Zhang et al.1 responded to our paper2 in which we showed that bitter taste receptors (TAS2Rs) mediate relaxation of airway smooth muscle by increasing the concentration of intracellular calcium ([Ca2+]i). We are pleased to see that they also found TAS2R agonists to markedly relax airways, with similar efficacies to those that we found2. In our study we showed that TAS2R function in airways was mediated by [Ca2+]i release on the basis of the concordance of relaxation potency and efficacy with measured [Ca2+]i release, the loss of TAS2R-mediated relaxation when [Ca2+]i release was blocked and the stimulation of spatially and temporally distinct [Ca2+]i events evoked by TAS2R agonists in intact airway smooth muscle. We considered that large-conductance Ca2+-activated K+ channels (BKCa) were activated, and thus played some part in the relaxation response, on the basis of the sensitivity of TAS2R-mediated relaxation of mouse airways and individual airway smooth muscle cells to the highly specific BKCa-channel inhibitor iberiotoxin (IbTx). However, we showed that IbTx only partially blocked relaxation (Fig. 3b,c of the paper2) to bitter tastant, leaving open the possibility of additional mechanisms.
To further address the concerns of Zhang et al.1, we first examined whether blockade or direct activation of BKCa by IbTx or NS1619, respectively, results in the expected changes in airway smooth muscle tone. In mouse trachea studied ex vivo, 100 nM IbTx caused a 15% ± 5.3% increase in force over baseline (P = 0.01, n = 14), whereas 100 μM NS1619 caused a 91% ± 6.0% decrease in force of methacholine-contracted airways (P < 0.01, n = 4). These results indicate that BKCa activation status is capable of regulating the airway smooth muscle contractile state in a dynamic fashion and in a direction consistent with receptor-mediated channel opening resulting in bronchodilation. Next, we reexamined the effects of IbTx on methacholine-contracted mouse airways using the TAS2R agonist chloroquine, the same agent used by Zhang et al.1. Mouse (FVB/N strain) tracheas in the ex vivo model were studied with full dose-response studies to ascertain potential nonspecific effects of chloroquine.
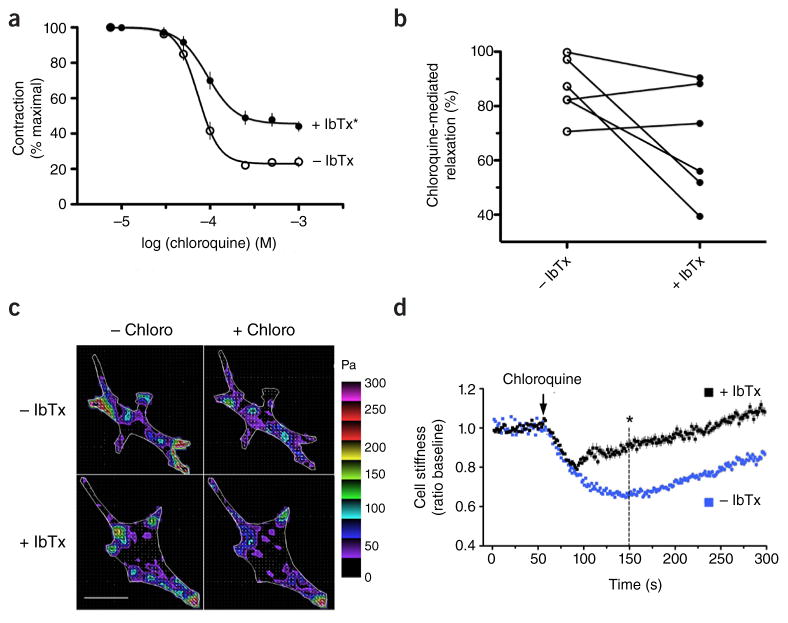
Consistent with our original report2, IbTx partially blocked the relaxation effect of this TAS2R agonist in mouse trachea, with an ~35% decrease in efficacy (Fig. 1a). This effect is not ambiguous and is highly reproducible. It is unclear why Zhang et al.1 find no effect of IbTx, but potential differences due to mouse strains3,4 or methodology might explain this discrepancy. We used 100 μM methacholine versus the 10 μM concentration used by Zhang et al.1 to contract the rings, and we performed studies starting with low concentrations of chloroquine and increasing them in a dose-response fashion (as opposed to a single high dose), which might have a priming effect. Finally, it has been established that mouse airway physiological and [Ca2+]i responses are highly dependent on the anatomical location of the segment5. Studies of human airways can be helpful to assure the applicability of findings in mice. And, indeed, there are many known differences in receptor responsiveness to G protein–coupled receptor agonists between mouse and human lung6,7. In our original paper2 (as well as in this reply), we used human airway smooth muscle cells or airways whenever possible because of this potential issue. We have now studied IbTx sensitivity of bitter tastant responses in rings from fourth- and fifth-order human bronchi. We found between-sample variation in relaxation and the extent of the IbTx effect, with the most apparent IbTx effect being noted at 500 μM doses of chloroquine (Fig. 1b). The variability may be due to underlying pathology, exposure history or differing anatomical regions of the airway, but overall these findings demonstrate that blockade of BKCa with IbTx results in physiologically relevant loss of TAS2R-mediated relaxation in human airways (Fig. 1b).
