Abstract
Background
There is evidence that exposure to chlorinated solvents may be associated with childhood medulloblastoma and primitive neuroectodermal tumor (M/PNET) risk. Animal models suggest genes related to detoxification and DNA repair are important in the carcinogenicity of these pollutants, however, there have been no human studies assessing the modifying effects of these genotypes on the association between chlorinated solvents and childhood M/PNET risk.
Procedure
We conducted a case-only study to evaluate census tract-level exposure to chlorinated solvents and the risk of childhood M/PNET in the context of detoxification and DNA repair genotypes. Cases (n = 98) were obtained from Texas Children’s Hospital and MD Anderson Cancer Center. Key genotypes (n = 22) were selected from the Illumina Human 1M Quad SNP Chip. Exposure to chlorinated solvents (methylene chloride, perchloroethylene, trichloroethylene, and vinyl chloride) was estimated from the U.S. EPA’s 1999 Assessment System for Population Exposure Nationwide (ASPEN). Logistic regression was used to estimate the case-only odds ratios and 95% confidence intervals (CIs).
Results
There were 11 significant gene-environment interactions associated with childhood M/PNET risk. However, after correcting for multiple comparisons, only the interaction between high trichloroethylene levels and OGG1 rs293795 significantly increased the risk of childhood M/PNET risk (OR = 9.24, 95% CI: 2.24, 38.24, Q = 0.04).
Conclusions
This study provides an initial assessment of the interaction between ambient levels of chlorinated solvents and potentially relevant genotypes on childhood M/PNET risk. Our results are exploratory and must be validated in animal models, as well as additional human studies.
Keywords: Hazardous air pollutants, chlorinated solvents, DNA repair genes, detoxification genes, childhood medulloblastoma and primitive neuroectodermal tumor
INTRODUCTION
Central nervous system (CNS) tumors are the second most common pediatric malignancy and the most common solid tumor in children [1]. Medulloblastoma and primitive neuroectodermal tumor (M/PNET) account for approximately 24% of childhood CNS tumor cases and have an overall incidence rate of 6.6 per 1,000,000 person-years [1]. Although 5-year survival has improved in recent years, the long-term consequences of treatment are significant [2]. In spite of the clinical importance of M/PNET, the etiology of these conditions remains unclear. Therefore, it is critical to identify modifiable risk factors in order to lessen the impact of M/PNET. Although environmental toxicants have been associated with the development of M/PNET [3]; much work remains to fully elucidate the relationship between specific groups of toxicants and M/PNET risk, including the assessment of gene-environment interactions.
The U.S. Clean Air Act of 1990 identified 189 environmental toxicants as hazardous air pollutants (HAPs). These pollutants are particularly important because: 1) they are known or suspected to cause a range of adverse health outcomes, including cancer [4]; 2) their levels are increasing in communities throughout the U.S. [5]; and 3) there are currently no national air quality standards for HAPs, as there are for the “criteria” air pollutants (e.g., carbon monoxide and ozone) as defined by the U.S. Environmental Protection Agency (EPA) [6]. Although HAPs are considered to have important health consequences, population-scale individual-level measurements of HAPs are nonexistent and monitoring of HAPs in communities throughout the U.S. is very limited. Consequently, the U.S. Environmental Protection Agency (EPA) has developed the Assessment System for Population Exposure Nationwide, or ASPEN model, to estimate annual concentrations of HAPs at the census tract level [7]. Independent assessments, including our own study in Texas [8], have concluded that estimates of selected HAPs obtained using the ASPEN model are a good surrogate for exposure measures based on personal and outdoor monitoring [9].
HAPs are a heterogeneous grouping of toxicants including chlorinated solvents (e.g., vinyl chloride), aromatic solvents (e.g., benzene), and heavy metals (e.g., cadmium). Among these pollutants, there is limited and equivocal evidence that chlorinated solvents are associated with adult [10, 11] and childhood brain tumors [3, 12, 13]. Chlorinated solvents are a family of chemical compounds that are commonly used in many industrial settings. Their uses include paint stripping (methylene chloride), dry cleaning (perchloroethylene), metal degreasing (trichloroethylene), and the production of polyvinyl chloride (vinyl chloride). The principal route of environmental exposure is inhalation of ambient air [14–16]. Many of these compounds have been identified as known (e.g., vinyl chloride), probable (trichloroethylene, perchloroethylene) or possible (methylene chloride) human carcinogens by the International Agency for Research on Cancer (IARC) [14–16].
The suspected mechanism of carcinogenicity for several chlorinated solvents (e.g., trichloroethylene) follows a well-established paradigm [17]. After exposure, these toxicants are either excreted or bioactived by Phase I and/or Phase II detoxification enzymes. Phase I reactions generally involve the introduction of a functional group, whereas Phase II reactions involve direct conjugation [18]. If xenobiotics, such as chlorinated solvents, are bioactivated by Phase I or Phase II enzymes, the reactive intermediates can form DNA adducts leading to strand breaks and other cytotoxic effects [17]. Because of this, studies assessing environmental exposure to these and similar toxicants should also account for genotypes related to detoxification of these exposures and DNA repair capacity (particularly base excision repair and nucleotide excision repair). Therefore, we conducted an exploratory study to evaluate exposure to environmental levels of chlorinated solvents and the risk of childhood M/PNET in the context of detoxification and DNA repair genotypes using a case-only approach.
