Abstract
Subclinical inflammation is frequently associated with obesity. Here, we aim to better define the acute inflammatory response during fasting. To do so, we analyzed representatives of immune-related proteins in circulation and in tissues as potential markers for adipose tissue inflammation and modulation of the immune system. Lipopolysaccharide treatment or high-fat diet led to an increase in circulating serum amyloid (SAA) and α1-acid glycoprotein (AGP), whereas adipsin levels were reduced. Mouse models that are protected against diet-induced challenges, such as adiponectin-overexpressing animals or mice treated with PPARγ agonists, displayed lower SAA levels and higher adip-sin levels. An oral lipid gavage, as well as prolonged fasting, increased circulating SAA concurrent with the elevation of free FA levels. Moreover, prolonged fasting was associated with an increased number of Mac2-positive crown-like structures, an increased capillary permeability, and an increase in several M2-type macrophage markers in adipose tissue. This fasting-induced increase in SAA and M2-type macrophage markers was impaired in metabolically challenged animals. These data suggest that metabolic inflexibility is associated with a lack of “immunological fitness.”
Keywords: adipose tissue, diabetes, inflammation, macrophages, obesity, vascular biology, acute phase reactants, metabolic flexibility
Obesity is associated with infiltration of proinflammatory macrophages in adipose tissue and with a low-grade state of inflammation as judged by systemic and tissue measurements of proinflammatory cytokines and acute-phase reactants (1). Numerous studies indicate that increased levels of free FAs (FFAs) are critical for the pathogenesis of the metabolic syndrome (2). For instance, palmitate can get channeled into increased biosynthesis of ceramides, which can cause insulin resistance, inflammation, and apoptosis (3–5). Furthermore, intermediates of FFAs, through their conversion to triglycerides (lysophosphatidic acid, phosphatidic acid and diacylglycerol), can activate proinflammatory kinases (6). FFAs may also enhance inflammation through binding and signaling through Toll-like receptor-4 (TLR4) (7). Thus, failure of adipose tissue to expand and/or buffer excess FFAs may be the starting point for obesity- and lipodystrophy-related metabolic disturbances. In obesity, as well as in critically ill patients, FFAs are chronically elevated and can induce a vicious proinflammatory cycle over the course of which adipocytes and macrophages further aggravate local and systemic inflammation (8). In support of this hypothesis, intensive insulin treatment that inhibits lipolysis and hyperglycemia has been successful in the reduction of inflammation and, more importantly, mortality of critically ill patients (9). In healthy individuals, lipolysis is efficiently suppressed by insulin in the fed state. However, recent studies by Kosteli et al. (10) suggest that weight loss induces a transient state of inflammation in adipose tissue. This is also consistent with a central role for FFAs in metabolic inflammation. In fact, the highest levels of FFAs in the system are found close to the site of release and reesterification, i.e., near adipocytes. We expected that the systemic increase in FA oxidation during fasting would limit a generalized proinflammatory response, owing to the high turnover of FFAs under those conditions. In contrast to our expectations, however, we found that fasting is associated with a systemic increase in the acute-phase reactant serum amyloid A (SAA) (9). Circulating SAA is thought to originate mainly from the liver, but adipose tissue can also express very high levels under some conditions (11). Prolonged fasting increases hepatic steatosis, suggesting that the uptake of lipids exceeds the FA oxidation in this organ. We therefore hypothesized that adipose tissue and the liver are at risk for a fasting-induced inflammatory response and aimed to study the fasting-induced immune response in more detail. We identified vascular endothelial growth factor (VEGF) as a potential local mediator of fasting-induced inflammatory and metabolic effects. In contrast to generic metabolically unfavorable conditions, fasting induces a response in adipose tissue and in the liver that involves macrophages that could operationally be categorized as “M2”, i.e., macro-phages that display a somewhat reduced inflammatory potential and are prone to promoting tissue repair and remodeling (12). This response is seen in metabolically fit animals only, and is lost in metabolically challenged animals. This expands the previously established concept of “metabolic fitness” to an additional area that involves “immunological fitness” that is lost in the context of metabolic dysfunction.
MATERIALS AND METHODS
Animals
Pure wild-type FVB and C57B6 mice were used for all studies. Adiponectin transgenic (adipo tg) mice (13) were used for plasma measurements as a model of resistance to high-fat diet (HFD)-induced metabolic dysfunction, whereas ob/ob mice were used to collect blood from a mouse model with extreme dyslipidemia and hyperglycemia. The fasting response was also compared between adipo tg, adiponectin ko mice (adipo-null) (14) and wild-type mice. Mice were maintained on a 12 h light/dark cycle and housed in groups of two to four with unlimited access to water, chow (No. 5058, Lab-Diet), or HFD (No. D12492, Research Diets, Inc.). A subset of adipo-null and wild-type mice on C57B6 background were fed a high-fat high-sucrose diet (40% lipid, 40% carbohydrates, and 20% protein) with or without Rosiglitazone at a dose of 10 mg/kg/day. The Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center, Dallas approved all animal experiments.
Development of ELISAs
Specific sandwich ELISAs were developed by Millipore Corporation to measure plasma adipsin (EZMADPSN-22K), SAA (EZMSAA3-12K), and α1-acid glycoprotein (AGP) (EZMAGP-23K). The sensitivity of the mouse adipsin ELISA was 0.039 μg/ml with a dynamic standard range of 0.039–2.5 μg/ml and it utilizes 40 μl of a 1/8-diluted serum/plasma sample. There was no significant cross-reactivity with known acutephase proteins, cytokines, and adipokines, except for glutathione S-transferase tagged mouse lipocalin-2 (1.5%) and mouse AGP (4.0%). Intra-assay (n = 8) and inter-assay (n = 8) Coefficient of Variation(CV) at 0.09 and 0.75 μg/ml concentrations were <7 and <13%, respectively. The mean recovery of exogenously spiked adipsin (0.156, 0.313, and 0.325 μg/ml) in serum and plasma samples (n = 5) were 96.9, 96.3, and 101.3% of the expected values, respectively. Linearity of dilutions in serum and plasma samples (n = 5) ranged between 83.9% and 122.7%, respectively. For SAA ELISAs, the sensitivity was 0.078 μg/ml, dynamic range was 0.078–5.0 μg/ml, with 10 μl sample volume required. There was no significant cross-reactivity with known acute-phase proteins, cytokines and adipokines. However, SAA3 is very similar to SAA1 and -2, and we cannot rule out that this assay detects all of the isoforms of SAA. Intra-assay and inter-assay CV at 0.2 and 1.8 μg/ml concentrations were <14 and <13%, respectively. Recovery of exogenously spiked SAA3 (0.313, 0.625, and 1.25 μg/ml) were 97, 96, and 101%, and linearity of dilutions ranged between 80.0% and 107%. For AGP ELISA, the sensitivity was 6.9 pg/ml, dynamic range was 6.9–5,000 pg/ml, and 20 μl of a 1/3,000 diluted serum or plasma sample is required. Intra-assay and inter-assay CV at 250 and 1,750 μg/ml concentrations were <8% and <10%, respectively. Recovery of spiked AGP (123 pg/ml and 370 pg/ml) were 95% and 83%, and linearity of dilutions ranged between 91% and 121%.
Blood chemistry
Specific ELISAs were developed for SAA, AGP, and adipsin levels (Millipore). FFA levels were measured with NEFA-HR (2) (Wako Pure Chemical Industries, Japan).
Quantitative real-time RT-PCR
Tissues were collected in RNAlater (Ambion) and stored at −80°C until Trizol reagent (Invitrogen) extraction followed by RNA purification using the RNeasy Mini Kit and RNase-Free DNase (Qiagen). RNA was reverse transcribed to cDNA by the iScript cDNA synthesis kit (Bio-Rad); IQ SYBR Green Supermix (Bio-Rad) was used for the quantitative PCR reactions, and β-actin was used as endogenous control. Primer sequences used are presented in supplementary Table I.
Vascular permeability
Evan's Blue Dye was tail vein-injected at a dose of 25 mg/kg to fed and 24 h fasted wild-type FVB mice. Two hours after the injection, the mice were anesthetized and completely perfused with PBS. The adipose tissue was thoroughly minced, and 0.1 ml tissue was transferred to 0.175 ml trichloroacetic acid and incubated at 37°C until the tissue pieces were completely dissolved. The homogenates were centrifuged at 1,000 g for 30 min, and the permeability, as judged by the blue color in the supernatant, was quantified spectrophotometrically at 610 nm.