Figure 1.
Airway smooth muscle responses to the TAS2R agonist chloroquine are sensitive to the BKCa antagonist IbTx. (a) Mouse airway relaxation in response to chloroquine in the absence (control) or presence of 100 nM IbTx. Results are from nine independent experiments. *P < 0.05 versus control. (b) Human airway relaxation due to 500 μM chloroquine in the absence (control) or presence of 100 nM IbTx. Shown are the individual results from six rings derived from two subjects. Data are scaled to the maximal response in the group. For experiments in a and b, airway rings were studied in a lateral isometric myograph, with baseline passive tension set at 5 mN, and then contracted with 100 μM methacholine. (c) Representative traction maps of single human airway smooth muscle cells determined by Fourier transform traction microscopy. The colors represent the magnitude of the regional tractions in Pa indexed to the color bar at the right. Results are representative of four experiments. Scale bar, 40 μm. (d) Quantitative measurements of cell relaxation ascertained by magnetic twisting cytometry. Results are from individual measurements from 717 cells exposed to 1.0 mM chloroquine alone (−IbTx) and 808 cells pretreated for 30 min with 100 nM IbTx followed by exposure to 1.0 mM chloroquine. Data are from five independent experiments and are shown as mean ± s.e.m. *P < 0.001 versus −IbTx at 90 s after chloroquine exposure (the 150 s point of the y axis). These studies were approved by the Institutional Animal Care and Use Committee of the University of Maryland and the Institutional Review Board of Johns Hopkins University. Tissues in b–d were from discarded surgical specimens.
We performed additional studies with isolated airway smooth muscle cells, again derived from human samples, examining mechanics in the absence of any influence from the epithelium. We used Fourier transform traction microscopy8,9 to measure spatiotemporal changes in the contractile stress generated by an individual human airway smooth muscle cell. Figure 1c shows representative traction fields before and after exposure to chloroquine, in the absence or presence of IbTx. The cell boundary is shown by the white line, and colors show the magnitude of the tractions in Pascals (Pa). In most of these intracellular areas, traction was markedly reduced by chloroquine (Fig. 1c, top). Furthermore, IbTx treatment blocked some, but not all, areas of the chloroquine-mediated traction responses (Fig. 1c, bottom), indicative of partial blockade of the relaxation effect of the TAS2R agonist in human airway smooth muscle. We performed additional quantitative studies using magnetic twisting cytometry. In five independent experiments representing >1,500 airway smooth muscle cell measurements (Fig. 1d), we found that chloroquine relaxed human airway smooth muscle cells to a maximum of 34% ± 0.8% (n = 717) at 90 s after exposure (the 150 s point of Fig. 1d), whereas in the presence of IbTx relaxation at that same time point was 14% ± 1.6% (n = 808, P < 0.001). As we previously described2, there is an initial relaxation response to chloroquine that is not sensitive to the toxin (Fig. 1d), and this may represent an additional component that is manifested in the coordinated relaxation of the intact airway that is IbTx insensitive.
