Abstract
Introduction and Aims
Cigarette smoking occurs frequently among individuals with methamphetamine dependence. Preclinical and clinical evidence has suggested that the common co-abuse of methamphetamine and cigarettes represents a pharmacologically meaningful pattern.
Methods
The present study is a secondary analysis of a randomised, placebo-controlled trial of bupropion treatment for methamphetamine dependence (bupropion n=36; placebo n=37). A hierarchical logistic modelling approach assessed the efficacy of bupropion for reducing MA use separately among smokers and non-smokers. Among smokers, relations between cigarettes smoked and MA use were assessed.
Results
Smoking status did not affect treatment responsiveness in either the bupropion condition or the placebo condition. In the placebo condition, increased cigarette use was associated with an increased probability of methamphetamine use during the same time period. This effect was not observed in the bupropion condition.
Discussion and Conclusions
Initial smoking status did not impact treatment outcomes. Among smokers, results suggest that bupropion may dissociate cigarette and methamphetamine use. The effect was modest and a precise pharmacologic mechanism remains elusive. Cholinergic systems may be relevant for methamphetamine use outcomes. Future studies should continue to assess the role of smoking in methamphetamine treatment outcomes.
Keywords: bupropion, methamphetamine dependence, nicotine, cigarettes, comorbidity
Introduction
Methamphetamine (MA) users smoke cigarettes at rates typically exceeding 80% [1]. Converging preclinical and clinical evidence suggests a specific and meaningful link between smoking cigarettes and MA use. Cholinergic interneurons are found in brain regions relevant to the reinforcing effects of MA and preclinical data suggests that the modulation of nicotinic acetylcholine receptors exerts important dopaminergic effects [2-6]. In humans, methylphenidate at doses of 10 milligrams or more increased smoking among non-treatment seeking smokers [7] and d-amphetamine increased cigarette consumption in a dose-dependent manner [8]. Medications modulating cholinergic transmission have shown some promise in mitigating cardiovascular and subjective effects of MA [9]. Generally, the evidence suggests a positive association between nicotine and MA use, but some evidence suggests that nicotine can substitute for MA thereby reducing MA self-administration [10, 11].
This report is a secondary data analysis of a randomised, placebo-controlled trial of bupropion for MA dependence [12]. Bupropion is a dopamine and norepinephrine reuptake inhibitor [13] and acts as an antagonist at several nicotinic acetylcholine receptor sites [14]. The current analyses examined whether smoking status moderated treatment outcomes in either of the treatment conditions and examined the hypothesis that a decrease in cigarette use would be associated with decreased MA use.
Methods
Study participants were 73 adult treatment-seeking outpatients meeting DSM-IV-TR criteria for current MA dependence without significant medical or psychiatric comorbidity. Complete methodological details are provided in the original report [12]. The study used a randomised, double-blind, placebo-controlled clinical trial design (bupropion 300 mg daily) in conjunction with behavioural therapy. Urine samples were analysed onsite for MA metabolites using radioimmunoassay. Cigarette smoking was assessed weekly using the time-line follow back methods [15]. Primary analyses, using the intention-to-treat approach, accommodated the nesting of data points within subjects by implementing hierarchical logistic modelling with random intercepts. The MA use outcome was modeled as a Bernoulli random variable with the response probability as a function of subject-specific effects and the influence of covariates. The first models were fit in the smokers and non-smokers separately to determine if bupropion had different MA use benefits across the smoking groups. Among smokers, a second hierarchical logistic model was fit to estimate the relationship between cigarette use and MA use. In this second set of analyses, age, gender and baseline MA use were included as covariates.
Results
Seventy-three participants were randomised to bupropion (N=36) or placebo (N=37). Twenty-five participants (34%) completed the trial. Table 1 reports the demographic and MA treatment outcomes for smokers and nonsmokers in each medication condition.
Table 1.
