Abstract
We describe the development of a method for the extraction and analysis of 62 sterols, oxysterols, and secosteroids from human plasma using a combination of HPLC-MS and GC-MS. Deuterated standards are added to 200 μl of human plasma. Bulk lipids are extracted with methanol:dichloromethane, the sample is hydrolyzed using a novel procedure, and sterols and secosteroids are isolated using solid-phase extraction (SPE). Compounds are resolved on C18 core-shell HPLC columns and by GC. Sterols and oxysterols are measured using triple quadrupole mass spectrometers, and lathosterol is measured using GC-MS. Detection for each compound measured by HPLC-MS was ∪ 1 ng/ml of plasma. Extraction efficiency was between 85 and 110%; day-to-day variability showed a relative standard error of <10%. Numerous oxysterols were detected, including the side chain oxysterols 22-, 24-, 25-, and 27-hydroxycholesterol, as well as ring-structure oxysterols 7α- and 4β-hydroxycholesterol. Intermediates from the cholesterol biosynthetic pathway were also detected, including zymosterol, desmosterol, and lanosterol. This method also allowed the quantification of six secosteroids, including the 25-hydroxylated species of vitamins D2 and D3. Application of this method to plasma samples revealed that at least 50 samples could be extracted in a routine day.
Keywords: cholesterol/biosynthesis, cholesterol/metabolism, lipidomics, mass spectrometry, oxysterols, steroid hormones, vitamin D
Sterols play essential roles in the physiological processes of virtually all living organisms. Members of this lipid class are integral building blocks in the cellular membranes of animals and have important functions in signaling, regulation, and metabolism (1). To date, the majority of studies have focused on cholesterol, the most abundant sterol in mammals that serves as both a precursor and product to a host of important molecules, including steroid hormones, bile acids, oxysterols, and intermediates in the cholesterol biosynthetic pathway (2–4). Although cholesterol has gained significant notoriety due to the compound's negative impact on health, other sterols, such as plant-derived phytosterols, are thought to offer potential human health benefits by lowering circulating cholesterol levels (5, 6).
Novel functions for sterols and secosteroids continue to be identified. For example, the cholesterol metabolites 25-hydroxycholesterol and 7α,25-dihydroxycholesterol have recently been shown to play important signaling roles in the immune system (7–9). 24-hydroxycholesterol has been shown to be involved in cholesterol turnover in the brain, and it plays a role in memory (10) and glucose metabolism (11). Alterations in vitamin D levels arising from the lack of sun exposure and/or use of sunscreen are postulated to have a negative impact on health (12, 13). These findings together with the large number of sterols suggest that there may be undiscovered roles for sterols in biology, and they highlight the need for continued research into the biochemical pathways associated with these compounds.
The extraction and analysis of sterols in human plasma present a unique challenge due to their virtual insolubility, sequestration within lipoproteins, and dramatic differences in the levels of individual sterols. Cholesterol is the most abundant sterol, with circulating levels on the order of 1 to 3 mg/ml, whereas 25-hydroxycholesterol is a million-fold less abundant at 1 to 3 ng/ml (14, 15). The circulating form of sterols in humans is primarily as steryl esters, in which a fatty acid is esterified to carbon 3 of the sterol; however, a small variable percentage of free sterols also exist. Enzymatically formed oxysterols are typically between 59% and 91% esterified (15). This duality poses additional analytical challenges because free versus esterified sterols must be isolated or measured separately, or steryl esters must first be converted to free sterols. Representative structures of a sterol, steryl ester, and secosteroid are shown in Fig. 1. Other lipids, such as triglycerides, phospholipids, and sphingolipids that are present in the plasma at concentrations similar to cholesterol, further confound the analysis of sterols.
Fig. 1.
Structures of (A) cholesterol, (B) 25-hydroxyvitamin D3, and (C) cholesteryl ester with positional numbering system.
Traditionally, the extraction of sterols has relied on one of two classic methods: Bligh/Dyer method or Folch method (16, 17). Both make use of a mixture of chloroform and methanol to simultaneously disrupt lipoproteins and solubilize lipids. An alkaline hydrolysis step is employed to cleave sterol-fatty acid conjugates; a strong base in alcohol is added to the extract, which is then often incubated at room temperature or elevated temperature (60–100°C) for 1–2 h (18–20). Hydrolysis also serves to degrade other abundant lipid classes, such as triglycerides and phospholipids, which reduces sample complexity. Alternatively, if the goal is to measure only free, unesterified sterols, the alkaline hydrolysis step is eliminated. Lastly, solid-phase extraction (SPE) is utilized to isolate sterols from other components. Ideally, cholesterol could be isolated from the sample because it would simplify subsequent instrumental analysis of other less abundant sterol species; however, SPE does not yet have the inherent resolution or reproducibility to quantitatively isolate cholesterol from all other sterols (18, 21). Although many variations of these extraction procedures have been reported in the literature, the fundamental steps of the various methods are the same.
Instrumental analysis of sterols and related compounds in plasma has typically focused on either a single analyte or a limited group of analytes. Historically, these methods have employed GC and GC-MS for the analysis of select sterols (18, 22, 23). GC and GC-MS have limitations with regard to sample composition, injection volume, sensitivity, and mass spectral scanning functions. Despite these limitations, GC and GC-MS are still widely used for sterol analysis due to their chromatographic resolving capacity, ease of use, and relative low cost of the acquisition and operation of the instruments.
Recently, methods have been developed for sterol analysis using LC-MS (24–26). Like GC and GC-MS, these methods are typically optimized for the detection of one or two analytes. In 2007, our laboratory described a method for analyzing a diverse group of sterols and oxysterols using LC-MS (21). The method was optimized for detection of 12 common sterols and oxysterols in a single extraction. Since then, high performance liquid chromatography (HPLC) has become more widely used in the analysis of sterols and is often coupled to triple quadrupole mass spectrometers for quantitative analysis to enhance sensitivity and selectivity. Similarly, improvements in ionization and ion transfer into the mass spectrometer have enhanced the ability to measure low-level metabolites in biological systems.
Here we report a method for the quantitative analysis of sterols, oxysterols, and secosteroids in plasma. Using a streamlined extraction procedure and a combination of LC-MS (electrospray ionization and atmospheric pressure chemical ionization), this method allows for the analysis of approximately 60 sterols from a single extraction of 200 μl of plasma. The extraction procedure is flexible and can be tailored to the sample type and information sought. As many sterols are positional isomers, chromatographic resolution remains crucial for the analysis of sterols and related compounds because the MS cannot differentiate between isobaric compounds. The instrumental analysis used in this method employs recent advances in HPLC column technology that provide increased resolution and sensitivity, and the throughput is such that 50 samples can be readily assayed per day. The method lends itself to translational research involving large cohorts of clinically well-characterized patients and can be adapted to serum, tissue, or other biological matrices.
