Abstract
Autoantibodies specific for malondialdehyde-modified LDL (MDA-LDL) represent potential biomarkers to predict cardiovascular risk. However, MDA-LDL is a high variability antigen with limited reproducibility. To identify peptide mimotopes of MDA-LDL, phage display libraries were screened with the MDA-LDL-specific IgM monoclonal Ab LRO4, and the specificity and antigenic properties of MDA mimotopes were assessed in vitro and in vivo. We identified one 12-mer linear (P1) and one 7-mer cyclic (P2) peptide carrying a consensus sequence, which bound specifically to murine and human anti-MDA monoclonal Abs. Furthermore, MDA mimotopes were found to mimic MDA epitopes on the surface of apoptotic cells. Immunization of mice with P2 resulted in the induction of MDA-LDL-specific Abs, which strongly immunostained human atherosclerotic lesions. We detected IgG and IgM autoAbs to both MDA mimotopes in sera of healthy subjects and patients with myocardial infarction and stable angina pectoris undergoing percutaneous coronary intervention, and the titers of autoAbs correlated significantly with respective Ab titers against MDA-LDL. In conclusion, we identified specific peptides that are immunological mimotopes of MDA. These mimotopes can serve as standardized and reproducible antigens that will be useful for diagnostic and therapeutic applications in cardiovascular disease.
Keywords: atherosclerosis, oxidized LDL, oxidation-specific epitopes, autoantibodies, peptides, mimotopes, phage display, biomarker, myocardial infarction
Atherosclerosis is a chronic inflammatory disease modulated by innate and adaptive immunity. There is emerging interest to identify immunological factors as novel biomarkers for the stratification of cardiovascular risk. Several potential antigens are present in atherosclerotic lesions, and epitopes of oxidized LDL (OxLDL) appear to be prominent and immunodominant (1). The oxidative modification of LDL has been shown to result in the generation of various oxidation-specific epitopes (OSE) that are recognized by specific antibodies (Abs) in a hapten-specific manner. These include adducts with proteins of lipid peroxidation breakdown products, such as malondialdehyde (MDA), which forms complex condensation products, as well as the remaining “core aldehydes,” such as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC) (1, 2). For the assessment of specific immune responses to such OSEs of OxLDL, two model antigens are widely used, namely, Cu2+-oxidized LDL (CuOx-LDL), which contains many different OSEs, and malondialdehyde-modified LDL (MDA-LDL), which is generated by the derivatization of LDL with various MDA-type adducts.
Antibodies against these two models of OxLDL have been documented in lesions and plasma of patients and animal models of atherosclerosis (3–5). In animal studies of atherogenesis, antibody titers to OSEs correlate strongly with the progression and regression of atherosclerosis (6–8). In contrast, although many studies in human populations have also shown a positive correlation of such titers with manifestations of cardiovascular disease (CVD), others have not (discussed in Refs. 9–13). Furthermore, IgG titers appear to correlate positively, whereas IgM titers are inversely associated with CVD events. Undoubtedly, the heterogeneity and complexity of human immunological responses and the large variation in clinical manifestations studied account in part for these differential human results.
The quality and reproducibility of the antigens used may differ substantially between laboratories or even within the same laboratory over time. Indeed, preparation of reproducible CuOx-LDL is very difficult, and for this reason, we and many other laboratories have preferentially utilized MDA-LDL as a model antigen. We previously reported that MDA epitopes are highly expressed in atherosclerotic lesions (3–5) and that MDA-specific IgM antibodies account for more than 10% of all natural IgM antibodies in mice and represent an equally high percentage of natural IgM in humans (14). Moreover, in a series of studies, we and others demonstrated that immunization with autologous MDA-LDL reduces atherosclerotic lesion formation in rabbits and mice (15–20), and we recently showed that the expression of an MDA-specific human monoclonal antibody in cholesterol-fed LDLR−/− mice, by intraperitoneal injection or adenoviral-mediated expression, reduces lesion formation by 29–46% (21). These data suggest that MDA-type epitopes are very prominent, prevalent, and important in CVD, and thus represent key candidate antigens to characterize immune responses that are relevant to atherosclerosis. Even here, MDA-modification of LDL results in the formation of various neoepitopes, including the immunodominant malondialdehyde-acetaldehyde (MAA)-type adduct (22), with different densities and antigenic properties. All of these factors have made it difficult to standardize the measurements of autoAb titers to MDA for potential clinical applications, such as biomarkers for CVD, or the use of MDA-LDL as an immunogen in vaccine approaches to atherosclerosis (2, 23, 24).
Therefore, to develop highly reproducible OSEs that could be used as standard antigens to assess MDA-specific Ab responses, we identified and characterized MDA mimotopes by screening phage display peptide libraries using an MDA-specific monoclonal Ab. These MDA mimotopes were found to reflect epitopes on MDA-LDL and on apoptotic cells, and when used as immunogens, to induce MDA-specific Ab responses in mice that reacted with epitopes in human atherosclerotic lesions. Most importantly, we demonstrated that human plasma contains MDA mimotope-specific autoAbs and that titers to these mimotopes correlated well with titers against the traditional antigen MDA-LDL.
MATERIALS AND METHODS
Antigens and antibodies
Human native LDL, MDA-LDL, MAA-modified LDL, CuOx-LDL, and MAA-BSA were prepared as previously described (5, 14). BSA was purchased from Sigma-Aldrich (St. Louis, MO). LRO4 is a monoclonal IgM Ab that was cloned from the spleens of cholesterol-fed Ldlr−/− mice, and selected for binding to MDA-LDL using methods described previously (25). EO6 is an extensively described phosphocholine (PC)-specific murine IgM mAb that binds to the PC of oxidized phospholipids (26). MDA2 is a murine IgG monoclonal Ab raised against murine MDA-LDL in BALB/c mice (27). IK17 is a human IgG Fab fragment specific for an MDA epitope on MDA-LDL and CuOx-LDL (28).
Phage library and biopanning
For the identification of peptide mimotopes of MDA epitopes in MDA-LDL, commercial random phage display peptide libraries purchased from New England Biolabs (NEB; Beverly, MA) were screened using the MDA-specific IgM mAb LRO4. Phage display peptide libraries (Ph.D.-C7C and Ph.D.-12) consisted of a linear 12-mer (Ph.D.-12) or a cyclic 7-mer peptide flanked by cysteine residues (Ph.D.-C7C) fused to the outer minor phage coat protein (pIII) of M13 phage. Library screening was performed according to NEB's instructions with some modifications (supplementary Fig. I depicts the steps involved in this procedure). Three rounds of biopanning were performed, in which high specificity was obtained by increasing the concentration of Tween 20 (Sigma-Aldrich) in washing buffers as well as the number of washing steps with each round of biopanning. Furthermore, specificity was increased by decreasing the concentration of coated LRO4 mAb as well as the incubation time of phages with LRO4.
Briefly, LRO4 or a control isotype anti-KLH IgM Ab (C48-6; BD Bioscience-Pharmingen) was coated in buffer containing 0.1 M NaHCO3 (pH 8.5) on ELISA plates (Nunc Maxisorp, Roskilde, Denmark) overnight (ON) at 4°C at a concentration of 100 μg/ml for the first, 50 μg/ml for the second, and 10 μg/ml for the third biopanning round. The next day, wells were washed with TBS containing 0.05% v/v Tween 20 (TBS-T) and incubated with blocking buffer containing 0.1 M NaHCO3 (pH 8.6) and 5 mg/ml BSA for 1 h at room temperature (RT). After further washing with TBS-T, 10 μl of a library solution (containing 2 × 1012 phages) diluted in 100 μl of blocking buffer were first added to wells coated with control IgM (negative selection), and then unbound phages were transferred to wells coated with LRO4 (positive selection) and incubated at RT. The incubation time for negative or positive selection was reduced from 60 min for the first round to 30 min for the second and third biopanning rounds. Thereafter, plates were washed five times with TBS-T to remove unbound phages. To further elute nonspecifically bound phages, wells were incubated with 100 μl of 100 μg/ml of native LDL for 1 h at RT. After washing up to five times with TBS-T, bound phages were eluted with 100 μL of elution buffer containing 0.2 M glycine-HCl (pH 2.2) and 1 mg/ml BSA. Eluates were transferred to microfuge tubes and neutralized with 15 μl of 1 M Tris-HCl (pH 9.1). The eluted phages were then titrated and amplified for the next round of panning as described (29). The second and third biopanning rounds were carried out using amplified eluates from the first and second rounds as input phages, respectively. To remove nonspecifically bound phages and to increase the affinity in the subsequent biopanning rounds, wells were washed 15 and 30 times with 0.1% TBS-T in the second round and 0.5% TBS-T in the third round of biopanning, respectively. In the last biopanning round, LRO4-bound phages were competitively eluted with increasing concentrations (3–150 μg/ml) of MDA-LDL diluted in blocking buffer, followed by elution using elution buffer.
