Abstract
Spore-forming bacteria are of particular concern in the context of planetary protection because their tough endospores may withstand certain sterilization procedures as well as the harsh environments of outer space or planetary surfaces. To test their hardiness on a hypothetical mission to Mars, spores of Bacillus subtilis 168 and Bacillus pumilus SAFR-032 were exposed for 1.5 years to selected parameters of space in the experiment PROTECT during the EXPOSE-E mission on board the International Space Station. Mounted as dry layers on spacecraft-qualified aluminum coupons, the “trip to Mars” spores experienced space vacuum, cosmic and extraterrestrial solar radiation, and temperature fluctuations, whereas the “stay on Mars” spores were subjected to a simulated martian environment that included atmospheric pressure and composition, and UV and cosmic radiation. The survival of spores from both assays was determined after retrieval. It was clearly shown that solar extraterrestrial UV radiation (λ≥110 nm) as well as the martian UV spectrum (λ≥200 nm) was the most deleterious factor applied; in some samples only a few survivors were recovered from spores exposed in monolayers. Spores in multilayers survived better by several orders of magnitude. All other environmental parameters encountered by the “trip to Mars” or “stay on Mars” spores did little harm to the spores, which showed about 50% survival or more. The data demonstrate the high chance of survival of spores on a Mars mission, if protected against solar irradiation. These results will have implications for planetary protection considerations. Key Words: Planetary protection—Bacterial spores—Space experiment—Simulated Mars mission. Astrobiology 12, 445–456.
1. Introduction
Planetary missions involved in the detection of signatures of life or the return of planetary samples to Earth are subjected to internationally accepted standards for planetary protection, as established by the Committee on Space Research (COSPAR) (COSPAR, 2011). According to the scientific interest and goals of missions to different targets in our solar system, COSPAR Planetary Protection guidelines group missions into five categories. Missions landing on Mars belong to category IV, which refers to those target bodies “of chemical evolution and/or origin of life interest and for which scientific opinion provides a significant chance of contamination which could compromise future investigations.” This “implies the presence of environments where terrestrial organisms could survive and replicate, and some likelihood of transfer to those places by a plausible mechanism” (quoted from COSPAR, 2011). Depending on the subcategory being considered, Planetary Protection requirements for category IV missions include trajectory biasing, clean room assembly, bioburden reduction with monitoring, possible partial sterilization of hardware that may come in direct contact with the body, and a bioshield for that hardware. COSPAR defines “Bioburden constraints…with respect to the number of aerobic microorganisms that survive a heat shock of 80°C for 15 minutes (hereinafter ‘spores') and are cultured on TSA at 32°C for 72 hours” (quoted from COSPAR, 2011). Lander systems that do not carry instruments for the investigations of extant martian life are grouped in category IVa and are restricted to a total surface bioburden level of≤3×105 spores and an average of≤300 spores/m2 (Nicholson et al., 2009). These limits have been met by all Mars surface missions, for example, the Mars Exploration Rover (MER) twin landers, which accounted at launch for a surface bioburden of 1.2×105 spores (MER A) and 1.3×105 spores (MER B), respectively, with a mean density of 74 spores/m2 (Horneck et al., 2007).
For most Mars missions (e.g., the orbiters Mars Odyssey and Mars Express, and the landers Viking, Pathfinder, Beagle 2, MER, and Phoenix), the residual contamination was also controlled via characterization of the microbial diversity of the cultivable organisms (Cooper et al., 2011) from the spacecraft or lander (Puleo et al., 1977; La Duc et al., 2003, 2007; Rettberg et al., 2006; Ghosh et al., 2009; Stieglmeier et al., 2009). In most cases, spore formers constituted a dominant fraction of those microorganisms cultivated after heat-shock treatment according to the Planetary Protection standard procedure (COSPAR, 2011); however, spore formers constituted only ∼10% of the cultivable microorganisms that were collected from the Phoenix lander (Ghosh et al., 2009). Because spores are highly resistant to a variety of environmental extremes, including certain sterilization procedures (Nicholson et al., 2000; Link et al., 2004) and the harsh environment of outer space (Horneck et al., 1994, 2010) or the martian surface (Nicholson and Schuerger, 2005), these spore-forming microbes are of particular concern in the context of planetary protection (La Duc et al., 2003, 2004). Assuming they are capable of coping with the different environmental attacks imposed on them during a mission to Mars, spores may pose a serious hazard to the in situ life-detection experiments and to the efforts to maintain the surface of the target planet in pristine condition.
The EXPOSE-E mission of the European Space Agency (ESA) provided the opportunity to investigate in situ the hardiness of bacterial endospores on such a hypothetical mission to Mars. Mounted for 1.5 years on the balcony of the Columbus module of the International Space Station (ISS), the EXPOSE-E facility was used to expose bacterial spores as dried layers on spacecraft-qualified aluminum coupons either
to selected conditions of outer space and thereby simulate their journey to Mars as passengers on board a Mars probe—the “trip to Mars” samples, or
to simulated martian surface conditions and thereby simulate their arrival with the Mars probe and their long-term stay on Mars—the “stay on Mars” samples.
In the experiment PROTECT (Resistance of spacecraft isolates to outer space for planetary protection purposes), we selected as an assay system two representatives of bacterial endospores:
Spores of Bacillus pumilus SAFR-032, isolated from an air lock of the spacecraft assembly facility at the Jet Propulsion Laboratory (JPL) between the clean room and entrance floor (Venkateswaran et al., 2003a), which showed elevated resistance to UV radiation (Link et al., 2004) and hydrogen peroxide treatment (Kempf et al., 2005) compared to the wild-type strain.
Spores of B. subtilis 168 as a “space veteran,” about which most of the data on spore responses to the space environment have been obtained so far (Bücker et al., 1974; reviewed in Taylor, 1974; Horneck et al., 1984, 2001; Nicholson et al., 2000, Horneck et al., 2010).
After 1.5 years of exposure to the selected conditions of a hypothetical Mars mission, the samples on the EXPOSE-E facility were retrieved and subjected to an intense investigative program. In this paper, we report on the survival of the spores in the “trip to Mars” samples as well as the “stay on Mars” samples. Further in-depth analyses of the PROTECT flight samples are presented by Moeller et al., (2012 in this issue) on the mutagenic specificity of the test parameters, by Nicholson et al. (2012) on global transcription responses during germination of B. subtilis spores, and by Vaishampayan et al. (2012) on the UV resistance of the B. pumilus “space survivors.”
2. Material and Methods
2.1. Bacterial strains, culture media, and sample preparation
Endospores of the following two Bacillus species were used in the PROTECT space experiment: B. pumilus SAFR-032, an isolate from the JPL Spacecraft Assembly Facility (SAF) that possesses elevated resistance to environmental stresses (Venkateswaran et al., 2003a, 2003b) and the “space-veteran” Bacillus subtilis 168 (DSM 402) that has been used in space experiments since Apollo 16 (reviewed in Horneck et al., 2010). Identification of B. pumilus SAFR-032 was achieved via 16S rDNA sequencing, gyrB analysis, DNA-DNA hybridization, and whole genome sequencing (Venkateswaran et al., 2001, 2003a, 2003b; La Duc et al., 2003; Dickinson et al., 2004a, 2004b; Gioia et al., 2007). Sporulation was induced in B. pumilus SAFR-032 by incubation at 32°C in a nutrient broth sporulation medium; after 3 days, spores were harvested and purified as previously described (Schaeffer et al., 1965; Nicholson and Setlow, 1990; Vaishampayan et al., 2012). Spores of B. subtilis 168 were obtained by cultivation under vigorous aeration in double-strength liquid Schaeffer sporulation medium (Schaeffer et al., 1965), purified, and stored in distilled water at 4°C as described previously (Moeller et al., 2005, 2006).