EXPERIMENTAL PROCEDURES
Materials
Human plasma samples were obtained from 200 subjects of the Cooper Institute (Dallas, TX) following protocols approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. Written consent was obtained from each patient prior to sample collection. Pooled normal human plasma was purchased from Innovative Research (Novi, MI) for use as a control sample. Primary and deuterated sterol and secosteroid standards were obtained from Avanti Polar Lipids (Alabaster, AL), unless otherwise noted. A complete list of analytes is given in Table 1. Dichloromethane (DCM) and hexane were from Burdick and Jackson (Honeywell; Morristown, NJ), methanol, chloroform (CHCl3), acetonitrile (ACN), and water were from Fisher Scientific (Fair Lawn, NJ). Dulbecco's phosphate-buffered saline (DPBS) was from Mediatech (Manassas, VA). Ammonium acetate (NH4OAc) and butylated hydroxytoluene (BHT) were from Sigma-Aldrich (St. Louis, MO), 10N potassium hydroxide (KOH) was from Fisher Scientific, ammonium iodide (NH4I) was from Acros Organics (Geel, Belgium), and N-methyl-N[tert-butyldimethylsilyl trifluoroacetamide with 1% tert-butyldimethylchlorosilane (MTBSTFA with 1% TBDMCS) was from Restek (Bellefonte, PA). All solvents were HPLC-grade or better; all chemicals were ACS-grade or better.
TABLE 1.
Sterols, oxysterols, and secosteroids
| Common Name | LIPID MAPS Number | Elemental Formula | MW | Identifier | RRI | Source |
| Dehydroergosterol | LMST01031023 | C28H42O | 394.32 | A | 0.77* | AVT |
| Vitamin D2 | LMST03010001 | C28H44O | 396.34 | B | 0.80* | AVT |
| Vitamin D3 | LMST03020001 | C27H46O | 384.34 | C | 0.83* | AVT |
| Zymosterol† | LMST01010066 | C27H44O | 384.34 | D | 0.87* | AVT |
| Desmosterol† | LMST01010016 | C27H44O | 384.34 | E | 0.92* | AVT |
| 8(9)-Dehydrocholesterol | LMST01010242 | C27H44O | 384.34 | F | 0.97* | AVT |
| Ergosterol | LMST01030093 | C28H44O | 396.34 | G | 0.98* | SIG |
| 7-Dehydrocholesterol | LMST01010069 | C27H44O | 384.34 | H | 1.00* | AVT |
| 8(14)-Dehydrocholesterol | 1.00* | AVT | ||||
| Cholestenone (Δ4) | LMST01010015 | C27H44O | 384.34 | I | 1.00* | AVT |
| Cholestenone (Δ5) | LMST01010248 | C27H44O | 384.34 | J | 1.03* | STER |
| Brassicasterol | LMST01030098 | C28H46O | 398.35 | K | 1.05* | SIG |
| Lathosterol | LMST01010089 | C27H46O | 386.35 | L | 1.05*§ | SIG |
| Cholesterol† | LMST01010001 | C27H46O | 386.35 | M | 1.06*§** | AVT |
| 14-Demethyl-lanosterol† | LMST01010176 | C29H48O | 412.37 | N | 1.07* | AVT |
| Lanosterol | LMST01010017 | C30H50O | 426.39 | O | 1.09* | AVT |
| Dihydrocholesterol† | LMST01010077 | C27H48O | 388.37 | P | 1.12* | SIG |
| Campesterol | LMST01030097 | C28H48O | 400.37 | Q | 1.13* | SIG |
| Stigmasterol | LMST01040124 | C29H48O | R | 1.13* | AVT | |
| Cycloartenol | LMST01100008 | C38H50O | 426.39 | S | 1.13* | STER |
| β-Sitosterol† | LMST01040129 | C29H50O | 414.39 | T | 1.20* | SIG |
| 24, 25-Dihydrolanosterol | LMST01010087 | C30H52O | 428.40 | U | 1.22* | AVT |
| Stigmastanol | LMST01040128 | C29H52O | 412.37 | V | 1.27* | SIG |
| 1α, 25-Dihydroxyvitamin D2† | LMST03020660 | C27H44O3 | 416.33 | b | 0.35†† | SIG |
| 7 α, 25-Dihydroxycholesterol† | LMST04030166 | C27H46O3 | 4.18.34 | a | 0.33†† | AVT |
| 7 α, 27-Dihydroxycholesterol† | LMST04030178 | C27H46O3 | 418.34 | c | 0.38†† | AVT |
| 1α, 25-Dihydroxyvitamin D2 | LMST03010040 | C27H44O3 | 428.33 | d | 0.41†† | CRO |
| 3β, 27-Dihydroxy-5-cholesten-7-one | LMST04030180 | C27H44O3 | 416.33 | e | 0.44†† | AVT |
| 7α, 26-Dihydroxycholest-4-en-3-one | LMST04030157 | C27H44O3 | 416.33 | f | 0.44†† | AVT |
| 16α, 27-Dihydroxycholesterol | LMST04030179 | C27H46O3 | 418.34 | g | 0.44†† | STER |
| (20R)-17α, 20-Dihydroxycholesterol | LMST04030176 | C27H46O3 | 418.34 | h | 0.56†† | STER |
| 25-Hydroxyvitamin D2† | LMST03020246 | C27H44O2 | 400.33 | i | 0.77†† | AVT |
| 25-Hydroxyvitamin D3† | LMST03010030 | C28H46O2 | 412.33 | j | 0.83†† | AVT |
| 22R-Hydroxycholesterol† | LMST01010086 | C27H46O2 | 402.35 | k | 0.89†† | AVT |
| 25-Hydroxy-cholesterol† | LMST01010018 | C27H46O2 | 402.35 | l | 0.93†† | AVT |
| 24S-Hydroxy-cholesterol | LMST01010019 | C27H46O2 | 402.35 | m | 0.95†† | AVT |
| 24-Oxocholesterol | LMST01010133 | C27H44O2 | 400.33 | n | 0.98†† | STER |
| 27-Hydroxy-cholesterol† | LMST01010057 | C27H46O2 | 402.35 | o | 1.00†† | AVT |
| 15α-Hydroxycholestene | LMST01010267 | C27H46O2 | 402.35 | p | 1.00†† | AVT |
| 20-Hydroxycholesterol | LMST01010201 | C27H46O2 | 402.35 | q | 1.00†† | STER |
| 24(S), 25-Epoxycholesterol | LMST01010012 | C27H44O2 | 400.33 | r | 1.02†† | AVT |
| 5α, 6β-Dihydroxycholestanol | LMST01010052 | C27H48O3 | 420.36 | s | 1.02†† | STER |
| 15-Ketocholestene | LMST01010269 | C27H44O2 | 400.33 | t | 1.08†† | AVT |
| 15-Ketocholestane | LMST01010270 | C27H46O2 | 402.35 | u | 1.12†† | AVT |
| 3-Oxo-7α-hydroxycholesterol | LMST01010271 | C27H44O2 | 400.33 | v | 1.16†† | SIG |
| 7-Oxo-cholestenone | LMST01010272 | C27H42O2 | 398.32 | w | 1.19†† | STER |
| 7α-Hydroxy-cholesterol† | LMST01010013 | C27H46O2 | 402.35 | x | 1.18†† | AVT |
| 7β-Hydroxy-cholesterol† | LMST01010047 | C27H46O2 | 402.35 | 1.18†† | AVT | |
| 1α-Hydroxyvitamin D2 | LMST03010028 | C27H44O2 | 412.33 | y | 1.19†† | EMD |
| 1α-Hydroxyvitamin D3 | LMST03020231 | C27H44O2 | 400.33 | z | 1.20†† | EMD |
| 7-Oxocholesterol† | LMST01010049 | C27H44O2 | 400.33 | a | 1.20†† | AVT |
| Cholestan-6-oxo-3, 5-diol | LMST01010126 | C27H46O3 | 418.34 | b | 1.21†† | STER |
| 15β-Hydroxycholestane | LMST01010268 | C27H46O2 | 402.35 | c | 1.21†† | AVT |
| 15α-Hydroxycholestane | LMST01010273 | C27H48O2 | 404.37 | d | 1.21†† | AVT |
| 6-Ketocholestanol | LMST01010276 | C27H46O2 | 402.35 | e | 1.29†† | SIG |
| 6α-Hydroxycholestanol† | LMST01010135 | C27H48O2 | 402.35 | f | 1.27†† | AVT |
| 19-Hydroxycholesterol | LMST01010274 | C27H46O2 | 402.35 | g | 1.29†† | STER |
| 5, 6β-Epoxy-cholesterol† | LMST01010011 | C27H46O2 | 402.35 | h | 1.33†† | AVT |
| 5α-Hydroxycholesterol | LMST01010275 | C27H48O2 | 402.35 | i | 1.33†† | STER |
| 5, 6α-Epoxy-cholesterol | LMST01010010 | C27H46O2 | 402.35 | j | 1.36†† | AVT |
| 4β-Hydroxy-cholesterol† | LMST01010014 | C27H46O2 | 402.35 | k | 1.42†† | AVT |
LIPID MAPS numbers can be used to access additional information at lipidmaps.org. The alphabetical identifier corresponds to the chromatographs in Fig. 1. AVT, Avanti Polar Lipids (Alabaster, AL); EMD, EMD Chemicals (Rockland, MA); SIG, Sigma-Aldrich (St. Louis, MO); STER, Seraloids (Newport, RI). MW, molecular weight; RRI, relative retention index.