MATERIALS AND METHODS
Study subjects
The study population included 98 incident M/PNET cases recruited consecutively from Childhood Cancer Epidemiology and Prevention Center at Texas Children’s Hospital (Houston, TX) between 1986 and 2010. Cases were <18 years of age and histopathologically confirmed (ICD-O-3 codes 9470–9474). After written informed consent was obtained from the parent, we obtained a blood sample from each participant. These samples were used to obtain DNA for genotyping. Demographic and clinical data were abstracted from medical records. Only cases who were residing in the state of Texas at the time of diagnosis were included based on the exposure assessment. The study protocol was approved by the Baylor College of Medicine and MD Anderson Cancer Center Institutional Review Boards.
Environmental Exposure Assessment
Based on previous studies of brain tumors [14–16], as well as ambient levels in Texas [5, 8], we selected the following chlorinated solvents for analysis: methylene chloride, perchloroethylene, trichloroethylene, and vinyl chloride. Census tract-based estimates of ambient levels of the selected chlorinated solvents were obtained from the U.S. EPA’s 1999 ASPEN model [7, 19, 20]. The methods used for ASPEN have been described fully elsewhere [7, 20]. Briefly, ASPEN is part of the National Air Toxic Assessment (NATA) and is based on the EPA’s Industrial Source Complex Long Term Model. It takes into account emissions data; rate, location, and height of pollutant release; meteorological conditions; and the reactive decay, deposition, and transformation of pollutants. ASPEN model results were first generated for 1990 as part of the U.S. EPA Cumulative Exposure Project and then again for 1996, 1999, and 2002 as part of NATA. We opted to use the 1999 modeled estimates as the U.S. EPA advises against using multiple assessments (e.g., 1999 + 2002). This is due to differences in the methodology of successive assessments [21]. Additionally, 1999 is an approximate midpoint within our study period (1986–2010). Ambient air levels of chlorinated solvents (and other HAPs) are reported as annual concentrations in µg/m3 [20].
Residential air levels of the chlorinated solvents were estimated based on address at diagnosis as reported in medical records. Specifically, case addresses were mapped to their respective census tracts. This information was then linked to ASPEN census tract-level estimates of ambient levels of methylene chloride, perchloroethylene, trichloroethylene, and vinyl chloride.
Genotyping and SNP selection
Genotyping was performed using the Illumina Human 1M Quad SNP Chip according to manufacturer’s instructions (Illumina, San Diego, USA). Samples were excluded when fewer than 95% of genotypes were called overall. For quality assurance purposes, duplicate samples were genotyped in the same batches.
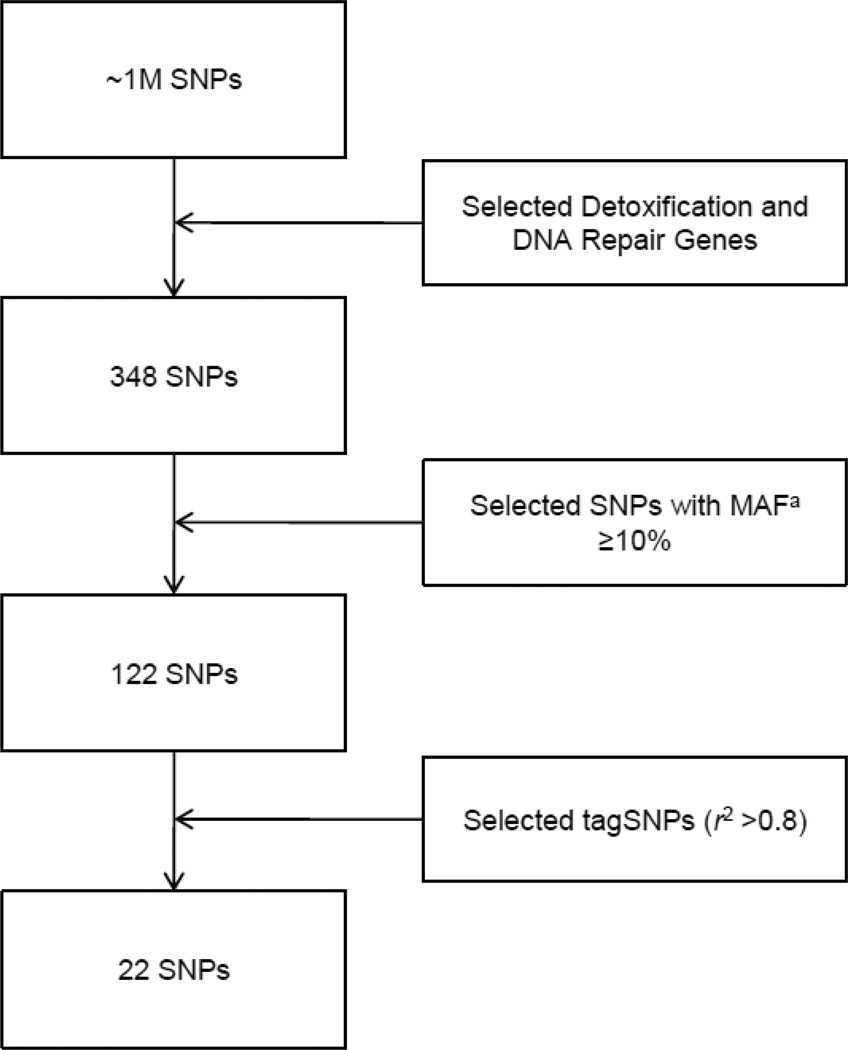
As we were interested in genetic modifiers of environmental exposure to chlorinated solvents, we compiled a list of the fifteen key detoxification and DNA repair genes (base excision repair and nucleotide excision repair) suspected in toxicant-induced carcinogenesis [14–16, 22]. These genes and the corresponding number of SNPs available on the Illumina Human 1M Quad SNP Chip are listed in Table I. From this list of candidate SNPs (n = 348), those with a minor allele frequency <10% (n = 226) were eliminated from the analysis. From the remaining 122 SNPs, tagSNPs with high linkage disequilibrium (r2 >0.8) were identified for each gene [23]. TagSNPs (n = 22) were selected for analysis (Figure 1) using the SNPStats program [24]. As population stratification may bias case-only studies [25], we also included an additional 212 ancestry informative markers to account for population structure [26].
