Abstract
Recombinant human prostaglandin endoperoxide H synthase-1 (huPGHS-1) was characterized. huPGHS-1 has a single high-affinity heme binding site per dimer and exhibits maximal cyclooxygenase (COX) activity with one heme per dimer. Thus, huPGHS-1 functions as a conformational heterodimer having a catalytic monomer (Ecat) with a bound heme and an allosteric monomer (Eallo) lacking heme. The enzyme is modestly inhibited by common FAs including palmitic, stearic, and oleic acids that are not COX substrates. Studies of arachidonic acid (AA) substrate turnover at high enzyme-to-substrate ratios indicate that nonsubstrate FAs bind the COX site of Eallo to modulate the properties of Ecat. Nonsubstrate FAs slightly inhibit huPGHS-1 but stimulate huPGHS-2, thereby augmenting AA oxygenation by PGHS-2 relative to PGHS-1. Nonsubstrate FAs potentiate the inhibition of huPGHS-1 activity by time-dependent COX inhibitors, including aspirin, all of which bind Ecat. Surprisingly, preincubating huPGHS-1 with nonsubstrate FAs in combination with ibuprofen, which by itself is a time-independent inhibitor, causes a short-lived, time-dependent inhibition of huPGHS-1. Thus, in general, having a FA bound to Eallo stabilizes time-dependently inhibited conformations of Ecat. We speculate that having an FA bound to Eallo also stabilizes Ecat conformers during catalysis, enabling half of sites of COX activity.
Keywords: prostaglandin, half of site, naproxen, aspirin, ibuprofen, celecoxib, palmitic acid, oleic acid, fatty acid, cyclooxygenase
Prostaglandin endoperoxide H synthases (PGHSs) catalyze the conversion of certain essential PUFAs to eicosanoid products (1–5). Of particular importance are the formation of 1-, 2- and 3-series prostaglandins from dihomo-γ-linolenic acid (DHLA), arachidonic acid (AA), and eicosapentaenoic acid (EPA), respectively. PGHSs are commonly called cyclooxygenases (COXs). There are two PGHS isoforms encoded by different genes and known as PGHS-1 and PGHS-2 or COX-1 and COX-2.
PGHSs catalyze both COX and peroxidase (POX) reactions that occur at distinct physical sites on the enzymes but sites that are electronically and mechanistically linked. The COX is a bis-oxygenase. During the COX reaction with AA, two O2 molecules are introduced into the carbon backbone of the substrate, and various rearrangements occur that yield a bicyclic endoperoxide called prostaglandin G2 (PGG2). The 15-hydroperoxyl group of PGG2 can be reduced to prostaglandin H2 by the POX activity. The POX and COX activities of PGHSs require hydroperoxides (4, 6). Hydroperoxides are immediate substrates for the POX activity of PGHS. Oxidation of the heme group at the POX site during the reduction of peroxides leads to the formation of a tyrosyl radical in the COX site. The tyrosyl radical abstracts a hydrogen from AA in the initial step in COX catalysis. Ovine (ov) PGHS-1 prefers primary and secondary alkyl hydroperoxides to H2O2 or bulky peroxides like t-butyl hydroperoxide (7, 8).
PGHSs are targets of COX inhibitors, including nonspecific nonsteroidal anti-inflammatory drugs (nsNSAIDs) that inhibit both isoforms and COX-2 inhibitors that are relatively more specific for the COX activity of PGHS-2; the more recently developed COX-2 inhibitors are often called coxibs. Mechanistically, COX inhibitors fall into two broad categories, time-dependent and time-independent/rapidly reversible inhibitors (4, 5, 9, 10). Inhibition of PGHS-2 is associated with decreased inflammation and analgesia (11–13). COX-2 inhibition may also decrease mortality from colon cancer (14, 15). Human PGHS-1 (huPGHS-1) is the target of low-dose aspirin (ASA) therapy that reduces the risk of recurrence of myocardial infarction and is used to treat unstable angina (16–21).
Our recent examination of PGHS-2 (22–24) has established that this isoform functions in an unusual manner as a conformational heterodimer (22–25). Although PGHS-2 is a homodimer comprising two tightly associated monomers having apparently identical primary structures, each monomer functions differently. One behaves as an allo-steric monomer, designated Eallo, whereas its partner monomer, called Ecat, performs the catalytic operations. There is also evidence that ovPGHS-1 functions as a conformational heterodimer (22, 25–28).
Because of its ease of isolation, most of what is known about the PGHS-1 isoform comes from studies of ovPGHS-1 (1–5). There is limited biochemical information about huPGHS-1 (29–33). However, examining the properties of the huPGHS-1 isoform is important because of its obvious pharmacologic importance and a now-growing recognition that there are potentially significant drug interactions involving ASA and other nsNSAIDs (5, 24, 34, 35), COX-2 inhibitors such as celecoxib (CBX) (35–37), and dietary FAs (22, 35).
In this report, we describe interactions of huPGHS-1 with COX inhibitors and nonsubstrate FAs. Our results provide support for the concept that huPGHS-1, like huPGHS-2, operates as a conformational heterodimer. Two additional, more-specific observations are a) that nonsubstrate FAs weakly inhibit huPGHS-1, and b) that responses of huPGHS-1 to nsNSAIDs and COX-2 inhibitors are potentiated by common FAs that are not COX substrates. A novel finding is that FAs, when combined with time-independent nsNSAIDs like ibuprofen (IBF) elicit a time-dependent inhibition. We speculate that this reflects an FA-induced kinetic delay that underlies the ability of PGHSs to exhibit half of site activity and to be regulated allosterically by ambient FAs in cells.
EXPERIMENTAL PROCEDURES
Materials
AA, oleic acid (OA; 18:1ω9), 11 cis-eicosaenoic acid (Δ11c 20:1), dihomo-γ-linolenic acid (DHLA), α-linoleic acid (LA), α-linolenic acid, 11 cis, 14 cis-eicosadienoic acid (EDA), adrenic acid (ADA; 8 cis, 11 cis, 14 cis, 17 cis-docosatetraenoic acid), EPA (5 cis, 8 cis, 11 cis, 14 cis, 17 cis-eicosapentaenoic acid), and docosahexaenoic acid (DHA; 4 cis, 7 cis, 10 cis, 13 cis, 16 cis, 19 cis-docosahexaenoic acid) were from Cayman Chemical Co. Lauric acid (12:0), myristic acid (14:0), pentadecanoic acid (15:0), palmitic acid (PA; 16:0), margaric acid (17:0), stearic acid (SA; 18:0), nonadecanoic acid (19:0), flurbiprofen (FBP), indomethacin (INDO), ASA, and naproxen (NAPX) were from Sigma. Hemin (heme) was from Frontier Scientific, Logan, UT. CelebrexR (CBX) was from physician samples. IBF was from Tocris Bioscience. Decyl maltoside (C10M), n-octyl β-d-glucopyranoside (β-OG), and C10E6 detergents were purchased from Anatrace (Maumee, OH). BCA protein reagent was from Pierce. Polyacrylic acid (sodium salt) 5100 was from Hampton Research Corp. [1-14C]AA (1.85 GBq/mmol) was from American Radiolabeled Chemicals. Hexane, isopropanol, and acetic acid were HPLC grade from Thermo Fisher Scientific, Inc. Complete protease inhibitor was from Roche Applied Science. Restriction enzymes were from New England Biolabs, Inc. Nickel-NTA (nitrilotriacetic acid) was from Qiagen. A sample of tobacco etch virus (TEV) protease was generously provided by Dr. Ming Lei (38). All other materials were analytical grade from Sigma Chemical Co.