Immunohistochemistry
The tissues were collected for immunohistochemical analysis, and deparaffinized adipose tissue and liver sections were stained for Mac2 (rat anti-mouse Mac2; Cedarlane), CD163 (rabbit anti-mouse CD163; Santa Cruz Biotechnology), and CD301 (rat anti-mouse CD301; Serotec) antigen. Antigen retrieval by boiling the slides for 15 min in antigen-unmasking solution (Vector Laboratories) was performed for adipose samples that were stained for of CD163 and CD206. Liver samples were incubated with Avidin/Biotin blocking system (Thermo Scientific) to block endogenous biotin/avidin. Endogenous peroxidase activity was blocked in all samples by Dual Endogenous Enzyme Block (Dako). Detection was performed by the Liquid DAB+ Substrate Chromogen System, using secondary anti-rat or anti-rabbit biotinylated anti-bodies and HRP-streptavidin (all reagents from Dako).
Flow cytometry analysis
Pooled inguinal and gonadal adipose tissues from fed and fasted FVB mice were minced and digested at 37°C for 40 min in DMEM (Mediatech) supplemented with 1% BSA and 1.5 mg/ml collagenase (from Clostridium histolyticum; Sigma). Undigested tissue was removed by filtration through a steel filter mesh, and the stromal-vascular fraction was pelleted by centrifugation at 1,000 g for 8 min. The pellet was dissolved and incubated in red blood cell lysis buffer (BD Pharm Lyse; BD Biosciences) for 2 min at room temperature. Additional buffer (1% BSA-supplemented DMEM) was added prior to centrifugation and resuspension in fluorescence-activated cell sorting (FACS) buffer (2% FBS in PBS). The cells were stained with 1/100-diluted FITC anti-mouse F4/80 (Serotec), 1/100 PerCP/Cy5.5 anti-mouse CD11b (clone M1/70; BioLegend), 1/100 phycoerythrin anti-mouse CD11c (clone N418; BioLegend), and 1/100 allophycocyanin anti-mouse (clone C068C2; BioLegend) for 30 min on ice. Cells were washed twice with FACS buffer, fixed for 10 min with 2% formalin in PBS, resuspended in FACS buffer, and run on an FACScalibur instrument. Analysis was performed using FlowJo software.
Statistical methods
Data are in generally expressed as mean ± SEM. Student's t-test was used for comparisons between groups, and P <0.05 was considered significant. Asterisks *, **, *** indicate p-values of <0.05, <0.01, and <0.001, respectively.
RESULTS
Circulating SAA is a marker for low-grade inflammation in mice
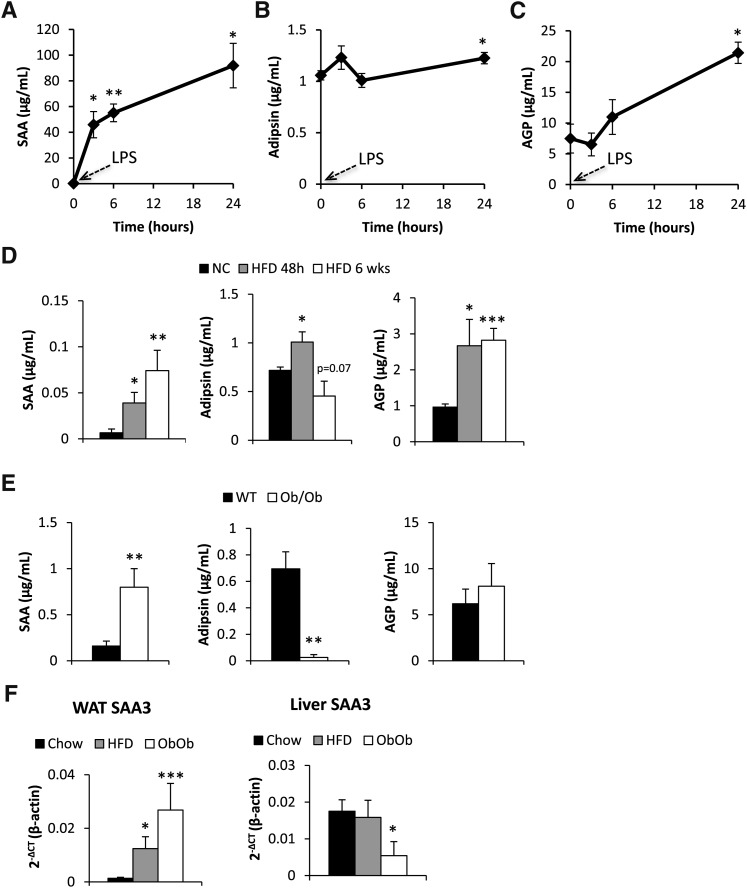
We initially sought to establish a circulating marker that appropriately reflects inflammation due to metabolic dysregulation in mice. To start with a general proinflammatory stimulus, mice were exposed to 100 ng/g body weight lipopolysaccharide (LPS). SAA, AGP, and adipsin (15, 16) levels were measured at various intervals post injection with newly developed ELISA assays. As expected, the classical acute-phase reactant SAA was dramatically upregulated (Fig. 1A). Adipsin levels increased slightly after 24 h in response to LPS (Fig. 1B). Similarly, AGP was upregulated, albeit to a much lesser extent than SAA and with slower kinetics (Fig. 1C).
Fig. 1.
Circulating serum SAA, adipsin, and AGP levels in response to 0.1 mg/kg LPS i.p. (A–C) or high-fat diet for 48 h or for 6 weeks (D), as well as in genetically obese ob/ob mice (E). SAA3 mRNA levels in gonadal adipose tissue (WAT) and in liver from chow-fed, 8 weeks high-fat diet-fed and ob/ob mice (F). [* Significant difference as compared with baseline(A–C), normal chow (D, F) or WT (E)].
To test whether we could induce an elevation in the context of metabolic dysfunction, we tested whether the levels of SAA, AGP, and adipsin are affected by excess nutrient exposure and thereby contribute to the subclinical proinflammatory conditions frequently observed in the obese state. We examined the relative degree of inflammation in an acute setting after 48 h post initial exposure to HFD as well as in the chronic state 6 weeks post initiation of the HFD. Within 48 h, SAA levels were significantly increased and go up even further in the chronic, 6 week HFD regimen. AGP levels were also increased in the acute setting, and then remain at this elevated level after 6 weeks, without a further increase compared with the 48-h time point. Adipsin levels increased slightly within 48 h of HFD, but then dropped below the baseline levels seen for chow-fed animals after 6 weeks HFD challenge (Fig. 1D).
The lack of leptin in the ob/ob mouse is one of the most potent metabolic challenges that a mouse can be exposed to. SAA levels were almost 5-fold upregulated under those conditions. In contrast, AGP levels were not elevated in ob/ob mice. However, adipsin was profoundly downregulated, consistent with previous observations (15) (Fig. 1E). At the transcriptional level, HFD-induced obesity, as well as genetically induced obesity in the ob/ob mice, was associated with increased SAA3 mRNA expression in adipose tissue (Fig. 1F). A similar regulation was also seen for SAA1 and SAA2 mRNA (see supplementary ). In contrast, obesity did not induce an increased expression of SAA3 mRNA in the liver. In fact ob/ob mice had even lower hepatic SAA3 mRNA levels compared with the chow-fed controls (Fig. 1G). Liver SAA1 mRNA levels were unaltered in the three groups, whereas there was about a 2-fold increase in hepatic SAA2 mRNA in the obese mice (see supplementary Fig. I).
