1. Structure
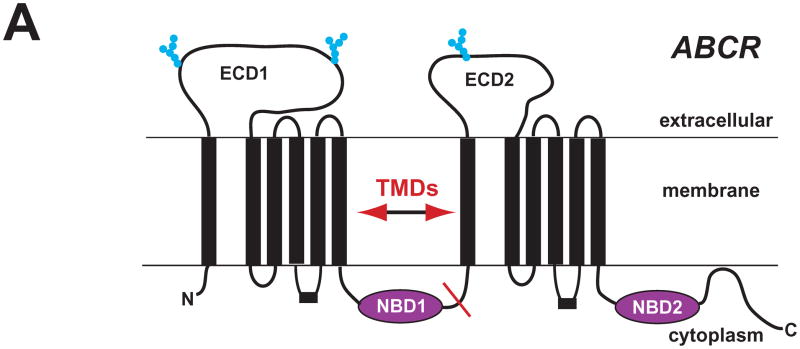
Previously known as the photoreceptor rim protein (RmP), human ABCA4 (ABCR) (NP000341.2) is a large (2,273 aa, 220 kD), retina-specific adenosine triphosphate (ATP)-binding cassette transporter (ABC). The proposed structure of ABCR, which is based on hydropathy plots and its homology to other ABCs, consists of two six transmembrane domains (TMD), two large glycosylated extracellular domains (ECD1, 602 aa, ECD2, 289 aa), and two conserved intracellular nucleotide binding domains (NBD1, NBD2, ~140 aa each) (Fig. 1A) (see Sun and Nathans, 2001; Molday 2007). When inserted into the membrane, the TMDs of ABCR are predicted to form a gated, barrel/hyperboloid structure that is permeable to retinoid ligands and controls channel access to extracellular/intracellular binding sites. In general, the TMDs of the ABC protein family are not highly conserved, an observation that is consistent with the diverse transport functions of this protein family. When ATP binds to the NBDs, ABCR undergoes a series of conformational changes that bring the NBDs together and rotates and tilts the TMDs thereby modulating ligand binding to the gated channel. The current proposed alternating access-release model for retinoid transport by ABCR is based on recent structural analyses of bacterial importer/exporter ABCs (Fig. 1B). The model proposes that retinoid transport occurs as a result of alternating exposure of external and internal TMD ligand binding site(s) located along the permeation channel that are gated by coupled structural changes in NBDs that themselves are modulated by the binding and hydrolysis of ATP.
Figure 1.
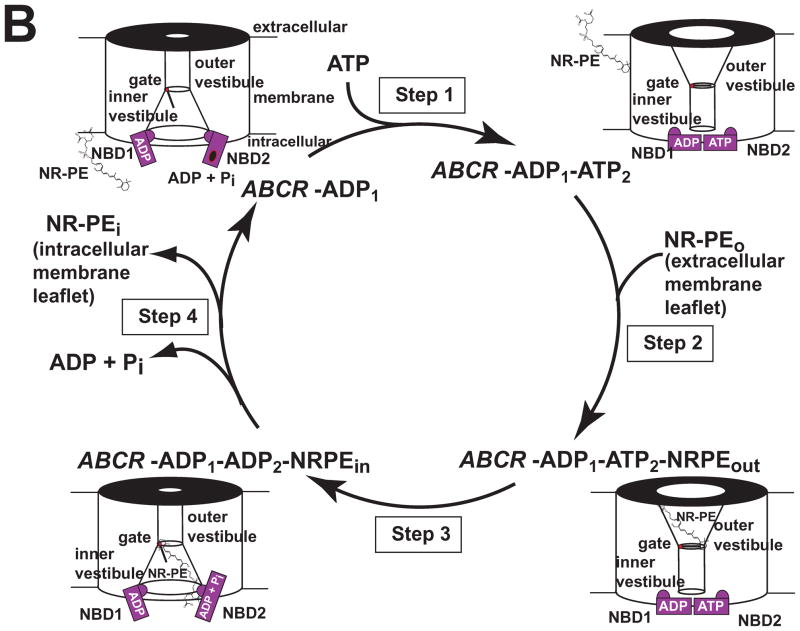
ABCR Structure and Function. (A) Secondary structure schematic is shown (adapted from Bungert et al. 2001, J Biol. Chem. 276: 23539-23546). Red line shows the approximate half point. (B) ABCR modeled as a retinoid flipase that transports retinoids (NRPE, ATR) from photoreceptor extracellular to cytoplasmic surfaces. An alternating access-release model for ABCR has the following steps (modeled schematically): 1) binding of adenosine-triphosphate (ATP) to NBD2 which promotes spatial NBD1/NBD2 interaction, and coupled kinetic transition of TMDs to expose outer vestibule high affinity binding site, 2) binding of NR-PE/ATR on extracellular side of gate, 3) ATP hydrolysis promotes gate opening and movement of NR-PE/ATR along the TMD channel to a low affinity binding site on intracellular side of gate, and 4) and release of adenosine diphosphate (ADP) and phosphate (Pi) to promote separation of NBDs and release of bound ligand.