Expression, purification, and assay of huPGHS-1
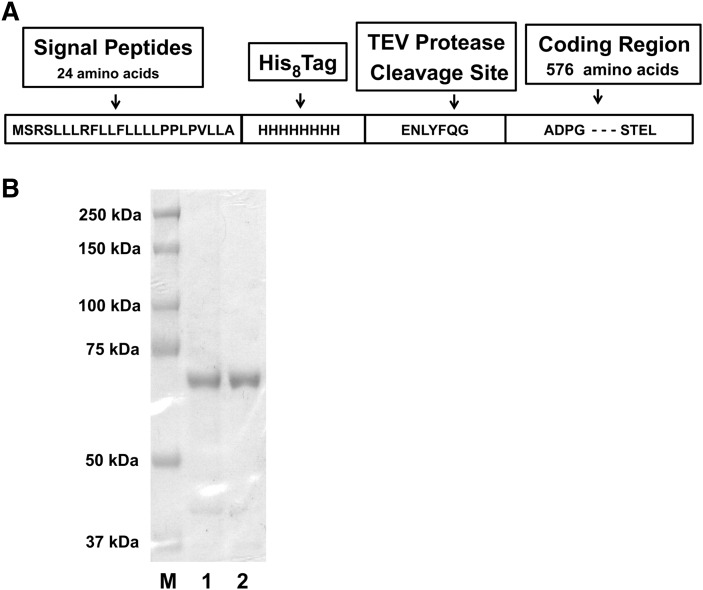
A recombinant huPGHS-1 was engineered with a sequence that contains a octa-histidine tag and a TEV protease cleavage site near the N-terminus but downstream of the signal peptide (Fig. 1). The coding region was incorporated into a baculovirus that was used to infect insect cells from which the recombinant enzyme was purified. The pFastBac expression vector was generated according to the instructions of the manufacturer for the Invitrogen Bac-to-Bac expression system. After 3–4 days of infection with the recombinant baculovirus, the cells were harvested, washed with PBS, and stored at −80°C. The method for the purification of huPGHS-1 was essentially the same as in a previous report for ovPGHS-1 (22). Protein was quantified using BCA reagent. The purity of the recombinant huPGHS-1 was determined by SDS-PAGE and by Western blot analysis. The specific activity of purified huPGHS-1 for the preparations used in the experiments reported here averaged 19 U/mg. One unit of COX activity is defined as 1 μmol of O2 consumed/min at 37°C in a standard assay mixture containing 100 μM AA.
Fig. 1.
Preparation of recombinant huPGHS-1. A: Predicted amino acid sequence of recombinant huPGHS-1 indicating the location of the signal peptide, the octa-histidine tag, and the TEV protease cleavage site. B: Coomassie Blue staining after SDS-PAGE of purified recombinant huPGHS-1 (lane 1), recombinant hexa-histidine-tagged ovPGHS-1 (22) (lane 2), and Bio-Rad protein molecular weight standards (lane M). The purity of huPGHS-1 in lane 1 is 92 ± 1.9%, and the purity of ovPGHS-1 in lane 2 is 94 ± 0.9% as determined by densitometry using ImageJ software (rsbweb.nih.gov/ij/).
The COX activity of huPGHS-1 was measured as described previously (39), except that huPGHS-1 was routinely preincubated with two molar equivalents of heme at 37°C for 10 min before being assayed. COX activity was measured polarographically at 37°C in glass chambers containing 3 ml of 0.1 M Tris HCl, pH 8.0, 100 μM AA, 1 mM phenol, and 5 μM heme. A Yellow Springs Instruments Model 53 oxygen monitor was used to monitor O2 consumption, and kinetic traces were recorded using DasyLab (DasyTec) software (22). Reactions were initiated by adding enzyme to the assay chamber, unless otherwise indicated. In experiments involving COX inhibitors, the enzyme preparations were usually preincubated with heme, then preincubated with the inhibitor plus or minus an FA, and an aliquot was then added to the O2 electrode assay chamber. The rates reported were maximal rates occurring after a lag phase. The lag time is defined as the time required for the COX activity to reach a maximum after initiating the reaction (24), which, under optimal conditions, is about 10 s.
Cleavage of huPGHS-1 by TEV protease
Purified recombinant huPGHS-1 [2.0 mg (1.0 mg/ml)] was treated with TEV protease (0.2 mg) at 4°C for 24 h in 20 mM Tris HCl, pH 8.0, containing 40 mM KCl, 5% glycerol, and 0.02% C10E6. The sample was mixed with Ni-NTA resin equilibrated in 20 mM Tris HCl, pH 8.0, containing 40 mM KCl, 5% glycerol, and 0.02% C10E6. The reaction solution was filtered slowly through the Ni-NTA resin twice, the resin was harvested and washed with 2.0 ml of buffer, and was harvested again. The yield of unbound huPGHS-1 was 1.1 mg.
HPLC analysis of the oxygenation of AA at high ratios of huPGHS-1 to AA
Oxygenation reactions at high enzyme/substrate ratios were conducted in 100 μl reaction mixtures containing 1 μM [1-14C]AA, 0.10–2.0 μM of huPGHS-1, 5 μM hematin, and 1 mM phenol in 0.1 M Tris HCl, pH 8.0, at 37°C essentially as described previously (24). Organic extracts of the reactions were subjected to HPLC on a Luna C18 (2) column (5 μm, 250 × 4.6 mm, Phenomenex) mounted on a Shimadzu HPLC system equipped with a radio-HPLC detector (IN/US system, β-RAM model 4), and the separated radioactive reaction products and starting material were quantified (24).
Titration of huPGHS-1 with heme
Difference absorption spectra of apo-huPGHS-1 titrated with heme were obtained using a Shimadzu UV-2501 PC scanning spectrophotometer as described previously (24). Aliquots of heme solutions in 20 mM Tris HCl, pH 8.3, containing 40 mM KCl, 0.02% C10E6, and 2% DMSO, were added to a quartz cuvette containing 200 μl of 5 μM apo-huPGHS-1 in the same buffer solution without DMSO at room temperature (40, 41). The increase in absorbance at 412 nm was transformed to a binding fraction and then plotted using GrapherTM 7 software in a linear form using the Scatchard equation in which the slope is equal to the −1/Kd for heme binding.
RESULTS
Characterization of recombinant huPGHS-1 and its interaction with heme
A recombinant huPGHS-1 was engineered with a sequence that contains both an octa-histidine tag and a TEV protease cleavage site near the N-terminus and downstream of the signal peptide (Fig. 1A). The coding region was incorporated into a baculovirus that was used to infect insect cells from which the recombinant enzyme was purified. Figure 1B shows an SDS-PAGE of the purified recombinant huPGHS-1. The electrophoretic mobility of the huPGHS-1 with respect to the standards corresponded to a protein with a molecular mass of 73 kDa, similar to that of ovPGHS-1 prepared from ovine seminal vesicles. The purity of the huPGHS-1 was estimated by densitometry to be greater than 90%. The average specific activity for three representative preparations of purified huPGHS-1 was 19 ± 1.8 U/mg, and the Km of huPGHS-1 for AA was 5.1 ± 0.8 μM. These values are in the range of those reported previously for other recombinant versions of huPGHS-1 (31–33).
Both the COX and POX activities of PGHSs require heme for activity. Purified huPGHS-1 had little or no activity when assayed in the absence of heme but underwent a relatively rapid time-dependent activation upon preincubation with heme (Fig. 2A). These results indicated that the purified enzyme is largely in an apo form, as is typically observed with purified PGHSs (24, 26, 42, 43) and is activated at relatively low heme/protein ratios. We analyzed in further detail the affinity and stoichiometry of heme binding to apo-huPGHS-1 (Fig. 2B–D). Titration of apo-huPGHS-1 with heme yielded a major UV/VIS spectral peak with a maximum at 412 nm, consistent with the formation of a heme/PGHS-1 complex (Fig. 2B) (24, 26, 42, 43). The data indicate that there is one high-affinity heme binding site per PGHS-1 dimer. In the representative titration shown in Fig. 2B, there was a higher affinity heme site with a Kd1 = 0.110 ± 0.005 μM (n = 3) and a second lower affinity site(s) (Kd2 = 6.7 ± 1.1 μM) (Fig. 2C, D). We presume that the higher affinity site represents binding of heme to a POX site of huPGHS-1 and that lower affinity binding occurs to the second POX site and/or another site(s); removal of the octa-histidine tag on the N-terminus of the recombinant enzyme using TEV protease had no significant effect on the heme binding spectra (data not shown). In parallel with the spectroscopic titrations, COX activity assays were performed with huPGHS-1 at different heme-to-protein ratios (Fig. 2E). Maximal COX activity occurred with about one heme per dimer (i.e., 0.95 mol heme/mol dimer). This is consistent with there being one high-affinity heme binding site per huPGHS-1 dimer. Similar results were found in earlier studies of ovPGHS-1 by Kulmacz and Lands (26) and more recently with huPGHS-2 in our laboratory (24).
Fig. 2.