Table I.
Candidate Detoxification and DNA Repair Genes
| Pathway | Gene Symbol | No. of SNPsa |
|---|---|---|
| Phase I Detoxification | CYP1A1 | 12 |
| CYP1A2 | 14 | |
| CYP1B1 | 29 | |
| CYP2E1 | 43 | |
| Phase II Detoxification | EPHX1 | 26 |
| GSTA4 | 41 | |
| GSTM1 | 5 | |
| GSTM3 | 12 | |
| GSTP1 | 16 | |
| NAT1 | 75 | |
| NAT2 | 24 | |
| DNA Repair | ||
| Base Excision Repair | OGG1 | 11 |
| XRCC1 | 21 | |
| Nucleotide Excision Repair | ERCC1 | 12 |
| XPA | 7 |
Based on the Illumina Human 1M Quad SNP Chip
Figure 1.
Detoxification and DNA Repair SNP Selection Process.
a Minor Allele Frequency
Statistical Analysis
Frequency distributions, means and standard deviations were determined for demographic and clinical variables among the case subjects. The means and standard deviations, as well as the 10th, 50th, and 90th percentiles for levels of methylene chloride, perchloroethylene, trichloroethylene, and vinyl chloride were compared between case census tracts and the state of Texas as a whole. Correlations between levels of methylene chloride, perchloroethylene, trichloroethylene, and vinyl chloride were determined using Spearman’s rank correlation.
A case-only design was used to examine the interaction between exposure to ambient levels of selected chlorinated solvents and tagSNPs in detoxification and DNA repair genes (i.e., gene-environment interactions). In a case-only design, the entire sample consists of those with disease (i.e., cases) [27]. Specifically, M/PNET subjects were used to assess the association between the environmental exposure and the genotype. Details of the case-only design have been presented previously [27–29], and the design has been used extensively to evaluate gene-environment interactions (e.g., [30]) as it offers better precision than traditional case-control approaches [28]. Briefly, the case-only odds ratio (COR) is the odds of an environmental exposure (E) given the presence of a genetic factor (G) divided by the odds of E given the absence of G. The COR is reversible [29], such that it is also the odds of G given the presence of E divided by the odds of G given the absence of E. Therefore, the COR is interpreted as the multiplicative interaction between G and E in causing diease [29]. An important assumption is that of independence between E and G in the general population [28]. Schmidt and Schaid demonstrated that when there is independence between the E and G in the general population, the COR is equivalent to the interaction estimation based on risk ratios in a given source population [31]. For this study assessing the association between air pollutant exposure and selected genotypes, we believed this assumption was reasonable [25].
We conducted an unconditional logistic regression to estimate the case-only ORs and 95% confidence intervals (CIs) for assessing the interaction between each chlorinated solvent and genotype on childhood M/PNET risk. We classified the genotypes for each SNP based on the number of minor alleles (i.e., 0, 1, or 2), and categorized exposure as high versus low based on the 90th percentile of the distribution of each pollutant separately. In other words, low exposure (reference) was the <90th percentile, whereas high exposure was ≥90th percentile. We did this based on the assumption that more extreme levels would be associated with higher risk [32]. In the unconditional logistic regression models, the exposure variable was the dependent variable and the genotype was the independent variable. We used Structure version 2.3.3 [33] to determine the percent ancestry from each of three ancestry subgroups (African, Amerindian, and European) among the case population [33, 34]. This information was utilized in the unconditional logistic regression models (as a continuous variable for each subgroup) to account for potential population stratification [25]. The logistic model expression for our analysis is presented in the following equation, where E is the environmental factor, G is the genetic factor, and C is the covariate for ancestry:
Because there were 88 pairwise interactions (four pollutants by 22 genotypes) examined in this study, the Benjamini and Hockberg method (false discovery rate) was used to calculate corrected P-value (i.e., Q-value) for the purpose of determining statistical significance [35]. We used Intercooled Stata, version 10.1 (StataCorp LP, College Station, TX) to conduct the statistical analyses.