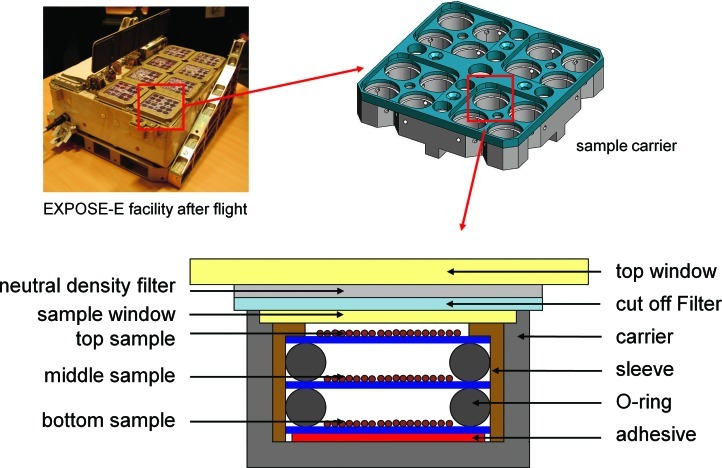
Flight samples were prepared from aqueous spore suspensions by transferring 20 μL onto discs of aluminum (Al 6061-T6) coupons as spacecraft-qualified material with a diameter of 13 mm and drying them overnight in air at room temperature. These samples simulate the presence of microbial cells on spacecraft materials at the time of launch, for example, on a mission to Mars. The air-dried layers consisted of 1.1×107 and 8.5×107 spores (B. pumilus SAFR-032) or 3.6×108 spores (B. subtilis 168), resulting in monolayers for the low concentrations of B. pumilus SAFR-032 spores and multilayers for the high concentrations of B. pumilus SAFR-032 (about 2–3 spore layers) and for B. subtilis 168 (about 5–10 spore layers). The thickness of the spore layer was geometrically estimated from the mean spore size, the number of spores per sample, and the sample area. The flight samples were accommodated in two trays of EXPOSE-E (Fig. 1) as stacks of three sample carriers each (Fig. 2). A total of 288 flight samples were prepared for PROTECT, and the same number of ground control samples were prepared for the EXPOSE-E mission ground reference (MGR), which was run at the Planetary and Space Simulation facilities at the Deutsches Zentrum für Luft- und Raumfahrt (DLR) (Rabbow et al., 2009, 2012). The MGR simulated the flight conditions as closely as possible. Because the first flight data set arrived quite late and some time was needed for processing these data, the MGR started 7 months after the launch of EXPOSE-E (Rabbow et al., 2012). In addition, laboratory controls were prepared at the same time and from the same batch of spores as the flight and MGR samples, and they were stored in the dark under ambient laboratory conditions (temperature 20±2°C and relative humidity 33±5%).
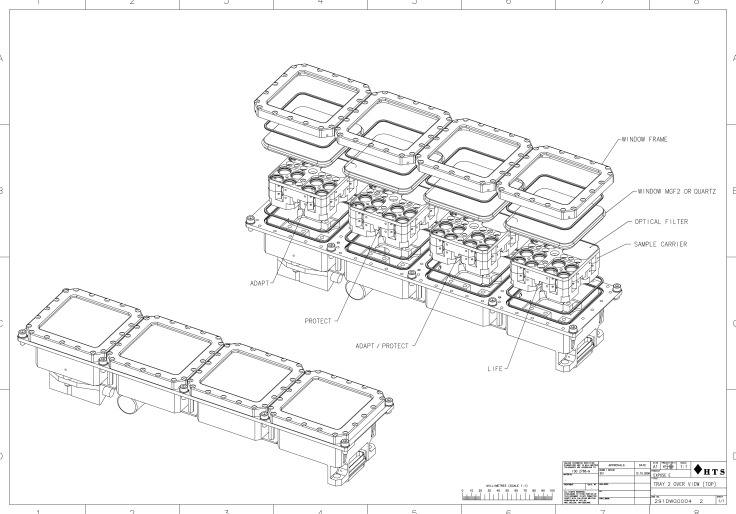
FIG. 1.
Exploded view of one tray of the EXPOSE-E facility.
FIG. 2.
EXPOSE-E facility after the mission, sample carrier, and scheme of a sample stack (air-dried layers of spores on aluminum coupons) (credit ESA, RUAG, KT, and Marko Wassmann, DLR). Color images available online at www.liebertonline.com/ast
2.2. Spaceflight protocol
The stacks of the PROTECT flight samples were accommodated in two exposure trays of the EXPOSE-E facility (Fig. 1) that was part of the European Technology Exposure Facility (EuTEF) attached to the European Columbus module of the ISS. Tray 1 was designed to test the effects of outer space conditions during a simulated interplanetary trajectory, for example, from Earth to Mars, with access to space vacuum, cosmic radiation, and—for the top layer of the sample stack—solar extraterrestrial radiation at wavelengths of λ>110 nm. Martian surface conditions were simulated in tray 2, including atmospheric pressure (103 Pa) and composition (1.6% argon, 0.15% oxygen, 2.7% nitrogen in CO2), cosmic radiation and—for the top layer of the sample stack—the UV radiation climate of Mars at wavelengths of λ>200 nm (Table 1). To maintain the simulated martian atmospheric conditions, tray 2 was sealed during the entire mission. EXPOSE-E was assembled at the DLR, transferred to Kennedy Space Center, Florida, USA, for integration into EuTEF, and launched on Space Shuttle STS-122 to the ISS on 7 February 2008. EuTEF was mounted on the balcony of Columbus by extravehicular activity on 15 February 2008, and the exposure of the EXPOSE-E samples started on 20 February 2008. After 1.5 years in space, EXPOSE-E was retrieved by extravehicular activity on 2 September 2009 and returned to Earth by STS-128 on 12 September 2009. Sample de-integration and distribution of the samples to the co-investigators began at the DLR on 3 December 2009. The MGR served as ground control and was performed with a second set of trays and samples that was identical to the flight experiment. The MGR began on 12 September 2008 and ended on 12 April 2010. The flight protocol was followed to the best extent possible under the limits of the Earth-bound simulation facilities (Table 2) (Rabbow et al., 2009, 2012). In total, there were five sets of samples: (i) spaceflight “trip to Mars” samples, either Sun-exposed or kept in the dark; (ii) spaceflight “stay on Mars” samples, either Sun-exposed or kept in the dark; (iii) MGR samples in simulated space environment, either irradiated (solar simulator) or kept in the dark; (iv) MGR samples in simulated martian environment, either irradiated (solar simulator) or kept in the dark; and (v) laboratory controls.
Table 1.
Test Parameters of PROTECT Spaceflight Experiment, Exposure Time 559 Days (Rabbow et al., 2012), Radiation Dose Data from Berger et al. (2012)
| |
|
|
|
Solar radiationa |
Cosmic radiation |
Temperature rangec |
|
|
||
|---|---|---|---|---|---|---|---|---|---|---|
| Tray / compartment | Test environment | Pressure | Exposure condition | Spectrum | Earth s.c.h | UV fluence | Doseb | °C | Bacillus sp. | Sample n |
| 1/2 | Outer space simulating interplanetary trajectory | ≈ 10−4 Pa | Sun-exposed 100% T | >110 nm | 1631 | 550 MJ/m2 | 155 mGy | −20 to +59 | B. pumilus | 4 |
| B. subtilis | 4 | |||||||||
| ≈ 10−4 Pa | Sun-exposed 0.1% T | >110 nm | 797 kJ/m2 | 155 mGy | −20 to +59 | B. pumilus | 4 | |||
| B. subtilis | 4 | |||||||||
| ≈ 10−4 Pa | dark | — | — | — | 140 mGy | −20 to +59 | B. pumilus | 40 | ||
| B. subtilis | 40 | |||||||||
| 1/3 | Outer space simulating interplanetary trajectory | ≈ 10−4 Pa | Sun-exposed 100% T | >110 nm | 1803 | 608 MJ/m2 | 168 mGy | −20 to +59 | B. subtilis | 4 |
| ≈ 10−4 Pa | Sun-exposed 0.1% T | >110 nm | 882 kJ/m2 | 158 mGy | −20 to +59 | B. subtilis | 4 | |||
| ≈ 10−4 Pa | dark | — | — | — | 152 mGy | −20 to +59 | B. subtilis | 40 | ||
| 2/2 | Simulated martian climate | 103 Pa Mars atm. | Sun-exposed 100% T | >200 nm | 1319 | 398 MJ/m2 | 155 mGy | −20 to +59 | B. pumilus | 4 |
| B. subtilis | 4 | |||||||||
| 103 Pa Mars atm. | Sun-exposed 0.1% T | >200 nm | 526 kJ/m2 | 153 mGy | −20 to +59 | B. pumilus | 4 | |||
| B. subtilis | 4 | |||||||||
| 103 Pa Mars atm. | dark | — | — | — | 142 mGy | −20 to +59 | B. pumilus | 40 | ||
| B. subtilis | 40 | |||||||||
| 2/3 | Simulated martian climate | 103 Pa Mars atm. | Sun-exposed 100% T | >200 nm | 1429 | 432 MJ/m2 | 165 mGy | −20 to +59 | B. subtilis | 4 |
| 103 Pa Mars atm. | Sun-exposed 0.1% T | >200 nm | 569 kJ/m2 | 180 mGy | −20 to +59 | B. subtilis | 4 | |||
| 103 Pa Mars atm. | dark | — | — | — | 134 mGy | −20 to +59 | B. subtilis | 40 | ||
s.c.h., solar constant hours; T, transmission of optical filter system.