Interaction of heme with huPGHS-1. A: Time-dependent activation of huPGHS-1 by heme. Purified, recombinant huPGHS-1 (1.25 μM) was preincubated for the indicated times at 37°C with two molar equivalents of heme (2.50 μM). The enzyme was then added to a standard COX assay mixture containing 5 μM heme, and COX activity was monitored polarographically as described in Experimental Procedures. B: Absorption spectra of apo-huPGHS-1 (5.0 μM) titrated with heme (0–12.5 μM) at room temperature as described in Experimental Procedures. The data shown in B are representative of three separate experiments with different enzyme preparations. C: Absorbance values at 412 nm for different heme concentrations from B. D: Determination of the Kd values for heme binding to the enzyme using a Scatchard analysis in which the slope is equal to –1/Kd (GrapherTM 7 software); the value of Kd1 in this experiment was 0.11 ± 0.005 μM, and the value of Kd2 was 6.7 ± 1.1 μM. E: huPGHS-1 (0.98 μM) was preincubated with the indicated concentrations of heme for 10 min at 37°C, and then the COX activity was measured using an O2 electrode assay; in this case, 5 μM heme was not included in the standard assay mixture. Values in E are from triplicate determinations. Error bars indicate ± SD.
FA substrate specificity of huPGHS-1
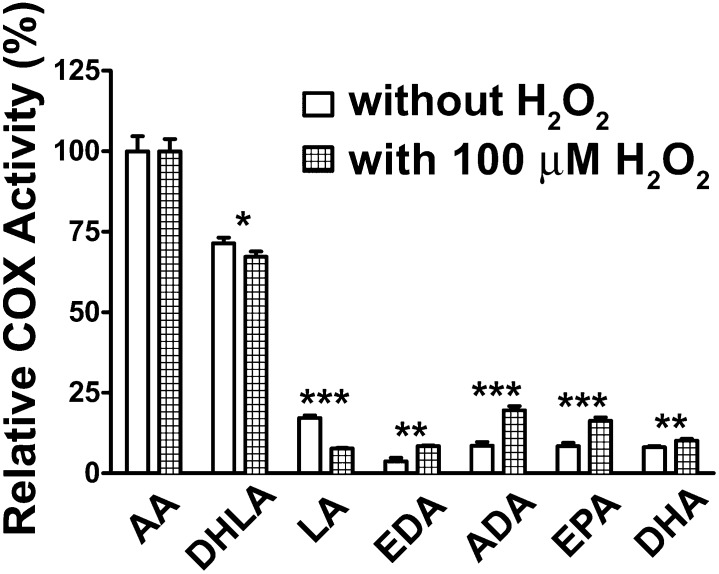
A number of PUFAs are oxygenated by PGHSs (6, 22, 31). Characterization of the products has shown that DHLA, AA, EPA, and ADA are converted to endoperoxides and monohydroxy FAs in different proportions, whereas α-LA, γ-linolenic acid, 11cis, 14cis-eicosadienoic acid (EDA), and DHA are converted primarily to monohydroxy FAs (22, 23, 31, 44–46). PGHS-1 has a more-restrictive specificity than PGHS-2 (5, 47). As shown in Fig. 3, huPGHS-1 will oxygenate a number of PUFAs. The specificity of huPGHS-1 is similar to that of ovPGHS-1 (22). AA and DHLA are oxygenated with greatest efficiencies. Addition of 100 μM H2O2 to the COX assay mixtures caused increases in the rate of oxygenation of EDA, ADA, EPA, and DHA, as has been observed for EPA previously with PGHSs from various sources (6).
Fig. 3.
COX specificity of huPGHS-1 toward different PUFA substrates in the presence and absence of 100 μM H2O2. Purified apo-huPGHS-1 was activated by preincubation with two molar equivalents of heme at 37°C for 10 min and then added to an oxygen electrode assay chamber containing the indicated FAs (100 μM) without H2O2 (open bars) or with 100 μM H2O2 (hatched bars). In addition to AA, substrate FAs are DHLA, LA, EDA, ADA (8cis, 11cis, 14cis, 17cis-docosatetraenoic acid), 5cis, 8cis, 11cis, 14cis, 17cis-eicosapentaenoic acid (EPA), and 4cis, 7cis, 10cis, 13cis, 16cis, 21cis-docosahexaenoic acid (DHA). The results are from one experiment involving triplicate determinations. The error bars indicate ± SD. Asterisks denote significant differences with versus without H2O2 as determined using GraphPad Prism 5 software. *P < 0.05, **P < 0.01, ***P < 0.001.
The effects of nonsubstrate FAs on the COX activity of huPGHS-1
Nonsubstrate FAs have either no major effect or a substantial stimulatory effect on huPGHS-2 (22, 24). Members of this same group of FAs were generally found to have a slight inhibitory effect on the COX activity of huPGHS-1 (Fig. 4). OA is the most-effective inhibitor, causing 25–30% inhibition. That nonsubstrate FAs cause only incomplete inhibition at relatively high nonsubstrate FA-to-AA ratios suggests that nonsubstrate FAs do not compete effectively with AA for binding to the COX active site of the enzyme.
Fig. 4.
Nonsubstrate FAs modestly inhibit AA oxygenation by huPGHS-1. The indicated concentrations of nonsubstrate FAs (5.0 μM or 20 μM) were included along with 2.0 μM AA in an O2 elec-trode assay chamber, and COX activities were measured following addition of an aliquot of huPGHS-1 that had been activated by preincubation for 10 min with two molar equivalents of heme. The control sample contained 2.0 μM AA alone in the chamber. The results are from a single experiment, with each value derived from triplicate determinations. The error bars indicate ± SD. There were no statistically significant differences between values obtained with 5 μM versus 20 μM nonsubstrate FAs. Statistically significant differences between the average rates with each of the nonsubstrate FAs (i.e., the average of the values with 5.0 μM and 20.0 μM FAs) versus the control rate are indicated with asterisks. *P < 0.05, **P < 0.01, ***P < 0.001.
Studies of heme and inhibitor binding to ovPGHS-1 (26, 27), huPGHS-2 (24), and huPGHS-1 (below) suggest that huPGHS-1 behaves as a conformational heterodimer with Eallo and Ecat monomers. To further examine whether nonsubstrate FAs may affect huPGHS-1 allosterically, we performed studies of AA oxygenation at high enzyme-to-substrate ratios (Tables 1, 2) similar to those performed previously with huPGHS-2 (24).
TABLE 1.
RP-HPLC analysis of the extent of AA oxygenation at high ratios of huPGHS-1 to AA
| Unreacted [1-14C]Arachidonic Acid Remaining After 8 Min(% of Starting Radioactivity) |
||||
| Reaction Components | Reaction 1 | Reaction 2 | Reaction 3 | Average ± SD |
| 1 μM huPGHS-1 + 1 μM [1-14C]AA no unlabeled AA after 4 min | 10 | 12 | 9.7 | 11 ± 1.2 |
| 11 | 10 | 12 | ||
| 1 μM huPGHS-1 + 1 μM [1-14C]AA 60 μM unlabeled AA after 4 min | 2.4 | 2.5 | 2.3 | 2.4 ± 0.06a |
| 0.1 μM huPGHS-1 + 1μM [1-14C]AA 60 μM unlabeled AA after 4 min | 0.88 | 1.1 | 1.0 | 1.0 ± 0.12a |
| 2 μM huPGHS-1 + [1-14C]AA 60 μM unlabeled AA after 4 min | 4.8 | 4.9 | 4.9 | 4.9 ± 0.09a |
[1-14C]AA (1 μM) was incubated with the indicated concentration of huPGHS-1 at 37°C for 4 min. Where indicated, 60 μM unlabeled AA was added 4 min after initiating the reaction, and the incubation was continued for another 4 min. The reactions were stopped by the addition of ethyl acetate/acetic acid (20:1), and an aliquot of the organic phase was subjected to radio-reverse-phase (radio-RP-HPLC) to separate the radioactive products and unreacted AA, as described in Experimental Procedures. The results are shown as the percentage of 14C label remaining in the HPLC fraction eluting with unreacted AA.
Significant difference from control to which no unlabeled AA was added at 4 min in Student t-test (P < 0.005).
TABLE 2.
RP-HPLC analysis of the extent of AA oxygenation at a high ratio of huPGHS-1 to AA in the presence or absence of unlabeled AA, PA, or OA
| Fatty Acid Added Four Min After Initiating the Reaction | Unreacted [1-14C]Arachidonic Acid Remaining After 8 min (Ave. ±SD from Three Reactions(% of Starting Radioactivity) |
| Control (no FA added) | 11 ± 1.2ab |
| 60 μM AA | 2.4 ± 0.06ac |
| 1 μM PA | 8.7 ± 1.7c |
| 5 μM PA | 6.4 ± 1.2c |
| 25 μM PA | 3.0 ± 0.90c |
| 2 μM OA | 3.9 ± 0.72c |
| 10 μM OA | 2.9 ± 1.1b,c |
[1-14C]AA (1 μM) was incubated with huPGHS-1 (1 μM) at 37°C for 4 min, then unlabeled AA, PA, or OA was added, and the incubation was continued for another 4 min. The reactions were stopped by the addition of ethyl acetate/acetic acid (20:1), and an aliquot of the organic phase was subjected to radio-RP-HPLC to separate the radioactive products and unreacted AA, as described in Experimental Procedures. The results are shown as the percentage of 14C label remaining in the HPLC fraction eluting with unreacted AA.