Relative to cholestenonoe (Δ4) (Identifier I).
Deuterated analog available from Avanti Polar Lipids.
Lathosterol and cholesterol coelute in an actual plasma extract.
Cholesterol signal shown 0.1× scale.
Relative to 27-hydroxycholesterol (o).
Extraction, hydrolysis, and isolation of sterols and secosteroids from human plasma
Frozen plasma samples (stored at −80°C) were equilibrated to room temperature for approximately 30 min. To ensure homogeneity, each sample was pipetted three times with a 1 ml air-displacement pipette (fitted with a disposable 1 ml barrier tip). Then 200 μl of plasma were added drop-wise to 3 ml of 1:1 DCM:methanol in a 16 × 100 glass tube placed in a 30°C ultrasonic bath. The tube also contained 20 μl of a deuterated sterol and secosteroid standard cocktail and BHT at 50 μg/ml (see supplementary data for standard amounts). The tube was flushed with N2 for several seconds to displace oxygen, sealed with a PTFE-lined screw cap, and incubated at 30°C in the ultrasonic bath for 10 min. Following incubation, the sample was centrifuged at 3,500 rpm for 5 min at 25°C to pellet protein and other insoluble material. The supernatant from each sample was decanted into a 16 × 100 mm glass screw-cap tube and set aside. A 1:1 DCM:methanol solution (3 ml) was added to the pelleted material, and then the tube was capped and vigorously agitated until the pellet was dislodged and disrupted. The sample was centrifuged at 3,500 rpm for 5 min at 25°C, and the resulting supernatant was decanted back into the tube containing the supernatant from the initial extraction.
Hydrolysis was performed by adding 300 μl of 10N KOH to each tube and flushing with N2 for several seconds, followed by placement in a water bath at 35°C for 1.5 h. Following hydrolysis, 3 ml of DPBS was added to each sample, the tubes were capped, and the samples were agitated for several seconds. Samples were centrifuged at 3,500 rpm for 5 min at 25°C. The organic (lower) layer was removed with a 9″ Pasteur pipette and transferred to a 16 × 100 glass tube and set aside. DCM (3 ml) was added to the remaining sample, and then the tube was capped, vortexed for several seconds, and centrifuged at 3,500 rpm for 5 min at 25°C. Using a 9″ Pasteur pipette, the organic layer (lower layer) was removed and transferred to the 16 × 100 glass culture tube containing the initial sample. To maximize extraction efficiency, the same pipette was used for each liquid-liquid step, and the pipette was placed into a separate glass culture tube between steps. Hydrolyzed samples were then dried under N2 using a 27-port drying manifold (Pierce; Fisher Scientific, Fair Lawn, NJ).
Sterols and secosteroids were isolated using 200 mg, 3 ml aminopropyl SPE columns (Biotage; Charlotte, NC). The column was rinsed and conditioned with 2 × 3 ml of hexane. The extracted and dried plasma sample was dissolved in approximately 1 ml of hexane and gently swirled for several seconds. The sample (and any insoluble material) was then transferred to the SPE column using a 6″ Pasteur pipette and eluted to waste. The extract tube was rinsed with 1 ml of hexane and gently swirled, and the rinse was transferred to the column and eluted to waste. Again, to minimize sample loss, the same Pasteur pipette was used at each step. Following the addition of sample, the column was rinsed with 1 ml of hexane to elute nonpolar compounds. Sterols were then eluted from the column with 4.5 ml (1.5 column volumes) of 23:1 CHCl3:methanol into a new 16 × 100 glass culture tube. The eluted sample was dried under N2.
To prepare the sample for instrumental analysis, 400 μl of warm (37°C) 90% methanol was added, and the tube was placed in an ultrasonic bath for 5 min at 30°C. The sample was then transferred to an autosampler vial containing a 500 μl deactivated insert (Restek, Bellefonte, PA) and 20 μl of d6-6α-hydroxycholestanol as an internal standard. If particulate matter was observed in the sample during dissolution, then the vial insert was carefully transferred to a standard microcentrigure tube using fine-point tweezers and centrifuged at room temperature for 5 min at 6,000 rpm in a microcentrifuge. The vial insert was then carefully transferred back to the autosampler vial and stored at room temperature until analysis.
A 50 μl aliquot of the sample was removed and transferred to a new autosampler vial for analysis by GC-MS. The sample was dried under N2. Derivatization of sterols was performed by adding 100 μl of 1:1 pyridine:MTBSTFA (1%TBDMCS) with 2 mg/ml NH4I (dissolved first in pyridine), flushing the sample with N2, capping the vial, and incubating at 70°C for 30 min. The derivatized sample was dried under N2, dissolved in 150 μl of hexane, vortexed for 5 min, and stored at room temperature until analysis.
Instrumental analysis
Quantitative analysis of sterols, oxysterols, and secosteroids was performed using a combination of techniques that included HPLC-MS-APCI, HPLC-MS-ESI, and GC-MS. Oxysterols were analyzed using a tertiary Shimadzu LC-20XR HPLC system (Shimadzu Scientific Instruments, Columbia, MD) equipped with an ultrasonic degasser, column oven, and autosampler. The HPLC was coupled to an AB Sciex API-5000 MS equipped with Turbo V ESI source (Foster City, CA). Oxysterols were resolved with a binary solvent gradient using a Kinetex C18 HPLC column (150 × 2.1 mm, 2.6 μm particle size; Phenomenex, Torrance, CA). The mobile phases were (A) 70% ACN with 5 mM NH4OAc (dissolved in water first) and (B) 1:1 IPA:ACN with 5 mM NH4OAc (dissolved first in IPA for 10–12 h). Following preparation, mobile phases were allowed to come to thermal equilibrium overnight at 25°C. After injection of 30 μl of extract, the gradient was started at 0% B and ramped to 100% B over 7 min, held at 100% B for 3 min, and returned to 0% B for a 2 min equilibration. The flow rate was 0.5 ml/min, and the column was maintained at 18°C.
