SUMMARY
While insomnia is a well-established risk factor for the initial onset, recurrence or relapse of affective disorders, the specific characteristics of insomnia that confer risk remain unclear. Insomnia patients with an evening chronotype may be one particularly high-risk group, perhaps due to alterations in positive affect and its related affective circuitry. We explored this possibility by comparing diurnal patterns of positive affect and the activity of positive affect-related brain regions in morning- and evening-types with insomnia. We assessed diurnal variation in brain activity via the relative regional cerebral metabolic rate of glucose uptake by using [18F]fluorodeoxyglucose positron emission tomography during morning and evening wakefulness. We focused on regions in the medial prefrontal cortex and striatum, which have been consistently linked with positive affect and reward processing. As predicted, chronotypes differed in their daily patterns in both self-reported positive affect and associated brain regions. Evening-types displayed diurnal patterns of positive affect characterized by phase delay and smaller amplitude compared to those of morning-types with insomnia. In parallel, evening-types showed a reduced degree of diurnal variation in the metabolism of both the medial prefrontal cortex and the striatum, as well as lower overall metabolism in these regions across both morning and evening wakefulness. Taken together, these preliminary findings suggest that alterations in the diurnal activity of positive affect-related neural structures may underlie differences in the phase and amplitude of self-reported positive affect between morning and evening chronotypes, and may constitute one mechanism for increased risk of mood disorders among evening-type insomniacs.
Keywords: insomnia, chronotype, positive affect, diurnal variation, brain metabolism, position emission tomography
INTRODUCTION
Substantial evidence suggests that the pathophysiologies of insomnia and affective disorders are intertwined. Insomnia symptoms confer an increased risk of initially developing an affective disorder (Baglioni et al., 2011), persist in the wake of remission from the affective disturbance (Carney et al., 2007), and are risk factors for recurrence or relapse (Perlis et al., 1997). Nevertheless, it remains unclear whether certain subtypes of insomnia are associated with the greatest risk for affective disorders. For some vulnerable individuals, hyperarousal may be both a central feature of insomnia and a chronic stressor that eventually leads to depression (Riemann et al., 2010); however, other pathways from insomnia to depression are also plausible. For instance, several lines of evidence suggest that insomnia patients with an evening chronotype may represent one particularly high-risk group.
Chronotype, or morningness-eveningness, may moderate the risk of mood disorders in insomnia patients. Chronotypes reflect individual differences in both the circadian clock and the homeostatic sleep drive (Mongrain et al., 2006). The evening chronotype carries negative consequences for sleep. Eveningness is not only associated with later sleep timing, but also worse sleep quality, shorter sleep duration, greater daytime sleepiness, and more irregular sleep/wake schedules (e.g.,(Taillard et al., 1999, Giannotti et al., 2002)), including larger weekend-weekday differences in sleep timing (Randler, 2008, Wittmann et al., 2006). Thus, evening types may be more susceptible than morning types to “social jet lag,” a mismatch between biological timing and the timing of sociocultural demands such as school and work (Wittmann et al., 2006).
Eveningness has also been consistently linked to affective disturbance, both in unipolar depression (Drennan et al., 1991, Hasler et al., 2010a), and in bipolar disorder and subclinical mania (Mansour et al., 2005, Soehner et al., 2007). Evening-type insomniacs report greater depressive symptoms than morning- or intermediate-types (Ong et al., 2007), as well as more total sleep time, more time in bed, more variable out-of-bed times, and more dysfunctional sleep-related cognitions. The elevated depression among evening-type insomniacs remains statistically significant even after controlling for total sleep time, suggesting the depression is not solely due to the severity of their insomnia. Thus, eveningness may be an under-recognized risk factor for the development of affective disorders among individuals with insomnia.
The association between affective disorders and eveningness may be mediated by positive affect (PA) and reward function. Circadian rhythms appear to modulate PA and reward processing, as well as their underlying neural systems (Hasler et al., 2010a, Boivin et al., 1997, McClung, 2007, Murray et al., 2009, Webb et al., 2009). Positive affect (but not negative affect) and reward motivation show 24-hour cyclicity, and are modulated by both circadian phase and homeostatic sleep drive (Murray et al., 2009, Boivin et al., 1997). Thus, chronotype should predict timing differences in PA rhythms, and limited evidence suggests this is the case (Porto et al., 2006). However, chronotype appears to influence more than phase differences. For instance, the association between eveningness and depression may be mediated through lower mean PA and reduced reward responsiveness (Hasler et al., 2010a). Depression is characterized not simply by lower overall PA, but also by blunted rhythms in PA and various physiological rhythms (Murray, 2007, Souetre et al., 1989). Taken together, this suggests evening-types with depression have relatively delayed and blunted PA rhythms.
We used a cross-method, cross-domain approach to explore chronotype differences in sleep and affective functioning in a sample of adults with primary insomnia. In particular, we focused on diurnal patterns of self-reported PA and the activity of PA-related brain areas in morning- and evening-types with insomnia. As in previous studies by our group (Buysse et al., 2004, Germain et al., 2007), diurnal variation in brain activity was assessed via relative regional cerebral metabolic rate of glucose uptake (rCMRglu) by using [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) during morning and evening wakefulness. We focused on rCMRglu in the medial prefrontal cortex (mPFC) and striatum, as these two regions figure prominently in theoretical models of PA and reward processing (Forbes et al., 2010, Haber and Knutson, 2010)) and show evidence of modulation by sleep and circadian factors (e.g., (Forbes et al., in press, Holm et al., 2009). According to these models, observed PA and reward function are orchestrated through the relative balance between cortical (mPFC) and subcortical (striatum) elements of the reward circuit, with the mPFC providing top-down modulation of the striatum.
Based on the well-established chronotype and sleep literature, we hypothesized that evening-type insomniacs would report more sleep disturbance and more variable sleep/wake timing than morning-type insomniacs. Given the more nascent literature on chronotype differences in affect and brain metabolism, we made general predictions in the service of exploratory hypothesis-generating analyses to set the stage for more refined hypotheses in future work. Specifically, we predicted that compared to morning-types, evening-types would have reduced mean levels of self-reported PA, and diurnal patterns of PA characterized by reduced amplitude and delayed phase; and compared to morning-types, evening-types would demonstrate reduced diurnal variation in rCMRglu in PA-related neural structures and reduced rCMRglu in these same regions.