Value is from Table 1.
Value shown is average ± SD for six determinations, and other values shown are average ± SD for triplicate determinations.
Significant difference from the control value (no FA added at 4 min) in Student t-test (P < 0.05).
The average specific activity of purified huPGHS-1 is about 20 U/mg, so under optimal, saturating substrate conditions, 1 mol of enzyme can convert about 1,500 mol of AA to product per min. However, as shown in Table 1, when 1 μM [1-14C]AA was incubated with 1 μM huPGHS-1 for 8 min at 37°C, 11% of the radioactive AA remained in an unreacted form. If a bolus of unlabeled AA was added to the reaction mixture 4 min after the reaction had been initiated, the amount of unreacted radioactive AA decreased to 2.4%. Thus, the enzyme remained active, but was unable to oxygenate all of the AA. Table 1 also shows that, 1.0%, 2.4%, and 4.9% of the AA remains when 1 μM [1-14C]AA is incubated for 4 min with 0.1, 1.0, or 2.0 μM huCOX-1, respectively, and 60 μM cold AA is added after the initial 4 min (and the reaction continued for another 4 min). These latter data show that the amount of unreacted AA is proportional to and increases with the amount of huPGHS-1 added to the reaction mixture. The results are consistent with a model (24) in which unreacted [1-14C]AA is bound to the enzyme in a latent form.
In Table 2, we paired 1 μM [1-14C]AA with 1 μM huPGHS-1 in the presence or absence of unlabeled AA or a nonsubstrate FA (i.e., PA or OA). When unlabeled AA, PA, or OA was added to the reaction mixture, the amount of unreacted [1-14C]AA remaining decreased. This suggests that the presence of these FAs can lead to a net displacement of [1-14C]AA from a latent, unreactive pool, enabling the [1-14C]AA to be oxygenated. Collectively, the results shown in Fig. 4 and Tables 1 and 2 are consistent with the idea that both AA and nonsubstrate FAs bind to an allosteric (Eallo) site of huPGHS-1 and that when added in excess, nonsubstrate FAs can replace AA in the allo-steric site. Thus, it appears that huPGHS-1, like huPGHS-2 (24), has a high-affinity Eallo site to which AA can bind but where oxygenation does not occur. When nonsubstrate FAs such as PA or OA bind the allosteric monomer of huPGHS-1, they slightly decrease the catalytic efficiency of the partner, catalytic monomer toward AA.
Heme and nonsubstrate FAs potentiate inhibition of huPGHS-1 by ASA
ASA causes irreversible inhibition of PGHS-1 by acetylating the sidechain of Ser-530 in one monomer of ovPGHS-1 (35). A similar experiment with purified holo-huPGHS-1 and [14C]acetyl-salicylic acid indicated that ASA acetylates a single monomer of huPGHS-1 (data not shown). Earlier reports showed that heme is required for the efficient acetylation of ovPGHS-1 by ASA (48). Similar results were observed in comparing the inhibition of apo- and holo-huPGHS-1 by ASA (Fig. 5). For example, treatment of apo-huPGHS-1 (Fig. 5A) versus holo-huPGHS-1 (Fig. 5B, C) with 0.5 mM ASA under similar conditions caused 20% and >80% COX inhibition, respectively. We recently found that nonsubstrate FAs affect the responses of huPGHS-2 to various inhibitors including ASA. PA, OA, SA, and Δ11c 20:1 potentiated the inhibition of holo-huPGHS-1 by ASA (Fig. 5D).
Fig. 5.
ASA inhibition of huPGHS-1 is potentiated by heme and by nonsubstrate FAs. A: Effect of ASA on apo-huPGHS-1. Purified apo-huPGHS-1 was incubated with ASA at 37°C for 60 min, and COX activities were measured polarographically using a standard assay mixture with 100 μM AA, as detailed in Experimental Procedures. The results are shown as a percentage of starting COX activity. B: Effect of preincubation with heme on huPGHS-1 COX activity. Purified apo-huPGHS-1 (2.4 μM) was preincubated with two molar equivalents of heme at 37°C for 10 min, then incubated with 0, 0.5, or 4.0 mM ASA at 37°C for 60 min and assayed as in A. C: Time-dependent inactivation of huPGHS-1 by ASA. Purified apo-huPGHS-1 (1.25 μM) was preincubated with two molar equivalents of heme as in B, then incubated with 0 or 0.5 mM ASA at 37°C for the indicated times and then assayed for COX activity as in A. D: Effects of various nonsubstrate FAs on inhibition of huPGHS-1 by ASA. Purified apo-huPGHS-1 was activated by preincubation with heme, as in B, incubated with 50 μM ASA with or without a nonsubstrate FA at 37°C for 30 min, and then assayed for COX activity, as in A. The control contained neither ASA nor a nonsubstrate FA. The results are from a single experiment involving triplicate determinations. The error bars indicate ± SD. Asterisks denote significant differences between ASA plus FA versus ASA alone in the Student t-test. *P < 0.05, **P < 0.01, ***P < 0.001. PA, palmitic acid; SA, stearic acid, OA, oleic acid.
Effects of ASA on huPGHS-1 are attenuated by IBF, CBX, and NAPX
IBF (9, 30) and CBX (49) are time-independent (i.e., rapidly reversible inhibitors) of ovPGHS-1. It is known that IBF and CBX can interfere with the effect of ASA on ovPGHS-1 (35). Not surprisingly, this also occurs with purified huPGHS-1, as illustrated in Fig. 6. NAPX is a time-dependent inhibitor of ovPGHS-1 (50) that also attenuates the action of ASA.
Fig. 6.
ASA inhibition of huPGHS-1 is attenuated by IBF, CBX, and NAPX. Purified apo-huPGHS-1 was preincubated with two molar equivalents of heme at 37°C for 10 min, then incubated with 100 μM ASA with or without an inhibitor (100 μM IBF, 0.78 μM CBX, or 100 μM NAPX) at 37°C for 30 min. Finally, an aliquot of the preincubation mixture was added to an oxygen electrode assay chamber, and COX activities were assayed polarographically, as described in Experimental Procedures, with 100 μM AA as substrate. Addition of the preincubation mixture to the assay chamber resulted in a 75-fold dilution of the contents of the preincubation mixture. The results are presented as a percentage of the COX activity of the heme-activated huPGHS-1. The control is without ASA or inhibitor. The results are from a single experiment involving triplicate determinations. The error bars indicate ± SD. Significant differences between ASA plus inhibitor versus ASA alone are denoted with asterisks. ***P < 0.001, ****P < 0.0001.
Effects of nonsubstrate FAs on responses of huPGHS-1 to time-dependent COX inhibitors
FBP, DICLO, NAPX, INDO, and meclofenamic acid (MECLO) are time-dependent inhibitors of huPGHS-1 (30). As shown in Fig. 7A, various nonsubstrate FAs modestly potentiate the inhibition by FBP of huPGHS-1. Similarly, PA increases the time-dependent inhibition of huPGHS-1 by DICLO and NAPX (Fig. 7B, C) but has no effect on the response of huPGHS-1 to INDO or MECLO (Fig. 7D, E). For an FA to potentiate the effect of an inhibitor (e.g., FBP, DICLO, and NAPX) or at least not to interfere with the action of an inhibitor (e.g., INDO and MECLO), the FA must bind to a different site on the enzyme than the inhibitor. It should be noted that when tested at concentrations three to five times higher than those used in Fig. 7, each inhibitor by itself caused a complete, time-dependent loss of COX activity.
Fig. 7.
Nonsubstrate FAs modestly potentiate time-dependent inhibition by FBP, diclofenac, and NAPX but not by INDO or MECLO. A: Interactions between various nonsubstrate FAs and FBP. B: Interactions between PA and DICLO. C: Interactions between PA and NAPX. D: Interactions between PA and INDO. E: Interactions between PA and MECLO. Purified apo-huPGHS-1 was preincubated with heme as described in the legend to Fig. 3, and then with the indicated concentrations of nonsubstrate FAs and/or inhibitor at 37°C for 30 min. Finally, COX activities were assayed polarographically as described in Experimental Procedures with 100 μM AA as substrate. The results are presented as a percentage of the COX activity of the heme-activated huPGHS-1. The control in each panel is without any nonsubstrate FA or inhibitor. The results in each panel are from a single experiment involving triplicate determinations. The error bars indicate ± SD. Significant differences between results obtained with an inhibitor alone versus the inhibitor plus a nonsubstrate FA are denoted with asterisks. *P < 0.05, **P < 0.01.