RESULTS
Of the M/PNET cases, 60% had a diagnosis of medulloblastoma (n = 59), whereas 40% had a PNET diagnosis (n = 39) (Table II). The majority of the cases were male (71%, n = 70) and the mean age at diagnosis was 7.2 years. Among M/PNET cases, 46% (n = 45) were non-Hispanic White, 14% were non-Hispanic Black (n = 14), 36% were Hispanic (n = 35), and 4% reported another race/ethnicity (n = 4).
Table II.
Characteristics of Childhood Medulloblastoma and Primitive Neuroectodermal Tumor Cases
| Characteristic | Childhood M/PNETa Cases (N = 98) |
|---|---|
| Sex, no. (%) | |
| Male | 70 (71.4) |
| Female | 28 (28.6) |
| Age at Diagnosis (years), mean (SD) | 7.2 (4.8) |
| Race/ethnicity, no. (%) | |
| Non-Hispanic White | 45 (45.9) |
| Non-Hispanic Black | 14 (14.3) |
| Hispanic | 35 (35.7) |
| Other | 4 (4.1) |
| Histology, no. (%) | |
| Medulloblastoma | 59 (60.2) |
| PNET | 39 (39.8) |
Medulloblastoma and primitive neuroectodermal tumor
Based on the 1999 U.S. EPA ASPEN Model, the annual estimated mean and median levels of methylene chloride, perchloroethylene, trichloroethylene, and vinyl chloride were higher in case census tracts compared to all Texas census tracts (Table III). The 90th percentile of levels was slightly higher among case census tracts compared to all Texas census tracts for methylene chloride (0.76 µg/m3 versus 0.75 µg/m3), trichloroethylene (0.13 µg/m3 versus 0.12 µg/m3), and vinyl chloride (0.13 µg/m3 versus 0.10 µg/m3).
Table III.
Characteristics of Methylene Chloride, Perchloroethylene, Trichloroethylene, and Vinyl Chloride levels (in µg/m3) from the 1999 U.S. EPA ASPEN Model among M/PNET Case and Texas Census Tracts
| Pollutant | Cases | Texas | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 10th | 50th | 90th | Mean | SD | 10th | 50th | 90th | |
| Methylene Chloride | 0.49 | 0.24 | 0.09 | 0.51 | 0.76 | 0.44 | 0.29 | 0.09 | 0.43 | 0.75 |
| Perchloroethylene | 0.21 | 0.12 | 0.02 | 0.22 | 0.33 | 0.18 | 0.14 | 0.01 | 0.17 | 0.33 |
| Trichloroethylene | 0.09 | 0.07 | 0.05 | 0.09 | 0.13 | 0.08 | 0.03 | 0.05 | 0.08 | 0.12 |
| Vinyl Chloride | 0.08 | 0.07 | 0.0003 | 0.08 | 0.13 | 0.06 | 0.04 | 0.00006 | 0.06 | 0.10 |
Levels of methylene chloride, perchloroethylene, trichloroethylene, and vinyl chloride were significantly correlated with one another (P <0.001) (Table IV). However, some correlations were stronger (e.g. methylene chloride-trichloroethylene, ρ = 0.93) than others (perchloroethylene-vinyl chloride, ρ = 0.43). These correlations were not consistent across census tracts. For instance, the correlation between methylene chloride and trichloroethylene was not significant in those census tracts with the highest (i.e., ≥90th percentile) trichloroethylene levels (ρ = 0.36, P = 0.30).
Table IV.
Correlations Between Methylene Chloride, Perchloroethylene, Trichloroethylene, and Vinyl Chloride from the 1999 U.S. EPA ASPEN Model for Texas Census Tracts
| Methylene Chloride | Perchloroethylene | Trichloroethylene | Vinyl Chloride | |
|---|---|---|---|---|
| Methylene Chloride | 1.00 | |||
| Perchloroethylene | 0.89 | 1.00 | ||
| Trichloroethylene | 0.93 | 0.78 | 1.00 | |
| Vinyl Chloride | 0.57 | 0.43 | 0.57 | 1.00 |
P <0.001
Selected gene-environment interactions are presented in Table V. There were 11 significant interactions involving each of the chlorinated solvents (methylene chloride, n = 3; perchloroethylene, n = 1; trichloroethylene, n = 3; vinyl chloride, n = 4). However, after correcting for multiple comparisons, only one interaction remained statistically significant. Specifically, the case-only OR for those residing in census tracts with the highest estimated trichloroethylene levels (i.e., ≥90th percentile) and had the minor allele of OGG1 rs293795 was statistically significant (OR = 9.24, 95% CI: 2.24, 38.24, Q = 0.04). Additionally, two interactions had borderline significance (i.e., Q <0.10). M/PNET cases who resided in census tracts with the highest estimated trichloroethylene levels and the minor allele of OGG1 rs159150 had an increased risk (OR = 6.50, 95% CI: 1.70, 24.81, Q = 0.07), whereas the minor allele of NAT1 rs13253389 was protective among those living in census tracts with the highest estimated vinyl chloride levels (OR = 0.05, 95% CI: 0.01, 0.39, Q = 0.09).