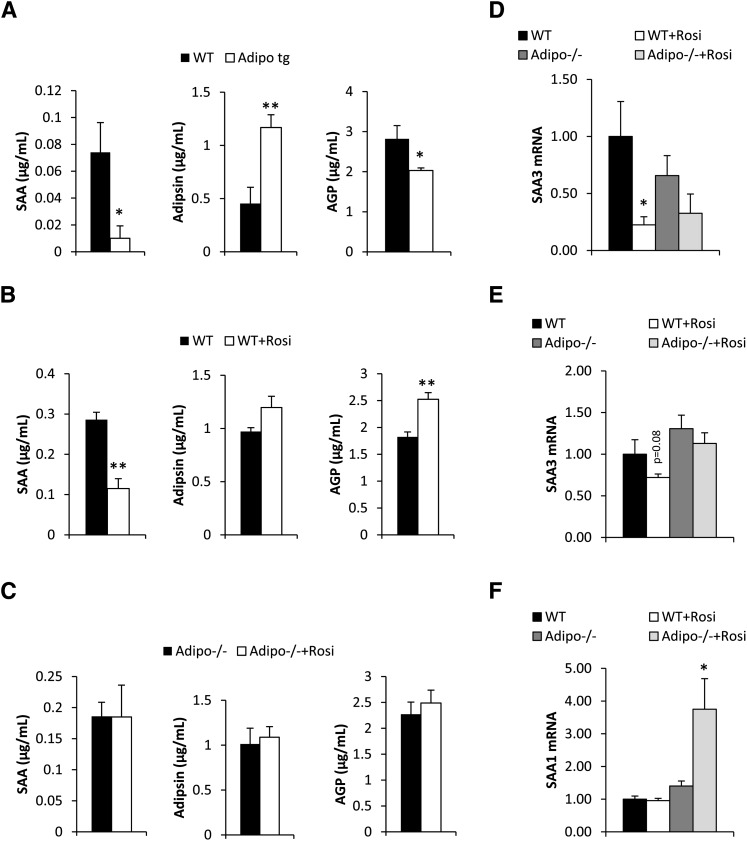
We have previously demonstrated that mice overexpressing adiponectin are protected against the negative metabolic impact of a HFD (13, 17, 18). This was achieved, at least in part, through the anti-inflammatory properties that adiponectin can exert on multiple cell types. At baseline, there was, however, a slight trend toward increased SAA in the adiponectin-overexpressing mice (0.007 ± 0.004 vs. 0.04 ± 0.03 μg/ml in wild-type vs. transgenic mice, P = 0.14) and a trend toward increased AGP levels (0.96 ± 0.09 vs. 1.48 ± 0.50 μg/ml in wild-type vs. transgenic mice, P = 0.24). In contrast (and consistent with the anti-inflammatory effects of adiponectin), the adiponectin-transgenic mice were protected against the HFD-induced increase in SAA and AGP. Adipsin levels were elevated upon adiponectin overexpression, even at baseline (0.72 ± 0.03 vs. 1.24 ± 0.05 μg/ml in wild-type vs. transgenic mice, P < 0.001), and remained significantly higher after 6 weeks on a HFD in adiponectin-overexpressing mice (Fig. 2A).
Fig. 2.
Circulating serum SAA, adipsin, and AGP levels in high-fat diet-fed wild-type and adipo tg mice (A), in wild-type (B), and adipo−/− mice (C) on high-fat high-sucrose diet with or without rosiglitazone treatment (11 days of treatment) and inguinal adipose SAA3 mRNA (D), liver SAA1 (E), and liver SAA3 expression (F) in the same mice as used in panels B and C (* significant difference as compared with WT).
Chronic administration of a PPARγ agonist (rosiglitazone) to HFD-fed mice significantly increases adiponectin levels (19) and improves metabolic parameters, as demonstrated by many previous reports (20). Along with improvements in glucose and lipid parameters (data not shown), PPARγ agonists also have potent anti-inflammatory properties and significantly suppressed SAA levels. In addition, there was also a trend toward increased adipsin levels. Somewhat unexpectedly, AGP levels were also increased in PPARγ agonist-treated mice relative to controls. Given that PPARγ agonists substantially increase adiponectin levels, we wanted to test whether adiponectin is involved in the modulation of SAA and adipsin in this context. We found that untreated HFD-fed adiponectin-deficient mice displayed lower SAA levels (0.29 ± 0.02 vs. 0.19 ± 0.02 μg/ml in wild-type vs. adiponectin knockout mice, P < 0.05), a trend toward higher AGP levels (1.89 ± 0.09 vs. 2.27 ± 0.12 μg/ml in wild-type vs. adiponectin knockout mice, P = 0.15), while adipsin levels were comparable (0.97 ± 0.04 vs. 1.0 ± 0.17 μg/ml in wild-type vs. adiponectin knockout mice, P = 0.84). Importantly, the PPARγ agonist-mediated effects on these proteins were completely lost in mice lacking adiponectin, i.e., SAA, adipsin, and AGP levels in adiponectin-deficient mice were not thiazolidinediones responsive (Fig. 2C). In line with the systemic protein measurements of SAA, SAA3 mRNA was reduced in adipose tissue depots upon TZD exposure (Fig. 2D). A similar trend was observed for SAA3 in the liver (Fig. 2E). However, in the PPARγ agonist-treated adiponectin-deficient mice, this effect was not observed. With respect to hepatic SAA1 and -2, neither liver SAA1 (Fig. 2F) nor SAA2 mRNA (data not shown) was affected by PPARγ agonist in wild-type mice, whereas PPARγ agonist-treated adiponectin-deficient mice displayed elevated levels of liver SAA1/2 mRNA. This indicates that adiponectin is important for mediating the suppressive effects of PPARγ agonist treatment on acute-phase reactant markers in circulation. This is consistent with our previous observations that demonstrated a reduced efficiency in achieving PPARγ agonist-mediated metabolic improvements in the absence of adiponectin (14).
Fasting induces a systemic acute-phase response
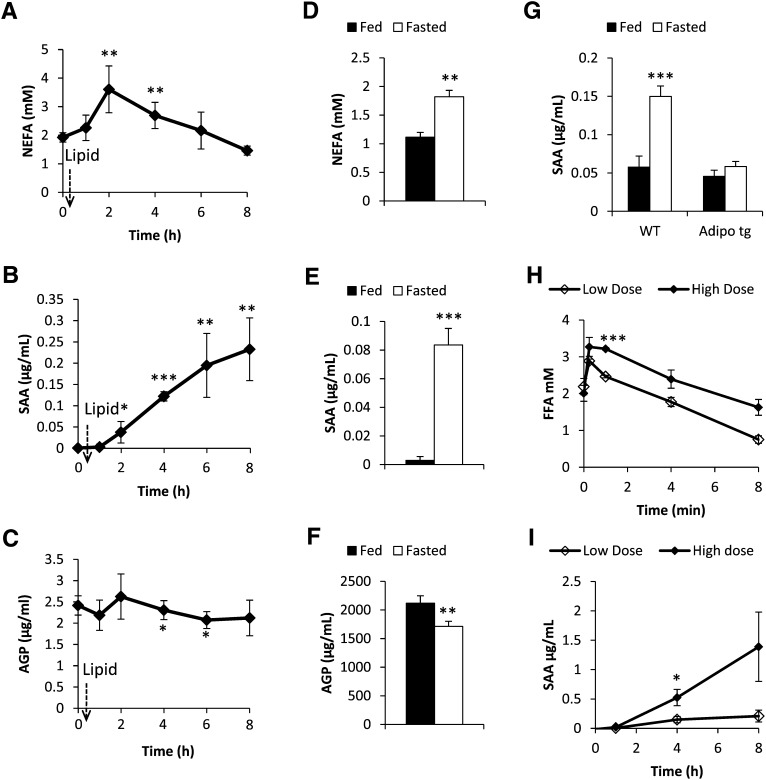
Exposure to as little as 48 h of HFD feeding causes an increase in SAA and AGP levels. This further argues for an essential role of lipids as a link between metabolic dysfunction and systemic inflammation. We therefore sought to investigate the effects of FFAs on SAA and AGP levels in more detail by manipulating the FFA levels through an oral administration of a lipid load (olive oil, 15 μl/g body weight), as well as testing the effect of fasting in healthy wild-type mice, a condition associated with elevated lipolysis and elevated local FFA levels in adipose tissue. FFA levels increased 2 h post the oral lipid gavage and remained significantly elevated for an additional 2 h (Fig. 3A). Concomitant with the change in FFAs, SAA levels increased within 2 h and remained high throughout the 8 h study period (Fig. 3B). In contrast, there was a small but significant decrease in AGP levels (Fig. 3C). A similar response was observed after a 24 h fast (Fig. 3D–F). In contrast, adiponectin tg mice did not display a significant increase in SAA levels after a 24 h fast despite similar FFA levels, further highlighting the potent anti-inflammatory role of adiponectin (Fig. 3G). To further explore the relationship between FFA and SAA, we induced lipolysis pharmacologically by using a low dose (0.1 mg/kg) and a high dose (1.0 mg/kg) of β3 adrenergic receptor-agonist (β3AR-agonist). Indeed, β3AR-agonist dose-dependently generated both higher FFA and SAA levels in healthy chow-fed mice (Fig. 3H, I).
Fig. 3.
Circulating serum FFA, SAA, and AGP levels in wild-type mice in response to an oral load of lipids (A, B, C) or in response to a 24 h fast (D, E, F). Serum SAA in fed and fasted wild-type mice in comparison to adiponectin-overexpressing mice (G). H, I: Circulating FFA and SAA levels, respectively, in response to a low dose (0.1 mg/kg i.p.) and a high dose (1.0 mg/kg i.p.) of β3AR-agonist. [* Significant difference as composed with baseline (A–C), fed (D–G) or low dose (H–I)].
