Pharmacol. 130, 1131–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andreka P., Aiyar N., Olson L. C., Wei J. Q., Turner M. S., Webster K. A., Ohlstein E. H., Bishopric N. H. (2002) Bucindolol displays intrinsic sympathomimetic activity in human myocardium. Circulation 105, 2429–2434 [DOI] [PubMed] [Google Scholar]
- 39. Jasper J. R., Michel M. C., Insel P. A. (1988) Molecular mechanism of beta-adrenergic receptor blockers with intrinsic sympathomimetic activity. FASEB J. 2, 2891–2894 [DOI] [PubMed] [Google Scholar]
- 40. Jasper J. R., Michel M. C., Insel P. A. (1990) Amplification of cyclic AMP generation reveals agonistic effects of certain beta-adrenergic antagonists. Mol. Pharmacol. 37, 44–49 [PubMed] [Google Scholar]
- 41. Joseph S. S., Lynham J. A., Molenaar P., Grace A. A., Colledge W. H., Kaumann A. J. (2003) Intrinsic sympathomimetic activity of (-)-pindolol mediated through a (-)-propranolol-resistant site of the beta1-adrenoceptor in human atrium and recombinant receptors. Naunyn Schmiedebergs Arch. Pharmacol. 368, 496–503 [DOI] [PubMed] [Google Scholar]
- 42. Baker J. G., Hall I. P., Hill S. J. (2003) Agonist actions of “β-blockers” provide evidence for two agonist activation sites or conformations of the human β1-adrenoceptor. Mol. Pharmacol. 63, 1312–1321 [DOI] [PubMed] [Google Scholar]
- 43. Baker J. G., Hall I. P., Hill S. J. (2003) Agonist and inverse agonist actions of “β-blockers” at the human β2-adrenoceptor provide evidence for agonist-directed signalling. Mol. Pharmacol. 64, 1357–1369 [DOI] [PubMed] [Google Scholar]
- 44. Baker J. G. (2005) Sites of action of β-ligands at the human β1-adrenoceptor. J. Pharmacol. Exp. Ther. 313, 1163–1171 [DOI] [PubMed] [Google Scholar]
- 45. Bundkirchen A., Brixius K., Bölck B., Schwinger R. H. (2002) Bucindolol exerts agonistic activity on the propranolol-insensitive state of beta1-adrenoceptors in human myocardium. J. Pharmacol. Exp. Ther. 300, 794–801 [DOI] [PubMed] [Google Scholar]
- 46. Donaldson J., Brown A. M., Hill S. J. (1988) Influence of rolipram on the cyclic-3′,5′-adenosine monophosphate response to histamine and adenosine in slices of guinea-pig cerebral cortex. Biochem. Pharmacol. 37, 715–723 [DOI] [PubMed] [Google Scholar]
- 47. Pauwels P. J., Gommeren W., Van Lommen G., Janssen P. A., Leysen J. E. (1988) The receptor binding profile of the new antihypertensive agent nebivolol and its stereoisomers compared with various beta-adrenergic blockers. Mol. Pharmacol. 34, 843–851 [PubMed] [Google Scholar]
- 48. Pauwels P. J., Van Gompel P., Leysen J. E. (1991) Human beta 1- and beta 2-adrenergic receptor binding and mediated accumulation of cAMP in transfected Chinese hamster ovary cells. Profile of nebivolol and known beta-adrenergic blockers. Biochem. Pharmacol. 42, 1683–1689 [DOI] [PubMed] [Google Scholar]
- 49. Azzi M., Charest P. G., Angers S., Rousseau G., Kohout T., Bouvier M., Pineyro G. (2003) β-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. U. S. A. 100, 11406–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Galandrin S., Oligny-Longpre G., Bonin H., Ogawa K., Gales C., Bouvier M. (2008) Conformational rearrangements and signaling cascades involved in ligand-biased mitogen-activated protein kinase signaling through the beta1-adrenergic receptor. Mol. Pharmacol. 74, 162–172 [DOI] [PubMed] [Google Scholar]
- 51. Galandrin S., Bouvier M. (2006) Distinct signaling profiles of â1 and â2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol.Pharmacol. 70, 1575–1584 [DOI] [PubMed] [Google Scholar]
- 52. Arch J. R. (2004) Do low-affinity states of beta-adrenoceptors have roles in physiology and medicine? Br. J. Pharmacol. 143, 517–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Granneman J. G. (2001) The putative beta4-adrenergic receptor is a novel state of the beta1-adrenergic receptor. Am. J. Physiol. Endocrinol. Metab. 280, E199–E202 [DOI] [PubMed] [Google Scholar]
- 54. Kaumann A. J., Molenaar P. (2008) The low-affinity site of the beta1-adrenoceptor and its relevance to cardiovascular pharmacology. Pharmacol. Ther. 118, 303–336 [DOI] [PubMed] [Google Scholar]
- 55. Molenaar P. (2003) The ‘state’ of beta-adrenoceptors. Br. J. Pharmacol. 140, 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maack C., Böhm M., Vlaskin L., Dabew E., Lorenz K., Schäfers H. J., Lohse M. J., Engelhardt S. (2003) Partial agonist activity of bucindolol is dependent on the activation state of the human beta1-adrenergic receptor. Circulation 108, 348–353 [DOI] [PubMed] [Google Scholar]
- 57. Walter M., Lemoine H., Kaumann A. J. (1984) Stimulant and blocking effects of optical isomers of pindolol on the sinoatrial node and trachea of guinea pig. Role of beta-adrenoceptor subtypes in the dissociation between blockade and stimulation. Naunyn Schmiedebergs Arch. Pharmacol. 327, 159–175 [DOI] [PubMed] [Google Scholar]