Solar irradiance was measured by different UV sensors (four sensors located at each corner of the EXPOSE-E platform and the R3DE experiment). Due to roughly 20% data loss, these measurements needed to be complemented by model calculations. By using shadow maps with respect to the upper side of EXPOSE-E and the Composite Solar Irradiance Reference Spectra (http://wwwsolar.nrl.navy.mil/solar_spectra.html), the fluence of the biologically effective UV range 200–400 nm was calculated for each compartment (Rabbow et al., 2012).
The total dose of cosmic radiation and the dose gradient in the sample stacks was measured by thermoluminescence dosimetry (Berger et al., 2012). In addition, the time profile of cosmic radiation exposure was measured with the R3DE experiment (Dachev et al., 2012).
During the mission, EXPOSE-E experienced about 10 periods of relatively high temperatures (about 0°C to 40°C) alternating with 10 periods of relatively low temperatures (about −12°C to 0°C). This periodicity was caused by the changing orientation of the ISS to the Sun. In addition, for each orbit the temperature varied with a periodicity of 90 minutes (60 min sunshine and 30 min shadow) around 5–10°C. At one time during the second half of the mission, the temperature increased up to +59°C, which lasted for less than 1 day. The lowest temperature of −21°C was recorded a few times during the mission (see Fig. 5 in Rabbow et al., 2012).
Table 2.
Test Parameters of PROTECT MGR Experiment, Exposure time 559 days (Rabbow et al., 2012)
| |
|
|
|
Sun simulator radiation |
Background radiation |
Temperature rangea |
|
|
|
|---|---|---|---|---|---|---|---|---|---|
| Tray / compartment | Test environment | Pressure | Exposure conditions | Spectrum | UV fluence | Dose | °C | Bacillus sp. | Sample n |
| 1/2 | Simulated space | 1.7×10−3 Pa | irradiated 100% T | >200 nm | 528 MJ/m2 | 1 mGy | −20 to +59 | B. pumilus | 4 |
| B. subtilis | 4 | ||||||||
| 1.7×10−3 Pa | irradiated 0.1% T | >200 nm | 628 kJ/m2 | 1 mGy | −20 to +59 | B. pumilus | 4 | ||
| B. subtilis | 4 | ||||||||
| 1.7×10−3 Pa | dark | — | — | 1 mGy | −20 to +59 | B. pumilus | 40 | ||
| B. subtilis | 40 | ||||||||
| 1/3 | Simulated space | 1.7×10−3 Pa | irradiated 100% T | >200 nm | 583 MJ/m2 | 1 mGy | −20 to +59 | B. subtilis | 4 |
| 1.7×10−3 Pa | irradiated 0.1% T | >200 nm | 694 kJ/m2 | 1 mGy | −20 to +59 | B. subtilis | 4 | ||
| 1.7×10−3 Pa | dark | — | — | 1 mGy | −20 to +59 | B. subtilis | 40 | ||
| 2/2 | Simulated martian climate | 103 Pa Mars atm. | irradiated 100% T | >200 nm | 382 MJ/m2 | 1 mGy | −20 to +59 | B. pumilus | 4 |
| B. subtilis | 4 | ||||||||
| 103 Pa Mars atm. | irradiated 0.1% T | >200 nm | 409 kJ/m2 | 1 mGy | −20 to +59 | B. pumilus | 4 | ||
| B. subtilis | 4 | ||||||||
| 103 Pa Mars atm. | dark | — | — | 1 mGy | −20 to +59 | B. pumilus | 40 | ||
| B. subtilis | 40 | ||||||||
| 2/3 | Simulated martian climate | 103 Pa Mars atm. | irradiated 100% T | >200 nm | 432 MJ/m2 | 1 mGy | −20 to +59 | B. subtilis | 4 |
| 103 Pa Mars atm. | irradiated 0.1% T | >200 nm | 569 kJ/m2 | 1 mGy | −20 to +59 | B. subtilis | 4 | ||
| 103 Pa Mars atm. | dark | — | — | 1 mGy | −20 to +59 | B. subtilis | 40 | ||
T, transmission of optical filter system.
Time profile of temperature variations was adjusted to the spaceflight data.
2.3. Analysis of spore survival
After flight, the spore layers were removed from the aluminum coupons by applying the PVA-stripping method three times as described in Horneck et al. (2001). In brief, spore layers were covered by a 10% aqueous polyvinyl alcohol (PVA) solution. After drying, the spore-PVA layer was stripped off, and the spores were suspended in 1 mL sterile distilled water, which resulted in 95% recovery of the spores. The stripping procedure was repeated three times for maximal spore recovery. The procedure does not affect spore viability. Spore survival was determined from appropriate dilutions by their ability to form macroscopic visible colonies on nutrient agar plates after incubation for 24 h at 37°C (B. subtilis) or for 24 h at 32°C (B. pumilus). The surviving fraction was determined from the quotient N/N0, with N=the number of colony-forming units of the flight sample and N0 that of the original sample before launch.
2.4. Numerical and statistical analysis
There were at least four parallel samples for each exposure condition (Tables 1 and 2). The data for each exposure condition are expressed as averages±standard deviations (Tables 3 and 4). The spore survival data were compared statistically by using the Student t test. Values were analyzed in multigroup pairwise combinations, and differences with P values of≤0.05 were considered statistically significant (Moeller et al., 2005).
Table 3.
Survival of Spores of B. subtilis 168 after Spaceflight during the EXPOSE-E Mission, after MGR, and Laboratory Controls
| Colony formers (N) | Survival (N/N0) | |||||||
|---|---|---|---|---|---|---|---|---|
| Spaceflight experiment | ||||||||
| Lab control (before)a | Lab control (after) | Flight unit Sun-exposed | Flight unit Sun-exposed | Flight unit darkc | Lab control (after) | Flight unit Sun-exposed | Flight unit Sun-exposed | Flight unit darkc |
| 100% Tb | 0.1% Tb | 100% Tb | 0.1% Tb | |||||
| Outer space simulating interplanetary trajectory: “trip to Mars” samples | ||||||||
| (3.6±0.5)×108 | (3.9±0.5)×108 | (3.2±2.0)×105 | (3.8±1.7)×106 | (2.0±0.2)×108 | (1.1±0.1) | (8.8±1.4)×10−4 | (1.1±0.1)×10−2 | (5.5±1.0)×10−1 |
| Simulated martian climate: “stay on Mars” samples | ||||||||
| (3.6±0.5)×108 | (3.9±0.5)×108 | (1.8±0.7)×107 | (7.3±0.9)×107 | (2.7±0.4)×108 | (1.1±0.1) | (4.9±0.9)×10−2 | (2.0±0.3)×10−1 | (7.4±1.3)×10−1 |
| Mission ground reference | ||||||||
| Lab control (before)a | Lab control (after) | MGR unit irradiated | MGR unit irradiated | MGR unit dark | Lab control (after) | MGR unit irradiated | MGR unit irradiated | MGR unit darkc |
| 100% Tb | 0.1% Tb | 100% Tb | 0.1% Tb | |||||
| Simulated space environment | ||||||||
| (3.6±0.5)×108 | (3.9±0.5)×108 | (1.0±0.2)×106 | (1.6±0.3)×107 | (1.8±0.4)×108 | (1.1±0.1) | (2.8±0.6)×10−3 | (4.5±0.6)×10−2 | (4.8±1.0)×10−1 |
| Simulated martian climate | ||||||||
| (3.6±0.5)×108 | (3.9±0.5)×108 | (3.2±0.4)×107 | (6.3±0.8)×107 | (2.4±0.2)×108 | (1.1±0.1) | (9.0±1.2)×10−2 | (1.7±0.4)×10−1 | (6.7±1.2)×10−1 |
n=5, taken as untreated control N0.
n=3, T=transmission of optical filter system.
n=21.
Table 4.