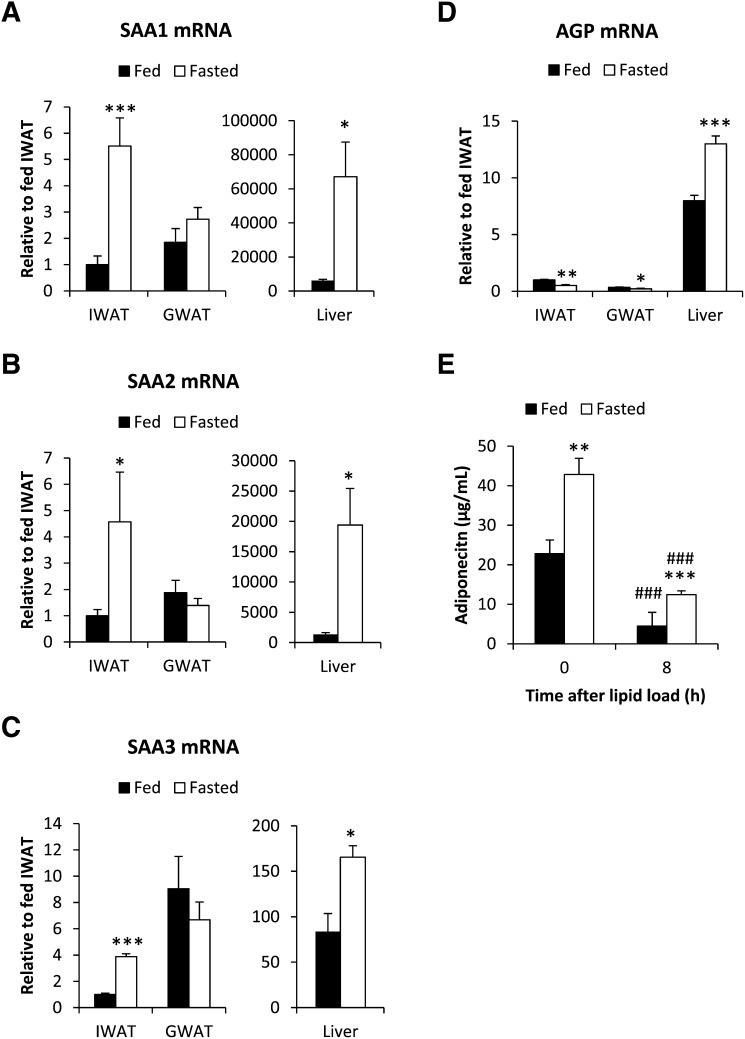
Prolonged fasts, such as a 24 h food deprivation in mice, are associated with a general repression of the immune defense and an increase in whole-body FA oxidation. However, fasting induces hepatic steatosis (21), indicating that the increase in hepatic FA uptake exceeds the FA oxidation. During those conditions, the exposure to FFA is expected to be particularly high locally in the adipocyte microenvironment. Therefore, we hypothesized that fasting induces a transient proinflammatory response in both the liver and in adipose tissue. In support of that, fasting increases the mRNA expression of SAA1, -2, and -3 in the liver and in inguinal adipose tissue. Surprisingly however, no changes were observed in gonadal adipose tissue (Fig. 4A–C). As anticipated, SAA3 is the main SAA isotype in adipose tissue (about 100-fold higher than SAA1 and -2) (22). However, SAA1, -2, and -3 were all expressed at a much higher degree in the liver, suggesting that the liver may be a significant source of circulating SAA during fasting conditions. In contrast, the fasting-induced reduction in circulating AGP correlated better with the transcriptional changes in adipose tissue. We found reduced expression of AGP in adipose tissue, whereas the expression was increased in the liver of fasted mice (Fig. 4D). As a control, we also measured mRNA expression of PEPCK and SREBP1c, genes well known to be regulated by fasting. As expected, fasting caused increased PEPCK and reduced SREBP1c levels in both adipose and liver (see supplementary Fig. IA, B). We also tested whether this fasting-induced SAA mRNA expression could be mimicked by an oral load of lipid. However, the SAA1, -2, and -3 gene expression in liver was unaffected by the exogenous lipid load. In adipose tissue, there was even a tendency toward reduced SAA3 mRNA levels 8 h post lipid gavage, as compared with PBS-gavaged control mice (data not shown). Thus, exogenous lipids may increase circulating SAA levels by a mechanism other than the fasting-induced release of FFAs. Possibly, the gut may be the major source of increased SAA levels after an oral lipid loading (23). We have summarized the SAA mRNA responses in liver and adipose tissue, as well as plasma protein, under the different conditions in Table 1. On the basis of these results, we conclude that the local levels of FFAs in the adipocyte microenvironment differ between fasted and oral lipid-loaded, fed conditions. In the fasted state, there is a dramatic increase in FFAs both locally in adipose tissue and systemically, while blood flow to the gut is downregulated. Oral lipid loading also increases the FFA levels, but the esterification of FFA to triglyceride is, at the same time, very efficient. Therefore, the local levels of FFA in adipose tissue and liver may not reflect the circulating levels. Interestingly, we found that fasting and lipid loading have the opposite effect on circulating adiponectin levels (Fig. 4E). Fasting increases adiponectin levels, whereas lipid loading leads to a reduction in adiponectin levels. This is consistent with the idea that circulating adiponectin reflects changes in lipid metabolism and energy state in adipose tissue.
Fig. 4.
SAA1 (A), SAA2 (B), SAA3 (C), and AGP (D) mRNA expression in inguinal adipose tissue (IWAT), gonadal adipose tissue (GWAT), and liver of fed and 24 h-fasted wild-type FVB mice. E: Circulating adiponectin levels in fed and overnight-fasted wild-type FVB mice before and after an oral lipid load. (* Significant difference as compared with fed.)
TABLE 1.
Obesity, fasting, and lipid-induced changes in circulating SAA and transcriptional regulation of SAA1-3 mRNA in inguinal adipose tissue (WAT) and liver
| Obese vs. lean |
Fasted vs. fed |
Lipid load vs. PBS | |||
| HFD | ob/ob | Chow | HFD | ||
| Circulating SAA | ↑↑ | ↑↑↑ | ↑ | (↑) | ↑↑↑ |
| Liver SAA1 mRNA | ↑ | – | ↑↑↑ | (↑) | – |
| Liver SAA2 mRNA | (↑) | ↑ | ↑↑↑ | (↑) | – |
| Liver SAA3 mRNA | – | ↓ | ↑ | (↓) | – |
| WAT SAA1 mRNA* | – | ↑↑ | ↑↑ | (↓) | (↓) |
| WAT SAA2 mRNA* | – | ↑↑ | ↑↑ | (↓) | (↓) |
| WAT SAA3 mRNA | ↑↑ | ↑↑↑ | ↑ | – | (↓) |
Arrows represent the relative changes under the studied conditions; an arrow in parenthesis represents changes that did not reach significance; an asterisk* indicates transcripts that are expressed only at very low levels; – indicates no change.