A combination of a time-independent COX inhibitor and a nonsubstrate FA induces a time-dependent inhibition of huPGHS-1
The observation that nonsubstrate FAs can potentiate the effects of some time-dependent COX inhibitors led us to investigate potential interactions between FAs and time-independent inhibitors. We were surprised to find that when PA was preincubated with IBF, mefenamic acid (MEF), or CBX, an inhibition was observed in each case that was incomplete but exhibited time dependence (Fig. 8); similar results were obtained when OA was substituted for PA (Fig. 8C). For example, when huPGHS-1 was preincubated with PA or IBF alone and then assayed upon dilution into an assay mixture containing 100 μM AA, greater than 95% of the starting COX activity remained. In contrast, preincubation of huPGHS-1 with PA and IBF together led to a nonadditive, approximately 20% inhibition; additionally, when huPGHS-1 pretreated in this way was assayed in the presence of 100 μM AA plus 100 μM IBF, the COX activity was also decreased by about 25% over that observed with enzyme that had been preincubated with PA or IBF alone. The time dependence elicited by preincubation of huPGHS-1 with PA and CBX or MEF, while apparent, was less pronounced than that observed with IBF. In the cases of IBF (Fig. 8A) and CBX (Fig. 8C), both of which can interfere with ASA inhibition of huPGHS-1 (Fig. 6), the overall evidence is consistent with these inhibitors functioning by binding to the Ecat monomer of huPGHS-1.
Fig. 8.
A time-dependent inhibition of huPGHS-1 COX activity occurs when IBF, MEF, or CBX are preincubated with a nonsubstrate FA. A: Interactions between PA and IBF. B: Interactions between PA and MEF. C: Interactions between PA and CBX. Purified apo-huPGHS-1 was activated with heme as described in the legend to Fig. 3, then incubated with the indicated concentrations of nonsubstrate FAs and/or inhibitor at 37°C for 30 min. Finally, COX activities were assayed polarographically as described in Experimental Procedures, with 100 μM AA as substrate and, where shown, the indicated concentration of COX inhibitor was included in the assay chamber; note that addition of the preincubation mixture to the assay chamber resulted in a 75-fold dilution of the contents of the preincubation mixture. The results are presented as a percentage of the COX activity of the heme-activated huPGHS-1. The control in each panel is without inhibitor or nonsubstrate FA. The results in each panel are from a single experiment involving at least triplicate determinations. The error bars indicate ± SD. Significant differences between results obtained with an inhibitor alone versus the inhibitor plus nonsubstrate FA are denoted with asterisks. *P < 0.05, **P < 0.01.
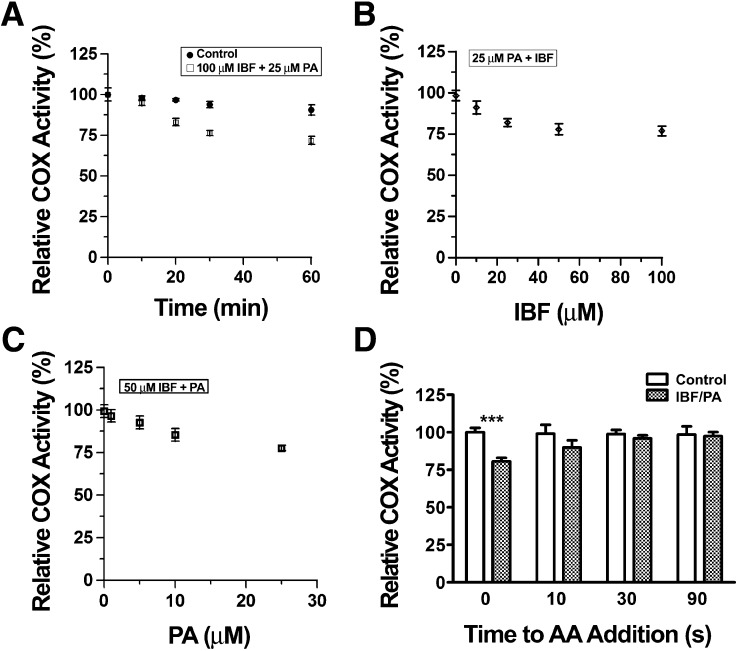
We investigated the unusual time dependence seen with IBF plus nonsubstrate FAs in greater detail first by performing preincubations of enzyme with IBF plus PA for 0–60 min (Fig. 9A). An obvious time dependence occurred during the first 30 min of the preincubation of 100 μM IBF plus 25 μM PA, with a maximum decrease in activity of 20–25% of control values. There was a dose dependence in the effects of both PA and IBF (Fig. 9B, C). Increasing the concentration of IBF beyond 100 μM or the concentration of PA beyond 25 μM did not affect the magnitude of the inhibition; however, the critical micelle concentration of PA is approximately 25 μM under the preincubation conditions (24).
Fig. 9.
Time-dependence characteristics of COX inhibition of huPGHS-1 by IBF plus PA. A: Time dependence with IBF plus PA. Purified apo-huPGHS-1 was activated with heme as described in the legend to Fig. 3, then 1 μM holo-huPGHS-1 was preincubated with no inhibitor (control) or 100 μM IBF plus 25 μM PA at 37°C. At the indicated times, an aliquot of the sample was added to a standard O2 electrode assay mixture containing 100 μM AA to measure COX activity. Addition of enzyme sample to the assay chamber involved a 75-fold dilution of the preincubation mixture. B: Inhibition of huPGHS-1 by different concentrations of IBF plus a fixed concentration of PA. huPGHS-1 (1 μM) was activated with heme and then preincubated at 37°C for 30 min with the indicated concentrations of IBF plus PA (25 μM), and the samples were assayed as in A. C: Inhibition of huPGHS-1 by different concentrations of PA plus a fixed concentration of IBF. huPGHS-1 (1 μM) was activated with heme, then preincubated at 37°C for 30 min with the indicated concentrations of PA plus IBF (50 μM), and then assayed as in A. With respect to B and C, the relative COX activities were 101 ± 2.3% and 98.6 ± 2.8%, respectively, when 100 μM IBF or 25 μM PA was preincubated alone with huPGHS-1 for 30 min at 37°C. D: Recovery of COX activity following pretreatment with IBF plus PA. huPGHS-1 (1 μM) was activated with heme and then preincubated at 37°C for 30 min with 100 μM IBF plus 25 μM PA. Aliquots of the sample were added to a standard assay solution to which AA (100 μM final concentration) was added 0, 10, 30, or 90 s later. This involved a 75-fold dilution of the preincubation mixture. Significant differences between results at 0 s and later times are denoted with asterisks. ***P < 0.001.
Further substantiating the time-dependent feature of the combined IBF/PA inhibition was the observation that the time-dependent inhibition was reversed in a time-dependent manner. In the experiment depicted in Fig. 9D, huPGHS-1 was preincubated with IBF plus PA, and the enzyme sample was then added to an assay chamber to which AA was included at zero time or added 10, 30, or 90 s later. The COX activity was completely recovered within 30 s. We would emphasize that there is a 10 s kinetic lag time before maximal COX activity can be measured, even when AA is present in the assay mixture when the enzyme is added. Thus, the 0 s time point in Fig. 9D actually corresponds to a 10 s delay during which COX activity, if inhibited, could be recovered. We suspect, based on the rapid recovery of activity observed between 0 and 30 s (Fig. 9D), that were we able to measure activity at a real zero time, the enzyme would be inhibited by considerably more than 20–25%.
DISCUSSION
Comparisons of huPGHS-1, ovPGHS-1, and huPGHS-2
There is greater than 90% sequence identity between ovPGHS-1 and huPGHS-1 (5, 51). Thus, it is not surprising that the two enzymes are functionally and quantitatively similar with respect to heme stoichiometry and affinity (26), stoichiometry of ASA acetylation (35), dependence on heme for efficient ASA inhibition (48), and partial inhibition by nonsubstrate FAs including PA, SA, and OA (22). There are modest differences in substrate specificities, with huPGHS-1 exhibiting relatively less activity toward EDA, ADA, and DHA and more activity toward EPA (22).