We previously used voltage-sensitive dyes to ascertain changes in membrane potential2 on the basis of reports of correlations with other forms of whole-cell measurements, including patch-clamp technology10,11. We agree, though, that voltage-sensitive dyes do not represent direct measurements and may be less useful for dissecting complex pathways, particularly when new and paradoxical signaling events seem to be at play.
Given the physiologic responses that we observed in four different models, we find that blockade of BKCa substantially impairs, but does not ablate, TAS2R-mediated airway relaxation. We conclude that BKCa activation by localized Ca2+ events promoted by TAS2R agonists is one part of a complex mechanism by which these receptors relax airway smooth muscle and that this mechanism is required for the full physiological effect of receptor activation. Additional studies will be required to ascertain other partners in this multifactorial end-organ response. Nevertheless, TAS2Rs remain attractive targets as direct bronchodilators for the treatment of obstructive airway disease. Finally, we propose that, whenever possible, studies in this area should be performed in human rather than mouse airway smooth muscle cells and airways, as the receptor and effector responses of airways are differentially manifested (often markedly so6,7) between the species. Additionally, it is well recognized that, between human and mouse bitter taste receptor families, there are multiple expressed receptors without genetic or pharmacologic homologs12.
Acknowledgments
Supported by the US National Institutes of Health grants HL071609 and HL107361.
This is a reply to the commentary Zhang CH, Chen C, Lifshitz LM, Fogarty KE, Zhu MS, ZhuGe R. Activation of BK channels may not be required for bitter tastant-induced bronchodilation.. Nat Med. 2012 5 4;18(5):648-50.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Zhang CH, et al. Nat Med. 2012;18:648–650. doi: 10.1038/nm.2733. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande DA, et al. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levitt RC, Mitzner W. FASEB J. 1988;2:2605–2608. doi: 10.1096/fasebj.2.10.3384240. [DOI] [PubMed] [Google Scholar]
- 4.Levitt RC, Mitzner W. J Appl Physiol. 1989;67:1125–1132. doi: 10.1152/jappl.1989.67.3.1125. [DOI] [PubMed] [Google Scholar]
- 5.Bai Y, Zhang M, Sanderson MJ. Am J Respir Cell Mol Biol. 2007;36:122–130. doi: 10.1165/rcmb.2006-0036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin TR, Gerard NP, Galli SJ, Drazen JM. J Appl Physiol. 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 7.Held HD, Martin C, Uhlig S. Br J Pharmacol. 1999;126:1191–1199. doi: 10.1038/sj.bjp.0702394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolić-N⊘rrelykke IM, Butler JP, Chen J, Wang N. Am J Physiol Cell Physiol. 2002;283:C1254–C1266. doi: 10.1152/ajpcell.00169.2002. [DOI] [PubMed] [Google Scholar]
- 9.An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Am J Respir Cell Mol Biol. 2006;35:55–64. doi: 10.1165/rcmb.2005-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallos G, et al. Am J Physiol Lung Cell Mol Physiol. 2012;302:L248–L256. doi: 10.1152/ajplung.00131.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baxter DF, et al. J Biomol Screen. 2002;7:79–85. doi: 10.1177/108705710200700110. [DOI] [PubMed] [Google Scholar]
- 12.Shi P, Zhang J, Yang H, Zhang YP. Mol Biol Evol. 2003;20:805–814. doi: 10.1093/molbev/msg083. [DOI] [PubMed] [Google Scholar]