Plaque-forming assays and phage ELISA
The plaque-forming units (pfu) assay was carried out as described in NEB's instructions. The number of LRO4-reactive phages from each elution step was determined by infection of Escherichia coli bacteria (strain 2738; NEB) and was subsequently plated on X-gal/IPTG (isopropyl β-D-1-thiogalactopyranoside / 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside; Sigma-Aldrich) agar plates. Then the resulting blue plaques were counted to obtain phage titers. (29, 30).
Phage ELISA was performed as described by NEB with minor modifications. Ninety-six-well ELISA plates (Nunc Maxisorp) were coated with 5 μg/ml LRO4 mAb or control IgM mAb in NaHCO3 buffer (pH 8.6) at 50 μl/well ON at 4°C. Wells were washed with TBS containing 0.5% Tween 20 and then blocked with blocking buffer (TBS-T containing 1% BSA) at 200 μl/well for 1 h at RT. After further washing, 1010 pfu/ml of phage amplificates diluted in blocking buffer were added to the wells at 50 μl/well for 2 h at RT. Wells were washed again, and an HRP-labeled anti-M13 mAb conjugate (no. 27-9421-01; GE Healthcare, Amersham, UK) diluted 1:1,000 in blocking buffer was added for 1 h at RT, followed by the addition of an 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS; Sigma-Aldrich) substrate solution for 1 h at RT. The binding reactivity of selected phage clones was measured at OD 405–490 nm using a BioTek Synergy 2 plate reader.
For competitive phage ELISA, plates were coated with 5 μg/ml LRO4 and binding of 25 μl of phage solution at 2 × 1010 pfu/ml was tested in the presence of 25 μl of MDA-LDL at 100 μg/ml. Bound phages were detected as described above and data expressed as values obtained in presence of competitor (B) divided by the values obtained in the absence of competitor (B0). A reciprocal competition assay was performed in which 50 μl of 5 μg/ml MDA-LDL was coated on microtiter wells, and binding of LRO4 that was preincubated for 30 min at RT with a solution containing either no or 1 × 1010 pfu/ml phages with or without peptide was tested by chemiluminescent ELISA as described (14, 31).
Phage sequencing and peptide synthesis
Single-stranded phage DNA from amplified single-phage clones was prepared using the Qiaprep spin M13 kit (Qiagen, Hilden Germany). The DNA content was electrophoresed on a 1.2% agarose gel containing 0.01% ethidium bromide in Tris-Borate-EDTA buffer (TBE-buffer) and was visualized by UV illumination. DNA sequencing was performed by VBC Biotech Service using 96 gIII sequencing primers (NEB) corresponding to the phages’ minor coat protein (pIII) gene sequence. Peptide sequences were deduced from DNA sequences. Dodecamer and heptamer peptide sequences were aligned by the Clustal W program to obtain consensus sequences. A dodecamer linear peptide P1 (HSWTNSWMATFL), a cysteine-constrained heptamer cyclic peptide P2 (AC-NNSNMPL-C) and scrambled peptide of P2 (AC-SPNLNMN-C), and a control irrelevant peptide (IMGVGAVGAGAI) were synthesized by Peptide 2.0 Inc. (Chantilly, VA). A spacer (GGGS or GGGC or GGGK)-CONH2 was added at each C terminus. The purity of all the peptides was between 89–95% as assessed by high performance liquid chromatography and mass spectral analysis. For evaluation of its immunogenicity, P2 peptides were conjugated to BSA via the C-terminal cysteine.
Chemiluminescent ELISA
Binding of mAb as well as plasma Abs to respective antigens was measured by chemiluminescent ELISA as previously described (14, 31, 32). Antigens were coated at 5 μg/ml in PBS/EDTA (pH 7.4). Synthetic peptides were directly coated at 10 μg/ml (P1) or 5 μg/ml (P2) in 0.1 M NaHCO3 buffer (pH 8.6), unless indicated differently. Biotinylated peptides were immobilized at indicated concentrations on wells precoated with 10 μg/ml neutravidin (Pierce, Rockford, IL). Ab binding was measured using alkaline phosphatase (AP) labeled secondary Abs (described below), followed by chemiluminescent detection. For the detection of human autoAbs, a 1:400 plasma dilution was used. For human assays, internal controls consisting of high and low standard plasma samples were included on each microtiter plate to detect potential variations between microtiter plates. The intra-assay coefficients of variation for all assays were 10–14%.
The following secondary Abs were used: alkaline phosphatase (AP)-labeled goat anti-mouse IgM (μ-chain specific), IgG (γ-chain specific), IgG1 (Sigma-Aldrich), and IgG2c (Southern Biotech). For the detection of human autoAbs, AP-labeled goat anti-human IgG (γ-chain specific) and IgM (μ-chain specific; Sigma-Aldrich) were used. Biotin-conjugated Abs were detected with AP-conjugated neutravidin (1:10,000; Pierce).
Competition immunoassays
The specific binding of LRO4 to mimotopes was determined by competition immunoassays as described previously (14, 31, 33). Fifty microliters of 0.5 μg/ml MAA-BSA or 100 ng/ml of biotinylated peptides were plated as described above. LRO4 antibody was incubated in 1% BSA-TBS with or without increasing concentrations of competitors (BSA, MAA-BSA, P1, P2, and control peptide) for 30 min at RT. Binding of LRO4 was determined by chemiluminescent ELISA as described. The specificity of human plasma Ab binding to P1 was determined in a similar manner. In preliminary experiments, aliquots of pooled plasma samples (n = 26) from a published cohort of healthy subjects (34) were diluted in 1% BSA-TBS to yield a limiting plasma dilution. Diluted plasma (1:1,000) and increasing concentrations of competitors were incubated overnight at 4°C. Samples were then centrifuged at 15,800 g for 45 min at 4°C to pellet immune complexes, and supernatants were analyzed for binding to plated P1 (10 μg/ml) by chemiluminescent ELISA as described above.
Immunization studies
Two groups (n = 3/group) of 5-week-old male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were immunized with either a mimotope P2-BSA conjugate or BSA alone. For the primary immunization, 100 μg of antigen in PBS was emulsified in equal volumes of complete Freund's adjuvant (CFA; Sigma-Aldrich) and 50 μl of the homogenized suspension was injected subcutaneously at two sites. For the subsequent four booster immunizations every 2–3 weeks afterwards, 100 μg of antigen was emulsified in incomplete Freund's adjuvant (IFA; Sigma-Aldrich), and 100 μl was injected intraperitoneally. Blood was taken on days 0 (preimmune serum), 21, 35, 47, and 70, and plasma was stored at −80°C for further analyses. The experimental protocol was approved by the Animal Subjects Committee of the University of California, San Diego.