Table V.
Significant Gene-Environment Interactions Associated with Childhood M/PNET
| Pollutant | Gene | RefSNP | ORa | 95% CI | P-value | Q-valueb |
|---|---|---|---|---|---|---|
| Methylene chloride | OGG1 | rs293795 | 4.48 | 1.35, 14.87 | 0.01 | 0.17 |
| NAT2 | rs4271002 | 4.88 | 1.36, 17.58 | 0.02 | 0.17 | |
| GSTM3 | rs7483 | 2.93 | 1.06, 8.06 | 0.03 | 0.28 | |
| Perchloroethylene | NAT2 | rs4271002 | 4.40 | 1.22, 15.91 | 0.02 | 0.23 |
| Trichloroethylene | OGG1 | rs293795 | 9.24 | 2.24, 38.24 | 0.002 | 0.04 |
| OGG1 | rs159150 | 6.50 | 1.70, 24.81 | 0.006 | 0.07 | |
| ERCC1 | rs1007616 | 0.19 | 0.05, 0.78 | 0.02 | 0.16 | |
| Vinyl chloride | NAT1 | rs13253389 | 0.05 | 0.01, 0.39 | 0.004 | 0.09 |
| CYP1B1 | rs1800440 | 4.15 | 1.17, 14.67 | 0.03 | 0.27 | |
| EPHX1 | rs1051740 | 0.17 | 0.03, 0.95 | 0.04 | 0.27 | |
| ERCC1 | rs1007616 | 0.29 | 0.08, 0.99 | 0.04 | 0.27 |
Abbreviations: CI, confidence interval; OR, odds ratio;
Adjusted for population stratification using ancestry informative markers;
P-value corrected for multiple comparisons using false discovery rate
DISCUSSION
We found a significant gene-environment interaction between OGG1 rs293795 and exposure to ambient levels of the chlorinated solvent trichloroethylene, as estimated from the 1999 U.S. EPA ASPEN model, on the risk of childhood M/PNET. This association was seen in a relatively small study population after adjusting for population stratification and multiple comparisons. Interactions between trichloroethylene and other DNA repair gene SNPs were also observed, as well as interactions involving methylene chloride, perchloroethylene, vinyl chloride, and various detoxification and DNA repair genes on the risk of childhood M/PNET, however, these associations did not remain significant after correcting for multiple comparisons.
Trichloroethylene is a volatile compound used primarily for metal degreasing. The largest source is from industrial facilities and waste sites, and environmental exposure is most likely to occur via ambient air [36]. Trichloroethylene attains high concentrations (compared to blood) in the brain, which appears to be an important organ of toxicity [14]. Furthermore, IARC has identified trichloroethylene as a probable human carcinogen [14]. According to a recent risk assessment conducted in Southeast Texas using the U.S. EPA provisional inhalation unit risk estimate for trichloroethylene (1.7 × 10−6 µg/m3) [37], ambient levels found in the census tracts with high exposure (i.e., ≥0.13 µg/m3 in this study population) would be defined as having a possible cancer risk [5].
The OGG1 gene encodes 8-oxoguanine glycosylase, which is involved in base excision repair. Specifically, the OGG1 enzyme is responsible for the excision of 8-oxoguanine, a mutagenic base byproduct, which is the result of exposure to reactive oxygen [38]. To our knowledge, OGG1 genotypes have not been assessed previously in epidemiologic studies of trichloroethylene exposure. However, trichloroethylene and its metabolites have been shown to result in the formation of reactive oxygen species [39], which lead to the formation of 8-oxoguanine–DNA adducts and corresponding cytotoxic effects [38, 39]. Therefore, it is possible that trichloroethylene exposure is modified by OGG1 genotypes. OGG1 rs293795 is an intronic (non-coding) SNP and is not in a repeat region [40]. Although OGG1 rs293795 has not been implicated in childhood brain tumor risk, this SNP does appear to be associated with several other tumors including breast [41] and prostate cancer [42]. As in our study, the minor allele of OGG1 rs293795 (A>G) conferred risk for both breast and prostate cancer.
There is limited epidemiologic evidence for the association between trichloroethylene exposure and childhood M/PNET risk. Some parental occupational studies have indicated an association between solvent exposure and childhood brain tumor risk [13, 43]. For instance, fathers employed in motor vehicle-related occupations, where exposure to trichloroethylene is possible, were at a greater risk of having children with PNET (OR = 2.70, 95% CI: 1.11, 6.60) [44]. Additionally, mothers employed in professions where solvent exposure is likely to occur (electronic parts and components manufacturing; textile and garment workers) were at a greater risk of having children with brain tumors (OR = 13.78, 95% CI: 1.47, 129.05 and OR = 7.25, 95% CI: 1.42, 37.03; respectively) [43]. Whereas other parental occupational studies have found little evidence of an association between solvent exposure and childhood brain tumor risk (reviewed in [45]). There have been a few studies assessing environmental exposures to solvents and M/PNET risk. In one study assessing parental hobbies, there was suggestive evidence of an association between solvent-related exposures and M/PNET risk (OR = 1.4, 95% CI: 0.8, 2.6) [3]. Equivocal findings in previous studies of trichloroethylene (and other solvent exposures) and M/PNET risk may be due to differences in exposure assessment, when exposure was assessed (e.g., during pregnancy or at diagnosis), heterogeneous and small case groups, as well as not accounting for genotypes relevant to the metabolism of these toxicants.