METHODS
Participants
These data come from an ongoing study designed to examine mood, arousal, and pharmacologic treatment response in individuals with Primary Insomnia (PI) and good sleeper controls (MH24652). This study was approved by the University of Pittsburgh, Institutional Review Board and the Human Use Subcommittee of the Radioactive Drug Safety Committee, and all subjects provided informed consent.
One hundred participants with PI (M age = 36.7, range = 21–51 years), including 55 women, were included in the full sample. A subsample of 27 participants with FDG-PET data (M age = 37.8, range = 22–51 years), including 15 women, were included in the PET analyses. Study participants had to meet DSM-IV criteria for current PI and were required to have a mean wake after sleep onset (WASO) value of ≥30 minutes and sleep efficiency (SE) ≤85% on their screening sleep diary. Participants with concurrent sleep onset insomnia (mean sleep latency [SL] ≥30 minutes) were not excluded. Exclusion criteria included significant or unstable medical conditions; current major syndromal mood, anxiety, psychotic, or substance use disorder; current sleep disorder (other than PI) as defined by an apnea–hypopnea index >15 or periodic limb movement (PLM) arousal index >15 on one night of screening polysomnography; use of medications or substances known to affect sleep; coffee consumption (or equivalent) of >4 cups/24 h; and alcohol consumption of >14 drinks per week. Ten participants had past histories of major depression but were in remission for a minimum of 6 months.
Study design
The current study hypotheses were tested using a combination of laboratory assessment and ecological momentary assessment (EMA) methods, a technique well-suited to investigating dynamic processes in a naturalistic, ecologically valid setting. Participants were assessed on a variety of self-report measures under naturalistic conditions for 4–15 days. Self-reported affect was assessed four times per day: upon awakening, at noon, at 5 p.m., and upon retiring to bed at night. Sleep was assessed with sleep diaries. Following the EMA protocol, participants underwent brain magnetic resonance (MR) imaging studies prior to the PET assessments. The PET scans were scheduled within a 3-night sleep study at the University of Pittsburgh Neuroscience Clinical & Translational Research Center (N-CTRC). An initial polysomnographic (PSG) screening study was conducted at participants' usual sleep times to rule out sleep disorders and to serve as an accommodation night. (PSG data not reported here.) A second night of sleep studies for baseline sleep measures was conducted the following night. On the third morning, 2 to 4 hours after awakening, participants underwent the morning waking [18F]FDG PET study. On the evening of the third night, participants completed the evening waking [18F]FDG PET study at their habitual bedtime. Participants were compensated for their time. Data were collected between March 2002 and May 2010 (PET scans were completed between March 2006 and May 2010).
Baseline measures of chronotype, sleep quality, and depression
Composite Scale of Morningness (CSM; (Smith et al., 1989)
This 13-item self-report measure assesses chronotype (i.e., morningness-eveningness) using a Likert-type response format. The score is obtained by the sum of the items and ranges from 13 (extreme eveningness) to 55 (extreme morningness). The scale has acceptable internal consistency (Cronbach's alpha = 0.83; (Smith et al., 1989)) and good test-retest reliability and predictive validity (Guthrie et al., 1995). In order to maximize the group sizes for the subsample with PET data, we used a median split of the CSM data for the full sample (median = 34) to divide the group into evening-types (score = 13–33) and morning-types (34–55). Notably, the median CSM score coincided with the midpoint of the CSM's scoring range. For the sake of consistency, we applied this same categorization approach to the full sample for the PA analyses.
Pittsburgh Sleep Quality Index (PSQI; (Buysse et al., 1989))
The PSQI is a 19-item self-rated questionnaire for evaluating subjective sleep quality over the previous month. The 19 questions are combined into seven clinically derived component scores, each weighted equally from 0 to 3. The seven component scores are added to obtain a global score ranging from 0 to 21, with higher scores indicating worse sleep quality.
Inventory of Depressive Symptomatology - Self Report (Rush et al., 1986))
The IDS-SR is a 30-item self-report questionnaire for assessing the severity of depressive symptoms over the past 7 days. The content of the IDS is designed to assess all the criterion symptom domains of a DSM-IV diagnosis of a major depressive episode.
Repeated EMA measures
The Positive and Negative Affect Schedule (PANAS; (Watson et al., 1988))
Affect was assessed four times per day using the PANAS, a 20-item self-report scale that taps two broad dimensions: positive affect (PA) and negative affect (NA). The PA dimension taps the extent to which a person feels generally enthusiastic, active and alert and is anchored by high energy, full concentration, and pleasurable engagement on the one end and by lethargy and sadness on the other. The NA dimension taps the extent to which a person feels general subjective distress and unpleasurable engagement and is anchored on the one end by a variety of aversive mood states including anger, contempt, disgust, guilt, fear, and nervousness and anchored on the other end by a state of calmness and serenity (Watson et al., 1988). The PANAS demonstrates internal consistency reliability coefficients above 0.85 (Watson et al., 1988). This version of the scale asks subjects to report how they feel “right now”.
Pittsburgh Sleep Diary (Monk et al., 1994)
The Pittsburgh Sleep Diary is a daily self-report measure including items on bed time, lights out (the time participants close their eyes with the intention of falling asleep, sleep onset, sleep offset, sleep latency (SL), wakefulness after sleep onset (WASO), number of awakenings, and sleep quality (visual analog scale). Participants were instructed to complete these items each morning as soon as possible after rising.
Magnetic Resonance and PET Study Procedures
All participants received a brain magnetic resonance (MR) scan prior to PET studies to screen for pathology and to provide a high-resolution anatomical image for co-registration with PET scan images. Magnetic resonance imaging was performed at the Pittsburgh MR Research Center initially using a GE 1.5T Signa scanner, and then switching to a Siemens 3T Trio when the facilities were upgraded. Participants were positioned in a standard head coil and a brief scout T1-weighted image obtained. The following axial series oriented to the plane connecting the anterior and posterior commissures (AC-PC line) was acquired to screen participants for unexpected pathology: fast spin-echo T2-weighted (effTE/TR = 102/2500; 3T:104/4660), proton density weighted images (effTE/TR = 17/2000; 3T:23/4050) and fast fluid-attenuated inversion recovery (FLAIR) (effTE/TR/TI = 56/9002/2200; 3T:90/9160/2500). A field-of-view of 24 cm, image matrix of 256 × 192 pixels, 1 NEX, and 5mm section thickness/1mm inter-section gap was used for all axial MR series. A volumetric SPGR (MPRAGE on 3T) sequence with parameters optimized for maximal contrast among gray matter, white matter, and CSF was acquired in the coronal (sagittal for 3T) plane (1.5T/3T: TE = 5/2.98, TR = 25/23, flip angle = 40/9 degrees, NEX = 1, slice thickness = 1.5/1.2mm, 0mm interslice). Slices with 0 mm gap generated a 256×256×192 (3T: 256×240×160) matrix with a 24×18cm (3T: 24×25.6cm) FOV. This high-resolution anatomical sequence was for MR-guided ROI placement. MR data was transferred to the PET Facility over the electronic network and registered with PET data using Automated Image Registration (AIR).