Sterols were analyzed using a binary Shimadzu LC-10ADvp system with a column oven and CTC LC PAL autosampler (Leap Technologies, Carrboro, NC) coupled to an AB Sciex 4000 QTrap mass spectrometer equipped with a Turbo V APCI source. Sterols were resolved with a two-step isocratic elution using a Poroshell 120 SB-C18 HPLC column (150 × 2.1 mm, 2.7 μM particle size; Agilent Technologies, Wilmington, DE). The mobile phases were (A) 96% methanol with 0.1% acetic acid (AA) and (B) methanol with 0.1% AA. The autosampler was equipped with a 5 μl injection loop that was loaded with 15 μl of extract. The elution began at 100% A for 7.5 min, increased to 100% B at 8 min, was held at 100% B for 6 min, and returned to 100% A for a 6 min equilibration. The flow rate was 0.4 ml/min, and the column was maintained at 30°C.
Both MS instruments were operated in scheduled multiple-reaction monitoring (SMRM) mode. Transitions for each compound were optimized by flow injection via syringe pump using a representative solvent flow rate of 1:1 A:B for each instrument. MRM transitions for oxysterols in ESI mode were primarily optimized for the ammonium adduct [M+NH4]+ or the protonated molecular ion [M+H]+ generated through the loss of NH3. The transitions included the loss of one or more −OH groups as water, depending on the specific compound. Sterols in APCI mode were optimized for either the protonated adduct [M+H]+ or the loss of −OH as water [M+H−H2O]+. The transition includes a nondescript, low-mass fragment often at m/z 81, 95, or 109, although there were several exceptions in which fragments of various masses were generated. A complete list of transitions for sterols and oxysterols and a detailed description of instrument settings and parameters for both HPLC-MS analyses are provided in supplementary data.
Lathosterol was analyzed using an Agilent 7890 GC coupled to an Agilent 5975C mass-selective detector (MSD; Wilmington, DE). The instrument was equipped with a 40 m Agilent J and W DB-5MS column (180 μm i.d., 0.18 μm film thickness; Folsom, CA). The injection port was maintained at 300°C, and 1 μl injections were performed using splitless conditions with a purge to split vent at 0.5 min. Hydrogen was used as the carrier gas and was generated with a Parker Balston H2PD-150 hydrogen generator (Haverhill, MA) at an average linear velocity of 55 cm/s in constant flow mode. The temperature program began at 150°C for 1 min, then was ramped at 10°C/min to 325°C and held for 6.5 min. The transfer line was heated to 300°C, the ion source to 230°C, and the quadrupole to 150°C. Electron ionization was used at 70 eV, and selected ion monitoring (SIM) was used for data acquisition. A complete description of instrument configuration and settings is provided in the supplementary data.
Aliquots of plasma were sent to a commercial clinical chemistry laboratory for measurement of total cholesterol.
An extensive and detailed discussion of each step of the extraction and instrumental analysis is provided in supplementary data. Here the reader can gain useful tips and tricks as well as read discussion explaining how and why each step of the procedure was implemented.
Quantitation
Integration of areas under elution curves for LC-MS and GC-MS was done using Analyst™ 1.5.1 and Chemstation™ software, respectively. Quantitative values were calculated using isotope dilution and single-point calibration through a relative response factor (RRF) calculation. The RRF standard was run at the beginning, middle, and end of each sample set; typically, 54 samples per set. Deuterated analogs were not available for every analyte; thus, a deuterated compound of similar chemical composition was substituted when necessary. A table of primary compounds and the respective deuterated analogs used for quantitation is given in the supplementary data.
RESULTS
Chromatography
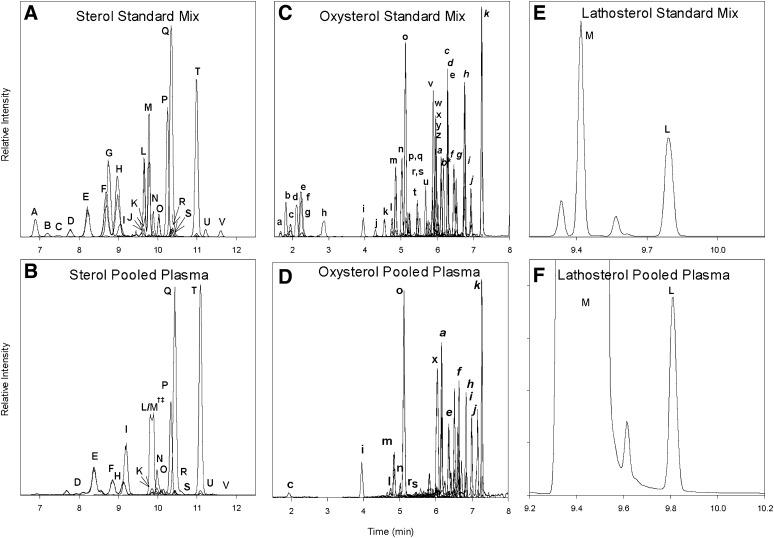
Reconstructed ion chromatograms (RIC) for standard mixes and extracts of a pooled plasma sample for sterols, oxysterols, and lathosterol are shown in Fig. 2. To improve the clarity of data presented in the figure, deuterated analogs are not included in the RICs. The chromatographic peaks are labeled alphabetically and correspond to compounds listed in Table 1.
Fig. 2.
(A–F) LC-MS chromatograms of standard mixes and extracts of a pooled plasma samples for sterols, oxysterols, and lathosterol.
The 22 sterol and sterol-derived compounds routinely detected in plasma by this method are either resolved or have unique masses that allow them to be uniquely identified by mass spectrometry. Sterols are labeled with capital letters and correspond to labels given in Table 1. Sterols elute between 6.5 and 11.5 min from the HPLC columns. Less-polar compounds, such as zymosterol (identifier D) and desmosterol (identifier E), elute early, and methylated sterols, such as sitosterol (identifier T) and 24,25-dihydrolanosterol (identifier U), elute later in the chromatogram. Use of the core-shell HPLC column resulted in chromatographic peak widths on the order of 6–8 s at full width-half maximum (FWHM). Fig. 2E depicts the resolution of lathosterol (identifier L) and cholesterol (identifier M) by GC-MS; however, in a plasma extract, the physiological levels of cholesterol overwhelm the lathosterol signal, causing inadequate resolution (Fig. 2F). To maintain a common scale, the cholesterol signal in Fig. 2B is plotted at 0.1× scale. The split peak shown in Fig. 2B is not an unresolved lathosterol and cholesterol signal but, rather, the result of the extreme abundance of cholesterol causing splitting and overload of the HPLC column.
Oxysterols elute between 1.5 and 7.5 min in three general groups as shown in Fig. 2C, D. The use of core-shell HPLC columns yielded chromatographic peak widths on the order of 3–6 s FWHM. Polar hydroxylated sterols such as 7α,27-dihydroxycholesterol (identifier c) elute in the first group between 1.5 and 3 min. The 25-hydroxylated vitamin D2 and D3 compounds (identifiers i and j) elute with the side-chain oxysterols 24-, 25-, and 27-hydroxycholesterol (identifiers l, m, and o) between 4 and 5.5 min. The least-polar compounds, ring structure oxysterols, such as 7α- and 4β-hydroxycholesterol (identifiers x and k), elute in the third group between 5.5 and 7.5 min.