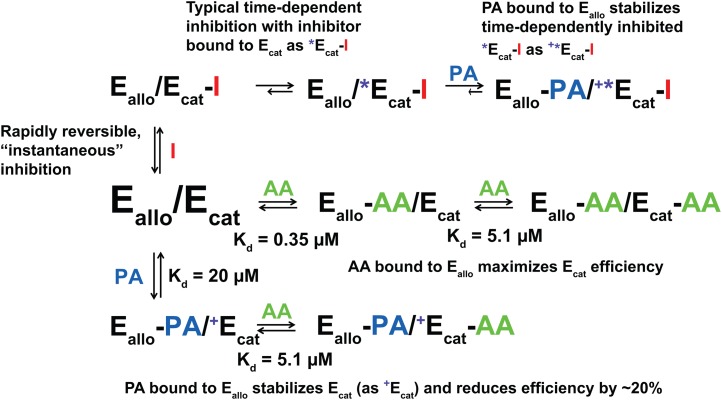
We recently provided evidence that both huPGHS-2 (22–25) and ovPGHS-1 (22, 25, 28) function as conformational heterodimers of the Eallo/Ecat form. Our present results with huPGHS-1 are consistent with and expand upon these and earlier studies (26, 27), indicating that heme, nonsubstrate FAs, and certain COX inhibitors bind with different affinities to one of the two monomers comprising each huPGHS-1 dimer. Using the general model developed for huPGHS-2 (24), one monomer binds heme and is denoted as the catalytic subunit (Ecat), and the other monomer is the allosteric subunit (Eallo) (Fig. 10).
Fig. 10.
Model depicting interactions among huPGHS-1 and COX inhibitors and nonsubstrate FAs. Eallo/Ecat represents a huPGHS-1 heterodimer where Ecat is the catalytic subunit to which heme is bound and Eallo is the allosteric subunit that lacks bound heme. AA is arachidonic acid, PA is palmitic acid, a representative nonsubstrate FA, I is a generic COX inhibitor that can bind to Ecat. Initially, all inhibitors bind Eallo/Ecat reversibly to form Eallo/Ecat-I. With typical time-dependent inhibitors, Eallo/Ecat-I undergoes a conformation change to a more-stable Eallo/*Ecat-I form that reconverts to Eallo/Ecat-I only slowly. However, the rates for the interconversion of Eallo/Ecat-I and Eallo/*Ecat-I are unique to each inhibitor, with the equilibrium lying in the direction of Eallo/Ecat-I with “reversible” inhibitors like IBF and toward Eallo/*Ecat-I with “irreversible” inhibitors like INDO. The presence of nonsubstrate FAs such as PA stabilizes Eallo/*Ecat-I. We speculate that this occurs through a process involving formation of an Eallo-FA/+*Ecat-I complex. We further suggest that nonsubstrate FAs must stabilize Eallo-FA/+*Ecat-I for all inhibitors, including those previously considered to be rapidly reversible, time-independent inhibitors (e.g., IBF). The AA in the Ecat-AA can be oxygenated to form PGG2. Additional explanations of the various forms and their relative activities are indicated in the figure. Kd values for AA and PA binding to Ecat and Eallo were calculated as described in the text. We make the assumption that nonsubstrate FAs do not bind efficiently to Ecat (i.e., Kd > 50 μM)
Effects of nonsubstrate FAs on huPGHS-1 catalysis
Nonsubstrate FAs have a modest inhibitory effect on AA oxygenation by huPGHS-1. The magnitude of the inhibition (15–30%) is similar for all the FAs tested. There is no dose-dependent increase in inhibition at concentrations above 5 μM FA, suggesting that the inhibition of huPGHS-1 by nonsubstrate FAs does not involve direct competition with AA at the Ecat site of huPGHS-1 but rather involves binding of nonsubstrate FAs to Eallo (Fig. 10) That PA and OA can replace bound, unreacted AA from Eallo of huPGHS-1 (Tables 1, 2) is further evidence that nonsubstrate FAs compete with AA for binding to the Eallo subunit of huPGHS-1.
Unlike nonsubstrate FAs, AA can also bind effectively to Ecat. Using the model shown in Fig. 10, the Km value for AA and the values shown in Table 2 for unreacted, Eallo bound AA at various nonsubstrate FA concentrations, we can calculate the Kd values for the binding of AA and nonsubstrate FAs to Eallo and Ecat, as described previously (24). We estimate the Kd for AA binding to Eallo to be 0.35 μM, similar to the value (0.26 μM) determined for Eallo of huPGHS-2 (24). The Kd value for the binding of PA (and OA) to Eallo is calculated to be about 60 times higher than that for AA, or approximately 20 μM. This estimate is based on the concentrations of PA (and OA) that causes 50% inhibition of AA binding to Eallo when the effective concentration of AA is about 0.0758 μM (Table 2). This Kd value for PA is in the same range (7.5 μM) as that estimated for PA binding to Eallo of huPGHS-2 (24). The Km for AA with huPGHS-1 was determined to be 5.1 μM, which, based on our model, is the Kd for AA binding to Ecat; again, we assume that FAs do not bind efficiently to Ecat because huPGHS-1 is not completely inhibited, even at high concentrations of nonsubstrate FAs.
We speculate that nonsubstrate FAs are important in the coordinate regulation of PGHS-1 versus PGHS-2 activities when the two isoforms are coexpressed at comparable levels in cells. Thus, at low AA concentrations and ambient levels of the major nonsubstrate FAs (i.e., PA, SA, and OA), huPGHS-1 activity would be expected to be somewhat depressed; in contrast, huPGHS-2 activity would be potentiated (22, 24). At relatively higher AA concentrations, such as those seen immediately following activation of cytosolic PLA2, huPGHS-1 activity would be increased relative to that of PGHS-2 (52). Obviously, this concept needs to be tested in intact cells.
Effects of nonsubstrate FAs on responses of huPGHS-1 to ASA and other time-dependent COX inhibitors
Nonsubstrate FAs either modestly potentiate or have no effect on the time-dependent inhibitory actions of the six time-dependent inhibitors tested in our study. The results with ASA, FBP, DICLO, and NAPX, whose actions are promoted by nonsubstrate FAs, are easily rationalized if the nonsubstrate FAs act allosterically to facilitate the binding and/or the subsequent actions of inhibitors on huPGHS-1 (24) (Fig. 10). Although less definitive, the fact that nonsubstrate FAs fail to interfere with the actions of INDO or MECLO suggests that FAs do not compete with the site to which either inhibitor binds; furthermore, we observed that slightly higher concentrations of INDO and MECLO (as well as ASA, FBP, DICLO, and NAPX) cause complete time-dependent inhibition of the enzyme, consistent with these inhibitors acting directly on Ecat (24). That NAPX functions via Ecat of huPGHS-1 is also consistent with the observation that NAPX can interfere with the action of ASA, which acts via Ecat. In contrast to what is observed with huPGHS-1, NAPX, and FBP appear to function as allosteric inhibitors of huPGHS-2 by binding to Eallo. We speculate that all time-dependent COX inhibitors of PGHS-1 function by binding Ecat, whereas with PGHS-2, at least some time-dependent COX inhibitors (e.g., NAPX and FBP) function via Eallo.
Combining IBF, MEF, or CBX with a nonsubstrate FA induces a time-dependent inhibition of huPGHS-1
IBF, MEF, and CBX are time-independent inhibitors of ovPGHS-1 (35, 53). IBF and MEF are reported to be time-independent inhibitors of huPGHS-2 (34), whereas CBX is a time-dependent inhibitor of this isoform (11). Empirically, time dependence means that the COX activity of the enzyme can be decreased in a time-dependent manner by preincubation with the inhibitor; typically, dilution of the enzyme inhibitor complex leads to a time-dependent restoration of activity. Using these criteria, we find that when tested alone, IBF, MEF, CBX, and nonsubstrate FAs are time-independent inhibitors of huPGHS-1 (Fig. 8). Thus, a surprising finding was that when huPGHS-1 was preincubated with a combination of IBF and PA, the enzyme lost 20% of its activity, and the degree of inhibition with IBF plus PA was enhanced during a 30 min preincubation period. Moreover, when enzyme that had been preincubated with PA plus IBF was diluted into an assay chamber lacking the AA substrate and then AA was added 30 s later, the activity was recovered; thus, the time-dependent inhibition was time dependently reversible.
The maximal inhibition that we could detect with IBF plus PA was incomplete (20–25%). However, because the inhibition was rapidly reversed (i.e., within 10–30 s) and the lag time required to achieve maximal activity is about 10 s, we suspect that the maximal time-dependent IBF inhibition was significantly more than 25%. One plausible explanation is that PA is acting via Eallo to expose the time-dependent component of IBF inhibition of huPGHS-1 that normally cannot be observed in the time frame (≥1 min) in which time dependence has usually been measured (24) (Fig. 10). We speculate that preincubating huPGHS-1 with IBF plus PA converts the enzyme to a time dependently inhibited conformer represented by the Eallo-PA/+*Ecat-IBF form (Fig. 10). When diluted into an assay chamber containing 100 μM AA, PA and then IBF dissociate, leading to formation of a maximally active Eallo-AA/Ecat-AA form.