Survival of Spores of B. pumilus SAFR-032 after Spaceflight during the EXPOSE-E Mission, after MGR, and Laboratory Controls
| Colony formers (N) | Survival (N/N0) | |||||||
|---|---|---|---|---|---|---|---|---|
| Spaceflight experiment | ||||||||
| Lab control (before)a | Lab control (after) | Flight unit Sun-exposed | Flight unit Sun-exposed | Flight unit darkc | Lab control (after) | Flight unit Sun-exposed | Flight unit Sun-exposed | Flight unit darkc |
| 100% Tb | 0.1% Tb | 100% Tb | 0.1% Tb | |||||
| Outer space simulating interplanetary trajectory: “trip to Mars” samples | ||||||||
| (1.1±0.1)×107 | (4.7±0.8)×106 | 5.0×100 | (1.4±0.9)×104 | (1.7±0.6)×106 | (4.3±0.7)×10−1 | (4.5±2.7)×10−7 | (1.3±0.8)×10−3 | (1.6±0.6)×10−1 |
| Simulated martian climate: “stay on Mars” samples | ||||||||
| (1.1±0.1)×107 | (4.7±0.8)×106 | 4.50×100 | (2.8±0.5)×105 | (7.7±1.8)×106 | (4.3±0.7)×10−1 | 4.1×10−7 | (2.5±0.5)×10−2 | (7.0±1.6)×10−1 |
| Mission ground reference | ||||||||
| Lab control (before)a | Lab control (after) | MGR unit irradiated | MGR unit irradiated | MGR unit dark | Lab control (after) | MGR unit irradiated | MGR unit irradiated | MGR unit darkc |
| 100% Tb | 0.1% Tb | 100% Tb | 0.1% Tb | |||||
| Simulated space environment | ||||||||
| (1.1±0.1)×107 | (1.5±0.1)×107 | (3.6±0.0)×103 | (3.3±1.8)×103 | (5.9±1.6)×106 | (1.4±0.1)×100 | (3.3±0.0)×10−4 | (3.0±1.6)×10−4 | (5.4±1.4)×10−1 |
| Simulated martian climate | ||||||||
| (1.1±0.1)×107 | (1.5±0.1)×107 | (1.6±0.6)×104 | (3.7±2.1)×103 | (4.9±2.7)×107 | (1.4±0.1)×100 | (1.4±1.2)×10−3 | (3.4±1.9)×10−4 | (4.5±2.5)×100 |
n=3, taken as untreated control N0.
n=2, T=transmission of optical filter system.
n=2.
3. Results
The PROTECT experiment was designed to assess the survivability of spacecraft contaminants on a landed mission to Mars. During the 559-day mission, bacterial endospores, attached to spacecraft-qualified aluminum coupons, were subjected either to a simulated Earth-to-Mars trajectory (tray 1: “trip to Mars” spores) or to simulated martian surface conditions (tray 2: “stay on Mars” spores). Their survival was determined after retrieval.
3.1. “Trip to Mars” spores
In EXPOSE-E, the spore layers were stacked in triplicate (Fig. 2), and the majority of samples (240 out of 288) were kept in the dark (Table 1). In tray 1, about 50% of these “dark” flight spores of B. subtilis in multilayers survived the 559-day exposure to the combined action of cosmic radiation, space vacuum, and temperature fluctuations (Tables 1 and 3). A similar survival value was obtained for the MGR spores of B. subtilis, which were treated with comparable simulated space conditions (Tables 2 and 3). The laboratory control of B. subtilis did not show any inactivation. Approximately 15% of the B. pumilus “dark” flight spores survived the 559-day exposure to the combined action of cosmic radiation, space vacuum, and temperature fluctuations, whether exposed as monolayers or multilayers (Tables 1 and 4). A higher survival (about 50%) was measured for the comparable MGR spores as well as for the laboratory control of B. pumilus (Tables 2 and 4).
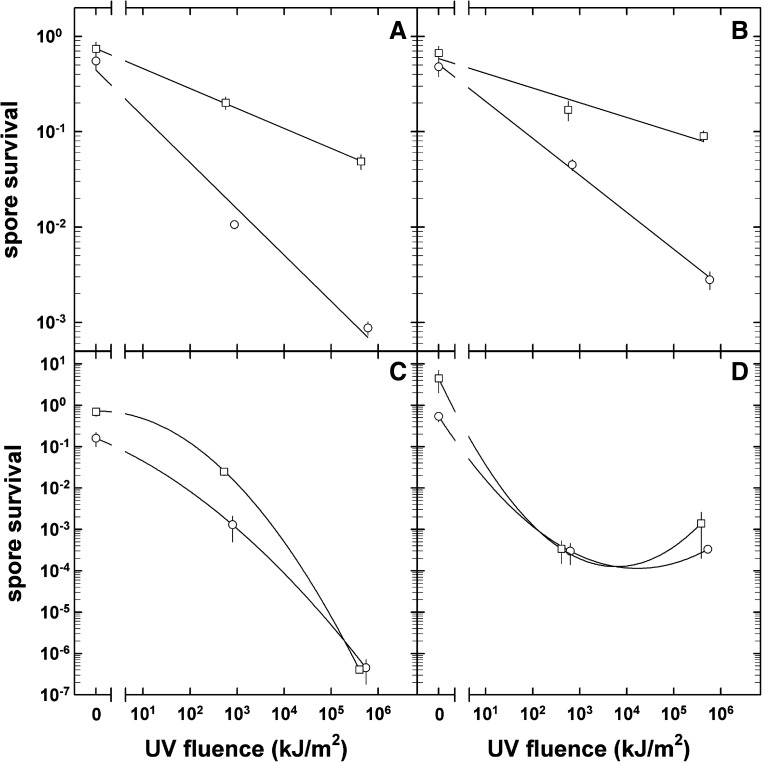
In addition to the space parameters to which the “dark” flight spores were subjected, in tray 1 solar electromagnetic radiation at wavelengths λ≥110 nm was also experienced by the top disk of spores in each stack (Tables 1 and 2). This additional space parameter led to significant inactivation of the spores. B. subtilis spores (in multilayers) were further inactivated by about 3 or 2 orders of magnitude, if exposed in addition to full extraterrestrial solar electromagnetic radiation or a 1000 times attenuated one, respectively (Table 3 and Fig. 3A). In those top sample layers that were exposed to the full extraterrestrial solar radiation (λ≥110 nm, 100% transmission), the color of the spore layer had turned from off-white to brownish (Fig. 4A). This change in color was unique for the top layers exposed to full sunlight and was not observed for samples under the 0.1% transmission neutral density filter (Fig. 4B) or for the dark flight samples. During the MGR, where a solar simulator provided radiation at wavelengths λ≥200 nm, B. subtilis spore survival was slightly higher (about a factor of 3–4) at fluences comparable to those of the flight samples (Table 3 and Fig. 3B). Under those conditions, no change in color was observed after the treatment.
FIG. 3.
Fluence survival curves of bacterial spores exposed to extraterrestrial solar radiation during the EXPOSE-E mission (open circles, “trip to Mars” spores; open squares, “stay on Mars” spores). (A) B. subtilis 168 flight samples; (B) B. subtilis 168 MGR samples; (C) B. pumilus SAFR-032 flight samples; (D) B. pumilus SAFR-032 MGR samples. Data are expressed as averages and standard deviations; error bars for survival data that are not visible were smaller than the symbol.
FIG. 4.