The known enzymatically formed oxysterols are resolved from other isobaric species with several exceptions, the first being the separation of 7α- and 7β-hydroxycholesterol. Previous work has shown that 7β-hydroxycholesterol is present in human plasma at approximately 10% of the level of 7α-hydroxycholesterol (14). Although the two compounds can be resolved by GC-MS, we were not able to resolve them with the HPLC parameters described here; therefore, the values for 7α-hydroxycholesterol include any 7β-hydroxycholesterol present in the sample. The second set of coeluting compounds of biological importance are 8,(14)-dehydrocholesterol and 7,(8)-dehydrocholesterol. The origins of 8,(14)-dehydrocholesterol are not well understood, and it is unclear if the compound was present in our samples. Thus, 7,(8)-dehydrocholesterol may include unknown quantities of 8,(14)-dehydrocholesterol. The third set of coeluting compounds is 24,25-dihydrolanosterol and an unknown compound. The 24,25-dihydrolanosterol is poorly resolved and is further complicated by the very low signal level observed in most of the human plasma samples analyzed here. The unknown compound has an isobaric signal and elutes after 24,25-dihydrolanosterol; with manual integration, a reasonable estimate of the peak area can be achieved. The fourth set of coeluting compounds is composed of 20-hydroxycholesterol and 15α-hydroxycholestenone. The origin of these compounds is poorly understood. Their coelution does not appear to be of major significance; in the 200 plasma samples analyzed here, only trace-level signals were detected for their MRM pairs corresponding to the correct retention time.
Due to the similar structures of lathosterol (identifier L) and cholesterol (Δ7,8 versus Δ5,6) and considerably disparate concentration levels (cholesterol is 1,000× more abundant), the HPLC method used to resolve sterols does not sufficiently separate lathosterol from cholesterol; however, the GC-MS method described herein is able to resolve lathosterol from cholesterol (Fig. 2E, F). Separation is accomplished through the use of hydrogen as a carrier gas in conjunction with a very high resolving column. A chromatographic signal for lathosterol is produced with a peak width of 2 s at FWHM.
Mass spectrometry
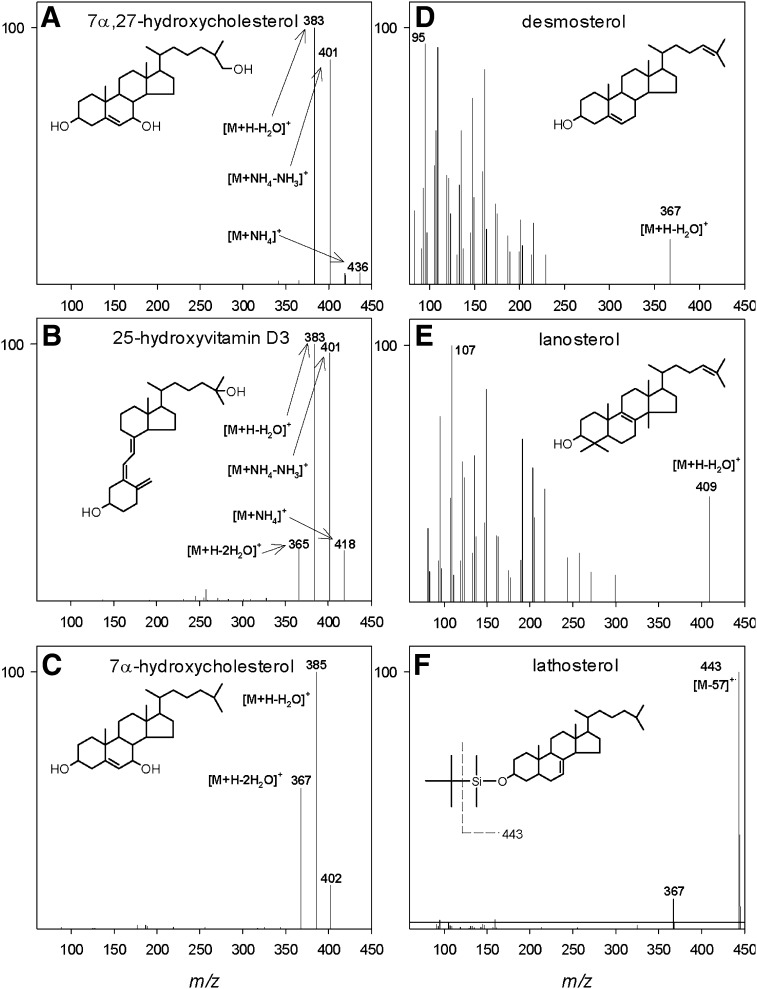
Representative mass spectra for selected sterols, oxysterols, and secosteroids are shown in Fig. 3. Examples, including 7α,27-dihydroxycholesterol, 25-hydroxyvitamin D3, and 7α-hydroxycholesterol, ionized under electrospray conditions are shown in Fig. 3A–C. The oxysterols are neutral and ionize poorly with electrospray alone; ammonium acetate is added to the mobile phase, which results in the formation of readily detectable ammonium adducts. All oxysterols show common features of ionization and fragmentation with collision-induced dissociation (CID) using mild collision energies (10–20 V). These features arise from both in-source decay and during CID. Mass spectral features common to most oxysterols include an ammonium adduct at [M+18]+, a protonated ion at [M+18-NH3]+, and ions derived from sequential losses of 17 and 18 mass units for each alcohol group, depending on the specific compound. Due to the four-ring structure and isooctyl side chain found in all oxysterols, no unique mass spectral information was obtained for isomeric compounds using CID. High-energy CID (50–60 V) yielded a heavily fragmented collision spectrum consisting of methylene groups spaced 14 mass units apart with no unique mass spectral information. For quantitative purposes, it was determined that mild CID conditions yielded better oxysterol signal intensity in conjunction with MRM.
Fig. 3.
(A–C) ESI product-ion mass spectra of representative oxysterols and the secosteroid 25-hydroxyvitamin D3. (D, E) APCI product-ion mass spectra of representative sterols. (F) EI mass spectrum of derivatized lathosterol. Selected ions are identified and structures are included in each panel for reference.
Respective mass spectra for two sterols, desmosterol and lanosterol, are shown in Fig. 3D, E. Like oxysterols, sterols are neutral but they are less polar and, therefore, more efficiently ionized by APCI. The pseudo-molecular ion formed for most sterols is the loss of the alcohol group as water at [M+H−H2O]+. Because the pseudo-molecular ion is a result of water loss, there are no additional alcohols to dissociate under mild CID conditions as with the oxysterols. Therefore, a high-energy CID is used (e.g., 50–60 V) to generate a heavily fragmented collision spectrum. For quantitation, the MRM transition was typically the pseudo-molecular ion and the most intense fragment ion, usually m/z 81, 95, or 109. Additional sterol mass spectra can be found at http://www.lipidmaps.org.
The electron ionization mass spectrum for MTBSTFA-derivatized lathosterol is shown in Fig. 3F. The base peak is observed at m/z 443, resulting from the loss of the isopropyl group at [M−57]+. A smaller signal is also present at m/z 367, which arises from the loss of the entire MTBS group with the oxygen at the C3 position, along with an additional unsaturation forming at an unknown location in the sterol molecule. The signal at m/z 443 was monitored with SIM for quantitation.