2. Function
The results of comparisons of mammalian ABCR to other ABCs, biochemical and cellular localization studies of ABCR, and analyses of ABCR knockout mice suggest that mammalian ABCR functions as an inward-directed retinoid flipase. Retinoid substrates imported by ABCR from the extracellular or intradiscal (rod) membrane surfaces to the cytoplasmic membrane surface are all-trans retinaldehyde (ATR) and N-retinyl-phosphatidylethanolamine (NR-PE), a covalent Schiff base adduct (-C=N-) of ATR with PE. Once transported to the cytoplasmic surface, ATR is reduced to vitamin A (VA) by trans-retinol dehydrogenase (tRDH) and then transferred to the retinal pigment epithelium (RPE) where it is converted to 11-cis-retinal.
The only biochemical assay that has been developed for ABCR function is enhanced ATP hydrolysis that occurs when ABCR, reconstituted (with PE) in membranes, is exposed to candidate ligands. No assay currently exists that measures actual substrate transfer across membranes. In support for an ABCR flipase function, certain retinoids stimulate ATPase activity, which occurs by simple Michaelis-Menten uncompetitive activation kinetics, as expected for a transporter with a single class of binding sites. Preferred ligands are protonated NR-PE (Kd ~ 4 μM) and ATR. Only NBD2 exchanges ATP with solution, while NBD1 has adenosine diphosphate (ADP) tightly bound.
ABCR is localized to outer segment disk edges of rods and cones. ABCR expression relative to rhodopsin is approximately 1:120 (1×106 copies/photoreceptor, ~103 copies/disk). Hydrophobic ATR formation is directly proportional to visual pigment bleaching, and upon release from opsins ATR can appear on both membrane surfaces to spontaneously and reversibly react with PE (NR-PE ⇔ ATR + PE). PE is more abundant in rod disk than plasma membranes, and is differentially distributed on cytoplasmic (>75%) and extracellular (<25%) surfaces. The ATR fraction that distributes to the cytoplasmic membrane surface is reduced to VA by tRDH. VA esterification in RPE can pull the equilibrium to NR-PE hydrolysis by mass action. Additional kinetic barriers emerge for the fraction of ATR solubilized on the extracellular/intradiscal (rods) membrane surfaces. While some may diffuse directly to the cytoplasmic leaflet, another fraction reacts with PE to become protonated NR-PE at physiological pH (pKa 6.0, unprotected Schiff base) and is trapped inside the disk as a charged species. ABCR could facilitate transfer of this population to the cytoplasmic surface.
The ABCR−/− knockout mouse has delayed dark adaptation but normal final rod threshold relative to controls (Weng et al., 1999). This suggests both ABCR dependent and independent (e.g. bulk transmembrane diffusion) pathways that remove ATR/NR-PE from extracellular membranes. After strong bleaches there is significant accumulation of ATR and NR-PE in outer segments, and decreases of VA and VA esters. Levels of the toxic cationic bis-pyridinium salt, N-retinylidene-N-retinyl-ethanolamine (A2E), increase with age but no retinal degeneration ensues. A2E forms from reaction of two molecules of ATR with PE. These findings support a role for ABCR to remove ATR/NR-PE from the extracellular photoreceptor surfaces during bleach recovery, and to suppress accumulation of A2E. No A2E accumulates in mice raised in darkness because visual pigment bleaching and turnover (visual retinoid cycle) is required to generate the ATR substrate needed for toxic A2E formation. The double knockout (ABCR−/− and rod tRDH (RDH8−/−)) severely constrains ATR clearance from rods. This causes large and rapid accumulations of both A2E and the toxic retinal dimer-PE conjugate (Ret-Di-PE) (another product of combining two ATR molecules and PE), formation of drusen, and RPE and photoreceptor degeneration and neovascularization. This model presents a full spectrum of human dry and wet age-related macular degeneration (AMD) (Maeda et al., 2008). The phenotype is suppressed by darkness and an RPE65 inhibitor, which indicates that cellular pathology ultimately originates from visual pigment bleaching, ATR formation, and retinoid visual cycle turnover. In the short mouse lifetime emergence of a lipofuscin-dependent atrophic and neovascular retinal degeneration required complete “system” bottleneck to stall conversion of ATR to VA.