At the start of each PET study (morning and evening), two intravenous catheters were placed, one in each arm, with normal saline infused at the minimal rate to keep the vein open. One catheter was used for injection of the radioligand, and the other was used to sample glucose and radioactivity. Participants were asked to lie supine with eyes closed, and wakefulness was continuously monitored with PSG by study personnel, per procedures originally described in (Nofzinger et al., 1998). After 20 minutes of EEG-monitored wakefulness, an intravenous bolus of [18F]-FDG (M dose = 6.52 ± 1.66 mCi) was injected via one of the indwelling catheters. Participants continued to lay quietly with EEG-monitored wakefulness for the 20-minute uptake period and then transported to the PET Center for a 10–15 minute transmission scan and a 60-minute emission scan. Six venous samples (one ml each) were drawn for radioactivity at 45, 55, 65, 75, 85 and 90 minutes following injection, and plasma glucose was assayed from the first and last samples.
PET studies were conducted at the University of Pittsburgh PET Facility on a Siemens/CTI ECAT HR+ PET scanner with a Neuro-insert (CTI PET Systems, Knoxville, TN) in 3D mode. This tomograph acquires 63 transaxial planes (2.4-mm thick) and has a final reconstructed in-plane resolution of approximately 6 mm full-width at half-maximum (FWHM). Data were acquired as subjects lie in the scanner with eyes closed. Subjects were positioned in the scanner parallel to the canthomeatal line such that the cerebellum is within the central 7 cm of the axial field of view.
Data Analysis
Analysis of clinical data
Two-tailed t-tests and one-way MANOVAs were to examine group differences in demographics, sleep, and affective functioning measures.
Analysis of PA data
A cosinor analysis variant of multilevel modeling (MLM) was employed to test for the presence of 24-hour diurnal rhythms in PA, as well as their moderation by chronotype. MLM can model periodic trends directly within-person (as compared to only aggregated across individuals), thereby providing a conceptually more adequate and statistically more powerful approach than the cosinor analysis techniques traditionally used to study circadian rhythms (e.g., (Yehuda et al., 1996)). In this special case of MLM, the presence of a 24-hour diurnal pattern is determined by a significant fit of the data to a sinusoidal curve, or sine wave, with a period of 24 hours. An equation representing a sinusoidal curve (i.e., the cosinor model) is used at Level-1 (Ching et al., 2006). The simplest form of this equation is f(t) = c0 + c1sin(2πt/P), with time (t) and period (P). The periodic function has its baseline value c0 at the origin (t = 0), rises to c0 + c1 at t = P/4, drops to c0 − c1 at t = 3P/4, and returns to baseline at t = P. This captures the periodic effect if the phase begins at t = 0. To allow a phase shift, a cosine term is included as regressor, resulting in f(t) = c0 + c1sin(2πt/P) + c2cos(2πt/P).
To analyze the 24-hour harmonic amplitude and phase, we fit a multilevel cosinor model with 24-hour period, fixed effects for chronotype, and random effects for individual subjects within chronotype. A fixed effect for age was included to control for possible confounding by age. The model was fit through maximum likelihood estimation in SAS. Wald-based inference for mean phases and amplitudes among E-types and M-types and for the differences in the mean phases and amplitudes between E-types and M-types were computed via an application of the delta method (Mikulich et al., 2003).
Analysis of neuroimaging data
The high-resolution structural MR images were aligned along the AC-PC line and medial longitudinal fissure using Statistical Parametric Mapping version 8 (http://www.fil.ion/ucl/ac/uk/spm/software/spm8/). They were then normalized to the ICBM 152 template (Montreal Neurological Institute) by means of the unified segmentation technique (Ashburner and Friston, 2005). The FDG images used were aligned and averaged over 60 to 90 minutes post-injection (six 5-minute frames) via established methods (Woods et al., 1993). The summed FDG images were co-registered to their corresponding structural MR and the previously obtained transformation parameters applied. The normalized FDG images were smoothed using a 10 mm FWHM Gaussian filter.
Analyses at the voxel-level were performed in SPM8. A full factorial model was used to test if there were the interaction effects between subject type (morning-type or evening type) and time of day (AM or PM) on glucose metabolism (measured as rCMRglc) in the two a priori regions of interest. Significant interactions were then decomposed using post-hoc analyses to compare the effects of group within time of day, and effects of time of day within each group. Age was used as a covariate in each model. Regression models were used to explore possible correlations between self-reported PA and rCMRglc, controlling for age. These analyses were restricted to the bedtime PA rating and evening PET scan in order to maximize variability in the main predictor (PA).
Given the focus of study, analyses were restricted to voxels within specific PA-related brain regions, rather than whole brain. Brain masks were constructed to represent these regions. The striatal region of interest (ROI) was anatomically-defined using the nucleus accumbens, caudate head, and putamen regions from WFU PickAtlas (v3.0.3). The mPFC ROI was constructed using the PickAtlas and defined as a 5,393-voxel sphere including medial Brodmann Area (BA) 10 and BA32. Because we had a priori hypotheses regarding specific ROIs, we used a less conservative statistical threshold—peak voxel-level significance level of p < 0.01 (uncorrected) and cluster size of greater than or equal to15 contiguous voxels—to identify loci of potential statistical significance and spatial extent within the resultant maximum intensity projections. We also corrected for multiple comparisons at the cluster level via Monte Carlo simulations conducted in the AlphaSim program within AFNI (http://afni.nih.gov/afni/docpdf/AlphaSim.pdf). Clusters meeting the minimum cluster size to achieve a cluster-level p<0.05 are noted in the text and marked in tables with an asterisk (*).
For the purpose of region localization, SPM8 results were converted to Talairach coordinates ([x y z]) using GingerALE, Version 2.0.4 (http://brainmap.org/ale/) and then localized using Talairach Client, Version 2.4.2 (http://www.talairach.org/client.html). Otherwise, data are reported using MNI coordinates.