Immunohistochemistry
Immunostaining of formal sucrose-fixed, paraffin-embedded sections of aortas of atherosclerotic Watanabe heritable hyperlipidemic (WHHL) rabbits and human carotid atherosclerotic endarterectomy lesions was performed as described previously (15). Sections of human or rabbit lesion were blocked with PBS containing 5% horse serum and stained with diluted (1:200 or 1:400) pre- and postimmune sera from P2-BSA- and BSA-immunized mice, followed by addition of a biotinylated horse anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). A Vectastain ABC-alkaline phosphatase kit and a Vector Red alkaline phosphatase chromogenic substrate (Vector Labs, Burlingame, CA) were used to visualize Ab staining. Slides were counterstained with Weigert's Iron Hematoxylin (Richard-Allan Scientific, Kalamazoo, MI). Immunostaining of adjacent sections in the absence of primary Abs or the MDA-specific mAb MDA2 (5 μg/ml) was used as a negative or positive control, respectively.
Flow cytometry
Binding of LRO4 IgM to apoptotic Jurkat T-cells was assessed by flow cytometry. Apoptosis of Jurkat cells was induced by UV irradiation (UV Stratalinker, Stratagene) at 20 mJ/cm2, followed by further incubation of the cells in serum free medium for 14–16 h. After washing in FACS buffer (PBS with 0.1% BSA), 106 cells were incubated with 1 μg LRO4 in 100 μl FACS buffer for 30 min at 4°C. In parallel experiments, cells were incubated with LRO4 in the presence of increasing concentrations of P1, P2, and an irrelevant control peptide. Following a washing step, cells were stained with FITC-labeled anti-mouse IgM (II/41; BD Biosciences-Pharmingen) in 100 μl staining buffer for 30 min at 4°C in darkness and washed again. Thereafter, cells were incubated with PE-labeled Annexin-V and 7-AAD (BD Bioscience-Pharmingen) in Annexin-V binding buffer for 15 min and immediately analyzed by flow cytometry using a BD LSR II analyzer (BD Bioscience, San Jose, CA). More than 5.0 × 104 cells were acquired per sample. Data analysis was performed using FlowJo software (Tree Star Inc., San Carlos, CA).
Human subjects
Human plasma samples from two independent studies were analyzed for the presence of anti-peptide Abs. Details on the study cohorts are described elsewhere (32, 35); research protocols were approved by the Human Research Protection Program at the University of California, San Diego. For these studies, autoAb titers to MDA-LDL as the antigen have already been reported (32, 35); some figures from the original papers are reproduced in supplementary Figs. V and VII to allow ready comparisons to the new data generated here. Study 1 included 18 healthy subjects and 7 patients with acute coronary syndrome treated with primary PCI. Blood was obtained at baseline, and at one, three, and seven months thereafter. In addition, plasma from acute coronary syndrome patients was obtained at time of hospital discharge (approximately four days after admission). Study 2 included 114 patients with stable angina pectoris undergoing elective PCI. Blood samples were obtained before PCI, immediately after PCI, and 6 and 24 h, three days, one week, and one, three, and six months after PCI (32).
Statistical analysis
Statistical analyses were performed with GraphPad Prism 5 for Windows (GraphPad Software Inc., San Diego, CA). Statistical analysis of two groups was performed using the Student t-test. For continuous variables, differences were evaluated by one-way ANOVA (ANOVA). Spearman correlations were calculated to summarize the association between mimotope and MDA-LDL-specific autoAbs. P ≤ 0.05 was considered statistically significant. Data are presented as mean ± SD, if not indicated otherwise.
RESULTS
Selection of phages that display peptide mimotopes of MDA epitopes
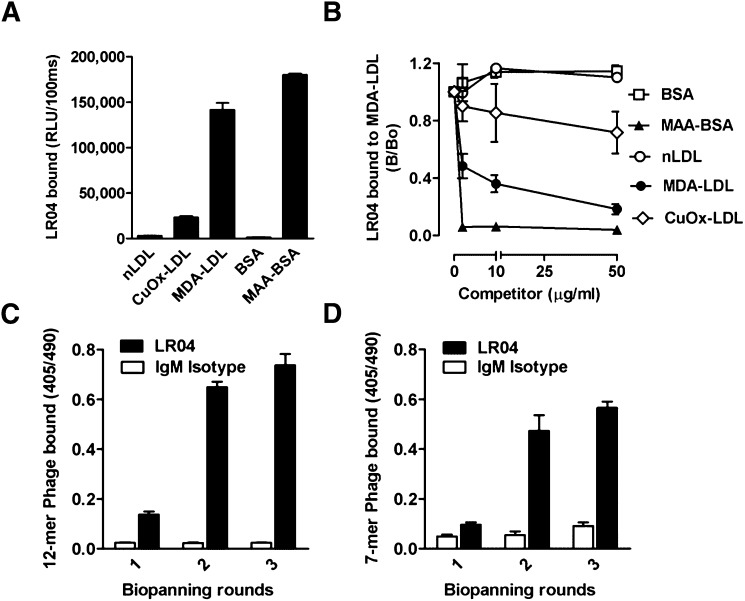
To generate peptide mimotopes of MDA epitopes present on MDA-LDL, peptide libraries were screened by biopanning for phages reactive with LRO4. LRO4 is a murine IgM mAb that was selected for its ability to bind to MDA-LDL. As shown in Fig. 1A, LRO4 binds to MDA-LDL and, to a much lesser degree, to CuOx-LDL, but not to native LDL. Moreover, LRO4 also bound to MAA-modified BSA but not to unmodified BSA, demonstrating specificity for advanced MDA adducts. (Fig. 1A). In immunocompetition assays, we further confirmed the high specificity of LRO4 for MDA adducts, as binding to coated MDA-LDL was efficiently competed by increasing concentrations of soluble MDA-LDL and MAA-BSA, but not native LDL or unmodified BSA (Fig. 1B). CuOx-LDL only showed moderate competition, consistent with the presence of minimal amounts of MDA-adducts in these preparations.
Fig. 1.
Biopanning of phage display peptide libraries with monoclonal antibody LRO4. (A) ELISA for the binding of the murine IgM mAb LRO4 (5 μg/ml) to native LDL (nLDL), CuOx-LDL, MDA-LDL, BSA, and MAA-modified BSA. Values are given as relative light units (RLU) per 100 ms and represent the mean ± SD of triplicate determinations. Data are representative of at least three independent experiments. (B) Competition immunoassay for the specificity of LRO4 binding to MDA-LDL. Data are expressed as a ratio of binding in the presence of competitor (B) divided by absence of competitor (B0) and represent the mean ± SD of triplicate determinations. Data are representative of three independent experiments. (C, D) ELISA for the binding of eluted phages to LRO4. After each round of biopanning, the binding of 1010 pfu/ml phage amplificates from the Ph.D.-12 library (C) and the Ph.D.-C7C library (D) to LRO4 (black bars) and an isotype control IgM (white bars) was tested as described in Materials and Methods. Values are given as absorbance measured at 405 and 490 nm and represent the mean ± SD of triplicate determinations.
Two phage peptide libraries, one displaying 12-mer linear peptides (Ph.D.-12) and one displaying 7-mer peptides constrained by flanking cysteines (Ph.D.-C7C), were screened for binding to coated LRO4 in three successive biopanning rounds. Enrichment for phages binding to LRO4, but not to a control IgM Ab, was confirmed by phage ELISA (Fig. 1C, D). A 336- and 400-fold enrichment of phage titers (determined by a plaque-forming units assay) was found after three rounds of selection with the Ph.D.-C7C library and the Ph.D.-12 library, respectively (supplementary Table I-A, B).