Fasting induces a local M2 type of inflammatory response in adipose tissue and the liver
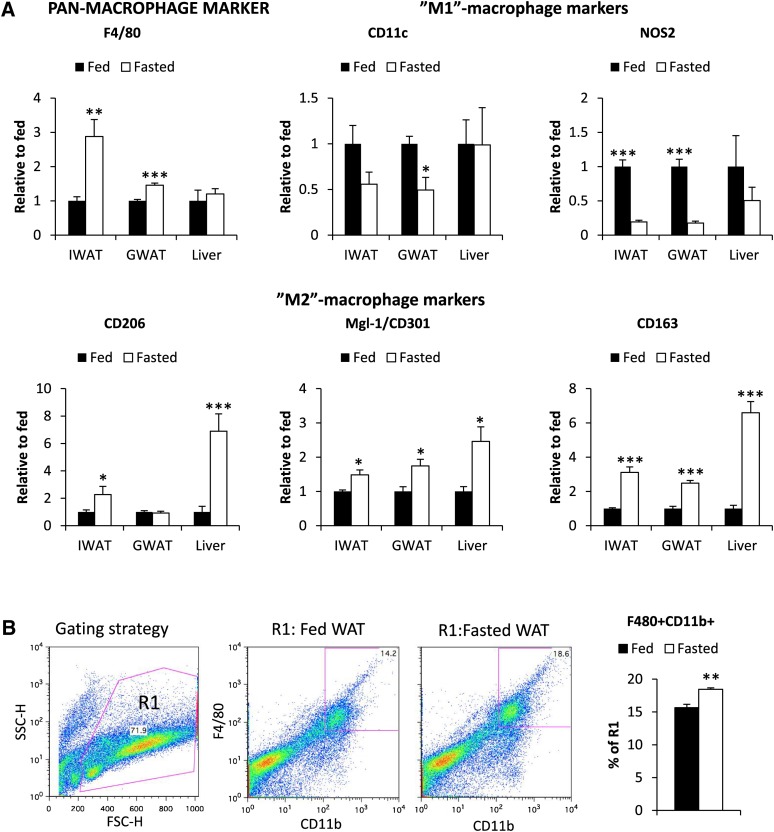
Inflamed adipose tissue, commonly seen in the context of obesity (1), is characterized by increased macrophage infiltration and the appearance of clusters of macrophages that are referred to as crown-like structures (CLSs). This is associated with a shift from an anti-inflammatory M2/Th2 population toward a proinflammatory M1/Th1 type of immune response. Kosteli et al. (10) have recently observed that fasting and weight loss is associated with a transient increase in macrophage infiltration. Here, we aim to further characterize this response by gene expression and immunohistochemistry analysis of harvested adipose tissue and livers of fed and fasted healthy wild-type animals. We found that the pan-macrophage marker F4/80 was upregulated in adipose tissue, but not in the liver (Fig. 5). While it is clear that the M1 and M2 categories of macrophages represent a continuum, comprising cells that have an overlapping marker distribution with many subtypes, this remains a useful general categorization describing general trends in the macrophage subpopulations. Under the conditions here, M1 markers were either unchanged or downregulated, while M2 markers were upregulated in adipose tissue and liver (Fig. 5A). Immunohistochemical visualization of Mgl2+/CD301+ and CD163+-positive cells further supports an increase of M2-type cells in adipose tissue and the liver (Fig. 6A, B). Flow analysis of the stromal vascular fraction of adipose tissue from fed and fasted mice is also in agreement with the quantitative real-time RT-PCR and immunohistochemistry data. Fasted mice indeed had increased levels of macrophages in adipose tissue (F4/80+CD11b+cells) (Fig. 5B). As representatives of M1 and M2 markers, we used CD11c and CD206 respectively, but we were unable to detect a significant difference between F4/80+CD11b+ cells from fed and fasted adipose tissue (data not shown). The expression of CD11c was, however, very low in both groups, indicating that most macrophages represent the M2 type in healthy mice and that fasting further increases the total number of M2 macrophages. Given the differential transcriptional regulation of SAA between fasted and lipid-gavaged conditions, we also wanted to explore M1/M2 markers in adipose tissue and liver in the context of an oral lipid exposure. In contrast to fasting, CD301 mRNA had a trend toward a decrease by 33% (P = 0.08), while NOS2 mRNA had a trend toward an increase by 18% (P = 0.07) in adipose of lipid-gavaged mice. The livers of the lipid-gavaged mice had a 30% decreased (P = 0.04) expression of CD163 mRNA as compared with controls, whereas other M1/M2 markers and SAA mRNA were unaltered. Interestingly, fasting induced a 60% increase in adiponectin expression in the inguinal depot (P < 0.05). A similar trend was seen in the gonadal depot, and this is reflected in an increase in the circulating adiponectin levels (Fig. 4E). Lipid loading, on the other hand, reduced adiponectin levels (Fig. 4E). Thus, our data are in full support of other reports showing that adiponectin shifts macrophages toward an M2 phenotype (24–26).
Fig. 5.
Expression of macrophage mRNA markers in inguinal adipose tissue (IWAT), gonadal adipose tissue (GWAT), and livers of fed and 24 h-fasted wild-type FVB mice (A). Analysis of the relative abundance of F480+CD11b+ cells of R1 (live cells) in the stromal vascular fraction from pooled IWAT and GWAT from fed and overnight-fasted wild-type FVB mice (B). (* Significant difference as compared with fed.)
Fig.6.
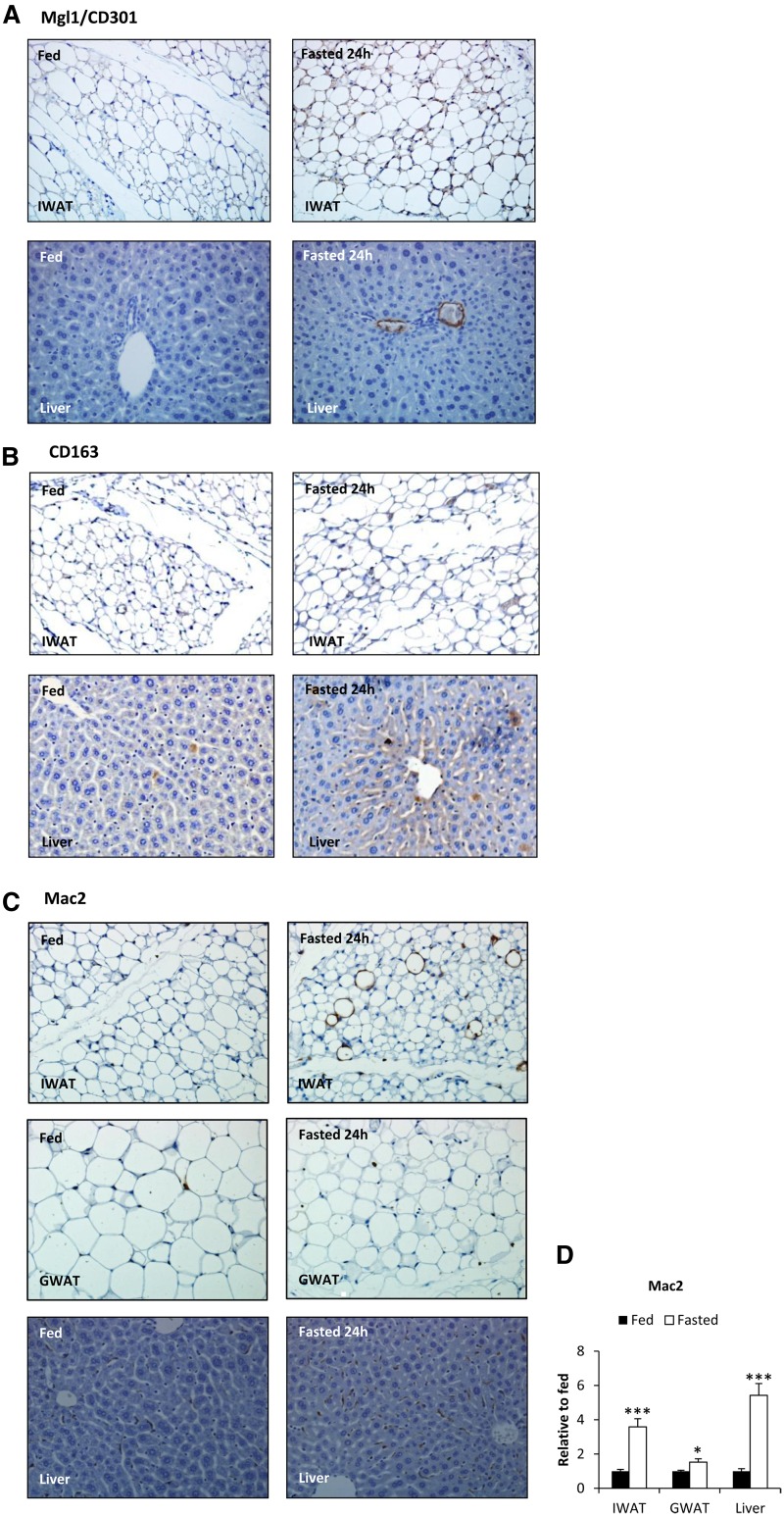
Immunohistochemical analyses of Mgl1/CD301 (A) and CD163 (B) expression in inguinal adipose tissue (IWAT) and livers of fed and 24 h-fasted wild-type FVB mice. Immunohistochemical analyses of Mac2 (C) expression in inguinal adipose tissue (IWAT), gonadal adipose tissue (GWAT), and in livers of fed and 24 h-fasted wild-type FVB mice. D: Mac2 mRNA expression levels from the same animals.
Lipid-gavaged mice also displayed a 64% reduction of Mac2 mRNA (a marker of phagocytically active macrophages) in adipose tissue compared with controls (P = 0.02). This finding was in contrast to fasted mice that displayed an increased number of Mac2+ cells in both adipose tissue and in the liver (Fig. 6C). Surprisingly, the fasting-induced Mac2+ CLSs were more common in inguinal adipose tissue, consistent with the gene expression data that indicated that fasting-induced inflammatory responses are more enhanced in inguinal relative to gonadal adipose tissue (Fig. 6D). We did not observe CD301+ or CD163+ cells in the CLSs, suggesting that CLS-associated Mac2+ cells represent a distinct type of macrophage.