Standardization of PA and PET timing
In order to compare the timing of aspects of the PA rhythms and PET scans across chronotype groups, we needed a way to account for individual differences in circadian and homeostatic timing. In the absence of a physiological circadian (e.g., melatonin) or homeostatic (e.g., slow wave activity) marker, we selected the diary-based mean sleep offset to serve as a proxy for circadian phase, referencing circadian time 0 (CT0) per convention.
RESULTS
Sample characteristics
The demographics and affective functioning for the sample are shown in Table 1. Consistent with other studies (Paine et al., 2006, Carrier et al., 1997), morning-types were significantly older than evening-types. Sex breakdowns were essentially the same across the two groups. The mean CSM scores significantly differed between the two groups, in accordance with the categorization approach. Although a median split on CSM was used for our analyses, the conventional scoring approach (Natale and Alzani, 2001) for the CSM yielded 22 morning-types, 20 evening-types, and 58 intermediate-types. Finally, seven of the 10 participants with past histories of depression were evening-types.
Table 1.
Sample demographics and affective characteristics
| Morning-types (n = 49) | Evening-types (n = 51) | t | p | |
|---|---|---|---|---|
| Age | 39.22 ± 8.82 | 34.34 ± 8.12 | 2.88 | 0.005 |
| Sex (m/f) | 22/27 | 23/28 | ||
| CSM | 41.18 ± 4.21 | 26.90 ± 4.63 | 16.12 | 0.000 |
| Positive affect (mean) | 2.39 ± 0.58 | 2.17 ± 0.61 | 1.79 | 0.08 |
| Negative affect (mean) | 1.31 ± 0.40 | 1.25 ± 0.30 | 0.88 | ns |
| IDS-SR | 17.20 ± 7.71 | 17.10 ± 6.38 | −0.039 | ns |
| IDS-SR (no sleep items) | 11.98 ± 7.13 | 11.24 ± 5.83 | 0.57 | ns |
Note: Values are mean ± SD unless otherwise stated
EMA data parameters
The final dataset included 1–15 days (median 7 days) of PA data per participant, resulting in a range of 6–51 assessments (mean 26 assessments) per participant. A majority (84%) of the sample had data for 3 or more assessments per day, and 30% had all 4 data points every day. The diary dataset was based on 4–7 diary entries per participant (median 7 entries), and 70.1% of the sample had data for all 7 days.
Timing of PET scans
To reiterate, morning scans were scheduled 2–4 hours after habitual wake-up time, and evening scans were scheduled at habitual bedtime. On average, the morning-types' morning PET scans (based on injection time) occurred at 8:31 (±0:46), significantly earlier than the evening-types' morning scans (9:19 ± 0:46; t = −2.65, p = 0.01). Mean scan times are shown in Figure 1. No chronotype differences were found between the clock times of the evening scans (22:54±0:55 and 23:22±0:46 for morning- and evening-types, respectively; t = −1.39, p = 0.18). After conversion to “circadian time” using each participant's diary-based sleep offset as CT0, we found no chronotype differences in the timing of the morning (2:13±0:49 and 2:19±1:04; t = −0.28, p = 0.78) or evening (16:36±1:00 and 16:22±1:21; t = 0.50, p = 0.62) scans.
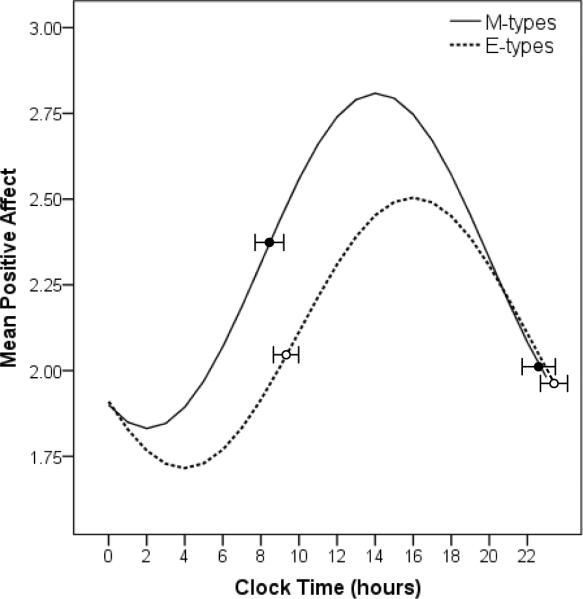
Figure 1.
Chronotype differences in diurnal rhythms of positive affect. The mean timing (± SD) of each group's morning and evening FDG-PET scans are indicated by the superimposed circles (filled circles = morning-types, open circles = evening-types).
Hypothesis 1: Evening-types will have more disturbed and irregular sleep than morning-types (Table 2)
Table 2.
Sleep-wake characteristics
| Morning-types (n = 49) | Evening-types (n = 51) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | p | |
| PSQI (0–3 for subscales) | (1,89) | |||||
| Sleep quality | 2.17 | 0.49 | 2.22 | 0.47 | 0.23 | ns |
| Sleep duration | 1.91 | 0.94 | 1.80 | 0.92 | 0.34 | ns |
| Habitual sleep efficiency | 1.91 | 1.03 | 1.93 | 1.12 | 0.01 | ns |
| Sleep disturbance | 1.41 | 0.50 | 1.27 | 0.45 | 2.17 | ns |
| Sleep latency | 1.74 | 1.06 | 2.36 | 0.80 | 9.72 | 0.002 |
| Sleep medication | 0.93 | 1.20 | 0.93 | 1.14 | 0.00 | ns |
| Daytime dysfunction | 1.30 | 0.79 | 1.62 | 0.61 | 4.62 | 0.03 |
| PSQI Total Score (0–21) | 11.39 | 3.26 | 12.13 | 3.00 | 1.28 | ns |
| Diary measures | (1,97) | |||||
| In-bed | 22:58 | 1:12 | 23:59 | 1:16 | 1.80 | 0.000 |
| Lights out | 23:19 | 1:05 | 0:24 | 1:10 | 23.17 | 0.000 |
| Sleep onset | 23:53 | 1:16 | 1:18 | 1:34 | 24.68 | 0.000 |
| Sleep offset | 6:14 | 1:02 | 7:49 | 1:26 | 39.79 | 0.000 |
| Sleep latency (min) | 33.7 | 28.8 | 53.9 | 46.5 | 6.68 | 0.011 |
| Wake after sleep onset (min) | 45.49 | 30.9 | 38.11 | 34.03 | 1.27 | ns |
| # of awakenings | 3.06 | 1.00 | 4.56 | 1.33 | 0.01 | ns |
| Sleep quality rating (1–10) | 6.24 | 1.04 | 7.82 | 1.42 | 0.64 | ns |
A one-way MANOVA for the PSQI component scales and total score was significant (F(7.38)=2.46, p = 0.024), indicating E-types generally reported worse subjective sleep quality. E-types reported significantly longer sleep latencies and worse daytime functioning on the respective component scales. The MANOVA for the diary data produced similar results (F(7,91)=6.32, p<0.001). All the sleep timing measures were significantly later among E-types, and as predicted, sleep latency was longer than that of M-types. Finally, based on a MANOVA for variability (SD) in timing of sleep (including in-bed, lights out, sleep onset, and sleep offset), E-types reported a trend towards more variable overall sleep timing (F (4, 94) = 2.02, p = .097), with significantly greater variability in the timing of sleep offset (F(1, 99) = 5.09, p = .03).