To identify individual LRO4-binding phages after the third round of biopanning, 132 single clones from the eluted phages of the Ph.D.-12 library and 30 clones from the eluted phages of the Ph.D.-C7C library were randomly selected and tested for reactivity with LRO4 and a control IgM by ELISA. Forty-two phage clones (25 dodecamers and 17 heptamers) showed significantly higher binding to LRO4 compared with control IgM (supplementary Table II-A, B). No binding of control phages (without a peptide insert) to LRO4 and control IgM was observed (data not shown). To prove the mimicry of selected phagotopes with MDA epitopes, a competitive phage ELISA was carried out, in which binding of LRO4 to MDA-LDL was tested in the absence and presence of selected phages. The ability of all clones to inhibit binding of LRO4 to MDA-LDL was greater than 75% (supplementary Table II-A, B). Control phages did not show any inhibition in this assay (data not shown). Selected phage clones with the highest binding capacity and specificity were amplified, sequenced, and amino acid sequences were deduced (supplementary Table II-A, B).
Binding characteristics of synthesized MDA mimotopes
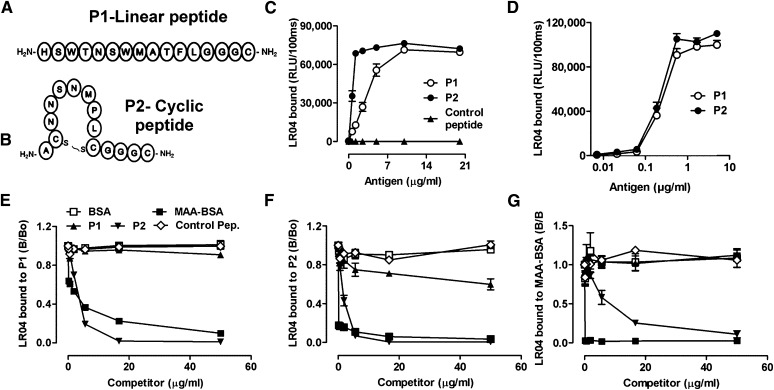
After alignment of all amino acid sequences in CLUSTAL W, we determined consensus sequences from each library and synthesized a linear dodecameric peptide P1 ( HSWTNSWMATFL ) (Fig. 2A) and a cyclic heptameric peptide P2 (AC NNSNMPL C) (Fig. 2B). Peptides were directly plated into microtiter wells at increasing concentrations, and the binding of LRO4 was tested. Both plated peptides P1 and P2 (but not an irrelevant control peptide IMGVGAVGAGAI ) were bound by LRO4 in a dose-dependent manner (Fig. 2C). A scrambled peptide of P2 (AC SPNLNMN C) was not recognized by LRO4 either (supplementary Fig. II-A). Binding of LRO4 to the plated peptides was similar to the binding to MDA-LDL and MAA-BSA (supplementary Fig. II-B). The PC-specific IgM mAb EO6 (26), which binds CuOx-LDL, did not react with either P1 or P2 (supplementary Fig. II-B).
Fig. 2.
Linear P1 and cyclic P2 peptides mimic a specific MDA epitope. (A, B) Schematic representation of synthesized peptides carrying the consensus sequence from phages identified with the Ph.D.-12 and Ph.D.-C7C libraries, respectively. P1(A) is a linear dodecameric peptide ( HSWTNSWMATFL ), and P2 (B) is a cyclic heptameric peptide (C NNSNMPL C). The peptides’ C terminus containing a GGGC-spacer was amidated. (C, D) ELISA for the binding of LRO4 to P1 and P2. (C) Binding of LRO4 to P1 and P2. Peptides P1, P2, and an irrelevant control peptide were coated at indicated concentrations. Binding of LRO4 (5 μg/ml) was determined by chemiluminescent ELISA. (D) Indicated concentrations of biotinylated peptides were captured on wells precoated with 10 μg/ml of neutravidin, and binding of LRO4 (5 μg/ml) was determined as described in Materials and Methods. Values are given as relative light units (RLU) per 100 ms and represent the mean ± SD of triplicate determinations. (E–G) Immunocompetition assays for the antigenic properties of P1 and P2. Binding of 0.5 μg/ml LRO4 to 100 ng/ml captured biotinylated peptides P1 (E) or P2 (F) or to 0.5 μg/ml coated MAA-BSA (G) was measured by ELISA in the presence of increasing concentrations of indicated competitors. Data represent the mean ± SD of triplicate determinations and are representative of three independent experiments.
To confirm the binding of LRO4 to the peptides in their native configuration, we designed peptides with a short spacer at the respective C-terminus (GGGK), which was linked to a biotin group. Biotinylated peptides were captured on neutravidin-coated wells and binding of LRO4 to increasing amounts of captured peptides was determined by ELISA. LRO4 recognized both peptides equally well, even at concentrations as low as 100 ng/ml (Fig. 2D).
To further test the binding specificity of LRO4 to P1 and P2 and to evaluate their function as peptide mimotopes of MDA epitopes, we performed a series of competition immunoassays. Binding of LRO4 to captured biotinylated P1 (Fig. 2E) and P2 (Fig. 2F) was fully competed by increasing concentrations of soluble MAA-BSA, but not unmodified BSA. Moreover, increasing concentrations of P2 fully competed for LRO4 binding to captured P2 as well as P1. Interestingly, soluble P1 did not compete for LRO4 binding to P1 or P2, respectively (Fig. 2E, F). Similarly, the irrelevant control peptide and scrambled peptide of P2 had no effect (Fig. 2E, F and supplementary Fig. II-C). In a reciprocal competition experiment, LRO4 binding to coated MAA-BSA was effectively competed by increasing concentrations of MAA-BSA as well as P2, whereas again, P1 did not have an effect (Fig. 2G). Thus, it appears that when plated, the linear peptide P1 serves as an excellent mimotope for MDA epitopes, but in solution, it loses this property, presumably due to conformational changes (36–38). In contrast, the cyclic peptide P2 is an excellent mimotope of MDA epitopes when plated and in solution.
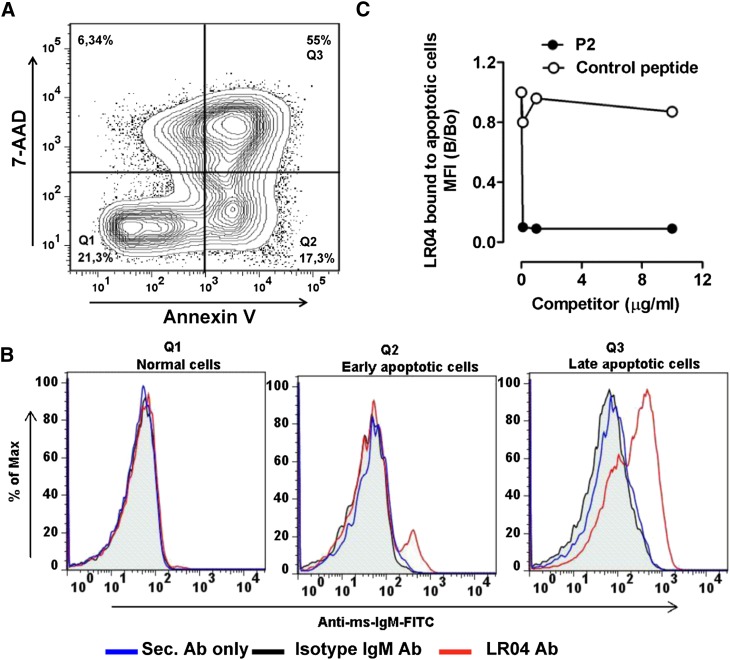
Peptides mimic an epitope present on apoptotic cells
To test whether the peptide mimotopes truly mimic epitopes occurring in vivo, we tested the ability of P2 to inhibit the binding of LRO4 to apoptotic cells. We previously showed that MDA epitopes are generated when cells undergo apoptosis and that MDA epitopes are a major OSE on the surface of apoptotic cells (14, 39). Consistent with these reports, we showed that LRO4 binds to the surface of early and late apoptotic cells, as indicated by Annexin-V and 7-AAD staining (Fig. 3A, B). Importantly, in the presence of increasing concentrations of soluble P2 (but not of an irrelevant control peptide), binding of LRO4 to apoptotic cells was fully inhibited (Fig. 3C). These data demonstrate that P2, the peptide mimotope of MDA, mimics epitopes on the surface of dying cells.