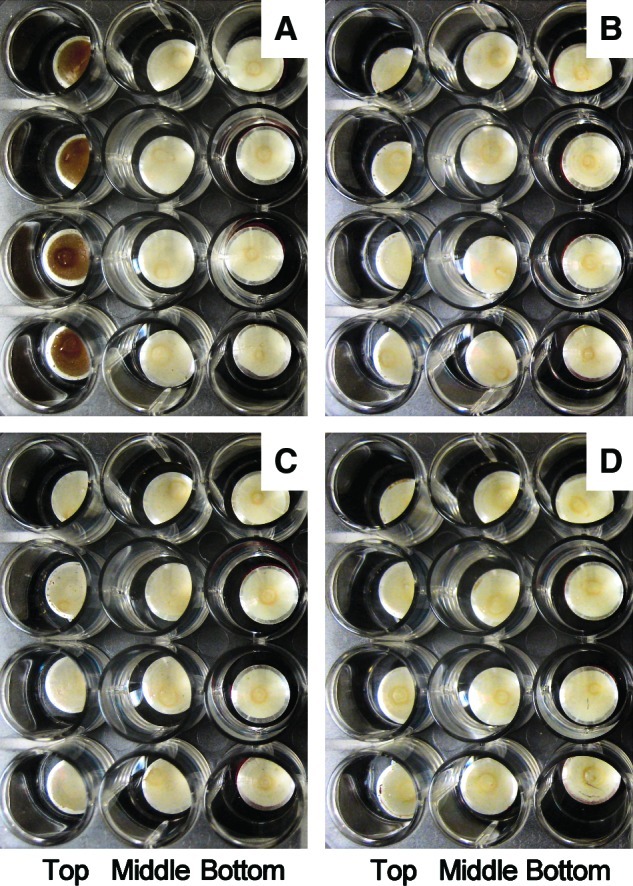
Photo of the multilayer of B. subtilis 168 spores on the aluminum coupon after spaceflight, taken during the de-integration of EXPOSE-E. (A) “Trip to Mars” spores, full (100%) Sun exposure (λ≥110 nm); (B) “Trip to Mars” spores, attenuated (0.1%) solar radiation (λ≥110 nm); (C) “Stay on Mars” spores, full (100%) Sun exposure (λ≥200 nm); (D) “Stay on Mars” spores, attenuated (0.1%) solar radiation (λ≥200 nm). Color images available online at www.liebertonline.com/ast
In the top disks of B. pumilus spores that were exposed in monolayers, extraterrestrial solar radiation caused a 6-log inactivation for λ≥110 nm, 100% transmission, and 2-log inactivation for λ≥110 nm, 0.1% transmission, respectively (Table 4, Fig. 3C). Comparable MGR samples of B. pumilus spores showed an inactivation that was about 3 orders of magnitude greater than that of the MGR dark samples (Table 4, Fig. 3D).
3.2. “Stay on Mars” spores
Tray 2 provided a partial simulation of martian surface conditions with respect to atmospheric composition and pressure, cosmic background radiation, and the martian environmental UV radiation spectrum (Table 1). Temperature fluctuations were identical to those of tray 1. As in tray 1, 240 out of 288 spore samples were kept in the dark. These dark “stay on Mars” samples exhibited 70–75% survival for both strains investigated (Tables 3 and 4). Similar survival values were obtained for the comparable MGR samples (Tables 3 and 4).
The optical filter system of tray 2 allowed access to solar electromagnetic radiation at wavelengths λ≥200 nm, which is comparable to the spectrum at the surface of Mars. Irradiation with this “martian” spectrum was less efficient at killing the top-layer spores of B. subtilis compared to the full extraterrestrial spectrum (Table 3 and Fig. 3A). There was no change in color of these irradiated top-layer “stay on Mars” spores of B. subtilis (Fig. 4C). Interestingly, the MGR samples of B. subtilis showed a similar difference in the UV responses of the “simulated space” and “simulated Mars” samples, although in both cases the UV spectrum and fluxes of the solar simulator were identical (λ≥200 nm) (Table 3 and Fig. 3B).
This difference in biological efficiency of the applied radiation between “space” and “Mars” samples was not observed in the spores of B. pumilus (Table 4 and Fig. 3C and 3D). This might be due to the very low survival rate (10−7) of the spores, which were exposed in monolayers, whereas B. subtilis spores were always exposed as multilayers.
4. Discussion
A variety of microorganisms were isolated from spacecraft and spacecraft assembly facilities, which included representatives from the Archaea, Bacteria, and Eukarya (fungi) that are common inhabitants of humans, soil, airborne dust, and buildings (La Duc et al., 2003; Rettberg et al., 2006; Nicholson et al., 2009). However, the current Planetary Protection Policy of COSPAR considers only aerobic bacterial endospores as the standard for assessing the bioburden of a spacecraft (COSPAR, 2011). This designation of bacterial spores as the international standard has been justified on the following grounds:
Spores are ubiquitous and common isolates from spacecraft and spacecraft assembly facilities (Nicholson et al., 2009; Conley, 2011; Nicholson and Moeller, 2011)
Spores are highly resistant to killing by chemical and physical disinfection procedures (Nicholson et al., 2000).
Spores have been used as the bioburden standard since the Viking missions in the 1970s (Conley, 2011).
The standard assay for bioburden determination is easy to perform and robust.
Some spore-forming species, for example B. pumilus, have been isolated from spacecraft and spacecraft assembly facilities that exhibit much higher spore resistance than common laboratory strains (La Duc et al., 2003; Link et al., 2004; Kempf et al., 2005; Gioia et al., 2007).
These considerations have motivated us to use bacterial endospores as a surrogate for a hypothetical bioburden of a robotic probe destined for Mars and to study spore hardiness during a simulated Earth to Mars transfer (“trip to Mars” samples) as well as after landing on the martian surface (“stay on Mars” samples). For these long-term exposure studies, the environment of the ISS in low-Earth orbit offered ideal conditions to mimic interplanetary space, in spite of some differences especially in the composition of the cosmic radiation field, the residual pressure, and the residual gas composition (Baglioni et al., 2007). The total exposure time was dictated by the EuTEF mission and lasted more than twice the general transfer time of a space probe from Earth to Mars (Fasoulas and Schmiel, 2007). Hence, PROTECT can be considered as a “worst case” scenario for a trip to Mars or a scenario for a hypothetical Mars sample return mission.
With regard to the “trip to Mars” samples, we studied two versions: (i) “dark flight,” which mimicked spores that were attached to the spacecraft but shielded from solar radiation, for example, located in the inner part of the spacecraft, and (ii) “Sun-exposed flight,” which mimicked spores that were exposed to sunlight during the trajectory, for example, located at the outer surface of the spacecraft. Both versions experienced identical levels of space vacuum, cosmic radiation, and temperature fluctuations. Results indicate that a substantial fraction of the “dark flight” spores (15–50%, depending on species and thickness of the spore layer) would survive a simulated trip to Mars. These observations confirm earlier long-term studies on the high survival rate of B. subtilis spores in space, if exposed as multilayers and shielded against extraterrestrial sunlight, such as the (67.2±10.2)% survival after the 2107-day flight on NASA's Long Duration Exposure Facility (LDEF) (Horneck et al., 1994), and the (45.5±0.1)% survival after the 327-day flight on ESA's European Retrievable Carrier (EURECA) mission (Horneck et al., 1995).
The results were much more dramatic for the Sun-exposed spores during the “trip to Mars.” Only in some samples were a few survivors detected from monolayers of B. pumilus, which decreased the survival rate to less than 10−6. These few survivors may be considered “lucky winners” that may have been located in pits or cracks of the aluminum surface and were thereby either shadowed or covered by upper, killed spores (Schuerger et al., 2005; Vaishampayan et al., 2012) that protected them from full UV radiation. This high lethality of unfiltered extraterrestrial solar UV radiation has already been demonstrated in previous short-term spaceflight studies, for example, on board Spacelab 1 (Horneck et al., 1984) and the ESA Biopan missions (Horneck et al., 2001). Ten seconds of exposure to unfiltered solar radiation in space killed 99% of spores of B. subtilis. Furthermore, space vacuum and solar UV radiation acted synergistically in spore inactivation. This synergism is also illustrated in Fig. 3B, where during the MGR the simulated space probes, which were irradiated under a vacuum of 1.7×10−3 Pa, were more efficiently inactivated than the simulated Mars probes, which were kept at 103 Pa, although both probes received the same irradiation at wavelengths of λ≥200 nm. This synergism is probably due to an altered DNA photochemistry of the spores that occurs in vacuum. Vacuum and extreme desiccation could induce changes in DNA conformation that lead to the production of bulky photoproducts, such as DNA-DNA or protein-DNA crosslinks that cannot be readily repaired during germination (Cadet and Douki, 2011). Actually, in spores that were UV irradiated in vacuum, DNA protein cross-linking and DNA strand breaks have been tentatively detected (Dose et al., 1995) as well as two thymine decomposition products, namely, the cis-syn and trans-syn cyclobutane thymine dimers, in addition to the so-called spore photoproduct di-pyrimidine 5,6-dihydro-5(α-thyminyl)thymine, which is normally induced in bacterial spores (Lindberg and Horneck, 1991; Nicholson et al., 2002). Action spectroscopy in space (Horneck et al., 1995) and in laboratory studies with synchrotron radiation (Munakata et al., 1991; Horneck and Rabbow, 2007) have demonstrated that vacuum UV radiation in the range of 125–175 nm effectively inactivates B. subtilis spores. The DNA is the most sensitive target molecule in the cell at these wavelengths. Photons of this highly energetic UV range are likely to ionize DNA components, including the sugar-phosphate backbone of the DNA (Vall-Ilosera et al., 2008; Cadet and Douki, 2011). The latter reaction is expected to lead to the formation of DNA strand breaks (Horneck and Rabbow, 2007). A third possibility is that broad-spectrum UV and vacuum might actually inactivate repair proteins themselves or other proteins needed for successful germination and growth. Hence, the lethality of solar UV radiation depends on three factors: the penetration depth of the radiation in the spore that is very limited for vacuum UV radiation, the type of DNA damage induced in the spore, and the repair capacity of each spore during germination. Shielding by the upper spore layers might be the reason for the much higher survival of B. subtilis spores in multilayers compared to spores of B. pumilus that were exposed in monolayers. The brownish color of those irradiated multilayers might be caused by photochemical reactions of the extraterrestrial vacuum UV radiation with the spore coat of the upper-layer spores, for example, through Maillard reactions (Cox, 1993). A similar change in color has been observed in the top layer of spores of B. subtilis after a nearly 6-year exposure to the space environment, including the full spectrum of extraterrestrial solar electromagnetic radiation during the LDEF mission of NASA (Horneck et al., 1994).