Hypothesis 2: Mean PA will be lower, and diurnal rhythms in PA will have later phase and smaller amplitude in evening-types
The evening-types showed a trend towards significantly lower mean PA (averaged across the entire EMA protocol), but did not differ from the morning-types in mean NA or severity of depression symptoms (Table 1). Positive affect showed robust diurnal rhythmicity in both groups (Figure 1), with a significant fit to the 24-hour sinusoid (sine term: B = −0.31, t (2532) = −9.56, p < 0.0001; cosine term: B = −0.58, t(2529) = −17.63, p < 0.0001) and significant group interaction terms (B = −0.13, t(2531) = −2.71, p = 0.007; B = 0.28, t(2528) = 6.31, p < 0.0001). As predicted, both phase and amplitude differed in the PA rhythms of E- and M-types (amp diff z-value = −2.49, p = 0.01; phase diff z-value = 6.89, p < 0.0001). The mean PA rhythm among E-types had a later phase (15:43, SE = 0:13) and lower amplitude (0.53, SE = 0.04) than that of M-types (13:52, SE = 0:10; 0.66, SE= 0.04, respectively). Morning-evening type differences remained after controlling for age. Notably, the troughs and peaks of the PA rhythms in Figure 1 occurred hours before the morning and evening PET scans, respectively.
When we ran parallel analyses using times standardized based on our sleep offset estimate of CT0, the acrophases of the PA rhythm were no longer significantly different between chronotypes, but the amplitude difference remained significant (see Supporting Information).
Hypothesis 3: E-types will show reduced diurnal variation in rCMRglu in PA-relevant brain areas, as well as lower mean levels of rCMRglu, particularly during morning wakefulness
Group × Time of Day Interaction Analyses
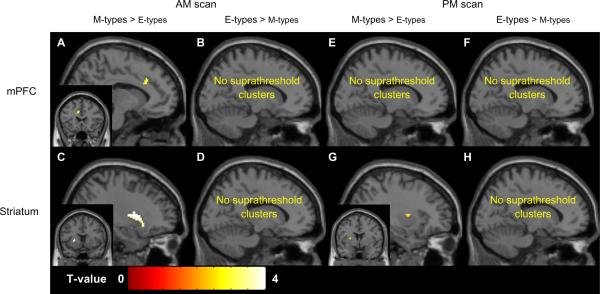
Compared to morning-types, evening-types showed smaller morning-to-evening increases in rCMRglu in a single cluster within the striatum; specifically, a 24 voxel cluster in the right putamen (peak voxel [36 −20 −6], t=3.80, p < 0.001). Evening-types showed smaller morning-to-evening decreases in rCMRglu in the mPFC only, compared to morning-types; specifically in a 143 voxel cluster in the left superior frontal gyrus (BA8; peak voxel [−16 46 38], t = 3.36, p = 0.001, survived AlphaSim correction). Based on these interactions, we ran post-hoc analyses to compare the effects of group within time of day, and the effects of time of day within each group.
Post-hoc comparisons of M- and E-types at the morning and evening wakefulness scans
Consistent with predictions, and regardless of time-of-day, M-types showed greater rCMRglu in subregions of both the mPFC and striatum relative to E-types (Table 3, Figure 2). During the morning scan, M-types had higher rCMRglu in the bilateral ACC/BA32 (Figure 2A) and in the left putamen (Figure 2C). The E-types did not show higher rCMRclu in any clusters within the mPFC or striatum.
Table 3.
Clusters showing morning- (n = 14) versus evening-type (n = 13) differences in relative regional glucose metabolism during morning and evening scans
| MNI coordinates of maximum voxel in cluster |
|||||||
|---|---|---|---|---|---|---|---|
| Region | Hemisphere | x | y | z | Cluster Size | t | p |
| AM Scan | |||||||
| M-type > E-type | |||||||
| ACC, BA32 | Left | −10 | 22 | 32 | 63 | 3.35 | 0.001 |
| ACC, BA32 | Right | 12 | 40 | 24 | 24 | 2.84 | 0.005 |
| Putamen | Left | −24 | 10 | 8 | 167 | 3.23 | 0.002* |
| PM Scan | |||||||
| M-type > E-type | |||||||
| Putamen | Left | −28 | 2 | 10 | 19 | 3.13 | 0.002 |
| Putamen | Left | −22 | 10 | −14 | 28 | 2.79 | 0.005 |
NOTE:
Clusters marked with an asterisk survived correction for multiple comparisons in Monte Carlo simulations.
Figure 2.
Chronotype differences in rCMRglu within the mPFC and striatum during morning and evening wakefulness. Only one representative cluster (with the highest t-value) is shown for each comparison. Coronal views are displayed left/right.
During the evening scan, when M-types showed higher rCMRglu in two clusters within the left putamen (Figure 2G). No chronotype differences in rCMRglu were found within the mPFC, and the E-types did not show higher rCMRclu in any clusters within the mPFC or striatum.
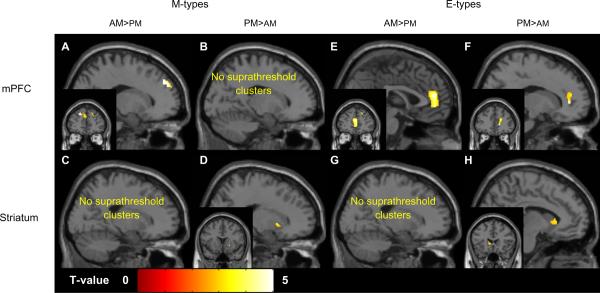
Post-hoc comparisons of the morning and evening wakefulness scans in M- and E-types
M-types showed a pattern of diurnal variation consistent with relatively greater rCMRglu in the mPFC during the morning, and relatively greater rCMRglu in the striatum during the evening (Table 4 and Figure 3). Among M-types, rCMRglu in the mPFC (peaks in bilateral superior frontal gyrus, BA8 and 9 and left ACC, BA32; Figure 3A) was greater in the morning, while rCMRglu in the striatum (bilateral putamen; Figure 3D) was greater in the evening.