Quality analysis/quality control
The recovery of sterols, oxysterols, and secosteroids from the solvent spike QC sample showed that for the 10 oxysterols reported in Table 2, recovery was between 99.6% and 109%, indicating virtually complete recovery. The 9 sterols detected had a slightly wider recovery range between 94.6% and 111%, with the exception of 7-dehydrocholesterol at 85%. The solvent spike gives an accurate measure of the extraction efficiency across a wide concentration range of sterols; however, this standard does not represent the complex chemical matrix found in a human plasma sample. Interference from this matrix may cause artificially high or low values due to chromatographic, ionization, or mass spectral issues.
TABLE 2.
Measurement of sterols, oxysterols, and secosteroids
| Average (RSE) | Median | Range | Pooled Plasma Average (RSE)† | NIST SRM Plasma Average | Spike Recovery (%)† | |
| Oxysterol (ng/ml)* | ||||||
| 7αHC (x) | 158.7 (3.2) | 153.6 | 33–435 | 56.86 (10.6) | 91.8 | 104.3 |
| 7α,27HC (c) | 10 (2.4) | 9.6 | 2–22.8 | 9.24 (5.3) | 9.6 | 99.6 |
| 25OH VD3 (i) | 24.3 (2.8) | 25.6 | 0–57.6 | 9.32 (1.4) | 20.7 | 97.1 |
| 25HC (l) | 11.8 (2.4) | 11.0 | 2.6–28.2 | 6.1 (3.9) | 5.7 | 106.3 |
| 24HC (m) | 56.1 (2.1) | 53.6 | 24–117 | 46.13 (3.3) | 44.6 | 101.4 |
| 27HC (o) | 151.4 (2.0) | 144.1 | 60–390 | 124.6 (2.0) | 131.0 | 100.9 |
| 4βHC (k) | 53.1 (2.4) | 48.9 | 16.7–144 | 43.9 (3.0) | 31.4 | 104.0 |
| 24oxoC (n) | 6.0 (2.2) | 5.6 | 2.6–12.1 | 5.1 (13.5) | 3.7 | 109.0 |
| 24/25EC (r) | 1.8 (4.4) | 1.5 | 0.2–9.1 | 1.5 (6.7) | 1.8 | 100.1 |
| 7oxoC (a) | 84 (4.5) | 66.5 | 25.7–360.8 | 40.54 (18.3) | 24.0 | 104.6 |
| Sterol (ng/ml)* | ||||||
| Chol (M)§ | 184.0 | 182.5 | 112–319 | 120.7 | 145.0 | N/A |
| Zymo (D) | 24.8 (6.5) | 16.9 | 0–139 | 19.7 (1.6) | 32.2 | 96.9 |
| Desm (E) | 713.4 (3.3) | 692.0 | 37–1,800 | 623 (0.8) | 722.2 | 97.1 |
| Lano (O) | 203.2 (6.4) | 151.2 | 35–1,480 | 101.2 (1.1) | 123.9 | 94.6 |
| 24-DiHL (U) | 20.8 (13) | 10.2 | 2.4–347 | 5.5 (1.6) | 8.5 | 96.0 |
| 7-DHC (H) | 803.2 (2.8) | 744.7 | 424–2,488 | 510 (1.1) | 1,137.8 | 85.0 |
| Cholestanol (P) | 2,913.9 (2.3) | 2827.6 | 160–6,200 | 2,526 (0.6) | 2,581.1 | 95.9 |
| 14-DML (N) | 517 (3.6) | 456.0 | 14.5–1,915 | 305 (1.1) | 451.1 | 107.3 |
| Lath | 3,892 (7.1) | 3,388 | 166–14,460 | 3,078 (3.9) | 2,080 | 106.5 |
| Camp (Q) | 3,831.4 (3.8) | 3,546.1 | 808–16,400 | 2,502 (0.7) | 2,645.6 | 111.0 |
Results from a cohort of 200 subjects from the Cooper Institute in Dallas, TX. Data are nanograms per milliliter except for cholesterol, which is milligrams per deciliter. NIST SRM, pooled plasma, and spike recovery are included for comparison as well as for QA/QC analysis. Letters in parentheses correspond to chromatographic peak labels in Fig. 1. Error values in parentheses represent the relative standard error (RSE).
*N = 200.
†N = 6.
§Milligrams per deciliter measured by clinical chemistry assay.
To better assess the recovery of sterols from a plasma sample, we added one deuterated internal standard to the autosampler vial prior to transferring the final extract. This addition allowed the recovery of deuterated surrogates in each sample to be determined. We quantified the recovery of four representative compounds that cover the range of oxysterols analyzed. The recovery for (d6) 1,25-hydroxyvitamin D3, (d6) 27-hydroxycholesterol, (d6) 7α-hydroxycholesterol, and (d6) 4β-hydroxycholesterol ranged from 87.9% to 100.8% (Table 3). These values, combined with solvent spike recoveries, show that this extraction procedure is both thorough and robust, with excellent recovery values. Additionally, the final dissolution of the purified dried extract is done in a single step with no additional rinsing. A second rinse step would cause undesired dilution of the final extract.
TABLE 3.
Recovery of four deuterated surrogate standards
| Average Recovery (%)* | % Relative Standard Deviation | % Relative Standard Error | |
| (d6) 1,25 VD3 | 87.9 | 25.7 | 1.8 |
| (d6) 27HC | 90.7 | 18.1 | 1.3 |
| (d6) 7αHC | 100.8 | 20.3 | 1.4 |
| (d6) 4βHC | 100.3 | 21.8 | 1.5 |
*N = 200.
With each batch of 50 samples extracted, an aliquot of a commercially obtained, pooled plasma sample was analyzed to monitor and evaluate day-to-day variation in measurements. As seen in Table 2, reproducibility was excellent across extractions done on six different days. The relative standard errors (RSE) for oxysterols ranged between 1.4 and 10.6 for known enzymatically formed oxysterols with higher RSEs of 13.5% and 18.3% for two compounds that are potentially formed through oxidation and may be partially degraded during base hydrolysis, 24-, and 7-oxocholesterol. The RSEs for sterols in pooled plasma have less variability and ranged between 0.6% and 3.9%; however, all sterols reported here are enzymatically formed, and none are oxidation products or known to be susceptible to base hydrolysis. Included in Table 2 are data from analysis of the National Institute of Standards and Technology (NIST) human plasma standard reference material (SRM) for reference (15) and comparison with the commercially obtained pooled plasma used in this study.
The solvent blank that was included with each batch of 50 samples did not show a response for any compound measured here. Therefore, there was no evidence of carryover, cross-contamination, or isobaric interferences originating from the sample extraction.
The instrument detection limit (IDL) was estimated at ≤ 50 pg on-column for each compound. The method detection limit (MDL) for each compound was estimated at ≤ 1 ng/ml. Detector response was linear over at least four orders of magnitude from the instrument detection limit.
Results from the Cooper Institute Cohort
Quantitative results for a representative set of sterols from 200 human plasma samples are shown in Table 2. The data include average, median, standard deviation, and range of concentrations measured. For reference and comparison, the results from the commercially obtained pooled plasma used as a QA/QC sample and the results from the previously analyzed NIST SRM plasma are included (15). Lastly, the solvent spike recovery value is provided showing one metric of our quality control scheme. In Table 3, the recoveries of four representative deuterated oxysterol standards are shown.