Fig. 3.
Mimotopes mimic MDA epitopes on apoptotic cells. (A) Representative flow cytometry plot of apoptotic Jurkat T-cells. Apoptosis of Jurkat T-cells was induced by 20 mJ/cm2 UV irradiation, and cells were stained with PE-labeled Annexin-V and 7-AAD to determine Annexin-V− 7-AAD− viable cells (Q1), Annexin-V+ early apoptotic (Q2) and Annexin-V+ 7-AAD+ late (Q3) apoptotic cells. (B) Representative flow cytometry histogram plot for the binding of apoptotic cells by LRO4. Apoptotic cells were induced as described in panel A and stained with either LRO4, isotype IgM, or no primary Ab, followed by detection using a FITC-conjugated anti-mouse IgM Ab as “secondary Ab” (Sec. Ab). Subsequently, cells were stained with PE-labeled Annexin-V and 7-AAD. The left panel represents staining of viable cells (Q1), the middle panel staining of early apoptotic cells (Q2), and the right panel staining of late apoptotic cells (Q3). (C) Inhibition of LRO4 binding to apoptotic cells by P2. Apoptotic cells were stained with LRO4 in the absence or presence of increasing amounts of P2 or an irrelevant control peptide, as indicated. Data represent the ratio of LRO4 binding as mean fluorescence intensity (MFI) obtained in the presence or absence of competitors (B/B0). Results are representative of two independent experiments.
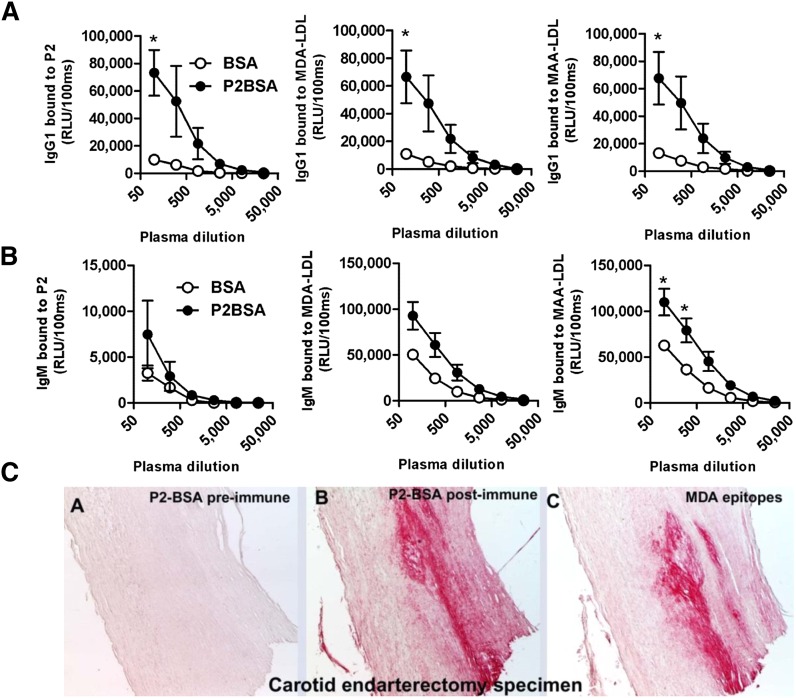
Mimotope immunization induces antibodies against MDA-LDL
On the basis of the preceding characterizations, we chose the cyclic P2 peptide to test for its immunogenic properties. P2 was conjugated to BSA (P2-BSA) as a carrier protein, which did not affect its reactivity and specificity for LRO4 (supplementary Fig. II-D, E). C57BL/6 mice were immunized with P2-BSA or BSA alone emulsified in Freund's adjuvant, and Ab titers were determined in the plasma obtained after the last boost. Mice immunized with P2-BSA developed high IgG1 titers to BSA and P2, whereas mice immunized with BSA developed only Ab titers to BSA (Fig. 4A and data not shown). Importantly, mice immunized with P2-BSA displayed a robust IgG1 response to MDA-LDL and MAA-LDL, which was not observed in control mice immunized with BSA alone (Fig. 4A), and a more modest IgG2c response (data not shown). In addition, the immunization with P2-BSA induced IgM titers to MDA-LDL and, more prominently, to MAA-LDL (Fig. 4B). Thus, immunization of mice with P2 mimotopes results in the induction of MDA-specific Abs, similar to that observed following immunization with homologous MDA-LDL (17).
Fig. 4.
MDA mimotope immunization induces MDA-specific Ab responses. (A, B) ELISA for the binding of plasma Abs of immunized mice to MDA-LDL, MAA-LDL, and P2. C57BL/6 mice were immunized with P2-BSA (n = 3) or BSA (n = 3) as described in Materials and Methods. Dilution curves of IgG1 and IgM binding to indicated antigens in pre- and postimmune plasma was determined by chemiluminescent ELISA. (A) Dilution curve of IgG1 binding. (B) Dilution curve of IgM binding. Values are given as relative light units (RLU) per 100 ms and represent the mean ± SEM of each group. (C) Immunohistochemical staining of human carotid endarterectomy specimens. Sections were stained with pooled preimmune (A) or postimmune plasma (B) of P2-BSA-immunized mice or with the MDA-LDL-specific mAb MDA2 (C). Positive staining is indicated by red color, and nuclei are counterstained with hematoxylin.
Antisera from mimotope-immunized mice recognize epitopes in atherosclerotic lesions
We immunostained human carotid endarterectomy specimens and lesions of WHHL rabbits with sera from immunized mice. Postimmune IgG of P2-BSA-immunized mice showed high reactivity in both human (Fig. 4C, panel B) and rabbit atherosclerotic lesions (supplementary Fig. III-B), similar to that achieved with the MDA-specific monoclonal Ab MDA2 (Fig. 4C, panel C). In contrast, postimmune IgG of BSA-immunized mice failed to stain (supplementary Fig. III-D). These data indicate that the Abs induced by mimotope immunization are specific for epitopes that occur in atherosclerotic lesions in vivo.
Mimotopes are recognized by human antibodies
We previously characterized the presence of autoAbs to MDA-LDL in humans in various clinical settings and demonstrated that these were hapten-specific (i.e., recognized MDA epitopes). Given the highly specific nature of P1 and P2 as MDA mimotopes in mice, we next asked whether the newly identified mimotopes would be equally recognized by human autoAbs, as was MDA-LDL. We first tested whether the human MDA-LDL-specific monoclonal Ab IK17, which was generated by Ab phage display, also binds the synthetic peptide mimotopes. Indeed, IK17 bound to MAA-BSA and P1 and P2 in a dose-dependent manner (supplementary Fig. IV), suggesting that both P1 and P2 could serve as MDA mimotopes for human autoAbs.
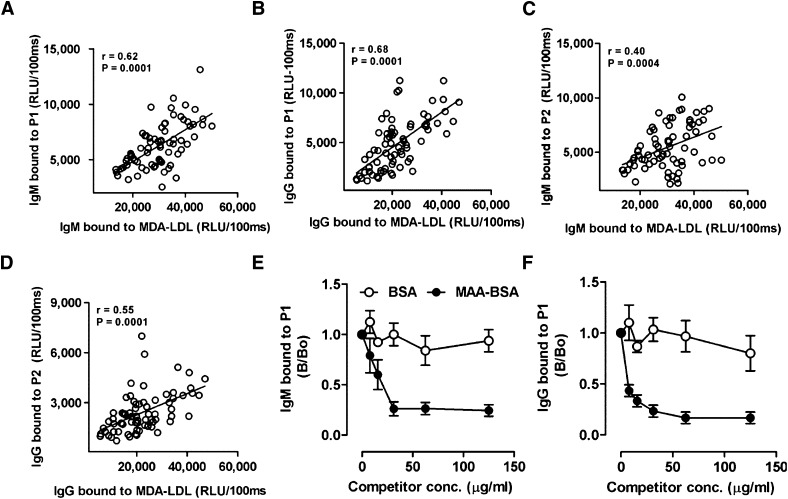
To directly validate this, we used plasma from a previous study (35) of middle-aged healthy volunteers (n = 18) sampled at four different time points over 210 days, and measured Ab binding to P1 and P2 as well as to MDA-LDL in parallel assays. Plasma of all individuals contained both IgG and IgM titers to P1 and P2, respectively (Fig. 5A–D). Moreover, the Ab titers to MDA-LDL in each plasma sample paralleled the titers to P1 and P2, resulting in significant correlations of IgG and IgM titers to P1 and P2 versus titers to MDA-LDL (Fig. 5). We further validated the specificity of P1-reactive Abs for MDA adducts in immunocompetition assays, showing that the binding of both IgM and IgG Abs to plated P1 was nearly completely inhibited by increasing concentrations of soluble MAA-BSA (Fig. 5E, F).