Sample characteristics and overall treatment outcomes of smokers and non-smokers
| Smokers (N=48) | Non-smokers (N=25) | |||
|---|---|---|---|---|
| Bupropion (N=29) |
Placebo (N=19) |
Bupropion (N=7) |
Placebo (N=18) |
|
| Age, mean | 35 | 33 | 34 | 36 |
| Gender, % (N) | ||||
| Male | 66% | 63% | 43% | 72% |
| Female | 34% | 37% | 57% | 28% |
| Ethnicity, % (N) | ||||
| White | 59% | 63% | 43% | 50% |
| Hispanic | 34% | 31% | 57% | 39% |
| Other | 3% | 5% | 0% | 11% |
| Educational, mean years | 12.8 | 12.5 | 13.7 | 13.0 |
| Days of MA use in past month, mean | 13a | 15 | 23a | 17 |
| Cigarettes per day at baseline | 11.4 | 11.7 | - | - |
|
| ||||
| Treatment Outcomes | ||||
|
| ||||
| Days retained, mean | 52 | 49 | 55 | 53 |
| End-of-Treatment Abstinence | 17% | 16% | 14% | 17% |
| Treatment Effectiveness Score (mean negative Urine Drug Screens) |
4.6 | 4.8 | 4.9 | 4.6 |
Group difference significant (p<.05)
There was no evidence that smokers (OR = 0.80, 95% CI = 0.47-1.27) benefitted more from bupropion over the course of treatment when compared with nonsmokers (OR = 0.71, 95% CI = 0.35-1.32). In the placebo condition, neither smokers (OR = 1.00, 95% CI = 0.67-1.45) nor non-smokers (OR = 0.95, 95% CI = 0.67-1.30) showed any change in their MA use over the treatment period.
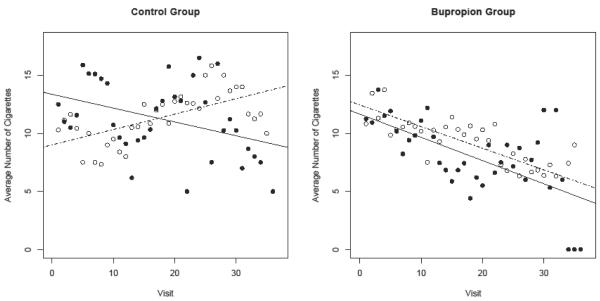
Next, a hierarchical logistic model estimated the probability, in each of the treatment conditions, of MA use depending on the number of cigarettes smoked. In the placebo group, the number of cigarettes smoked was positively associated with the probability of MA use, conferring a 4% increase in the odds of a positive screen for each additional cigarette smoked (OR = 1.04, 95% CI = 1.00-1.08, p=.05). In the bupropion condition, smoking was unrelated to MA use, although the strength of the association between the two substances in each of the conditions could not be distinguished statistically. Figure 1 depicts the relationship between smoking and MA use in the placebo and bupropion conditions by plotting the number of cigarettes smoked against MA urine drug screen results. In the control group, there is some suggestion of a non-linear effect, whereby smoking is positively associated with MA use during the first six weeks of treatment but becomes negatively associated with MA use during the second half of the trial. The positive odds ratio reported above reflects the diminished statistical weigh of the second half of the trial due to attrition.
Figure 1.
Average number of cigarettes smoked among individuals who screened positive for MA (black dots and black line representing linear trend) and individuals who screened negative for MA (hollow dots and dotted line representing linear trend) across the 12 week treatment period.
Discussion
The present study found no evidence that smokers and nonsmokers benefitted differently from treatment with bupropion in terms of MA use. Although statistical power (adequately powered to detect a very large effect) seriously limited our ability to detect relationships, effect sizes gave no indication that smokers fared worse. Analyses investigating the covariation between cigarette smoking and probability of MA use found a significant relationship in the placebo condition only. In the absence of active medication, more cigarettes were associated with increased probability of MA use. This effect was not observed in the bupropion condition, suggesting that bupropion may be effective in mitigating the escalation of co-occurring cigarette and MA use. However, the effect was modest and the pharmacologic mechanism responsible remains to be elucidated. Recently, we completed a randomised placebo-controlled pilot study of the cholinergic modulator, varenicline, which outperformed placebo in terms of retention and evidenced trends for improved MA abstinence (Swanson, Heinzerling, and Shoptaw, unpublished data). Future studies addressing the role of cholinergic modulation of dopaminergic-mediated drug effects are warranted.