These observations in fasted mice are in sharp contrast to adipose inflammation in the context of chronic metabolic dysfunction, during the course of which the gonadal fat pads play a much more significant role. The differential depot-specific fasting-induced activation of FFAs may form the underlying mechanistic basis for these differences. In insulin-resistant states, adipocytes fail to efficiently sequester excess FFAs due to impaired uptake and esterification of FAs as well as impaired suppression of lipolysis in the fed state. These defects are more commonly seen in visceral adipocytes, compared with often smaller-sized subcutaneous adipocytes. In the fasted state, lipolysis is driven primarily by the lack of insulin and, in at least the initial phase, also by increased adrenergic signaling. Smaller and healthier adipocytes are more sensitive to fasting-induced lipolysis, and the subsequent FFA release may be further enhanced by the higher surface-to-volume ratio. In support of a differential fasting-induced response in different fat pads, we observed that the inguinal adipose tissue was significantly more reduced than the gonadal adipose tissue compared with the fed controls (52.5% lighter inguinal, P = 0.01, and 37.3% lighter gonadal depot, P = 0.06 as compared with fed controls). Fasting therefore leads to a more-pronounced local increase of FFAs in the inguinal depot relative to the gonadal depot.
Fasting increases VEGF-A expression and vascular permeability
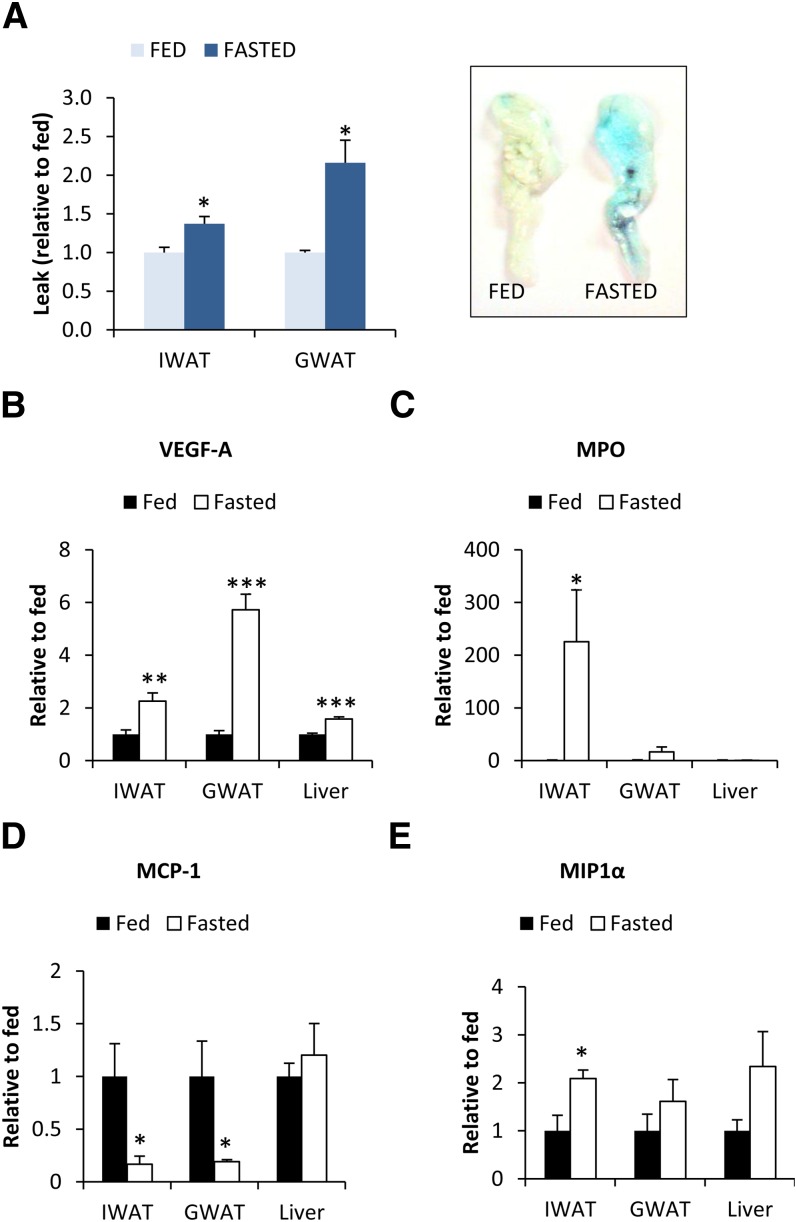
What are the underlying mechanism(s) that explain the increase in infiltrating leukocytes in adipose tissue? We examined vascular permeability in fed and fasted adipose tissue using an intravenous injection of Evans Blue dye. Fasting increased vascular permeability in the gonadal depot and, to a smaller extent, also in the inguinal depot (Fig. 7A). This phenomenon was associated with an increase in VEGF-A expression (Fig. 7B), a potent inducer of vascular permeability and a factor implicated in M2 polarization of macrophages. This could also enhance FFA exchange during the fasting period, a time of rapid FFA release from adipocytes. However, the inguinal adipose tissue displayed less-pronounced VEGF-A mRNA upregulation, but yet more Mac2+ CLSs and a dramatically increased myeoloperoxidase expression relative to the gonadal depot, arguing for additional contributing factors (Fig. 7C). MCP-1 was a strong candidate, given its established role for macrophage infiltration in obese adipose tissue (27, 28), but surprisingly, MCP-1 mRNA was downregulated in fasted adipose tissue (Fig. 7D). In contrast, we found a small upregulation of MIP-1α mRNA after fasting in the inguinal depot and a similar trend in the gonadal depot (Fig. 7E). MIP-1α has been shown to attract both neutrophils and macrophages and may therefore contribute toward fasting-induced adipose tissue inflammation.
Fig. 7.
Differences in vascular permeability as judged by the extracted amount of Evans Blue inguinal adipose tissue (IWAT) and gonadal adipose tissue (GWAT) after the tail vein administration of the dye to fed and fasted wild-type FVB mice (A). mRNA expression of VEGF-A, the neutrophil marker MPO, and the chemoattractants MCP-1 and MIP-1α in inguinal adipose tissue (IWAT), gonadal adipose tissue (GWAT) and livers of fed and 24 h-fasted wild-type FVB mice (B–E). (* Significant difference as compared with fed.)
Fasting of obese mice fails to induce a shift toward M2-polarized cells
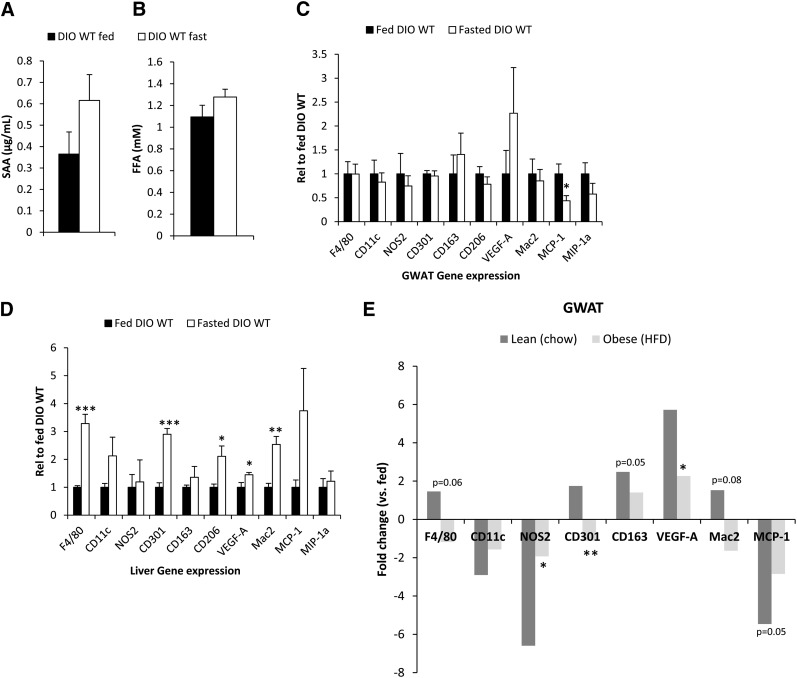
We have established that prolonged fasting leads to a phenotypic switch from the M1 to the M2 type of immune cell composition. This is in contrast to the trends seen in the context of obesity, which involves primarily a M1 type of immunity associated with a systemic and local proinflammatory state, thereby aggravating metabolic function. Therefore, we wanted to investigate whether prolonged fasting in diet-induced as well as in genetically-induced obese mice would overcome the potent M1 type of inflammation and lead to a similar shift toward an M2-polarized response, as observed in lean mice, thereby resetting the immune system to a less-proinflammatory state. As previously shown, a HFD induces a baseline elevation of SAA in circulation. Just like in lean mice, fasting induces a trend toward further elevation of SAA levels in HFD-exposed mice (Fig. 8A). The less-robust increase in SAA in the HFD-fed mice as compared with chow-fed mice may be a reflection of the blunted increase in fast-induced FFA (Fig. 8B). We also found that the expression levels of neither PEPCK, SREBP1c, nor AGP mRNA are significantly regulated by fasting in HFD-fed mice (see supplementary ). The fasting-induced increase in M2, the decrease in M1 markers, and the increase in VEGF are more-pronounced in lean animals. In contrast, these changes occur at a reduced level in adipose tissue of HFD-exposed animals (Fig. 8C). Changes at the level of the liver are seen under HFD conditions (Fig. 8D), but these changes remain much less significant than under chow-fed conditions. We have plotted a direct comparison of the fasting-induced changes in the lean versus the HFD-induced state in (Fig. 8E). Similar observations were made when we examined ob/ob mice in the fed and fasted state, i.e., these mice were also unable to mount a fasting-induced switch toward M2 macrophages (data not shown). Combined, we conclude that under chronically challenging conditions, the “normal” switch to the M2 phenotype is impaired. Therefore, the metabolic inflexibility characteristic of dysfunctional adipose tissue is associated with (and at least in part, may be due to) the lack of “immunological fitness” that is observed as part of a normal feeding/fasting cycle.