The two major limitations of this study are the small sample size (98 cases) and the single ecological assessment of chlorinated solvents based on 1999 ASPEN estimates. Due to the small sample size, this was primarily an exploratory analysis in which we were only able to detect strong effects. However, we did identify a significant interaction (even after adjusting for multiple comparisons) and potential candidate gene-environment interactions for investigation in larger studies of childhood M/PNET (a relatively rare condition). Regarding the exposure assessment, ASPEN data were not available for the entire study period; therefore we used 1999 as a surrogate for other years. Although this may result in exposure misclassification as levels are likely to change over time, we used a relative ranking of exposure (i.e., high versus low), therefore census tracts with high levels of chlorinated solvents in one year are likely to remain in the “high” category relative to census tracts with lower levels as the sources of chlorinated solvents (e.g., industrial facilities and waste sites) were unlikely to change during the study period [5, 19]. Furthermore, as there are no other sources of population-scale individual-level measurements of HAPs, ASPEN provides a cost-effective and exploratory method for evaluating the association between HAPs and adverse outcomes. Therefore, the ASPEN model has been used in previous studies of childhood cancer [46], birth defects [32], and autism [47], even when only one year of ASPEN data is available across multiple study years.
A second issue related to the exposure assessment is the use of address at diagnosis. This could introduce exposure misclassification if there is a great deal of residential mobility between the critical window of exposure and diagnosis. There have been no assessments of the impact of residential mobility on exposure assignment for childhood M/PNET. However, our own research on the impact of residential mobility during pregnancy (i.e., the period between preconception and delivery) indicated that maternal residential movement in Texas is generally within short distances, is typically not different between cases and controls, and does not significantly influence exposure assessment using ASPEN data [48]. Additionally, a study in California suggested changes in residence between delivery and diagnosis did not change urban/rural status for most children (>80%) with leukemia [49], an important predictor of exposure to HAPs [5, 8, 9, 48].
Another limitation is the inability to tease apart the effects of multiple chlorinated solvents on disease risk. This is especially true since levels of methylene chloride, perchloroethylene, trichloroethylene, and vinyl chloride were significantly correlated with one another across all census tracts. However, these correlations were not as strong in census tracts with the highest exposure level. For instance, levels of trichloroethylene were neither strongly nor significantly associated with levels of methylene chloride (ρ = 0.36, P = 0.30), perchloroethylene (ρ = 0.04, P = 0.92), or vinyl chloride (ρ = 0.40, P = 0.26) in census tracts where trichloroethylene levels were ≥90th percentile. Therefore, our results for trichloroethylene at the highest exposure level do appear to be distinct from any potential confounding effects by the other related chlorinated solvents.
A general limitation to this and other case-only studies is the inability to evaluate the independent effects of the exposures or genotypes in question [27]. However, we selected exposures and genotypes based on previously reported associations [36, 45]. Another limitation with the case-only design involves the assumption of independence between the environmental exposure and genotype in the general population [28]. As stated, for these and similar exposures (e.g., air pollution), we believed this assumption was reasonable [25].
Strengths of this study include a well-characterized population of M/PNET cases. We were also able to account for genotypes related to the metabolism of chlorinated solvents [38]. Furthermore, we used a tagSNP approach in order to reduce the potential number of comparisons [23]. Finally, as recent evidence suggests case-only studies are subject to the same concerns related to population stratification as case-control studies [25], we used 212 ancestry informative markers to account for population structure [26].
This study provides an initial assessment of the interaction between ambient levels of chlorinated solvents and potentially relevant genotypes on childhood M/PNET risk. Our analyses suggest an interaction between ambient levels of trichloroethylene and OGG1 rs293795 is associated with the risk of childhood M/PNET. We believe future investigations should include additional measures of exposure (e.g., air pollutant monitoring and biomarker data), larger sample sizes to determine moderate and modest interactions, as well as the inclusion of additional pollutants (e.g., heavy metals) and genotypes.
Supplementary Material
ACKNOWLEDGEMENTS
The genotyping for this work was supported by a Developmental Research Project (to M.E.S.) from the MD Anderson Brain and Spine Center and Brain SPORE, PI: Yung; P50CA127001. M.E.S. was also supported in part by an NCI Career Development Award, K07CA131505. The authors would also like to thank the families who participated in this study.
REFERENCES
- 1.Linabery AM, Ross JA. Trends in childhood cancer incidence in the US, (1992–2004) Cancer. 2008;112:416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 2.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 3.Rosso AL, Hovinga ME, Rorke-Adams LB, et al. A case-control study of childhood brain tumors and fathers' hobbies: a Children's Oncology Group study. Cancer Causes Control. 2008;19:1201–1207. doi: 10.1007/s10552-008-9189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US. EPA. About Air Toxics. [Accessed January 10, 2012]; http://www.epa.gov/ttn/atw/allabout.html. Published August 17, 2010.