DISCUSSION
Data
The dataset provided herein includes all compounds that were routinely detected in the 200 samples analyzed from the Cooper Institute. We assayed for approximately 60 compounds, but only a subset of 22 compounds was routinely detected. Not included in these data are values obtained for most known oxidation products, such as the 5/6(α/β)-epoxycholesterols (27), due in part to problems with the primary standard or deuterated analog, which have been subsequently resolved. Table 2 provides values for 25-hydroxyvitamin D3 and a select group of sterols and oxysterols measured in the 200 samples. These data were acquired using methods described here, but it should be noted that quantitative values for some compounds may vary, depending on specific extraction procedures employed. This method combines techniques from previously published work as well as novel aspects developed in our laboratory. The intent of this work was in part to develop a robust extraction procedure that generated values consistent with those in the literature and was capable of a reasonable throughput so it could be applied to large human cohorts. It is optimized toward enzymatically formed sterols, with secondary thought given to oxidation products of cholesterol. Investigators wishing to target these oxidation products may consider alternative extraction procedures.
Comparison of the data obtained from the Cooper Institute cohort with that obtained from the NIST SRM and commercially purchased pooled plasma provides insight into the variability observed between sample populations. In the Cooper Institute cohort, the average oxysterol concentrations for the 200 subjects ranged from 1.8 ng/ml for 24,25-epoxycholesterol to 158.7 ng/ml for 7α-hydroxy-cholesterol. The NIST SRM and the commercially purchased pooled plasma also showed 24,25-epoxycholesterol to be the least abundant of the oxysterols, with concentration levels of 1.5 and 1.8 ng/ml, respectively. In contrast to the 200 Cooper Institute samples, the most abundant oxysterol was determined to be 27-hydroxycholesterol at 124.6 ng/ml (NIST) and 131.0 ng/ml (pooled plasma). Average sterol concentrations ranged from 20.8 ng/ml for 24-dihydrolanosterol to 3,892 ng/ml for lathosterol. Cholesterol was measured by a non-MS method at an average of 1.84 mg/ml. Average observed sterol concentrations for the NIST SRM and pooled plasma were consistent with that observed in the Cooper Institute samples. The exception was campesterol (rather than lathosterol) as the most abundant sterol at ∼2,645 ng/ml. Cholesterol levels were lower in the NIST SRM than in the Cooper Institute samples; the average amount of cholesterol for the NIST SRM was 1.45 mg/ml and for the pooled plasma, 1.21 mg/ml.
The Cooper Institute samples originate from a largely Caucasian and affluent population. In contrast, the NIST SRM plasma was designed specifically to be representative of the broader US population in age, gender, and ethnicity. The only sample that was not well defined in terms of population was the commercially purchased pooled plasma, which was collected from anonymous donors. The only defined parameter for this sample is the age of the donors, ranging from 18 to 65 years of age. Although the populations showed differences in the measured values for some compounds, such as cholesterol and 7α-hydroxycholesterol (158.7 ng/ml for the Cooper samples, 91.8 ng/ml for the NIST SRM, and 56.9 ng/ml for the pooled plasma), most compounds were present in comparable levels (Table 2). For example, desmosterol levels were 713.4 ng/ml in the Cooper samples, 623.0 ng/ml in the pooled plasma, and 722.2 ng/ml in the NIST SRM. The variation observed among these samples may be attributable to population differences, such as the geographic region from which the pools were collected or the diet, age, gender, and/or ethnicity of the subjects.
Knowing the potential variability of sterol levels in different human populations provides an illustration in the nuances of comparing published values in the literature. Clearly the composition of the population can and will play a role in the measured levels of various compounds. This being written, the values we report for the Cooper Institute cohort compare favorably with other values in the literature (27), with the understanding that there can be variation between populations.
The diversity of sterols and secosteroids analyzed using these methods does comes with some compromises. The liquid chromatography methods described here are not able to resolve all stereoisomers (e.g., 7α- and 7β-hydro-xycholesterol; 4α- and 4β-hydroxycholesterol); thus, the values we report for these sterols in the Cooper Institute cohort include both isomers for each respective pair. Furthermore, although reasonable steps are taken to reduce nonenzymatic oxidation of sterols during isolation, we cannot rule out that at least some of the sterols measured here represent nonspecific oxidation products. A case in point is 7-oxocholesterol, which is readily detected but of unknown origin. It is likely that more aggressive measures, such as the removal of cholesterol, would mitigate the formation of oxidation products such as this oxosterol; however, the present method was developed to allow relative comparisons in sterol levels between subjects in large human cohorts. Because all samples were extracted with the same procedure, a relative analysis within the cohort is valid. The absolute value of each compound reflects the methods described here. Comparison of mean values for the Cooper Institute cohort with other cohort values that were obtained using different methods of extraction and analysis must be interpreted with care, especially for unresolved isomers and known oxidation products.
FUTURE DIRECTIONS
Although the HPLC methods described here are satisfactory for analyzing a wide range of sterols, oxysterols, and secosteroids, we believe further modifications to the methods may provide increased resolution and efficiency. For example, the dihydroxysterols (e.g., 7α,27-dihydroxycholesterol) and similar compounds are too polar for optimal elution and separation with the methods described here. The starting mobile phase of 70% ACN elutes this group of oxysterols early (around 2 min). Some of the chromatographic signals are broader than those signals for ring-structure and side-chain oxysterols, and some signals show tailing. To further optimize chromatographic conditions to resolve these polar sterols, we plan to test more aqueous mobile phases, such as those containing 50–60% ACN. The opposite problem is seen with respect to the sterol HPLC program. These compounds, especially the methylated, saturated sterols like 24,25-dihydrolanosterol, are highly retained at even 100% methanol. The shallow two-step isocratic gradient (96% methanol, 100% methanol) used here is not optimal, and employing a gradient elution may increase resolution and efficiency of separation. We will attempt to develop an HPLC program that makes use of a less retentive C8 column that may be more appropriate for sterol separation.
As we have discussed here, sterols are neutral molecules and therefore not well suited for ionization with ESI, although APCI yields sufficient sensitivity to measure physiological levels of sterols. Furthermore, the multi-ring structure of sterols does yield unique or useful fragment ions with CID. Recently, several groups have investigated derivatization schemes that “charge-tag” sterols, resulting in increases in ionization efficiency and or unique fragments obtained with CID (28–31). Despite the added steps required to perform this derivatization, the benefits of charge tagging are substantial and worth an increased effort in select situations. We plan to evaluate charge-tagging schemes to determine their suitability for the analysis of the broad set of sterols, oxysterols, and secosteroids we measured here.
Supplementary Material
Acknowledgments
The authors thank Jonathan Cohen for arranging access to samples from the Cooper Institute; Bonne Thompson, Daphne Head, and Aaron Ochoa for technical assistance in processing samples; and Stacy McDonald for technical review of the manuscript.
Footnotes
Abbreviations:
- CID
- collision-induced dissociation
- FWHM
- full width-half maximum
- MRM
- multiple-reaction monitoring
- NIST SRM
- National Institute of Standards and Technology standard reference material
- SIM
- selected ion monitoring
- SPE
- solid-phase extraction
This work was supported by the National Institute of General Medical Sciences Large Scale Collaborative “Glue” Grant U54-GM0-69338 (LIPID MAPS), National Institutes of Health Grant HL-20948, and Robert A. Welch Foundation Grant I-0971. A. R. Stiles is supported by National Institutes of Health Genetics Training Grant JT32-GM-03831. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five appendices.