Fig. 8.
Differences in SAA (A) and FFA levels (B) in fed and fasted animals after an 8 week HFD. Changes in critical macrophage markers in gonadal fat (C) and liver (D). A direct comparison between fasting-induced changes in adipose tissue is shown in E. [* Signifi cant difference as compared with fed (A-D) or lean (E).]
DISCUSSION
SAA3 is a member of a family of closely related proteins (29). In the mouse, SAA3 is the extra-hepatic member of this protein family, prominently expressed in adipocytes. We have previously shown that upon ablation of all functional adipocytes, SAA levels are reduced significantly upon a pro-inflammatory challenge (30), highlighting that adipocytes can significantly affect total circulating SAA levels, either directly or indirectly. In fact, a recent study by Clegg and colleagues (31) revealed that SAA3 is one of the most-differentially-regulated genes between male and female adipose tissue, even under unchallenged conditions.
SAA derived from adipocytes has garnered a lot of attention, with a number of publications focusing on the effects of SAA on a variety of metabolic processes, including its function as a mediator of the dialogue between hypertrophied adipocytes and macrophages through its regulation of adipocyte cholesterol efflux (32). The situation for SAA3 release from adipocytes into circulation seems to be rather complex. Chiba and colleagues (33) have recently shown that adipocyte-derived SAA3 may not reach the circulation. The changes in circulating SAA levels reported here are more consistent with a model that includes adipose tissue-derived SAA3 as one of the determinants of circulating levels under obese conditions, whereas the liver may contribute to a larger extent during fasted conditions. However, we do not know how the colon-derived SAA3 (another source of SAA3) behaves under these conditions (34). The lack of a transcriptional increase for SAA in both liver and adipose in mice that are given an oral load of lipids points indeed toward the colon as a potential source. Therefore, caution needs to be exerted in terms of singling out a specific tissue as the major determinant for circulating SAA3 levels.
Much less is known about the role of AGP in adipose tissue. It is well established that AGP is abundantly expressed in adipocytes; however, it is generally not regulated by inflammatory markers (22). Its exact function for adipocyte physiology therefore remains to be determined, but it is a protein that is constitutively expressed at rather high levels and may serve as an important carrier protein.
With respect to adipsin, progress in the field has been significantly hampered by the lack of widely available tools to measure this protein in plasma. After a series of exciting initial findings (15, 16, 35–37), adipsin has been the focus of studies revolving around acylation-stimulating protein (ASP) (38), where it is part of the protease complex that leads to the generation of ASP. While ASP has been demonstrated to potentially play an important role in cellular lipid handling in adipocytes (38), it is not clear whether adipsin exerts any other functions. However, the transcriptional regulation of adipsin bears many resemblances to the transcriptional regulation of adiponectin. These two proteins are the only known examples of adipokines that display an inverse correlation with fat mass, despite the fact that their expression is highly enriched in adipocytes.
We have used the availability of these assays as a segue into physiological studies at the interphase of feeding and fasting. In the initial phase of fasting, when there is an increased tone of the sympathetic nervous system that further enhances lipolysis and hepatic glucose output, there is also a concurrent activation of the immune system. In contrast, prolonged fasting is associated with a general suppression of the immune function and inflammation. One may argue that a higher proportion of FFAs is oxidized in the fasted state and that thereby their impact on inflammation is expected to be reduced. However, fasting induces hepatic steatosis, and the local FFA concentration surrounding adipocytes is elevated and able to signal through receptors, such as TLR4, and/or to contribute to ceramide synthesis. Indeed, we found that SAA levels are increased in circulation and transcriptionally in adipose tissue and in the liver. Furthermore, fasting-induced inflammation is associated with an increase in macrophage infiltration in a depot-specific manner.
The lack of fasting-induced SAA3 in adiponectin-overexpressing mice may rely on the potent local anti-inflammatory effect that adiponectin exerts. This anti-inflammatory action may be caused, at least in part, through the ceramide-lowering effects that we recently described for adiponectin (4). As a consequence, adipose tissue macrophages may therefore show a reduced response to lipids. Alternatively, adiponectin may also exert chronic effects on adipocyte lipid composition, causing a lower release of proinflammatory, saturated lipids. What is particularly intriguing is that the potent anti-inflammatory properties associated with PPARγ agonists result in a reduction of both circulating and adipose tissue SAA levels. Surprisingly, this SAA-suppressing effect of thiazolidinediones is completely lost in the absence of adiponectin, consistent with its powerful anti-inflammatory properties.
FFAs are widely accepted as inducers of inflammation. In that function, they exert potent effects in the context of metabolic dysregulation and are permissive for the infiltration of immune cells into adipose tissue. However, the observed polarization toward an M2 population requires additional factors. Similar observations may be relevant for mice on an intermittent fasting regimen. The ensuing switch from an M1 to an M2-leaning population of immune cells may explain the beneficial effects that an intermittent-fasting paradigm exerts on systemic metabolism. M2-type macrophages are generally associated with tissue remodeling and with expanding tumors. Thus, the M2 population of macrophages may play an integral role in the need for rapid adipose tissue remodeling during fasting-feeding transitions.
We do not know what the specific factors are that mediate the switch to an M2 type of macrophage population. Potential candidates are VEGF, leptin, and adiponectin. Adiponectin has been directly implicated in the M1-to-M2 transition of macrophages (25). Here, we observe distinct effects on macrophage polarization in response to fasting and in response to an oral load of lipids, i.e., both conditions characterized by elevated systemic FFA levels. Fasting is, however, associated with lipolysis, whereas excess lipid load is associated with an increased triglyceride synthesis. Interestingly, these two conditions provide opposite responses with respect to adiponectin levels. The dichotomous change observed for adiponectin under the two conditions are in line with the observed changes in macrophage polarization. Adiponectin levels are indeed decreased under unhealthy, obese conditions when there is a propensity toward increased M1-type macrophages. On the other hand, adiponectin levels and M2 polarization are phenomena that may depend on the differences in energy state of the adipocyte and local lipid metabolism.
In summary, we are proposing to use the term “immunological fitness” to indicate concomitant “metabolic flexibility,” both of which are parameters that are critical determinants of how well adipose tissue adapts to a changing metabolic environment.
Supplementary Material
Acknowledgments
The authors thank the Metabolic Phenotyping Core and the Molecular Pathology Core at UT Southwestern for their help at various stages of this work, as well as the rest of the Scherer, Unger, and Clegg laboratories for helpful discussions. Furthermore, we would like to thank Dr. Alan Chait and his research group for helpful comments on the SAA3 production in adipocytes.