- 5.Sexton K, Linder SH, Marko D, et al. Comparative assessment of air pollution-related health risks in Houston. Environ Health Perspect. 2007;115:1388–1393. doi: 10.1289/ehp.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US. EPA. Air Pollution Monitoring. [Accessed January 10, 2012]; http://epa.gov/airquality/montring.html. Published November 4, 2011.
- 7.Rosenbaum AS, Axelrad DA, Woodruff TJ, et al. National estimates of outdoor air toxics concentrations. J Air Waste Manag Assoc. 1999;49:1138–1152. doi: 10.1080/10473289.1999.10463919. [DOI] [PubMed] [Google Scholar]
- 8.Lupo PJ, Symanski E. A comparative analysis of modeled and monitored ambient hazardous air pollutants in Texas: a novel approach using concordance correlation. J Air Waste Manag Assoc. 2009;59:1278–1286. doi: 10.3155/1047-3289.59.11.1278. [DOI] [PubMed] [Google Scholar]
- 9.Payne-Sturges DC, Burke TA, Breysse P, et al. Personal exposure meets risk assessment: a comparison of measured and modeled exposures and risks in an urban community. Environ Health Perspect. 2004;112:589–598. doi: 10.1289/ehp.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heineman EF, Cocco P, Gomez MR, et al. Occupational exposure to chlorinated aliphatic hydrocarbons and risk of astrocytic brain cancer. Am J Ind Med. 1994;26:155–169. doi: 10.1002/ajim.4700260203. [DOI] [PubMed] [Google Scholar]
- 11.Yu CL, Wang SF, Pan PC, et al. No association between residential exposure to petrochemicals and brain tumor risk. Cancer Epidemiol Biomarkers Prev. 2005;14:3007–3009. doi: 10.1158/1055-9965.EPI-05-0594. [DOI] [PubMed] [Google Scholar]
- 12.Pan BJ, Hong YJ, Chang GC, et al. Excess cancer mortality among children and adolescents in residential districts polluted by petrochemical manufacturing plants in Taiwan. J Toxicol Environ Health. 1994;43:117–129. doi: 10.1080/15287399409531908. [DOI] [PubMed] [Google Scholar]
- 13.Peters FM, Preston-Martin S, Yu MC. Brain tumors in children and occupational exposure of parents. Science. 1981;213:235–237. doi: 10.1126/science.7244631. [DOI] [PubMed] [Google Scholar]
- 14.IARC Monographs on the evaluation of carcinogenic risks to humans. Vol. 63. Lyon, France: 1995.. International Agency for Research on Cancer; p. 551. [Google Scholar]
- 15.IARC Monographs on the evaluation of carcinogenic risks to humans. Vol. 71. Lyon, France: 1999. International Agency for Research on Cancer; p. 1050. [Google Scholar]
- 16.IARC Monographs on the evaluation of carcinogenic risks to humans. Vol. 97. Lyon, France: 2008. International Agency for Research on Cancer; p. 519. [Google Scholar]
- 17.Casarett L, Doull J. Casarett & Doull's Toxicology: The Basic Science of Poisons. Sixth ed. McGraw-Hill; 2001. p. 1236. [Google Scholar]
- 18.Murphy PJ. Xenobiotic metabolism: a look from the past to the future. Drug Metab Dispos. 2001;29:779–780. [PubMed] [Google Scholar]
- 19.US. EPA. 1999 National-Scale Air Toxics Assessment: 1999 Data Tables. [Accessed January 10, 2012]; http://www.epa.gov/ttn/atw/nata1999/index.html. Published April 15, 2010.
- 20.US. EPA. 1999 National-Scale Air Toxics Assessment. [Accessed January 10, 2012]; http://www.epa.gov/ttn/atw/nata1999/. Published April 15, 2010.
- 21.US. EPA. National Air Toxics Assessments. [Accessed January 9, 2012]; http://www.epa.gov/ttn/atw/natamain/index.html. Published October 27, 2011.