Two studies have found have found that bupropion outperforms placebo for users with light baseline use [12, 16]. Given its indication as a smoking cessation medication, it is plausible that these therapeutic effects were mediated primarily by reductions in cigarette smoking or that bupropion is only effective for MA dependent cigarette smokers. We found minimal evidence for this. Bupropion in conjunction with behavioural therapies may be efficacious for light baseline users regardless of smoking status.
This study should be interpreted in light of several limitations. Analyses were conducted in a post hoc manner and smoking status was not balanced across treatment condition. Statistical power limited our ability to make precise statistical estimates. Cigarette smoking was not biologically verified and data was collected weekly, thereby compromising the level of resolution in untangling the reciprocal effects of MA and cigarette smoking.
Acknowledgments
Funding source
This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (grant numbers P50 DA18185 [SS], T32 DA026400 [SS]). The National Institute on Drug Abuse had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
Footnotes
Declaration of interests.
All authors declare that they have no conflicts of interest.
References
- 1.Weinberger AH, Sofuoglu M. The Impact of Cigarette Smoking on Stimulant Addiction. The American Journal of Drug and Alcohol Abuse. 2009;35(1):12–17. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingstone PD, Wonnacott S. Nicotinic acetylcholine receptors and the ascending dopamine pathways. Biochemical Pharmacology. 2009;78(7):744–755. doi: 10.1016/j.bcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Jutkiewicz E, et al. Nicotine and amphetamine acutely cross-potentiate their behavioral and neurochemical responses in female Holtzman rats. Psychopharmacology. 2008;200(1):93–103. doi: 10.1007/s00213-008-1159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatch MB, Flores E, Forster MJ. Nicotine and methamphetamine share discriminative stimulus effects. Drug and Alcohol Dependence. 2008;93(1-2):63–71. doi: 10.1016/j.drugalcdep.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neugebauer NM, Harrod SB, Bardo MT. Nicotine elicits methamphetamine-seeking in rats previously administered nicotine. Drug and Alcohol Dependence. 2010;106(1):72–78. doi: 10.1016/j.drugalcdep.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camarasa J, et al. The involvement of nicotinic receptor subtypes in the locomotor activity and analgesia induced by methamphetamine in mice. Behavioural Pharmacology. 2009;20(7):623–630. doi: 10.1097/FBP.0b013e328331ba5b. [DOI] [PubMed] [Google Scholar]
- 7.Rush CR, et al. Methylphenidate increases cigarette smoking. Psychopharmacology (Berl) 2005;181(4):781–9. doi: 10.1007/s00213-005-0021-8. [DOI] [PubMed] [Google Scholar]
- 8.Tidey JW, O’Neill SC, Higgins ST. Psychopharmacology. 2000;153(1):85–92. doi: 10.1007/s002130000600. Without Title. [DOI] [PubMed] [Google Scholar]
- 9.De La Garza R, Shoptaw S, Newton TF. Evaluation of the cardiovascular and subjective effects of rivastigmine in combination with methamphetamine in methamphetamine-dependent human volunteers. Int J Neuropsychopharmacol. 2008;11(6):729–741. doi: 10.1017/S1461145708008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiranita T, et al. Nicotine Attenuates Relapse to Methamphetamine-Seeking Behavior (Craving) in Rats. Annals of the New York Academy of Sciences. 2004;1025(1):504–507. doi: 10.1196/annals.1316.062. [DOI] [PubMed] [Google Scholar]
- 11.Hiranita T, et al. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proceedings of the National Academy of Sciences. 2006;103(22):8523–8527. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoptaw S, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222–32. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley KF, DeSanty KP, Kast RE. Bupropion: pharmacology and therapeutic applications. Expert review of neurotherapeutics. 2006;6(9):1249–1265. doi: 10.1586/14737175.6.9.1249. [DOI] [PubMed] [Google Scholar]
- 14.Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. Journal of Pharmacology and Experimental Therapeutics. 2000;295(1):321–327. [PubMed] [Google Scholar]
- 15.Sobell MB, et al. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav. 1986;11(2):149–61. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- 16.Elkashef AM, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162–70. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]