1. Structure
Lysyl Oxidase (LOX, also called protein-lysine 6-oxidase) (Genbank accession numbers: Nucleotide NM_002317.5, Protein NP_002308.2, EC 1.4.3.13) is one of the five LOX family members (LOX and LOX Like1-4). The human LOX gene is located on chromosome 5q23.2. LOX is synthesized as a 50 kDa pre-protein containing 3 domains; the N-terminal signal peptide sequence (aa1–21), the N-terminal pro-peptide domain (aa 22–162), and the C-terminal catalytic domain (aa 162–417). The signal peptide is removed by cleavage at the Cys21-Ala22 bond and the pro-peptide domain is N-glycosylated in the endoplasmic reticulum and Golgi apparatus yielding a pro-enzyme, which is then secreted from cells as a catalytically inactive protein. The 32 kDa active enzyme (C-terminal domain) is released by proteolytic cleavage of the pro-peptide at Gly162–Asp163 by procollagen C-proteinase (bone morphogenetic protein 1; BMP-1). (Kagan and Li, 2003).
2. Function
LOX belongs to a family of amine oxidases. LOX was initially reported to be expressed and secreted by fibrogenic cells but is now known to be expressed in several other cell types. LOX oxidizes peptidyl lysine and hydroxylysine residues in collagen and lysine residues in elastin to produce peptidyl alpha-aminoadipic-delta-semialdehydes. These aldehyde modifications can spontaneously combine with vicinal peptidyl aldehydes or with epsilon-amino groups of peptidyl lysine to form covalent cross-links that stabilize and cause collagen and elastin fibers to be insoluble in the extracellular matrix (ECM). The significance of LOX was demonstrated in the LOX knockout mouse, which could not survive at birth, due to rupture of the aorta and diaphragm from incomplete elastin cross-linking. LOX is also essential for development of the distal and proximal airways, and the formation of alveoli in the lungs. LOX is also critical for notochord formation and muscle development. LOX affects differentiation of osteoblasts by forming cross-links in the surrounding collagen matrix. These results underline the importance of LOX in the morphogenesis and repair of connective tissues of the cardiovascular, respiratory, skeletal, and other organ systems (Fong SFT et. al., 2009). LOX can also induce crosslinking between lysines in histones in the nucleus (Kagan and Li, 2003). LOX has also been reported as a potent chemotactic agent for monocytes and vascular smooth muscle cells.
3. Disease involvement
Due to the range of LOX biological functions, abnormalities of LOX expression underlie the development of a number of pathological processes related with an imbalance in ECM synthesis/degradation. These include connective tissue fibrotic disorders of the heart (myocardial fibrosis), vasculature (arterial fibrosis), lungs (pulmonary fibrosis), skin (keloids, and scleroderma), kidney (nephritis), liver (liver stiffness preceding liver fibrosis), mouth (gingival atrophy), and colon (intestinal fibrotic disease). LOX is also associated with diseases such as rheumatoid arthritis, systemic sclerosis, Alzheimer’s disease, and several stages of breast cancer. (Rodriguez et al., 2008).
LOX is expressed in several ocular tissues, and the following diseases of the eye have been linked with LOX:
-
Keratoconus
Keratoconus is a corneal degeneration in which the extracellular matrix of the cornea loses its integrity, slowly changing from the normal round shape to a cone shape. One study reported that corneal LOX mRNA expression was significantly lower in keratoconus patients when compared to their age-matched controls. Another study showed that reducing copper levels in tears results in keratoconus in an animal model. Copper is an essential cofactor for LOX activity.
-
Diabetic retinopathy (DR)
There are a number of reports of lower expression of LOX co-factors in DR retina samples and lower LOX cross-linking activity in vitreous samples of patients with DR. However, the effect of high glucose on LOX expression and enzymatic activity in cultured human retinal endothelial cells is less clear, with conflicting evidence suggesting either increased or decreased LOX expression. Although discrepant, these studies indicate a potential involvement of LOX in the observed DR phenotype.
-
Rhegmatogenous retinal detachment (RRD)
RRD occurs when a tear in the retina leads to fluid accumulation beneath the retina that causes a separation of the neurosensory retina from its underlying RPE. Vitreous samples of normal vs. RRD patients revealed lower LOX activity in RRD vitreous samples, although the mechanisms of LOX down-regulation and RRD are unknown.