Fig. 5.
Mimotopes are recognized by human autoAbs and mimic MDA-LDL. (A–D) Correlation of mimotope and MDA-LDL-specific Ab titers in human plasma. Plasma of middle-aged healthy volunteers (n = 18) was obtained in a previous study (35) and Ab titers to P1, P2, and MDA-LDL were measured at 1:400 dilution by chemiluminescent ELISA as described in Materials and Methods. Correlation of IgM titers to MDA-LDL with IgM titers to P1 (A) and P2 (C). Correlation of IgG titer to MDA-LDL with IgG titers to P1 (B) and P2 (D). Values are given as relative light units (RLU) per 100 ms and represent the mean of triplicate determinations. Data points represent measurements of titers obtained from each of the 18 subjects at four different times: 0, 30, 120, and 210 days. Correlations were calculated by analyzing all data from all subjects using nonparametric Spearman's rank correlation (r = Spearman rank correlation coefficient). (E, F) Immunocompetition assays for specificity of IgG and IgM Abs to P1. Pooled human plasma was diluted 1:1,000 and incubated with or without increasing concentrations of BSA or MAA-BSA. Subsequently, samples were pelleted and binding of IgM (E) and IgG (F) Abs to coated P1 was determined in supernatants. Data are expressed as B/B0 and represent the mean ± SD of triplicate determinations.
We further analyzed disease-associated changes in MDA-specific Ab titers over a 7-month period using P1 and P2 as antigens. Previously, we measured the dynamics of OxLDL-specific Ab titers over time in patients with MI, and we found an initial 30% and 50% increase over baseline in anti-OxLDL IgG and IgM titers, respectively (35) (reproduced in supplementary Fig. V). When we tested these same plasma samples for Ab binding to P1 and P2, we found an even greater increase in both IgG and IgM titers to P1 and, to a lesser degree, to P2 (supplementary Fig. VI). Although IgM titers to P1 and P2 returned toward baseline after 210 days, IgG titers to both mimotopes remained increased even after 7 months.
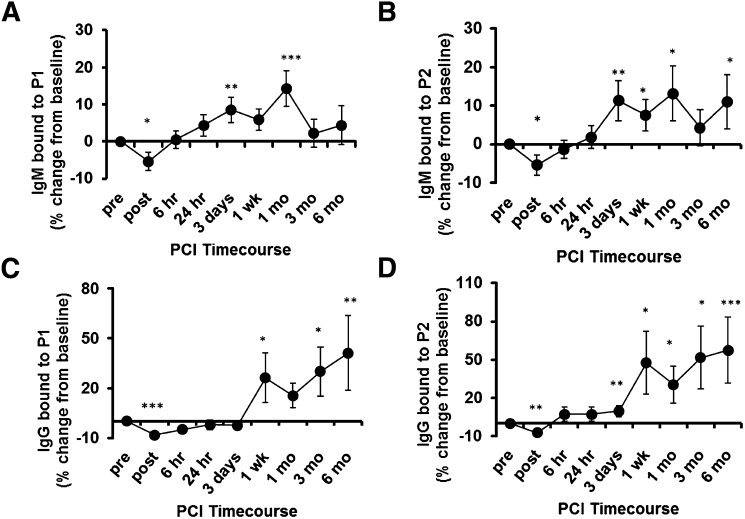
In another previous study, we examined autoAb titers in plasma from patients with stable angina collected immediately before and serially up to 6 months after PCI (32). Immediately after PCI, we found a significant drop in both IgG and IgM titers to MDA-LDL that was paralleled by an increase in immune complexes with apoB-containing lipoproteins. By 6 h, these values had returned to baseline, and over the next few weeks there was a rise in both IgG and IgM titers that persisted for up to 6 months (ref. 32 and reproduced in supplementary Fig. VII). Remarkably, utilizing the P1 and P2 mimotopes as antigens, we faithfully reproduced these patterns of changes in both IgG and IgM Ab responses (Fig. 6A–D). Anti-P1 IgM and IgG autoAbs positively correlated with previously (32) determined anti-MDA-LDL titers (r = 0.75, P < 0.0001 and r = 0.39, P < 0.0001, respectively), as did anti-P2 (r = 0.56, P < 0.0001 and r= 0.29, P < 0.0001, respectively).
Fig. 6.
Plasma antibody binding to mimotopes over time in patients undergoing PCI. A–D: ELISA for binding of plasma IgG and IgM to P1 and P2 in patients that underwent PCI. Sequential plasma samples were obtained following PCI (32). Samples were diluted 1:400 and binding of IgM (A, B) and IgG (C, D) to coated P1 (10 μg/ml; A, C) and P2 (5 μg/ml; B, D) was determined by chemiluminescent ELISA. Shown are relative mean percent changes in Ab binding compared with values obtained at baseline (pre-PCI). *P < 0.05, **P < 0.01, ***P < 0.001. These values are similar to titers against MDA-LDL as originally observed (32) and reproduced in supplementary Fig. VI.
Taken together, these data demonstrate that mimotope-specific Ab titers behave similarly to MDA-LDL-specific titers following an acute MI or PCI, and they suggest that as a consequence of MI or PCI, antigens are released that trigger an adaptive immune response, which cross-reacts with the newly identified peptide mimotopes. These findings demonstrate that P1 and P2 are mimotopes for relevant MDA antigens.
DISCUSSION
In this study, we report the identification and characterization of novel peptides mimicking the OSE MDA-LDL. On the basis of sequences obtained from three rounds of biopanning, we synthesized peptides carrying a consensus sequence for dodecameric (P1) and heptameric (P2) phagotopes, respectively. Both peptides were bound by the MDA-specific murine mAb LRO4 and the human IK17 Fab in a highly specific manner. Because we previously demonstrated that MDA-specific Abs bind to the surface of apoptotic cells (14, 39, 40), we confirmed the in vivo relevance of peptide mimotopes by demonstrating their ability to fully compete the binding of LRO4 to the surface of apoptotic cells. Moreover, immunization of mice with P2 mimotopes resulted in the induction of MDA-LDL-specific Ab titers, which specifically recognized epitopes in human carotid atherosclerotic lesions. Thus, the synthetic peptides mimic OSEs that occur in vivo and are enriched in atherosclerotic lesions.
These well-defined OSE mimotopes have significant potential in biotheranostic applications in humans by providing standardized, chemically defined antigens. As an example of their utility, we demonstrated the ability of P1 and P2 to act as surrogates for MDA-LDL in autoAb assays. Both sera of healthy subjects and patients with CVD contained autoAbs titers against the peptide mimotopes, which correlated positively with MDA-LDL-specific titers. We further showed that previously demonstrated dynamic changes in these titers to MDA-LDL following MI or PCI could be faithfully reproduced using P1 or P2 as antigens. For example, in response to PCI, there was an immediate drop in Ab titers, consistent with binding to OSE antigens released into the circulation, followed by long-term rises in Ab titers, consistent with anamnestic responses to OSEs, likely reflecting the extent of injury. These data exactly parallel our previous demonstration of the Ab dynamics against MDA-LDL as antigen (32, 35).