It is apparent from previous studies that there must be a transient time dependence associated with half of the sites of COX activity. For example, in order for the G533A/Native huPGHS-2 mutant heterodimer (having one monomer with a Gly533Ala mutation and a native monomer) to exhibit the same activity as the native homodimer (25), the mutant heterodimer would have to become at least transiently stabilized, with all of the G533A monomer in the Eallo form and all of the native subunit in the Ecat form (5). This could be facilitated were an FA bound to Eallo able to stabilize an Eallo-FA/Ecat complex long enough for it to bind substrate AA (Eallo-FA/Ecat-AA) without having the FA dissociate to Eallo/Ecat.
Interactions among COX-2 inhibitors and nsNSAIDs in their effects on huPGHS-1
As noted above, COX inhibitors fall into two broad mechanistic categories, time-dependent and time-independent. The difference is clinically relevant, inasmuch as it underlies the isoform-specific inhibition seen with selective COX-2 inhibitors (5, 9, 10, 49). The general model of an initial bimolecular binding event, followed by one or more unimolecular steps, adequately describes time-dependent inhibition for a single inhibitor (9). However, as illustrated in Fig. 10, it is now clear that there are previously unappreciated complexities to drug interactions and FA interactions that result from PGHSs operating as conformational heterodimers with functionally distinct Eallo and Ecat subunits (5, 22–28, 34, 35, 54).
The influence of COX-2 inhibitors and nsNSAIDs on the inhibition of human platelet PGHS-1 by ASA is of pharmacologic relevance because many individuals take low-dose ASA in combination with other NSAIDs (19, 55, 56). It has been shown previously that IBF, CBX, and NAPX are all capable of interfering with ovPGHS-1 activity (35, 50), that CBX interferes with the antiplatelet effects of ASA, at least in dogs (35), and that IBF and NAPX can interfere with the antiplatelet effects of ASA in humans (19, 57). We find that both CBX and IBF can interfere with ASA inhibition of huPGHS-1. Moreover, the combination of NAPX and ASA does not provide the level of inhibition seen with ASA alone, which is consistent with NAPX acting directly on Ecat to interfere with the action of ASA on huPGHS-1.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- ADA
- adrenic acid
- ASA
- aspirin
- CBX
- celecoxib
- COX
- cyclooxygenase
- DHA
- docosahexaenoic acid
- DHLA
- dihomo-γ-linolenic acid
- DICLO
- diclofenac
- EDA
- eicosadienoic acid
- EPA
- eicosapentaenoic acid
- FBP
- flurbiprofen
- hu
- human
- IBF
- ibuprofen
- INDO
- indomethacin
- MECLO
- meclofenamic acid
- LA
- linoleic acid
- MEF
- mefenamic acid
- NAPX
- naproxen
- nsNSAIDs
- nonspecific nonsteroidal anti-inflammatory drugs
- OA
- oleic acid
- ov
- ovine
- PA
- palmitic acid
- PGHS
- prostaglandin endoperoxide H synthase
- PGG2
- prostaglandin G2
- POX
- peroxidase
- SA
- stearic acid
- TEV
- tobacco etch virus
These studies were supported in part by National Institutes of Health Grant GM-068848. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Smith W. L., DeWitt D. L., Garavito R. M. 2000. Cyclooxygenases: structural, cellular and molecular biology. Annu. Rev. Biochem. 69: 145–182 [DOI] [PubMed] [Google Scholar]
- 2.Schneider C., Pratt D. A., Porter N. A., Brash A. R. 2007. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem. Biol. 14: 473–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouzer C. A., Marnett L. J. 2009. Cyclooxygenases: structural and functional insights. J. Lipid Res. 50 (Suppl.): 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai A. L., Kulmacz R. 2010. Prostaglandin H synthase: resolved and unresolved mechanistic issues. Arch. Biochem. Biophys. 493: 103–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith W. L., Urade Y., Jakobsson P. 2011. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 111: 5821–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W., Cao D., Oh S. F., Serhan C. N., Kulmacz R. J. 2006. Divergent cyclooxygenase responses to fatty acid structure and peroxide level in fish and mammalian prostaglandin H synthases. FASEB J. 20: 1097–1108 [DOI] [PubMed] [Google Scholar]
- 7.Ohki S., Ogino N., Yamamoto S., Hayaishi O. 1979. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J. Biol. Chem. 254: 829–836 [PubMed] [Google Scholar]
- 8.Liu J., Seibold S. A., Rieke C. J., Song I., Cukier R. I., Smith W. L. 2007. Prostaglandin endoperoxide H synthases: peroxidase hydroperoxide specificity and cyclooxygenase activation. J. Biol. Chem. 282: 18233–18244 [DOI] [PubMed] [Google Scholar]
- 9.Walker M. C., Kurumbail R., Kiefer J., Moreland K., Koboldt C., Isakson P., Seibert K., Gierse J. 2001. A three-step kinetic mechanism for selective inhibition of cyclo-oxygenase-2 by diarylheterocyclic inhibitors. Biochem. J. 357: 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blobaum A. L., Marnett L. 2007. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 50: 1425–1441 [DOI] [PubMed] [Google Scholar]
- 11.Gierse J. K., Zhang Y., Hood W. F., Walker M. C., Trigg J. S., Maziasz T. J., Koboldt C. M., Muhammad J. L., Zweifel B. S., Masferrer J. L., et al. 2005. Valdecoxib: assessment of cyclooxygenase-2 potency and selectivity. J. Pharmacol. Exp. Ther. 312: 1206–1212 [DOI] [PubMed] [Google Scholar]
- 12.Burian M., Geisslinger G. 2005. COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacol. Ther. 107: 139–154 [DOI] [PubMed] [Google Scholar]
- 13.Brune K., Furst D. E. 2007. Combining enzyme specificity and tissue selectivity of cyclooxygenase inhibitors: towards better tolerability? Rheumatology. 46: 911–919 [DOI] [PubMed] [Google Scholar]
- 14.Marnett L. J., DuBois R. N. 2002. COX-2: a target for colon cancer prevention. Annu. Rev. Pharmacol. Toxicol. 42: 55–80 [DOI] [PubMed] [Google Scholar]
- 15.Eisinger A. L., Prescott S. M., Jones D. A., Stafforini D. M. 2007. The role of cyclooxygenase-2 and prostaglandins in colon cancer. Prostaglandins Other Lipid Mediat. 82: 147–154 [DOI] [PubMed] [Google Scholar]
- 16.Antithrombotic Trialists’ Collaboration 2002. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 324: 71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capone M. L., Tacconelli S., Sciulli M. G., Grana M., Ricciotti E., Minuz P., Di Gregorio P., Merciaro G., Patrono C., Patrignani P. 2004. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation. 109: 1468–1471 [DOI] [PubMed] [Google Scholar]
- 18.Patrono C., Garcia Rodriguez L., Landolfi R., Baigent C. 2005. Low-dose aspirin for the prevention of atherothrombosis. N. Engl. J. Med. 353: 2373–2383 [DOI] [PubMed] [Google Scholar]
- 19.Renda G., Tacconelli S., Capone M., Sacchetta D., Santarelli F., Sciulli M., Zimarino M., Grana M., D'Amelio E., Zurro M., et al. 2006. Celecoxib, ibuprofen, and the antiplatelet effect of aspirin in patients with osteoarthritis and ischemic heart disease. Clin. Pharmacol. Ther. 80: 264–274 [DOI] [PubMed] [Google Scholar]
- 20.Grosser T., Yu Y., Fitzgerald G. A. 2010. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annu. Rev. Med. 61: 17–33 [DOI] [PubMed] [Google Scholar]
- 21.Eikelboom J. W., Hirsh J., Spencer F. A., Baglin T. P., Weitz J. I. 2012. Antiplatelet Drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141: e89S–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan C., Sidhu R. S., Kuklev D. V., Kado Y., Wada M., Song I., Smith W. L. 2009. Cyclooxygenase allosterism, fatty acid-mediated cross-talk between monomers of cyclooxygenase homodimers. J. Biol. Chem. 284: 10046–10055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma N. P., Dong L., Yuan C., Noon K. R., Smith W. L. 2010. Asymmetric acetylation of the cyclooxygenase-2 homodimer by aspirin and its effects on the oxygenation of arachidonic, eicosapentaenoic, and docosahexaenoic acids. Mol. Pharmacol. 77: 979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong L., Vecchio A. J., Sharma N. P., Jurban B. J., Malkowski M. G., Smith W. L. 2011. Human cyclooxygenase-2 is a sequence homodimer that functions as a conformational heterodimer. J. Biol. Chem. 286: 19035–19046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan C., Rieke C. J., Rimon G., Wingerd B. A., Smith W. L. 2006. Partnering between monomers of cyclooxygenase-2 homodimers. Proc. Natl. Acad. Sci. USA. 103: 6142–6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulmacz R. J., Lands W. E. 1984. Prostaglandin H synthase. Stoichiometry of heme cofactor. J. Biol. Chem. 259: 6358–6363 [PubMed] [Google Scholar]