Table 4.
Clusters showing diurnal variation in regional glucose metabolism
| MNI coordinates of maximum voxel in cluster |
|||||||
|---|---|---|---|---|---|---|---|
| Region | Hemisphere | x | y | z | Cluster Size | t | p |
| Morning-types | |||||||
| AM > PM | |||||||
| Superior frontal gyrus, BA8 | Left | –16 | 46 | 40 | 370 | 8.04 | 0.000* |
| ACC, BA32 | Left | –8 | 28 | 24 | 68 | 4.55 | 0.007 |
| Superior frontal gyrus, BA9 | Right | 22 | 48 | 32 | 32 | 3.79 | 0.001 |
| PM > AM | |||||||
| Putamen | Right | 20 | 14 | –2 | 17 | 3.56 | 0.002 |
| Putamen | Left | –30 | –6 | 4 | 18 | 3.16 | 0.004 |
| Evening-types | |||||||
| AM > PM | |||||||
| ACC, BA32 | Left | 0 | 46 | 16 | 382 | 4.86 | 0.000* |
| Medial frontal gyrus, BA9 | Right | 14 | 60 | 28 | 139 | 4.09 | 0.001* |
| PM > AM | |||||||
| ACC, BA32 | Right | 16 | 40 | 4 | 95 | 6.65 | 0.000 |
| Medial frontal gyrus, BA10 | Left | –18 | 48 | 6 | 17 | 3.39 | 0.003 |
| Caudate body | Left | –8 | 22 | 4 | 49 | 3.46 | 0.002 |
NOTE:
Clusters marked with an asterisk survived correction for multiple comparisons in Monte Carlo simulations.
Figure 3.
Diurnal variation in rCMRglu within the mPFC and striatum in M- and E-types with insomnia. Only one representative cluster (with the highest t-value) is shown for each comparison. Coronal views are displayed left/right.
Among E-types, the pattern was somewhat more complex. A large mPFC cluster (left ACC/BA32; Figure 3E) showed greater rCMRglu in the morning, as did a somewhat smaller cluster in the right medial frontal gyrus (BA9). A shift in laterality for the mPFC clusters was observed in the evening scans, when rCMRglu was greater in a large cluster in the right ACC/BA32 (Figure 3F), the left medial frontal gyrus (BA10), and the left caudate (Figure 3H).
Considering these data in context of the significant interactions noted in the initial full factorial model, findings suggest that diurnal variation in M-types manifests as decreasing rCMRglu in the mPFC throughout the course the day, while in parallel, rCMRglu in the striatum is showing relative increases. The E-types show relatively less diurnal variation in both regions; rCMRglu decreases in the mPFC, and rCMRglu increases in the striatum were both relatively smaller among E-types.
DISCUSSION
We investigated the role of self-reported chronotype in sleep and affective functioning of adults with primary insomnia, positing that eveningness would be particularly associated with worse sleep and self-reported PA, and altered neural function in brain regions associated with PA. As hypothesized, evening-types reported longer sleep onset latencies and more variable sleep timing. Also consistent with our predictions, evening-types with insomnia displayed diurnal patterns of self-reported PA characterized by phase delay and smaller amplitude compared to those of morning-types with insomnia. Exploratory analyses of FDG-PET data suggest that this pattern was roughly paralleled by that of relative glucose metabolism in brain regions associated with PA. Specifically, evening-types showed smaller morning-to-evening rCMRglu changes in both the mPFC and striatum, paralleling their pattern of reduced diurnal variation in self-reported PA. Likewise, evening-types showed lower relative glucose metabolism in the mPFC and striatum during both the morning and evening scans, paralleling the group differences in mean PA. Taken together, these preliminary findings suggest that alterations in the diurnal activity of PA-related neural structures may underlie differences in the phase and amplitude of self-reported PA between morning and evening chronotypes, and may constitute one mechanism for increased risk of mood disorders among insomniac evening-types.
The longer sleep latencies among the evening-types suggest that their insomnia is partly a consequence to trying to initiate sleep at too early a circadian phase, perhaps in the wake maintenance zone. The lack of chronotype differences in measures of sleep maintenance is consistent with other reports on the role of chronotype in insomnia (e.g., (Lack and Wright, 2007)). The evening-types' greater degree of variability in wake-times also might be expected given prior findings of more variable out-of-bed times (Ong et al., 2007), larger weekday-weekend differences in sleep timing (Wittmann et al., 2006) and more irregular social rhythms (Monk et al., 2004). These more irregular wake-times are consistent with the hypothesis of “social jet lag” among evening-types with insomnia as they struggle to accommodate work, school, or social schedules that are out-of-tune with their biological predispositions (Wittmann et al., 2006). Given that irregular timing and circadian misalignment are also associated with mood disorders (Emens et al., 2009, Frank et al., 2005, Hasler et al., 2010b), these patterns may put evening-types with insomnia at particular risk of developing depression.
The group differences in diurnal rhythms of PA are consistent with the broader literature on morningness-eveningness and affect dysregulation, wherein evening-types report less PA, more depression, and less reward responsiveness (Hasler et al., 2010a, Drennan et al., 1991). Evening-types also report greater substance use and sensation-seeking (Wittmann et al., 2006, Tonetti et al., 2010, Randler, 2008). Our data on the daily pattern of PA, not mean levels, complement these earlier findings and suggest plausible explanations for how these characteristics might be linked. For example, the blunted “highs” during the afternoon and evening among the evening-types may encourage compensatory sensation-seeking and increased drug and alcohol intake.
Although negative affect receives considerably more attention in the insomnia literature, these patterns in PA may also have particular relevance to insomnia. For example, blunted evening PA among evening-types may lead them to seek refuge in sleep and retire too early. Alternatively, blunted PA may be less effective at offsetting the tendency for elevated levels of anxiety and worry among many individuals with insomnia, thus exacerbating difficulty in falling asleep. The reduced amplitude of PA rhythms is also consistent with the presence of social jet lag or circadian misalignment among the evening-types. Misalignment typically results in reduced amplitude (Aschoff and Wever, 1981, Souetre et al., 1989). The lack of group differences in depression severity may be due to our exclusion of individuals with current mood disorders and/or extreme delays in sleep timing, potentially eliminating the portion of evening-types expected to be most depressed.