3. Disease Involvement
Stargardt macular dystrophy (STGD) is a hereditary juvenile macular degeneration (frequency 1/10,000) that emerges in the first two decades of life. STGD is clinically associated with yellow-white flecks at the RPE level causing focal, progressive macular RPE atrophy and associated photoreceptor loss. STGD is characterized by reductions in visual acuity and color vision, paracentral visual field changes, cone ERG changes, delayed dark adaptation late in disease, and substantial accumulation of autofluorescent RPE lipofuscin. Yellow lesions are due to clusters of RPE cells engorged with fluorescent lipofuscin pigments that contain toxic A2E/Ret-Di. The large human ABCR gene is mapped to 1p13-p21 (ID: 601691), and covers a span of approximately 130 kilo base pairs with fifty exons (processed mRNA: 7326 nt; open reading frame: 6819 nt). Known variants/mutations (>400) cause autosomal recessive STGD (STGD1, Mendelian Inheritance in Man (MIM): 248200), a later onset milder variant called fundus flavimaculatus (FF, MIM ID: 248200), recessive cone-rod dystrophy (CORD3, MIM: 604116), recessive retinitis pigmentosa (RP19, MIM: 601718), and contribute to AMD risk (ARMD2, MIM: 153800). ABCR mutations create a wide phenotypic spectrum (mild: ARMD2 → STGD1/FF → CORD3 → RP19: severe). In HEK293 cells expression of human ABCR variants leads to varied expression levels, ATP binding, and ATR- stimulated ATPase activity relative to normal. Broad clinical phenotypic heterogeneity (time of onset, clinical severity) is ultimately dependent upon the nature of the specific mutations involved. Mutations can affect the amount of remaining wild type ABCR activity, or cause toxic or dominant negative effects due to mutant proteins with abnormal folds.
ABCR may benefit vision on short and long time scales. Transducin is activated when opsin binds ATR noncovalently, at least in vitro, to elevate rod threshold by physiological “dark light”. Removal of ATR/NR-PE by ABCR appears important to normal bleach recovery kinetics and to mitigate persistent opsin signaling that drives photoreceptor death. ABCR could mitigate long term effects by mobilizing ATR to suppress irreversible addition of a second ATR molecule to NR-PE to form dihydro-N-retinylidene-N-retinyl-phosphatidylethanolamine (A2PE-H2). A2PE-H2 traps ATR into visually useless ligands, accumulates in outer segments, and is further oxidized to N-retinylidene-N-retinyl-phosphatidyl-ethanolamine (A2-PE). Upon disk shedding and phagocytosis of outer segment material by RPE cells, A2-PE is hydrolyzed in the RPE phagolysosome to form toxic A2E. A2E cannot be metabolized and accumulates as a major component of fluorescent RPE lipofuscin. Accumulating ATR also drives formation of toxic Ret-Di-PE complexes that, with A2E, exert cumulative or threshold toxicity at the primary RPE level with secondary photoreceptor demise in juvenile and age-related macular degenerations.
4. Future Studies
To advance ABCR knowledge a cellular assay is critically needed to measure actual transmembrane retinoid transport. Many important questions about ABCR structure, function, and system integration remain. What is the atomic structure under various conditions of NR-PE and ATP binding, what TMD residues constitute ligand binding sites, and what is the coupling mechanism of ATP binding/hydrolysis to conformational changes? What fraction of ATR is released from opsin on extracellular/intracellular membrane leaflets, does ABCR interface with recently proposed ATR exit sites on bleached rhodopsin, what fraction of the extracellular pool is mobilized by ABCR, and how are these pools modulated by bleaching level? What are the kinetics of ABCR performance? Can inhibitors and activators be identified (e.g. A2-PEH2)? Does ABCR interact with tRDH on the cytoplasmic surface, or with proteins in the disk rim or lumen, or subretinal space (e.g. IRBP)? Does phosphorylation, or other modulation, regulate function? What molecular and “system” problems underlie the diversity of clinical ABCR phenotypes?
Acknowledgments
The author acknowledges the support of Challenge and Unrestricted Grants to the University at Buffalo from Research to Prevent Blindness, and the National Eye Institute (R01 EY13433). Most of the current state of molecular knowledge of ABCR and its effects originated from studies in the labs of Drs. Rando Allikmets (Columbia Univ.), Robert Molday (Univ. of British Columbia), Jeremy Nathans (Johns Hopkins), Kris Palczewski (Case Western), Janet Sparrow (Columbia Univ.), and Gabriel Travis (Jules Stein-UCLA), which could not be fully referenced here.
ABBREVIATIONS
- A2E
N-retinylidene-N-retinyl-ethanolamine
- ABC
ATP-binding cassette
- ABCR
ATP-binding cassette retinoid protein, subfamily A, number 4 (ABCA4)
- ADP
adenosine diphosphate
- AMD
age-related macular degeneration
- ATP
adenosine triphosphate
- ATR
all-trans-retinal
- MIM
Mendelian Inheritance in Man
- NBD
nucleotide binding domain
- NR-PE
N-retinylidene-phosphatidyl-ethanolamine
- PE
phosphatidyl-ethanolamine
- Ret-Di
retinal dimer
- RPE
retinal pigment epithelium
- STGD
Stargardt macular dystrophy
- TMD
transmembrane domain
- tRDH
trans-retinol dehydrogenase
- VA
vitamin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS. ATP-binding cassette transporter ABCA4: molecular properties and role in vision and macular degeneration. J Bioenerg Biomembr. 2007;39:507–517. doi: 10.1007/s10863-007-9118-6. [DOI] [PubMed] [Google Scholar]
- Sun H, Nathans J. Mechanistic studies of ABCR, the ABC transporter in photoreceptor outer segments responsible for autosomal recessive Stargardt disease. J Bioenerg Biomembr. 2001;33:523–530. doi: 10.1023/a:1012883306823. [DOI] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]