Our findings also identify for the first time simple peptides that mimic lipid peroxidation-derived structures, although the exact nature of the molecular mimicry between nonprotein antigens and their mimicking peptides is not completely understood (41). This is noteworthy, as oxidized lipid derivatives are notoriously unstable structures. Peptide mimotopes of carbohydrates and PC of capsular polysaccharides have been described before (42–44). However, these are different from the MDA mimotopes described here. Importantly, MDA mimotopes are not recognized by the mAb EO6, which has specificity for PC. Thus, the identified peptides are highly specific mimics of MDA epitopes of MDA-LDL, which itself is a complex antigen with different types of MDA neoantigens. Recently, MAA epitopes have been identified as dominant epitopes of MDA modifications (Ref. 22 and unpublished data). Indeed, the newly found mimotopes seem to mimic MAA epitopes in MDA-LDL, as binding of autoAbs to P1 in pooled plasma of healthy subjects was inhibited by MAA-BSA, indicating that the mimotope-specific autoAbs in human plasma have specificity for MAA. These data suggest that the peptide mimotopes predominantly represent the fluorescent MAA-type epitopes that occur following MDA modification of proteins. Thus, not all MDA-LDL-reactive antibodies and/or proteins may bind to the mimotopes. Nevertheless, this fine specificity may be useful in future studies as they more specifically define a restricted set of “MDA-reactive” antibodies to an immunodominant set of MAA modifications.
We previously conducted extensive studies documenting the presence and predictive value of autoAbs to MDA-LDL in humans with CVD (e.g., Refs. 11, 13). One of the major limitations of such studies is preparing reproducible MDA-LDL, which is generated by MDA modification of freshly isolated LDL. The newly generated MDA mimotopes now identify for the first time a reliable surrogate for MDA-LDL and, more specifically, for MAA-type autoantibodies. The availability of these small peptide mimotopes will greatly enhance the reproducibility and facilitate the standardization of assays for the determination of such autoAbs. Indeed, the finding that we did not observe a strong positive correlation in all subjects between titers to MDA-LDL and to P1/P2 likely reflects subtle differences in disease-relevant autoAbs that are masked by the use of “MDA-LDL” as antigen. Future studies will be needed to determine whether the use of these peptides as antigens will refine the clinical utility of such antibody measurements as biomarkers of CVD or other inflammatory settings.
Other applications of “OSE mimotopes” may be use in molecular imaging and as immunogens in atheroprotective vaccines. In prior studies (45), we demonstrated that OSE in atherosclerotic lesions can be targeted and imaged with human and murine oxidation-specific antibodies carried by MRI nanoparticles. These nanoparticles accumulate within macrophages, allowing visualization of active lesions. In an analogous manner, “OSE mimotopes” may be bound to similar nanoparticles and used as imaging agents, where they would target scavenger receptors on activated macrophages. This approach may be complementary to using OSE-specific monoclonal antibodies, which would bind to extracellular epitopes, as for example, on OxLDL, whereas OSE mimotopes might more specifically image macrophages.
Another use would be as an immunogen in atheroprotective vaccines. We and others have demonstrated the atheroprotective effect of immunization strategies using MDA-LDL in animal models (24). Use of such an antigen in human studies would be impractical. In this study, we demonstrated that immunization with P2-conjugated BSA gave rise to anti-MDA-LDL Abs and that these Abs immunostained atherosclerotic lesions, suggesting its potential as an immunogen for such a vaccine. Future studies will be needed to evaluate its potential to induce atheroprotective immunity.
In conclusion, our studies have identified two peptides that mimic epitopes of MDA-LDL. We provide several lines of evidence documenting the capacity of these peptides to mimic epitopes relevant to atherogenesis. Because both mimotopes are recognized by MDA-specific autoAbs in human sera, they represent for the first time highly reproducible, standardized antigens for the determination of anti-MDA-LDL Abs. Thus, future studies using these mimotopes as antigens may provide a reliable assay to evaluate the role of MDA-specific Abs as biomarkers for CVD. Finally, in conjunction with different carriers, the newly identified peptides can also be used as immunogens to study the mechanistic role of MDA-specific immune responses in immunization studies, and they may provide the basis for potential atheroprotective vaccine preparations.
Acknowledgments
The authors thank Maria Ozsvar Kozma for excellent technical help and Dr. Krisztina Szalai for helpful discussions.
Footnotes
This work was supported by the doctoral program Cell Communication in Health and Disease (CCHD), funded by the Austrian Science Fund and the Medical University of Vienna; by the SFB Lipotox F30 of the Austrian Science Fund (C.J.B.); by National Institutes of Health Grants HL-088093 (C.J.B., S.T., J.L.W.) and RO1-HL-086599 (K.H., J.L.W.); and by the Fondation Leducq (C.J.B., S.T., J.L.W.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
Abbreviations:
- CuOx-LDL
- Cu2+-oxidized LDL
- CVD
- cardiovascular disease
- MAA-BSA
- malondialdehyde-acetaldehyde-modified BSA
- MDA-LDL
- malondialdehyde-modified LDL
- MI
- myocardial infarction
- OSE
- oxidation-specific epitope
- OxLDL
- oxidized LDL
- PC
- phosphocholine
- PCI
- percutaneous coronary intervention
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables and seven figures.
REFERENCES
- 1.Binder C. J., Chang M. K., Shaw P. X., Miller Y. I., Hartvigsen K., Dewan A., Witztum J. L. 2002. Innate and acquired immunity in atherogenesis. Nat. Med. 8: 1218–1226 [DOI] [PubMed] [Google Scholar]
- 2.Miller Y. I., Choi S. H., Wiesner P., Fang L., Harkewicz R., Hartvigsen K., Boullier A., Gonen A., Diehl C. J., Que X., et al. 2011. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 108: 235–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palinski W., Rosenfeld M. E., Yla-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. 1989. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. USA. 86: 1372–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ylä-Herttuala S., Palinski W., Butler S. W., Picard S., Steinberg D., Witztum J. L. 1994. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler. Thromb. 14: 32–40 [DOI] [PubMed] [Google Scholar]
- 5.Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. 1989. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Invest. 84: 1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsimikas S., Palinski W., Witztum J. L. 2001. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21: 95–100 [DOI] [PubMed] [Google Scholar]
- 7.Cyrus T., Pratico D., Zhao L., Witztum J. L., Rader D. J., Rokach J., FitzGerald G. A., Funk C. D. 2001. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein E-deficient mice. Circulation. 103: 2277–2282 [DOI] [PubMed] [Google Scholar]
- 8.Praticò D., Tangirala R. K., Hörkkö S., Witztum J. L., Palinski W., FitzGerald G. A. 2001. Circulating autoantibodies to oxidized cardiolipin correlate with isoprostane F(2alpha)-VI levels and the extent of atherosclerosis in ApoE-deficient mice: modulation by vitamin E. Blood. 97: 459–464 [DOI] [PubMed] [Google Scholar]
- 9.Gounopoulos P., Merki E., Hansen L. F., Choi S. H., Tsimikas S. 2007. Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease. Minerva Cardioangiol. 55: 821–837 [PubMed] [Google Scholar]
- 10.Tsimikas S. 2006. Oxidative biomarkers in the diagnosis and prognosis of cardiovascular disease. Am. J. Cardiol. 98: 9P–17P [DOI] [PubMed] [Google Scholar]