REFERENCES
- 1.Brown M. S., Goldstein J. L. 2009. Cholesterol feedback: from Schoenheimer's bottle to Scap's MELADL. J. Lipid Res. 50: S15–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myant N. B. 1981. The Biology of Cholesterol and Related Steroids. William Heinemann Medical Books, London. [Google Scholar]
- 3.Russell D. W. 2003. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72: 137–174 [DOI] [PubMed] [Google Scholar]
- 4.Dietschy J. M., Turley S. D. 2004. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 45: 1375–1397 [DOI] [PubMed] [Google Scholar]
- 5.Ostlund R. E., Jr, Racette S. B., Stenson W. F. 2003. Inhibition of cholesterol absorption by phytosterol-replete wheat germ compared with phytosterol-depleted wheat germ. Am. J. Clin. Nutr. 77: 1385–1389 [DOI] [PubMed] [Google Scholar]
- 6.Moruisi K. G., Oosthuizen W., Opperman A. M. 2006. Phytosterols/stanols lower cholesterol concentrations in familial hypercholesterolemic subjects: a systematic review with meta-analysis. J. Am. Coll. Nutr. 25: 41–48 [DOI] [PubMed] [Google Scholar]
- 7.Bauman D. R., Bitmansour A. D., McDonald J. G., Thompson B. M., Liang G., Russell D. W. 2009. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl. Acad. Sci. USA. 106: 16764–16769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannedouche, Zhang J., Yi T., Shen W., Nguyen D., Pereira J. P., Guerini D., Baumgarten B. U., Roggo S., Wen B., et al. 2011. Oxysterols direct immune cell migration via EBI2. Nature. 475: 524–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C., Yang X. V., Wu J., Kuei C., Mani N. S., Zhang L., Yu J., Sutton S. W., Qin N., Banie H., et al. 2011. Oxysterols direct B-cell migration through EBI2. Nature. 475: 519–523 [DOI] [PubMed] [Google Scholar]
- 10.Kotti T. J., Ramirez D. M. O., Pfeiffer B. E., Huber K. M., Russell D. W. 2006. Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proc. Natl. Acad. Sci. USA. 103: 3869–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki R., Lee K., Jing E., Biddinger S. B., McDonald J. G., Montine T. J., Craft S., Kahn C. R. 2010. Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metab. 12: 567–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross C. A., Taylor C. L., Yaktine A. L., Del Valle H. B., 2011. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press, Washington, DC [Google Scholar]
- 13.Kulie T., Groff A., Redmer J., Hounshell J., Schrager S. 2009. Vitamin D: an evidence-based review. J. Am. Board Fam. Med. 22: 698–706 [DOI] [PubMed] [Google Scholar]
- 14.Lund E. G., Diczfalusy U. 2003. Quantitation of receptor ligands by mass spectrometry. In Methods in Enzymology. D. W. Russell and D. J. Mangelsdorf, editors. Academic Press, San Diego, CA. 364: 24–37. [Google Scholar]
- 15.Quehenberger O., Armando A. M., Brown A. H., Milne S. B., Myers D. S., Merrill A. H., Bandyopadhyay S., Jones K. N., Kelly S., Shaner R. L., et al. 2010. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 51: 3299–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipide extraction and purification. Can. J. Biochem. Physiol. 37: 911–917 [DOI] [PubMed] [Google Scholar]
- 17.Folch J., Lees M., Stanley G. H. S. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509 [PubMed] [Google Scholar]
- 18.Dzeletovic S., Breuer O., Lund E., Diczfalusy U. 1995. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 225: 73–80 [DOI] [PubMed] [Google Scholar]
- 19.Lütjohann D., Breuer O., Ahlborg G., Nennesmo I., Siden A., Diczfalusy U., Björkhem I. 1996. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA. 93: 9799–9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu L., von Bergmann K., Lutjohann D., Hobbs H. H., Cohen J. C. 2004. Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J. Lipid Res. 45: 301–307 [DOI] [PubMed] [Google Scholar]
- 21.McDonald J. G., Thompson B. M., McCrum E. C., Russell D. W. 2007. Extraction and analysis of sterols in biological matrices by high performance liquid chromatography electrospray ionization mass spectrometry. In Methods in Enzymology. H. A. Brown, editor. Academic Press, San Diego, CA. 432: 145–170. [Google Scholar]
- 22.Axelson M., Mörk B., Sjövall J. 1988. Occurrence of 3 beta-hydroxy-5-cholestenoic acid, 3 beta,7 alpha-dihydroxy-5-cholestenoic acid, and 7 alpha-hydroxy-3-oxo-4-cholestenoic acid as normal constituents in human blood. J. Lipid Res. 29: 629–641 [PubMed] [Google Scholar]
- 23.Miettinen T. A., Ahrens E. H., Grundy S. M. 1965. Quantitative isolation and gas–liquid chromatographic analysis of total dietary and fecal neutral steroids. J. Lipid Res. 6: 411–424 [PubMed] [Google Scholar]
- 24.DeBarber A. E., Connor W. E., Pappu A. S., Merkens L. S., Steiner R. D. 2010. ESI-MS/MS quantification of 7[alpha]-hydroxy-4-cholesten-3-one facilitates rapid, convenient diagnostic testing for cerebrotendinous xanthomatosis. Clin. Chim. Acta. 411: 43–48 [DOI] [PubMed] [Google Scholar]
- 25.DeBarber A. E., Lütjohann D., Merkens L., Steiner R. D. 2008. Liquid chromatography-tandem mass spectrometry determination of plasma 24S-hydroxycholesterol with chromatographic separation of 25-hydroxycholesterol. Anal. Biochem. 381: 151–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulfer M. K., Taube C., Gelfand E., Murphy R. C. 2005. Ozone exposure in vivo and formation of biologically active oxysterols in the lung. J. Pharmacol. Exp. Ther. 312: 256–264 [DOI] [PubMed] [Google Scholar]
- 27.Griffiths W. J., Wang Y. 2009. Sterol lipidomics in health and disease: methodologies and applications. Eur. J. Lipid Sci. Technol. 111: 14–38 [Google Scholar]
- 28.Griffiths W. J., Hornshaw M., Woffendin G., Baker S. F., Lockhart A., Heidelberger S., Gustafsson M., Sjövall J., Wang Y. 2008. Discovering oxysterols in plasma: a window on the metabolome. J. Proteome Res. 7: 3602–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honda A., Yamashita K., Hara T., Ikegami T., Miyazaki T., Shirai M., Xu G., Numazawa M., Matsuzaki Y. 2009. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC-ESI-MS/MS. J. Lipid Res. 50: 350–357 [DOI] [PubMed] [Google Scholar]
- 30.Honda A., Yamashita K., Miyazaki H., Shirai M., Ikegami T., Xu G., Numazawa M., Hara T., Matsuzaki Y. 2008. Highly sensitive analysis of sterol profiles in human serum by LC-ESI-MS/MS. J. Lipid Res. 49: 2063–2073 [DOI] [PubMed] [Google Scholar]
- 31.Jiang X., Ory D. S., Han X. 2007. Characterization of oxysterols by electrospray ionization tandem mass spectrometry after one-step derivatization with dimethylglycine. Rapid Commun. Mass Spectrom. 21: 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.