Our FDG-PET findings are exploratory and must be interpreted with caution until replicated with a more definitive study. The timing of our scans appears less than ideal for capturing diurnal variation PA-related brain activity, given that the morning and evening scans each occurred several hours after the trough and peak of PA (Figure 1), or for examining chronotype differences in PA-related brain activity, given that there were minimal differences in PA at the evening scan. Indeed, an alternative explanation for our findings is that the two groups' PET scans occurred at different times relative to the PA rhythms. That said, the timing of self-reported affective experience need not necessarily reflect 1:1 correspondence with the activation patterns in these specific brain regions. Other exogenous factors (e.g., social interactions) and biological factors (e.g., homeostatic drive) may lead to a less than perfect correspondence, or the observed PA rhythms could reflect a complex interaction of diurnal patterns across multiple brain regions that is not captured by simple morning-evening comparisons in the two regions of interest examined here.
Despite these issues, our data suggest that brain areas implicated in PA show daily changes in rCMRglu that broadly parallel those of self-reported PA and could plausibly play a functional role in PA rhythms. Although evidence on the neural underpinnings of PA remains limited, as PA is difficult to effectively elicit in laboratory settings, at least two studies have reported associations between PA or related affective constructs and subregions of the mPFC or striatum. Forbes and colleagues (Forbes et al., 2010) also used an EMA protocol to assess PA in a sample of healthy adolescents, and reported that fMRI-assessed striatal reactivity to reward anticipation and outcome correlated with mean PA under naturalistic conditions. Similarly, a dispositional measure of positive emotionality correlated with metabolic rate (based on FDG-PET) in the anterior cingulate and orbitofrontal cortices (Volkow et al., 2011).
These preliminary FDG-PET results suggest that the specific patterns of diurnal variation in the mPFC and striatum may differ between chronotypes, differences that we speculate may relate to differential influences of circadian and homeostatic processes. In the morning-types, the decreasing metabolic activity in the mPFC from morning-to-evening may predominantly reflect accumulating homeostatic pressure (Franken and Dijk, 2009), while the increasing metabolic activity in the striatum may predominantly reflect circadian regulation, a possibility supported by accumulating evidence in the animal literature (e.g.,(Sleipness et al., 2007, Webb et al., 2009). Based on this conceptual framework, the evening-type's smaller morning-to-evening decreases in relative glucose metabolism in the mPFC, and smaller increases in relative glucose metabolism in both the mPFC and striatum, may be based on more global differences in the influence of circadian and homeostatic processes in evening-types, as reported elsewhere (Mongrain et al., 2006). Chronotype differences in circadian phase (and relative circadian alignment) could also potentially explain the disparate patterns of diurnal variation.
How do these reciprocal patterns of change map onto the observed patterns in self-reported PA? Theoretical models have converged on a core reward circuit comprised of prefrontal areas largely encompassed by the mPFC that provide top-down modulation, and subcortical areas, particularly the ventral striatum, that underlie approach motivation, reward processing, and the generation of PA (e.g., (Ernst and Fudge, 2009, Forbes and Dahl, 2005). These models posit that changes in reward function (as manifested by PA) may be due to shifts in the balance of control from the prefrontal cortex to the striatum, or vice versa. Thus, the increase in self-reported PA throughout the day is due to a combination of decreased regulatory control by mPFC's metabolic activity decreases, and the relatively enhanced influence of the striatum as its metabolic activity increases. This model fits well with the morning-type FDG-PET and PA findings. The blunted PA rhythm in the evening-types may be accounted for by continued top-down regulation of striatum by the mPFC during evening hours, by diminished circadian amplitude within the striatum due to circadian misalignment, or both.
Limitations
Our liberal threshold for significance in the analyses of the FDG-PET data may increase the potential for Type I errors. We employed this threshold in the interest of conducting exploratory analyses of data from a study not specifically designed to address the questions at hand, which could increase the potential for Type II errors. Most notably, the specific timing of our PET scans likely limited our ability to detect AM-PM or chronotype differences; although we standardized the scans relative to sleep timing, future studies should also consider scheduling the scans based on the temporal parameters of the PA rhythm. Likewise, our analyses linked a momentary, in vivo measure of PA with glucose metabolism in relevant brain regions over 20 minutes, in contrast with aforementioned studies using a more global measure of PA, or those directly eliciting PA in a task and looking at the associated brain activity. Furthermore, these brain regions have multiple functions, and not all these functions necessarily follow the same daily pattern as PA. Future studies could address these limitations by employing PA-eliciting tasks, fMRI methods, or using PET to focus on dopamine neurotransmission. In addition, the selection criteria in our study may have diminished our power to find statistically significant effects. Our exclusion of individuals with current mood disorders and or delayed sleep phase syndrome may have effectively eliminated the extreme evening-types reporting the worst sleep, lowest PA, and greatest depression. Finally, our findings are also limited by the smaller sample size in the PET analyses and the lack of comparison groups. These findings should be examined in other relevant populations (healthy individuals, mood disordered individuals) in order to determine which effects are a function of insomnia per se, and which are a function of chronotype.
Conclusion
These preliminary findings suggest that morning- and evening-type insomniacs differ in the daily patterns of both their affective experience and the activity of affective neural circuitry. Evening-types with insomnia not only display characteristic sleep disturbance, but they also show blunted patterns of PA with apparent parallels in the function of PA-related brain regions. More definitive studies using larger samples and neuroimaging sampling protocols temporally aligned to the diurnal rhythms in PA will be required to test the hypothesis that diurnal variation in PA-related brain regions underlies the rhythms in PA, and that this diurnal variation differs between chronotypes. Such research appears justified, as these differences may put evening-types with insomnia at particular risk of developing full-blown mood disorders, highlighting the need for more studies specifically directed at the links between chronotype, insomnia, and affect dysregulation.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institutes of Health (UL1-RR024153, R01-MH24652, and T32-HL082610). The views expressed in this article are those of the authors and do not necessarily reflect those of the funding agency. We would like to gratefully acknowledge the helpful feedback of Julie Price, PhD, during preparation of the manuscript.