Once the planetary landing probe has arrived at the surface of Mars, putative spore passengers are confronted with an environment as partially mimicked in tray 2 for the “stay on Mars” spores. While the applied atmospheric pressure and composition, the spectrum of UV radiation, and the level of cosmic radiation represented the martian conditions fairly well (Dartnell et al., 2007; Kminek et al., 2010), PROTECT did not simulate the martian diurnal variations of sunlight and temperature. Because the martian solar constant is only 45% that of Earth's (due to the difference in their distance from the Sun), the calculated Earth solar constant hours (s.c.h.) of PROTECT (Table 1 and Rabbow et al., 2012) correspond to about twice as much martian s.c.h. For example, the simulated martian UV fluence of 432 MJ/m2 in tray 2, compartment 3 (Table 1) would need, at Mars' distance from the Sun, an irradiation for 3144 h instead of 1429 h in Earth's orbit. This is an enormously long UV irradiation time, especially in view of the prediction that at the surface of Mars B. subtilis spores would be killed within about 30 s to 1 min (Cockell et al., 2000; Schuerger et al., 2003). For the simulated martian surface condition with more than 3000 h irradiation with a simulated martian UV spectrum, our results show about 5% survival of B. subtilis spores in multilayers; however, less than 10−6 B. pumilus spores survived when in monolayers. From these data, it follows that the survival of spores on Mars depends largely on the degree of shadowing, such as within multilayers or clumps, or in hidden cracks, than on the specific resistance of the bacterial strain under investigation. If shielded against martian UV radiation, 70–75% of the spores of both strains survived under simulated martian conditions.
5. Conclusions
The space experiment PROTECT of the EXPOSE-E mission provides experimental data on the responses of the most resistant microbial representatives, for example, spores of B. subtilis 168 and B. pumilus SAFR-032, to the conditions of a simulated “trip to Mars” and an extended “stay on Mars.” These data are important information for the Panel on Planetary Protection of COSPAR as well as for the major space agencies that are constantly revisiting the protocols of planetary protection with the aim of amending bioload measurements by adding recent innovations in molecular biology analysis and sterilization methods (e.g., taken from treatment of medical devices or pharmaceuticals). Since the specification of biological cleanliness depends on the type of model microbe, the microbial reduction methods require knowledge of the most resistant types of microorganisms that may be present.
Our studies have confirmed the high resistance of spores of two Bacillus species to the most adverse parameters encountered during a planetary mission, such as space vacuum, cosmic radiation, temperature fluctuations, long storage time, and martian atmospheric pressure and composition. However, these studies have also confirmed the enormous killing efficiency of solar UV radiation experienced on an Earth-to-Mars route as well as on the surface of Mars. Spores could only escape this harmful attack by hiding in cracks or pits of the spacecraft surface, protecting the inner layers in spore clumps, or shielding via the spacecraft itself (reviewed in Nicholson et al., 2005). However, because the landing probe is most likely encased in an entry shield or bioshield, putative spore passengers attached to the lander may well escape this irradiation during the Earth-to-Mars trajectory and thus survive the journey. Likewise, the chances of survival at the martian surface increase with the degree of shielding against martian UV radiation. Nevertheless, our data suggest that a substantial fraction of spores in multilayers could survive an exposure to martian UV irradiation for more than 3000 h.
By providing this information on the responses of the most resistant microbial representatives to the harsh environment of space and the martian surface, our investigation contributes to avoidance of false positives in life-detection experiments performed either in situ or on Mars samples returned to Earth, in the event that some of these resistant types of microbes escape sterilization treatment and are exported to Mars.
Acknowledgments
The PROTECT team thanks Martin Zell, Head of ESA ISS Utilisation and Astronaut Support Department; Pietro Baglioni and René Demets of ESA-ESTEC for their invaluable support in realizing the EXPOSE-E mission; the astronauts who were involved in the exposure and retrieval of EXPOSE-E; the NASA teams responsible for flights STS-122 and STS-128; the teams at ESA-ESTEC during the planning, operation, and evaluation period of EXPOSE-E; the teams at the companies KT and RedShift; and the team at MUSC DLR during the preparation, EVT, EST, and MGR of EXPOSE-E. The project was supported by DLR grant DLR-FuE-Projekt ISS-Nutzung in der Biodiagnostik, Programm RF-FuW, Teilprogramm 475 (to P.R., R.M., and E.R.), DLR contract no. D/316/67120270 (to G.H.), BMWi grant Fondsnummer 360 224, Förderkennzeichen 50 WB 0528 (to C.P.), NASA Planetary Protection grant NNA06CB58G (to W.L.N.), and NASA cooperative agreement NNX10AK33A (to R.L.M). The research described in this publication was partly carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration. This research was funded by a 2007 NRA ROSES grant (to K.V.). We are grateful to all the members of the JPL Biotechnology and Planetary Protection Group for technical assistance. K.V., W.L.N., and R.L.M. especially thank J. Rummel and C. Conley of the NASA Planetary Protection office for their unflagging support.
Author Disclosure Statement
No competing financial interest exists for Gerda Horneck, Ralf Moeller, Jean Cadet, Thierry Douki, Rocco L. Mancinelli, Wayne L. Nicholson, Corinna Panitz, Elke Rabbow, Petra Rettberg, Andrew Spry, Erko Stackebrandt, Parag Vaishampayan, or Kasthuri J. Venkateswaran.
Abbreviations
COSPAR, Committee on Space Research; DLR, Deutsches Zentrum für Luft- und Raumfahrt (German Aerospace Center); ESA, European Space Agency; EuTEF, European Technology Exposure Facility; ISS, International Space Station; JPL, Jet Propulsion Laboratory; LDEF, Long Duration Exposure Facility; MER, Mars Exploration Rover; MGR, mission ground reference; PVA, polyvinyl alcohol; SAF, Spacecraft Assembly Facility; s.c.h., solar constant hours.