-
Primary open angle glaucoma (POAG)
There are no direct reports of dysregulated LOX expression in primary open angle glaucoma. However, we recently reported that transforming growth factor beta 1–3 (TGFβ1–3) induce LOX and LOXL1-4 in human trabecular meshwork (TM) cells (Sethi et al. 2011). Both TGFβ1 and -2 have previously been reported to be profibrotic in the TM. There is evidence for the involvement of TGFβ2 in POAG and TGFβ1 in pseudoexfoliation (PEX) glaucoma. We also observed that gremlin, another profibrotic molecule that is elevated in glaucoma TM cells, induces expression of LOX genes in cultured human TM cells (unpublished data). Taken together, these data suggest a possible role for LOX in POAG and PEX pathogenesis. Another member of the LOX gene family, LOXL1, which is induced by TGFβ1, is associated with increased risk of developing PEX glaucoma and may play a role in the pathogenesis of PEX.
4. Further Studies
With most of the studies in eye research focusing on the genetic association of SNPs in LOXL1 gene in pseudoexfoliation syndrome, new findings of LOX and other LOX genes are still emerging. Compared to other systems, our understanding of LOX in the eye is very limited. Several questions need to be addressed in future studies. (1) Which LOX and LOXL genes play a role in ocular homeostasis and pathologies? (2) What cellular signaling mechanisms regulate expression of LOXs in ocular tissues? (3) Does LOX or other LOX genes regulate IOP and if so, what is the mechanism of this regulation? (4) Does LOX modulate physiology of other ECM-rich ocular tissues like optic nerve head? (5) Does altering LOX activity in cultured corneal epithelial cells regulate cellular elastin levels and what factors regulate LOX in keratoconus? (6) The cause and effect between LOX levels and RRD pathology should be evaluated. (7) How does high glucose affect LOX mRNA, protein, and enzyme activity in cultured retinal epithelial cells? (8) Will modulating ocular expression of LOX genes serve as a future therapy?
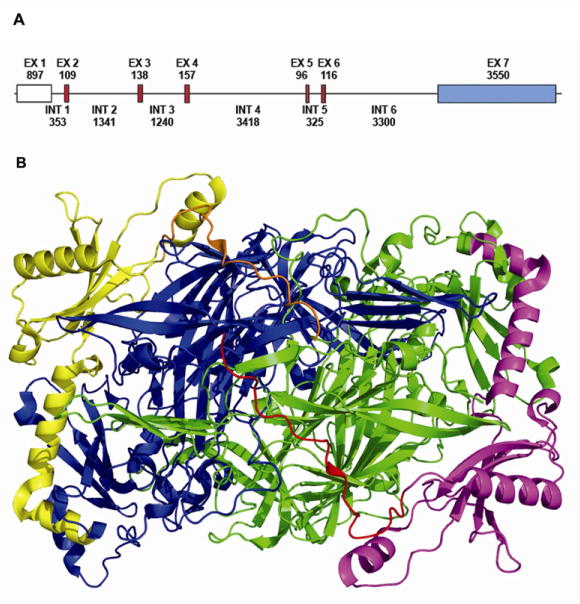
Figure 1. LOX gene and protein structure.
A, Exons are presented as boxes separated by introns (lines). The size of each exon and intron is shown in base pairs. The exons shaded in red encode amino acids sequences that are conserved in all lysyl oxidase family members. The exon shaded in blue contains the 3′ UTR sequence. B, 3D Crystal structure of Pichia pastoris LOX (Pymol). The molecule is presented as a dimmer with signal peptide domain (red and orange), pro-peptide (yellow and magenta) and C-terminal domain (green and blue) (Duff et al., 2003).
Acknowledgments
The authors would acknowledge Matthew Evans at UT Southwestern Medical Center for his assistance with generating 3D LOX structure. The authors would also like to acknowledge grant support from the National Institute of Health-National Eye Institute (EY-017374 awarded to RJW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Duff AP, Cohen AE, Ellis PJ, Kuchar JA, Langley DB, Shepard EM, Dooley DM, Freeman HC, Guss JM. The crystal structure of Pichia pastoris lysyl oxidase. Biochemistry. 2003;42:15148–15157. doi: 10.1021/bi035338v. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Rodriguez-Sinovas A, Martinez-Gonzalez J. Lysyl oxidase as a potential therapeutic target. Drug News Perspect. 2008;21:218–224. doi: 10.1358/dnp.2008.21.4.1213351. [DOI] [PubMed] [Google Scholar]
- Sethi A, Mao W, Wordinger RJ, Clark AF. Transforming Growth Factor-{beta} Induces Extracellular Matrix Protein Cross-Linking Lysyl Oxidase (LOX) Genes in Human Trabecular Meshwork Cells. Invest Ophthalmol Vis Sci. 2011;52:5240–5250. doi: 10.1167/iovs.11-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]