- 11.Ravandi A., Boekholdt S. M., Mallat Z., Talmud P. J., Kastelein J. J., Wareham N. J., Miller E. R., Benessiano J., Tedgui A., Witztum J. L., et al. 2011. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J. Lipid Res. 52: 1829–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopes-Virella M. F., Virella G. 2010. Clinical significance of the humoral immune response to modified LDL. Clin. Immunol. 134: 55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsimikas S., Brilakis E. S., Lennon R. J., Miller E. R., Witztum J. L., McConnell J. P., Kornman K. S., Berger P. B. 2007. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 48: 425–433 [DOI] [PubMed] [Google Scholar]
- 14.Chou M. Y., Fogelstrand L., Hartvigsen K., Hansen L. F., Woelkers D., Shaw P. X., Choi J., Perkmann T., Backhed F., Miller Y. I., et al. 2009. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Invest. 119: 1335–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palinski W., Miller E., Witztum J. L. 1995. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc. Natl. Acad. Sci. USA. 92: 821–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freigang S., Horkko S., Miller E., Witztum J. L., Palinski W. 1998. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler. Thromb. Vasc. Biol. 18: 1972–1982 [DOI] [PubMed] [Google Scholar]
- 17.Binder C. J., Hartvigsen K., Chang M. K., Miller M., Broide D., Palinski W., Curtiss L. K., Corr M., Witztum J. L. 2004. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 114: 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George J., Afek A., Gilburd B., Levkovitz H., Shaish A., Goldberg I., Kopolovic Y., Wick G., Shoenfeld Y., Harats D. 1998. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 138: 147–152 [DOI] [PubMed] [Google Scholar]
- 19.Fredrikson G. N., Soderberg I., Lindholm M., Dimayuga P., Chyu K. Y., Shah P. K., Nilsson J. 2003. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler. Thromb. Vasc. Biol. 23: 879–884 [DOI] [PubMed] [Google Scholar]
- 20.Zhou X., Caligiuri G., Hamsten A., Lefvert A. K., Hansson G. K. 2001. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 21: 108–114 [DOI] [PubMed] [Google Scholar]
- 21.Tsimikas S., Miyanohara A., Hartvigsen K., Merki E., Shaw P. X., Chou M. Y., Pattison J., Torzewski M., Sollors J., Friedmann T., et al. 2011. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J. Am. Coll. Cardiol. 58: 1715–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duryee M. J., Klassen L. W., Schaffert C. S., Tuma D. J., Hunter C. D., Garvin R. P., Anderson D. R., Thiele G. M. 2010. Malondialdehyde–acetaldehyde adduct is the dominant epitope after MDA modification of proteins in atherosclerosis. Free Radic. Biol. Med. 49: 1480–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binder C. J., Hartvigsen K., Witztum J. L. 2007. Promise of immune modulation to inhibit atherogenesis. J. Am. Coll. Cardiol. 50: 547–550 [DOI] [PubMed] [Google Scholar]
- 24.Amir S., Binder C. J. 2010. Experimental immunotherapeutic approaches for atherosclerosis. Clin. Immunol. 134: 66–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuominen A., Miller Y. I., Hansen L. F., Kesaniemi Y. A., Witztum J. L., Horkko S. 2006. A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 26: 2096–2102 [DOI] [PubMed] [Google Scholar]
- 26.Hörkkö S., Bird D. A., Miller E., Itabe H., Leitinger N., Subbanagounder G., Berliner J. A., Friedman P., Dennis E. A., Curtiss L. K., et al. 1999. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Invest. 103: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palinski W., Ylä-Herttuala S., Rosenfeld M. E., Butler S. W., Socher S. A., Parthasarathy S., Curtiss L. K., Witztum J. L. 1990. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 10: 325–335 [DOI] [PubMed] [Google Scholar]
- 28.Shaw P. X., Hörkkö S., Tsimikas S., Chang M. K., Palinski W., Silverman G. J., Chen P. P., Witztum J. L. 2001. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler. Thromb. Vasc. Biol. 21: 1333–1339 [DOI] [PubMed] [Google Scholar]
- 29.Perschinka H., Wellenzohn B., Parson W., van der Zee R., Willeit J., Kiechl S., Wick G. 2007. Identification of atherosclerosis-associated conformational heat shock protein 60 epitopes by phage display and structural alignment. Atherosclerosis. 194: 79–87 [DOI] [PubMed] [Google Scholar]
- 30.Petit M. A., Jolivet-Reynaud C., Peronnet E., Michal Y., Trepo C. 2003. Mapping of a conformational epitope shared between E1 and E2 on the serum-derived human hepatitis C virus envelope. J. Biol. Chem. 278: 44385–44392 [DOI] [PubMed] [Google Scholar]
- 31.Binder C. J., Hörkkö S., Dewan A., Chang M. K., Kieu E. P., Goodyear C. S., Shaw P. X., Palinski W., Witztum J. L., Silverman G. J. 2003. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 9: 736–743 [DOI] [PubMed] [Google Scholar]
- 32.Tsimikas S., Lau H. K., Han K. R., Shortal B., Miller E. R., Segev A., Curtiss L. K., Witztum J. L., Strauss B. H. 2004. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 109: 3164–3170 [DOI] [PubMed] [Google Scholar]
- 33.Friguet B., Chaffotte A. F., Djavadi-Ohaniance L., Goldberg M. E. 1985. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods. 77: 305–319 [DOI] [PubMed] [Google Scholar]
- 34.Faghihnia N., Tsimikas S., Miller E. R., Witztum J. L., Krauss R. M. 2010. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J. Lipid Res. 51: 3324–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsimikas S., Bergmark C., Beyer R. W., Patel R., Pattison J., Miller E., Juliano J., Witztum J. L. 2003. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J. Am. Coll. Cardiol. 41: 360–370 [DOI] [PubMed] [Google Scholar]
- 36.Shin J. S., Yu J., Lin J., Zhong L., Bren K. L., Nahm M. H. 2002. Peptide mimotopes of pneumococcal capsular polysaccharide of 6B serotype: a peptide mimotope can bind to two unrelated antibodies. J. Immunol. 168: 6273–6278 [DOI] [PubMed] [Google Scholar]
- 37.Dyson H. J., Wright P. E. 1991. Defining solution conformations of small linear peptides. Annu. Rev. Biophys. Biophys. Chem. 20: 519–538 [DOI] [PubMed] [Google Scholar]
- 38.Haro I., Gomara M. J. 2004. Design of synthetic peptidic constructs for the vaccine development against viral infections. Curr. Protein Pept. Sci. 5: 425–433 [DOI] [PubMed] [Google Scholar]
- 39.Chang M. K., Binder C. J., Miller Y. I., Subbanagounder G., Silverman G. J., Berliner J. A., Witztum J. L. 2004. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 200: 1359–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang M. K., Bergmark C., Laurila A., Hörkkö S., Han K. H., Friedman P., Dennis E. A., Witztum J. L. 1999. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. USA. 96: 6353–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson M. A., Pinto B. M. 2008. Structural and functional studies of peptide-carbohydrate mimicry. Top. Curr. Chem. 273: 55–116 [DOI] [PubMed] [Google Scholar]
- 42.Buchwald U. K., Lees A., Steinitz M., Pirofski L. A. 2005. A peptide mimotope of type 8 pneumococcal capsular polysaccharide induces a protective immune response in mice. Infect. Immun. 73: 325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris S. L., Dagtas A. S., Diamond B. 2002. Regulating the isotypic and idiotypic profile of an anti-PC antibody response: lessons from peptide mimics. Mol. Immunol. 39: 263–272 [DOI] [PubMed] [Google Scholar]
- 44.Monzavi-Karbassi B., Cunto-Amesty G., Luo P., Kieber-Emmons T. 2002. Peptide mimotopes as surrogate antigens of carbohydrates in vaccine discovery. Trends Biotechnol. 20: 207–214 [DOI] [PubMed] [Google Scholar]
- 45.Briley-Saebo K. C., Cho Y. S., Tsimikas S. 2011. Imaging of oxidation-specific epitopes in atherosclerosis and macrophage-rich vulnerable plaques. Curr. Cardiovasc. Imaging. Rep. 4: 4–16 [DOI] [PMC free article] [PubMed] [Google Scholar]