Footnotes
Abbreviations:
- AGP
- α1-acid glycoprotein
- ASP
- acylation-stimulating protein
- CLS
- crown-like structures
- FACS
- fluorescence-activated cell sorting
- FFA
- free FA
- HFD
- high-fat diet
- LPS
- lipopolysaccharide
- SAA
- serum amyloid
- tg
- transgenic
- TLR4
- Toll-like receptor-4
This work was supported by National Institutes of Health Grants R01-DK-55758, R01-CA-112023, RC1-DK-086629, and P01-DK-088761-01 (P.E.S.), DK-081182 (Jay Horton), and the Effie Marie Cain Scholarship in Angiogenesis Research (R.A.B.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. I.W.A. was also supported by a fellowship from the Throne-Holst Foundation and the Swedish Research Council (2006-3931) and by a VINNMER Fellowship from the VINNOVA Foundation. J.M.R. was supported by NIH Postdoctoral Fellowship F32-DK085935.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table and two figures.
REFERENCES
- 1.Sun K., Kusminski C. M., Scherer P. E. 2011. Adipose tissue remodeling and obesity. J. Clin. Invest. 121: 2094–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unger R. H., Scherer P. E. 2010. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol. Metab. 21: 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland W. L., Summers S. A. 2008. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr. Rev. 29: 381–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holland W. L., Miller R. A., Wang Z. V., Sun K., Barth B. M., Bui H. H., Davis K. E., Bikman B. T., Halberg N., Rutkowski J. M., et al. 2011. Receptor-mediated activation of ceramidase activity ini-tiates the pleiotropic actions of adiponectin. Nat. Med. 17: 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland W. L., Brozinick J. T., Wang L. P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., et al. 2007. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5: 167–179 [DOI] [PubMed] [Google Scholar]
- 6.Samuel V. T., Petersen K. F., Shulman G. I. 2010. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 375: 2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holland W. L., Bikman B. T., Wang L. P., Yuguang G., Sargent K. M., Bulchand S., Knotts T. A., Shui G., Clegg D. J., Wenk M. R., et al. 2011. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 121: 1858–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nduka O. O., Parrillo J. E. 2009. The pathophysiology of septic shock. Crit. Care Clin. 25: 677–702 [DOI] [PubMed] [Google Scholar]
- 9.Fahy B. G., Sheehy A. M., Coursin D. B. 2009. Glucose control in the intensive care unit. Crit. Care Med. 37: 1769–1776 [DOI] [PubMed] [Google Scholar]
- 10.Kosteli A., Sugaru E., Haemmerle G., Martin J. F., Lei J., Zechner R., Ferrante A. W., Jr 2010. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Invest. 120: 3466–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Y., Berg A. H., Iyengar P., Lam T. K., Giacca A., Combs T. P., Rajala M. W., Du X., Rollman B., Li W., et al. 2005. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J. Biol. Chem. 280: 4617–4626 [DOI] [PubMed] [Google Scholar]
- 12.Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. 2010. Development of monocytes, macrophages, and dendritic cells. Science. 327: 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Combs T. P., Pajvani U. B., Berg A. H., Lin Y., Jelicks L. A., Laplante M., Nawrocki A. R., Rajala M. W., Parlow A. F., Cheeseboro L., et al. 2004. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 145: 367–383 [DOI] [PubMed] [Google Scholar]
- 14.Nawrocki A. R., Rajala M. W., Tomas E., Pajvani U. B., Saha A. K., Trumbauer M. E., Pang Z., Chen A. S., Ruderman N. B., Chen H., et al. 2006. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 281: 2654–2660 [DOI] [PubMed] [Google Scholar]
- 15.Flier J. S., Cook K. S., Usher P., Spiegelman B. M. 1987. Severely impaired adipsin expression in genetic and acquired obesity. Science. 237: 405–408 [DOI] [PubMed] [Google Scholar]
- 16.Rosen B. S., Cook K. S., Yaglom J., Groves D. L., Volanakis J. E., Damm D., White T., Spiegelman B. M. 1989. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 244: 1483–1487 [DOI] [PubMed] [Google Scholar]
- 17.Kim J. Y., van de Wall E., Laplante M., Azzara A., Trujillo M. E., Hofmann S. M., Schraw T., Durand J. L., Li H., Li G., et al. 2007. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117: 2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asterholm I. W., Scherer P. E. 2010. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am. J. Pathol. 176: 1364–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Combs T. P., Wagner J. A., Berger J., Doebber T., Wang W. J., Zhang B. B., Tanen M., Berg A. H., O'Rahilly S., Savage D. B., et al. 2002. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 143: 998–1007 [DOI] [PubMed] [Google Scholar]
- 20.Toseland C. D., Campbell S., Francis I., Bugelski P. J., Mehdi N. 2001. Comparison of adipose tissue changes following administration of rosiglitazone in the dog and rat. Diabetes Obes. Metab. 3: 163–170 [DOI] [PubMed] [Google Scholar]
- 21.Guan H. P., Goldstein J. L., Brown M. S., Liang G. 2009. Accelerated fatty acid oxidation in muscle averts fasting-induced hepatic steatosis in SJL/J mice. J. Biol. Chem. 284: 24644–24652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y., Rajala M. W., Berger J. P., Moller D. E., Barzilai N., Scherer P. E. 2001. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J. Biol. Chem. 276: 42077–42083 [DOI] [PubMed] [Google Scholar]
- 23.Reigstad C. S., Lunden G. O., Felin J., Backhed F. 2009. Regulation of serum amyloid A3 (SAA3) in mouse colonic epithelium and adipose tissue by the intestinal microbiota. PLoS ONE. 4: e5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovren F., Pan Y., Quan A., Szmitko P. E., Singh K. K., Shukla P. C., Gupta M., Chan L., Al-Omran M., Teoh H., et al. 2010. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am. J. Physiol. Heart Circ. Physiol. 299: H656–H663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohashi K., Parker J. L., Ouchi N., Higuchi A., Vita J. A., Gokce N., Pedersen A. A., Kalthoff C., Tullin S., Sams A., et al. 2010. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 285: 6153–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandal P., Pratt B. T., Barnes M., McMullen M. R., Nagy L. E. 2011. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J. Biol. Chem. 286: 13460–13469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tateya S., Tamori Y., Kawaguchi T., Kanda H., Kasuga M. 2010. An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice. Endocrinology. 151: 971–979 [DOI] [PubMed] [Google Scholar]
- 28.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., et al. 2006. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116: 1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uhlar C. M., Whitehead A. S. 1999. Serum amyloid A, the major vertebrate acute-phase reactant. European J. Biochem. 265: 501–523 [DOI] [PubMed] [Google Scholar]
- 30.Pajvani U. B., Trujillo M. E., Combs T. P., Iyengar P., Jelicks L., Roth K. A., Kitsis R. N., Scherer P. E. 2005. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat. Med. 11: 797–803 [DOI] [PubMed] [Google Scholar]
- 31.Grove K. L., Fried S. K., Greenberg A. S., Xiao X. Q., Clegg D. J. 2010. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int. J. Obes. 34: 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poitou C., Divoux A., Faty A., Tordjman J., Hugol D., Aissat A., Keophiphath M., Henegar C., Commans S., Clement K. 2009. Role of serum amyloid a in adipocyte-macrophage cross talk and adipocyte cholesterol efflux. J. Clin. Endocrinol. Metab. 94: 1810–1817 [DOI] [PubMed] [Google Scholar]
- 33.Chiba T., Han C. Y., Vaisar T., Shimokado K., Kargi A., Chen M. H., Wang S., McDonald T. O., O'Brien K. D., Heinecke J. W., et al. 2009. Serum amyloid A3 does not contribute to circulating SAA levels. J. Lipid Res. 50: 1353–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meek R. L., Benditt E. P. 1986. Amyloid A gene family expression in different mouse tissues. J. Exp. Med. 164: 2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook K. S., Min H. Y., Johnson D., Chaplinsky R. J., Flier J. S., Hunt C. R., Spiegelman B. M. 1987. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 237: 402–405 [DOI] [PubMed] [Google Scholar]
- 36.Kitagawa K., Rosen B. S., Spiegelman B. M., Lienhard G. E., Tanner L. I. 1989. Insulin stimulates the acute release of adipsin from 3T3-L1 adipocytes. Biochim. Biophys. Acta. 1014: 83–89 [DOI] [PubMed] [Google Scholar]
- 37.Platt K. A., Min H. Y., Ross S. R., Spiegelman B. M. 1989. Obesity-linked regulation of the adipsin gene promotor in transgenic mice. Proc. Natl. Acad. Sci. USA. 86: 7490–7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cianflone K., Maslowska M., Sniderman A. D. 1999. Acylation stimulating protein (ASP), an adipocyte autocrine: new directions. Semin. Cell Dev. Biol. 10: 31–41 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.