- 27.Kulmacz R. J., Lands W. E. 1985. Stoichiometry and kinetics of the interaction of prostaglandin H synthase with anti-inflammatory agents. J. Biol. Chem. 260: 12572–12578 [PubMed] [Google Scholar]
- 28.Sidhu R. S., Lee J., Yuan C., Smith W. 2010. Comparison of cyclooxygenase-1 crystal structures: cross-talk between monomers comprising cyclooxygenase-1 homodimers. Biochemistry. 49: 7069–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnett J., Chow J., Ives D., Chiou M., Mackenzie R., Osen E., Nguyen B., Tsing S., Bach C., Freire J., et al. 1994. Purification, characterization and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochim. Biophys. Acta. 1209: 130–139 [DOI] [PubMed] [Google Scholar]
- 30.Laneuville O., Breuer D. K., Dewitt D. L., Hla T., Funk C. D., Smith W. L. 1994. Differential inhibition of human prostaglandin endoperoxide H synthases-1 and -2 by nonsteroidal anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 271: 927–934 [PubMed] [Google Scholar]
- 31.Laneuville O., Breuer D. K., Xu N., Huang Z. H., Gage D. A., Watson J. T., Lagarde M., DeWitt D. L., Smith W. L. 1995. Fatty acid substrate specificities of human prostaglandin-endoperoxide H synthase-1 and -2. Formation of 12-hydroxy-(9Z, 13E/Z, 15Z)- octadecatrienoic acids from alpha-linolenic acid. J. Biol. Chem. 270: 19330–19336 [DOI] [PubMed] [Google Scholar]
- 32.Gierse J. K., Hauser S. D., Creely D. P., Koboldt C., Rangwala S. H., Isakson P. C., Seibert K. 1995. Expression and selective inhibition of the constitutive and inducible forms of human cyclo-oxygenase. Biochem. J. 305: 479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith T., Leipprandt J., DeWitt D. L. 2000. Purification and characterization of the human recombinant histidine-tagged prostaglandin endoperoxide H synthases-1 and -2. Arch. Biochem. Biophys. 375: 195–200 [DOI] [PubMed] [Google Scholar]
- 34.Prusakiewicz J. J., Duggan K., Rouzer C., Marnett L. 2009. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochemistry. 48: 7353–7355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rimon G., Sidhu R. S., Lauver D. A., Lee J. Y., Sharma N. P., Yuan C., Frieler R. A., Trievel R. C., Lucchesi B. R., Smith W. L. 2010. Coxibs interfere with the action of aspirin by binding tightly to one monomer of cyclooxygenase-1. Proc. Natl. Acad. Sci. USA. 107: 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burde T., Rimon G. 2002. On the interaction of specific prostaglandin H synthase-2 inhibitors with prostaglandin H synthase-1. Eur. J. Pharmacol. 453: 167–173 [DOI] [PubMed] [Google Scholar]
- 37.Rosenstock M., Danon A., Rubin M., Rimon G. 2001. Prostaglandin H synthase-2 inhibitors interfere with prostaglandin H synthase-1 inhibition by nonsteroidal anti-inflammatory drugs. Eur. J. Pharmacol. 412: 101–108 [DOI] [PubMed] [Google Scholar]
- 38.Chen Y., Yang Y., Wang F., Wan K., Yamane K., Zhang Y., Lei M. 2006. Crystal structure of human histone lysine-specific demethylase 1 (LSD1). Proc. Natl. Acad. Sci. USA. 103: 13956–13961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thuresson E. D., Lakkides K. M., Rieke C. J., Sun Y., Wingerd B. A., Micielli R., Mulichak A. M., Malkowski M. G., Garavito R. M., Smith W. L. 2001. Prostaglandin endoperoxide H synthase-1: the functions of cyclooxygenase active site residues in the binding, positioning, and oxygenation of arachidonic acid. J. Biol. Chem. 276: 10347–10357 [DOI] [PubMed] [Google Scholar]
- 40.Duncan T., Osawa Y., Kutty R. K., Kutty G., Wiggert B. 1999. Heme-binding by Drosophila retinoid- and fatty acid-binding glycoprotein (RFABG), a member of the proapolipophorin gene family. J. Lipid Res. 40: 1222–1228 [PubMed] [Google Scholar]
- 41.Huber W. J., Backes W. 2008. Quantitation of heme oxygenase 1: heme titration increases yield of purified protein. Anal. Biochem. 373: 167–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Titus B. G., Kulmacz R., Lands W. 1982. Selective destruction and removal of heme from prostaglandin H synthase. Arch. Biochem. Biophys. 214: 824–828 [DOI] [PubMed] [Google Scholar]
- 43.Percival M. D., Ouellet M., Vincent C. J., Yergey J. A., Kennedy B. P., O'Neill G. P. 1994. Purification and characterization of recombinant human cyclooxygenase-2. Arch. Biochem. Biophys. 315: 111–118 [DOI] [PubMed] [Google Scholar]
- 44.Sprecher H., VanRollins M., Sun F., Wyche A., Needleman P. 1982. Dihomo-prostaglandins and -thromboxane. A prostaglandin family from adrenic acid that may be preferentially synthesized in the kidney. J. Biol. Chem. 257: 3912–3918 [PubMed] [Google Scholar]
- 45.Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R-L. 2002. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196: 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazan N. G., Calandria J. M., Serhan C. N. 2010. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J. Lipid Res. 51: 2018–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rouzer C. A., Marnett L. J. 2008. Non-redundant functions of cyclooxygenases: oxygenation of endocannabinoids. J. Biol. Chem. 283: 8065–8069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y. N., Marnett L. J. 1989. Heme prosthetic group required for acetylation of prostaglandin H synthase by aspirin. FASEB J. 3: 2294–2297 [DOI] [PubMed] [Google Scholar]
- 49.Gierse J. K., Koboldt C., Walker M., Seibert K., Isakson P. 1999. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem. J. 339: 607–614 [PMC free article] [PubMed] [Google Scholar]
- 50.Duggan K. C., Walters M. J., Musee J., Harp J. M., Kiefer J. R., Oates J. A., Marnett L. J. 2010. Molecular basis for cyclooxygenase inhibition by the non-steroidal anti-inflammatory drug naproxen. J. Biol. Chem. 285: 34950–34959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simmons D. L., Botting R. M., Hla T. 2004. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56: 387–437 [DOI] [PubMed] [Google Scholar]
- 52.Smith W. L. 2008. Nutritionally essential fatty acids and biologically indispensable cyclooxygenases. Trends Biochem. Sci. 33: 27–37 [DOI] [PubMed] [Google Scholar]
- 53.Rome L. H., Lands W. E. 1975. Structural requirements for time-dependent inhibition of prostaglandin biosynthesis by anti-inflammatory drugs. Proc. Natl. Acad. Sci. USA. 72: 4863–4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duggan K. C., Hermanson D., Musee J., Prusakiewicz J., Scheib J., Careter B., Banerjee S., Oates J., Marnett L. 2011. (R)-profens are substrate-selective inhibitors of endocannabinoid oxygenation by COX-2. Nat. Chem. Biol. 7: 803–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilner K. D., Rushing M., Walden C., Adler R., Eskra J., Noveck R., Vargas R. 2002. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J. Clin. Pharmacol. 42: 1027–1030 [PubMed] [Google Scholar]
- 56.Solomon S. D., Wittes J., Finn P. V., Fowler R., Viner J., Bertagnolli M. M., Arber N., Levin B., Meinert C. L., Martin B., et al. for the Cross Trial Safety Assessment 2008. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 117: 2104–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gladding P. A., Webster M., Farrell H., Zeng I., Park R., Ruijne N. 2008. The antiplatelet effect of six non-steroidal anti-inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteers. Am. J. Cardiol. 101: 1060–1063 [DOI] [PubMed] [Google Scholar]