EFERENCES
- Aschoff J, Wever R. The circadian system of man. In: ASCHOFF J, editor. Biological rhythms: Handbook of behavioral neurobiology. Plenum Press; New York: 1981. [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA, Dijk DJ, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997;54:145–52. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Nofzinger EA, Germain A, et al. Regional brain glucose metabolism during morning and Evening wakefulness in humans: Preliminary findings. Sleep. 2004;27:245–54. doi: 10.1093/sleep/27.7.1245. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatr Res. 1989;28 doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry. 2007;68:254–60. doi: 10.4088/jcp.v68n0211. [DOI] [PubMed] [Google Scholar]
- Carrier J, Monk TH, Buysse DJ, Kupfer DJ. Sleep and morningness-eveningness in the 'middle' years of life (20–59 y) J. Sleep Res. 1997;6:230–7. doi: 10.1111/j.1365-2869.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- Ching C, Fok T, Ramsay JO. Periodic trends, non-periodic trends, and their interactions in longitudinal or functional data. In: WALLS TA, SCHAFE JL, editors. Models for Intensive Longitudinal Data. Oxford University Press; New York: 2006. [Google Scholar]
- Drennan M, Klauber M, Kripke D, Goyette L. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. J Affect Disord. 1991;23:93–98. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatr Res. 2009;168:259–61. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci. Biobehav. Rev. 2009;33:367–82. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Dev Psychopathol. 2005;17:827–50. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE, Almeida JRE, et al. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biol Psychiatry. doi: 10.1016/j.biopsych.2011.10.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, et al. Healthy adolescents' neural response to reward: associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry. 2010;49:162–72. e1–5. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur J Neurosci. 2009;29:1820–9. doi: 10.1111/j.1460-9568.2009.06723.x. [DOI] [PubMed] [Google Scholar]
- Germain A, Nofzinger EA, Meltzer CC, et al. Diurnal variation in regional brain glucose metabolism in depression. Biol Psychiatry. 2007;62:438–45. doi: 10.1016/j.biopsych.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J. Sleep Res. 2002;11:191–99. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Guthrie JP, Ash RA, Bendapudi V. Additional validity evidence for a measure of morningness. J. Appl. Psychol. 1995;80:186–90. [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Allen JJB, Sbarra DA, Bootzin RR, Bernert RA. Morningness-eveningness and depression: Preliminary evidence for the role of BAS and positive affect. Psychiatr Res. 2010a;176:166–73. doi: 10.1016/j.psychres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Buysse DJ, Kupfer DJ, Germain A. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: Further evidence for circadian misalignment in non-seasonal depression. Psychiatr Res. 2010b;178:205–07. doi: 10.1016/j.psychres.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. J. Adolesc. Health. 2009;45:326–34. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack LC, Wright HR. Treating chronobiological components of chronic insomnia. Sleep Med. 2007;8:637–44. doi: 10.1016/j.sleep.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Mansour HA, Wood J, Chowdari KV, et al. Circadian phase variation in Bipolar I disorder. Chronobiol. Int. 2005;22:571–84. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- Mcclung CA. Circadian Rhythms, the Mesolimbic Dopaminergic Circuit, and Drug Addiction. TheScientificWorld J. 2007;7:194–202. doi: 10.1100/tsw.2007.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulich SK, Zerbe GO, Jones RH, Crowley TJ. Comparing linear and nonlinear mixed model approaches to cosinor analysis. Stat. Med. 2003;22:3195–211. doi: 10.1002/sim.1560. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. J. Sleep Res. 2006;15:162–6. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Potts JM, Degrazia JM, Kupfer DJ. Morningness-eveningness and lifestyle regularity. Chronobiol. Int. 2004;21:435–43. doi: 10.1081/cbi-120038614. [DOI] [PubMed] [Google Scholar]
- Monk TH, Reynolds CF, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J. Sleep Res. 1994;3:111–20. [PubMed] [Google Scholar]
- Murray G. Diurnal mood variation in depression: A signal of disturbed circadian function? J Affect Disord. 2007;102:47–53. doi: 10.1016/j.jad.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, et al. Nature's clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9:705–16. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Natale V, Alzani A. Additional validity evidence for the composite scale of morningness. Pers Indiv Differ. 2001;30:293–301. [Google Scholar]
- Nofzinger EA, Mintun MA, Price J, et al. A method for the assessment of the functional neuroanatomy of human sleep using FDG PET. Brain Res Brain Res Protoc. 1998;2:191–8. doi: 10.1016/s1385-299x(97)00042-1. [DOI] [PubMed] [Google Scholar]
- Ong JC, Huang JS, Kuo TF, Manber R. Characteristics of insomniacs with self-reported morning and evening chronotypes. J Clin Sleep Med. 2007;3:289–94. [PMC free article] [PubMed] [Google Scholar]
- Paine S-J, Gander PH, Travier N. The epidemiology of morningness/eveningness: Influence of age, gender, ethnicity, and socioeconomic factors in adults (30–49 years) J. Biol. Rhythms. 2006;21:68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997;42:209–12. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- Porto R, Duarte L, Menna-Barreto L. Circadian variation of mood: comparison between different chronotypes. Biol. Rhythm Res. 2006;37:425–31. [Google Scholar]
- Randler C. Differences between smokers and nonsmokers in morningness-eveningness. Soc Behav Pers. 2008;36:673–80. [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatr Res. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Res. 2007;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J. Appl.Psychol. 1989;74:728–38. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Soehner AM, Kennedy KS, Monk TH. Personality Correlates with Sleep-Wake Variables. Chronobiol. Int. 2007;24:889–903. doi: 10.1080/07420520701648317. [DOI] [PubMed] [Google Scholar]
- Souetre E, Salvati E, Belugou JL, et al. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatr Res. 1989;28:263–78. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- Taillard J, Philip P, Bioulac B. Morningness/eveningness and the need for sleep. J. Sleep Res. 1999;8:291–5. doi: 10.1046/j.1365-2869.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- Tonetti L, Adan A, Caci H, De Pascalis V, Fabbri M, Natale V. Morningness-eveningness preference and sensation seeking. Eur Psychiatry. 2010;25:111–5. doi: 10.1016/j.eurpsy.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, et al. Positive emotionality is associated with baseline metabolism in orbitofrontal cortex and in regions of the default network. Mol. Psychiatry. 2011;16:818–25. doi: 10.1038/mp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc.Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J. Biol. Rhythms. 2009;24:465–76. doi: 10.1177/0748730409346657. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J. Comput. Assisted Tomogr. 1993;17:536–46. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.