References
- Baglioni P. Sabbatini M. Horneck G. Astrobiology experiments in low Earth orbit. In: Horneck G., editor; Rettberg P., editor. Complete Course in Astrobiology. Wiley-VCH; Berlin: 2007. pp. 273–319. [Google Scholar]
- Berger T. Hajek M. Bilski P. Körner C. Vanhavere F. Reitz G. Cosmic radiation exposure of biological test systems during the EXPOSE-E mission. Astrobiology. 2012;12:387–392. doi: 10.1089/ast.2011.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücker H. Horneck G. Wollenhaupt H. Schwager M. Taylor G.R. Viability of Bacillus subtilis spores exposed to space environment in the M-191 experiment system aboard Apollo 16. Life Sci Space Res. 1974;12:209–213. doi: 10.1016/b978-0-08-021783-3.50033-7. [DOI] [PubMed] [Google Scholar]
- Cadet J. Douki T. Molecular effects of UV and ionizing radiation on DNA. In: Gargaud M., editor; López-García P., editor; Marin H., editor. Origins and Evolution of Life. Cambridge University Press; Cambridge, UK: 2011. pp. 359–374. [Google Scholar]
- Cockell C.S. Catling D. Davis W.L. Kepner R.N. Lee P.C. Snook K. McKay C.P. The ultraviolet environment of Mars: biological implications past, present and future. Icarus. 2000;146:343–359. doi: 10.1006/icar.2000.6393. [DOI] [PubMed] [Google Scholar]
- Conley C.A. Planetary protection. In: Gargaud M., editor; Amils R., editor; Cernicharo J., editor; Quintanilla H.J., editor; Cleaves W.M. III, editor; Irvine W.M., editor; Pinti D.L., editor; Viso M., editor. Encyclopedia of Astrobiology. Springer; Heidelberg: 2011. pp. 1279–1280. [Google Scholar]
- Cooper M. LaDuc M.T. Probst A. Vaishampayan P. Stam C. Benardini J.N. Piceno Y.M. Andersen G.L. Venkateswaran K. Assessing the cleanliness of surfaces: innovative molecular approaches vs standard spore assays. Appl Environ Microbiol. 2011;77:5438–5444. doi: 10.1128/AEM.00192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSPAR. COSPAR Planetary Protection Policy (20 October 2002, as amended 24 March 2011) COSPAR; Paris: 2011. [Google Scholar]
- Cox C.S. Roles of water molecules in bacteria and viruses. Orig Life Evol Biosph. 1993;23:29–36. doi: 10.1007/BF01581988. [DOI] [PubMed] [Google Scholar]
- Dachev T. Horneck G. Häder D.-P. Schuster M. Richter P. Lebert M. Demets R. Time profile of cosmic radiation exposure during the EXPOSE-E mission: the R3DE instrument. Astrobiology. 2012;12:403–411. doi: 10.1089/ast.2011.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnell L. Desorgher L. Ward J. Coates A. Modelling the surface and subsurface martian radiation environment: implications for astrobiology. Geophys Res Lett. 2007;34:L02207. [Google Scholar]
- Dickinson D.N. La Duc M.T. Haskins W.E. Gornushkin I.B. Winefordner J.D. Powell D.H. Venkateswaran K. Species differentiation of a diverse suite of Bacillus spores using mass spectrometry based protein profiling. Appl Environ Microbiol. 2004a;70:475–482. doi: 10.1128/AEM.70.1.475-482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D.N. La Duc M.T. Satomi M. Winefordner J.D. Powell D.H. Venkateswaran K. MALDI-TOFMS compared with other polyphasic taxonomy approaches for the identification and classification of Bacillus pumilus spores. J Microbiol Methods. 2004b;58:1–12. doi: 10.1016/j.mimet.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Dose K. Bieger-Dose A. Dillmann R. Gill M. Kerz O. Klein A. Meinert H. Nawroth T. Risi S. Stridde C. ERA-Experiment “Space Biochemistry.”. Adv Space Res. 1995;16:119–129. [PubMed] [Google Scholar]
- Fasoulas S. Astrodynamical and technological aspects of astrobiology missions in our Solar System. In: Schmiel T., editor; Horneck G., editor; Rettberg P., editor. Complete Course in Astrobiology. Wiley-VCH; Berlin: 2007. pp. 179–201. [Google Scholar]
- Ghosh S. Osman S. Vaishampayan P. Venkateswaran K. Recurrent isolation of extremotolerant bacteria from the clean room where Phoenix spacecraft components are assembled. Astrobiology. 2009;10:325–335. doi: 10.1089/ast.2009.0396. [DOI] [PubMed] [Google Scholar]
- Gioia J. Yerrapragada S. Qin X. Jiang H. Igboeli O.C. Muzny D. Dugan-Rocha S. Ding Y. Hawes A. Liu W. Perez L. Kovar C. Dinh H. Lee S. Nazareth L. Blyth P. Holder M. Buhay C. Tirumalai M.R. Liu Y. Dasgupta I. Bokhetache L. Fujita M. Karouia F. Moorthy P.E. Siefert J. Uzman A. Buzumbo P. Verma A. Zwiya H. McWilliams B.D. Olowu A. Clinkenbeard K.D. Newcombe D. Golebiewski L. Petrosino J.F. Nicholson W.L. Fox G.E. Venkateswaran K. Highlander S.K. Weinstock G.M. Paradoxical DNA repair and peroxide resistance gene conservation in Bacillus pumilus SAFR-032. PLoS One. 2007;2:e928. doi: 10.1371/journal.pone.0000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horneck G. Rabbow E. Mutagenesis by outer space parameters other than cosmic rays. Adv Space Res. 2007;40:445–454. [Google Scholar]
- Horneck G. Bücker H. Reitz G. Requardt H. Dose K. Martens K.D. Mennigmann H.D. Weber P. Microorganisms in the space environment. Science. 1984;225:226–228. doi: 10.1126/science.225.4658.226. [DOI] [PubMed] [Google Scholar]
- Horneck G. Bücker H. Reitz G. Long-term survival of bacterial spores in space. Adv Space Res. 1994;14:41–45. doi: 10.1016/0273-1177(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Horneck G. Eschweiler U. Reitz G. Wehner J. Willimek R. Strauch K. Biological responses to space: results of the experiment “Exobiological Unit” of ERA on EURECA I. Adv Space Res. 1995;16:105–118. doi: 10.1016/0273-1177(95)00279-n. [DOI] [PubMed] [Google Scholar]
- Horneck G. Rettberg P. Reitz G. Wehner J. Eschweiler U. Strauch K. Panitz C. Starke V. Baumstark-Khan C. Protection of bacterial spores in space, a contribution to the discussion on panspermia. Orig Life Evol Biosph. 2001;31:527–547. doi: 10.1023/a:1012746130771. [DOI] [PubMed] [Google Scholar]
- Horneck G. Debus A. Mani P. Spry J.A. Astrobiology exploratory missions and planetary protection requirements. In: Horneck G., editor; Rettberg P., editor. Complete Course in Astrobiology. Wiley-VCH; Berlin: 2007. pp. 353–397. [Google Scholar]
- Horneck G. Klaus D.M. Mancinelli R.L. Space microbiology. Microbiol Mol Biol Rev. 2010;74:121–156. doi: 10.1128/MMBR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf M.J. Chen F. Kern R. Venkateswaran K. Recurrent isolation of hydrogen peroxide-resistant spores of Bacillus pumilus from a spacecraft assembly facility. Astrobiology. 2005;5:391–405. doi: 10.1089/ast.2005.5.391. [DOI] [PubMed] [Google Scholar]
- Kminek G. Rummel J.D. Cockell C.S. Atlas R. Barlow N. Beaty D. Boynton W. Carr M. Clifford S. Conley C.A. Davila A.F. Debus A. Doran P. Hecht M. Heldmann J. Helbert J. Hipkin V. Horneck G. Kieft T.L. Klingelhoefer G. Meyer M. Newsom H. Ori G.G. Parnell J. Prieur D. Raulin F. Schulze-Makuch D. Spry J.A. Stabekis P.E. Stackebrandt E. Vago J. Viso M. Voytek M. Wells L. Westall F. Report of the COSPAR Mars special regions colloquium. Adv Space Res. 2010;46:811–829. [Google Scholar]
- La Duc M.T. Nicholson W. Kern R. Venkateswaran K. Microbial characterization of the Mars Odyssey spacecraft and its encapsulating facility. Environ Microbiol. 2003;6:977–985. doi: 10.1046/j.1462-2920.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- La Duc M.T. Satomi M. Venkateswaran K. Bacillus odysseyi sp nov., a round-spore-forming bacillus isolated from the Mars Odyssey spacecraft. Int J Syst Evol Microbiol. 2004;5:977–985. doi: 10.1099/ijs.0.02747-0. [DOI] [PubMed] [Google Scholar]