- 22.Qiu YL, Wang W, Wang T, et al. DNA repair gene polymorphisms and micronucleus frequencies in Chinese workers exposed to vinyl chloride monomer. Int J Hyg Environ Health. 2011;214:225–230. doi: 10.1016/j.ijheh.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Carlson CS, Eberle MA, Rieder MJ, et al. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institut Català d'Oncologia. SNPStats. [Accessed August 12, 2011]; http://bioinfo.iconcologia.net/snpstats/start.htm. Published 2006. [Google Scholar]
- 25.Wang LY, Lee WC. Population stratification bias in the case-only study for gene-environment interactions. Am J Epidemiol. 2008;168:197–201. doi: 10.1093/aje/kwn130. [DOI] [PubMed] [Google Scholar]
- 26.Tian C, Hinds DA, Shigeta R, et al. A genomewide single-nucleotide-polymorphism panel for Mexican American admixture mapping. Am J Hum Genet. 2007;80:1014–1023. doi: 10.1086/513522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoury MJ, Flanders WD. Nontraditional epidemiologic approaches in the analysis of gene-environment interaction: case-control studies with no controls! Am J Epidemiol. 1996;144:207–213. doi: 10.1093/oxfordjournals.aje.a008915. [DOI] [PubMed] [Google Scholar]
- 28.Piegorsch WW, Weinberg CR, Taylor JA. Non-hierarchical logistic models and case-only designs for assessing susceptibility in population-based case-control studies. Stat Med. 1994;13:153–162. doi: 10.1002/sim.4780130206. [DOI] [PubMed] [Google Scholar]
- 29.Gatto NM, Campbell UB, Rundle AG, et al. Further development of the case-only design for assessing gene-environment interaction: evaluation of and adjustment for bias. Int J Epidemiol. 2004;33:1014–1024. doi: 10.1093/ije/dyh306. [DOI] [PubMed] [Google Scholar]
- 30.Dennis J, Krewski D, Cote FS, et al. Breast cancer risk in relation to alcohol consumption and BRCA gene mutations--a case-only study of gene-environment interaction. Breast J. 2011;17:477–484. doi: 10.1111/j.1524-4741.2011.01133.x. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt S, Schaid DJ. Potential misinterpretation of the case-only study to assess gene-environment interaction. Am J Epidemiol. 1999;150:878–885. doi: 10.1093/oxfordjournals.aje.a010093. [DOI] [PubMed] [Google Scholar]
- 32.Lupo PJ, Symanski E, Waller DK, et al. Maternal exposure to ambient levels of benzene and neural tube defects among offspring: Texas: 1999-2004. Environ Health Perspect. 2011;119:397–402. doi: 10.1289/ehp.1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falush D, Hubisz M, Stephens M, et al. Structure. [Accessed August 2, 2011]; http://pritch.bsd.uchicago.edu/structure.html. Published 2011. [Google Scholar]
- 34.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes. 2007;7:574–578. doi: 10.1111/j.1471-8286.2007.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the False Discovey Rate: a Practical and Powerful Approach to Multiple Testing. J. R. Statist. Soc. B. 1995;57:289–300. [Google Scholar]
- 36.Agency for Toxic Substances and Disease Registry, Toxicological Profile for Trichloroethylene. 1997:335. [PubMed] [Google Scholar]
- 37.US. EPA. Trichloroethylene. [Accessed August 8, 2011]; http://www.epa.gov/ttn/atw/hlthef/tri-ethy.html. Published November 6, 2007.
- 38.Boiteux S, Radicella JP. The human OGG1 gene: structure, functions, and its implication in the process of carcinogenesis. Arch Biochem Biophys. 2000;377:1–8. doi: 10.1006/abbi.2000.1773. [DOI] [PubMed] [Google Scholar]
- 39.Youn CK, Song PI, Kim MH, et al. Human 8-oxoguanine DNA glycosylase suppresses the oxidative stress induced apoptosis through a p53-mediated signaling pathway in human fibroblasts. Mol Cancer Res. 2007;5:1083–1098. doi: 10.1158/1541-7786.MCR-06-0432. [DOI] [PubMed] [Google Scholar]
- 40.Genome Variation Server. GVS: Genome Variation Server, version 5.11. [Accessed September 9, 2011]; http://gvs.gs.washington.edu/GVS/index.jsp. Published February 26, 2010. [Google Scholar]
- 41.Rossner P, Jr, Terry MB, Gammon MD, et al. OGG1 polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:811–815. doi: 10.1158/1055-9965.EPI-05-0659. [DOI] [PubMed] [Google Scholar]
- 42.Lindstrom S, Zheng SL, Wiklund F, et al. Systematic replication study of reported genetic associations in prostate cancer: Strong support for genetic variation in the androgen pathway. Prostate. 2006;66:1729–1743. doi: 10.1002/pros.20489. [DOI] [PubMed] [Google Scholar]
- 43.Ali R, Yu CL, Wu MT, et al. A case-control study of parental occupation, leukemia, and brain tumors in an industrial city in Taiwan. J Occup Environ Med. 2004;46:985–992. doi: 10.1097/01.jom.0000138913.75380.13. [DOI] [PubMed] [Google Scholar]
- 44.Cordier S, Lefeuvre B, Filippini G, et al. Parental occupation, occupational exposure to solvents and polycyclic aromatic hydrocarbons and risk of childhood brain tumors (Italy, France, Spain) Cancer Causes Control. 1997;8:688–697. doi: 10.1023/a:1018419118841. [DOI] [PubMed] [Google Scholar]
- 45.Baldwin RT, Preston-Martin S. Epidemiology of brain tumors in childhood--a review. Toxicol Appl Pharmacol. 2004;199:118–131. doi: 10.1016/j.taap.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds P, Von Behren J, Gunier RB, et al. Childhood cancer incidence rates and hazardous air pollutants in California: an exploratory analysis. Environ Health Perspect. 2003;111:663–668. doi: 10.1289/ehp.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Windham GC, Zhang L, Gunier R, et al. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the san francisco bay area. Environ Health Perspect. 2006;114:1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lupo PJ, Symanski E, Chan W, et al. Differences in exposure assignment between conception and delivery: the impact of maternal mobility. Paediatr Perinat Epidemiol. 2010;24:200–208. doi: 10.1111/j.1365-3016.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 49.Urayama KY, Von Behren J, Reynolds P, et al. Factors associated with residential mobility in children with leukemia: implications for assigning exposures. Ann Epidemiol. 2009;19:834–840. doi: 10.1016/j.annepidem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.