- La Duc M.T. Dekas A. Osman S. Moissl C. Newcombe D. Venkateswaran K. Isolation and characterization of bacteria capable of tolerating the extreme conditions of clean room environments. Appl Environ Microbiol. 2007;73:2600–2611. doi: 10.1128/AEM.03007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg C. Horneck G. Action spectra for survival and spore photoproduct formation of Bacillus subtilis irradiated with short wavelength (200–300 nm) UV at atmospheric pressure and in vacuo. J Photochem Photobiol B. 1991;11:69–80. doi: 10.1016/1011-1344(91)80269-n. [DOI] [PubMed] [Google Scholar]
- Link L. Sawyer J. Venkateswaran K. Nicholson W. Extreme spore UV resistance of Bacillus pumilus isolates obtained from an ultraclean spacecraft assembly facility. Microb Ecol. 2004;47:159–163. doi: 10.1007/s00248-003-1029-4. [DOI] [PubMed] [Google Scholar]
- Moeller R. Horneck G. Facius R. Stackebrandt E. Role of pigmentation in protecting Bacillus sp endospores against environmental UV radiation. FEMS Microbiol Ecol. 2005;51:231–236. doi: 10.1016/j.femsec.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Moeller R. Horneck G. Rettberg P. Mollenkopf H.-J. Stackebrandt E. Nicholson W.L. A method for extracting RNA from dormant and germinating Bacillus subtilis strain 168 endospores. Curr Microbiol. 2006;53:227–231. doi: 10.1007/s00284-006-0099-1. [DOI] [PubMed] [Google Scholar]
- Moeller R. Reitz G. Nicholson W.L. Cadet J. Douki T. Mancinelli R.L. Panitz C. Rabbow E. Rettberg P. Spry A. Stackebrandt E. Vaishampayan P. Venkateswaran K.J. Horneck G. Mutagenesis in bacterial spores exposed to space and simulated martian conditions: data from the EXPOSE-E spaceflight experiment PROTECT. Astrobiology. 2012;12:457–468. doi: 10.1089/ast.2011.0739. [DOI] [PubMed] [Google Scholar]
- Munakata N. Saito M. Hieda K. Inactivation action spectra of Bacillus subtilis spores in extended ultraviolet wavelengths (50–300 nm) obtained with synchrotron radiation. Photochem Photobiol. 1991;54:761–768. doi: 10.1111/j.1751-1097.1991.tb02087.x. [DOI] [PubMed] [Google Scholar]
- Nicholson W.L. Moeller R. Spore. In: Gargaud M., editor; Amils R., editor; Quintanilla Cernicharo., editor; Cleaves H.J. III, editor; Irvine W.M., editor; Pinti D.L., editor; Viso M., editor. Encyclopedia of Astrobiology. Springer; Heidelberg: 2011. pp. 1565–1567. [Google Scholar]
- Nicholson W.L. Schuerger A.C. Bacillus subtilis spore survival and expression of germination-induced bioluminescence after prolonged incubation under simulated Mars atmospheric pressure and composition: implications for planetary protection and lithopanspermia. Astrobiology. 2005;5:536–544. doi: 10.1089/ast.2005.5.536. [DOI] [PubMed] [Google Scholar]
- Nicholson W.L. Setlow P. Sporulation, germination and outgrowth. In: Harwood C.R., editor; Cuttings S.M., editor. Molecular Biological Methods for Bacillus. John Wiley and Sons Inc.; Chichester, UK: 1990. pp. 391–450. [Google Scholar]
- Nicholson W.L. Munakata N. Horneck G. Melosh H.J. Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev. 2000;64:548–572. doi: 10.1128/mmbr.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W.L. Setlow B. Setlow P. UV photochemistry of DNA in vitro and in Bacillus subtilis spores at Earth-ambient and low atmospheric pressure: implications for spore survival on other planets or moons in the Solar System. Astrobiology. 2002;2:417–425. doi: 10.1089/153110702762470518. [DOI] [PubMed] [Google Scholar]
- Nicholson W.L. Schuerger A.C. Setlow P. The solar UV environment and bacterial spore UV resistance: considerations for Earth-to-Mars transport by natural processes and human spaceflight. Mutat Res. 2005;571:249–264. doi: 10.1016/j.mrfmmm.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Nicholson W.L. Schuerger A.C. Race M.S. Migrating microbes and planetary protection. Trends Microbiol. 2009;17:389–392. doi: 10.1016/j.tim.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Nicholson W.L. Moeller R. Cadet J. Douki T. Mancinelli R.L. Panitz C. Rabbow E. Rettberg P. Spry A. Stackebrandt E. Vaishampayan P. Venkateswaran K.J. Horneck G. Transcriptomic responses of germinating Bacillus subtilis spores exposed to 1.5 years of space and simulated martian conditions on the EXPOSE-E experiment PROTECT. Astrobiology. 2012;12:469–486. doi: 10.1089/ast.2011.0748. [DOI] [PubMed] [Google Scholar]
- Puleo J.R. Fields N.D. Bergstrom S.L. Oxborrow G.S. Stabekis P. Koukol R. Microbiological profiles of the Viking spacecraft. Appl Environ Microbiol. 1977;33:379–384. doi: 10.1128/aem.33.2.379-384.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbow E. Horneck G. Rettberg P. Schott J.-U. Panitz C. L'Afflitto A. von Heise-Rotenburg R. Willnecker R. Baglioni P. Hatton J. Dettmann J. Demets R. Reitz G. EXPOSE, an astrobiological exposure facility on the International Space Station—from proposal to flight. Orig Life Evol Biosph. 2009;39:581–598. doi: 10.1007/s11084-009-9173-6. [DOI] [PubMed] [Google Scholar]
- Rabbow E. Rettberg P. Barczyk S. Bohmeier M. Parpart A. Panitz C. Horneck G. von Heise-Rotenburg R. Hoppenbrouwers T. Willnecker R. Baglioni P. Demets R. Dettmann J. Reitz G. EXPOSE-E: an ESA astrobiology mission 1.5 years in space. Astrobiology. 2012;12:374–386. doi: 10.1089/ast.2011.0760. [DOI] [PubMed] [Google Scholar]
- Rettberg P. Fritze D. Verbarg S. Nellen J. Horneck G. Stackebrandt E. Kminek G. Determination of the microbial diversity of spacecraft assembly, testing and launch facilities: first results of the ESA project MiDiv. Adv Space Res. 2006;38:1260–1265. [Google Scholar]
- Schaeffer P. Millet J. Aubert J.-P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerger A.C. Mancinelli R.L. Kern R.G. Rothschild L.J. McKay C.P. Survival of Bacillus subtilis on spacecraft surfaces under simulated martian environments: implications for the forward contamination of Mars. Icarus. 2003;165:253–276. doi: 10.1016/s0019-1035(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Schuerger A.C. Richards J.T. Hintze P.E. Kern R.G. Characteristics of spacecraft components affect the aggregation of microorganisms and may lead to different survival rates of bacteria on Mars landers. Astrobiology. 2005;5:545–559. doi: 10.1089/ast.2005.5.545. [DOI] [PubMed] [Google Scholar]
- Stieglmeier M. Wirth R. Kminek G. Moissl-Eichinger C. Cultivation of strictly and facultatively anaerobic Bacteria from spacecraft associated clean rooms. Appl Environ Microbiol. 2009;75:3484–3491. doi: 10.1128/AEM.02565-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. Space microbiology. Annu Rev Microbiol. 1974;28:121–137. doi: 10.1146/annurev.mi.28.100174.001005. [DOI] [PubMed] [Google Scholar]
- Vaishampayan P.A. Rabbow E. Horneck G. Venkateswaran K.J. Survival of Bacillus pumilus spores for a prolonged period of time in real space conditions. Astrobiology. 2012;12:487–497. doi: 10.1089/ast.2011.0738. [DOI] [PubMed] [Google Scholar]
- Vall-Ilosera G. Huels M.A. Coreno M. Kivimäki A. Jakubowska K. Stankiewicz M. Rachlew E. Photofragmentation of 2-deoxy-D-ribose molecules in the gas phase. Chemphyschem. 2008;9:1020–1029. doi: 10.1002/cphc.200700635. [DOI] [PubMed] [Google Scholar]
- Venkateswaran K. Satomi M. Chung S. Kern R. Koukol R. Basic C. White D. Molecular microbial diversity of a spacecraft assembly facility. Syst Appl Microbiol. 2001;24:311–320. doi: 10.1078/0723-2020-00018. [DOI] [PubMed] [Google Scholar]
- Venkateswaran K. Hattori N. La Duc M.T. Kern R. ATP as a bio-marker of viable microorganisms in clean-room facilities. J Microbiol Methods. 2003a;52:367–377. doi: 10.1016/s0167-7012(02)00192-6. [DOI] [PubMed] [Google Scholar]
- Venkateswaran K. Kempf M. Chen F. Satomi M. Nicholson W. Kern R. Bacillus nealsonii sp nov., isolated from a spacecraft-assembly facility, whose spores are gamma-radiation resistant. Int J Syst Evol Microbiol. 2003b;53:165–172. doi: 10.1099/ijs.0.02311-0. [DOI] [PubMed] [Google Scholar]