Abstract
Background
Disturbed gait and balance are among the most consistent sequelae of chronic alcoholism. However, although a majority of alcoholics have never sought treatment, most investigations showing ataxia in alcohol dependent individuals have relied on samples drawn from treated populations. In addition, few studies have addressed the associations of codependence on other drugs with alcoholic gait and balance disturbance.
Methods
The present study employed the Walk-a-line Ataxia Battery (Fregly et al. 1972) to assess gait and balance in treatment-naive, actively drinking alcohol dependent men and women (TNA; n = 69) who were dependent on alcohol only (ALC; n = 43), or who also had a lifetime drug dependence (ALC+DRG; n = 26; i.e., methamphetamine, cocaine, opiates, and/or marijuana), compared with non-substance abusing controls (NSAC; n = 74). We also examined associations between lifetime alcohol use and age with gait and balance measures.
Results
Our main findings were 1) no evidence of disturbed gait and balance in ALC vs. NSAC and 2) significantly disturbed gait and balance in ALC+DRG, relative to both NSAC and ALC, along with steeper age-associated decline in gait and balance performance in ALC vs. ALC+DRG.
Conclusions
Our results provide evidence consistent with previous studies that TNA (without a lifetime drug codependence) may represent a population that is different and less impaired (including in gait and balance) than treated alcoholics. Additionally, we provide evidence that ALC+DRG, with greater alcohol use and family drinking density than ALC, have an accelerated effect of age on gait and balance disturbance compared to both NSAC and ALC. The ALC+DRG group likely represents a subset of TNA with different characteristics than ALC.
Keywords: active alcoholics, ataxia, gait and balance, drug codependence, cerebellum, treatment-naive alcoholics
Introduction
Disturbed gait and balance are among the most consistent and salient deficits in chronic alcoholism (Rosenbloom et al. 2007; Sullivan et al. 2000b). Ataxia may result from damage to one or more of several neural systems. Lesion studies (Victor et al. 1989) as well as behavioral and brain imaging studies (Rosenbloom et al. 2007; Sullivan et al. 2006) have demonstrated a strong association between cerebellar atrophy and impaired gait and balance, marked by lower limb ataxia. Ethanol-induced cerebellar damage may be caused by excitotoxicity, dietary factors (particularly thiamine depletion), glial abnormalities, oxidative stress, and impaired energy production (Baker et al. 1999; Jaatinen and Rintala 2008).
Imaging studies have also shown alcohol-induced degradation of white matter integrity (Fein et al. 2009b) and compromised fiber integrity in the corpus callosum (Pfefferbaum et al. 2006), both of which have been associated with postural instability (Starr et al. 2003). Other imaging studies (Sullivan et al. 2005) have found reduced striatal volumes in alcoholics and an association between alcohol dependence and blunted dopamine transmission in the ventral striatum (Martinez et al. 2005), potentially resulting in disturbed gait. Recent human positron emission tomography (PET) studies have found that dopaminergic denervation affects gait independent of age-related changes (Cham et al. 2008). Finally, ataxia has been associated with alcohol-related peripheral neuropathy (Melgaard and Ahlgren 1986). Most research reporting gait and balance impairments in chronic alcoholics has relied on convenience samples from inpatient or outpatient treatment settings, and it is questionable to what degree conclusions from these studies can be generalized to the population of individuals with chronic alcohol dependence (Fein and Landman 2005).
Findings from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC)(Cohen et al. 2007) indicate that, of the estimated 27 million Americans exhibiting alcohol use disorders at some time during their lives, only about 15% have ever received any treatment, and among those with a prior-to-past year alcohol dependence, about 25% have received treatment. A study by Fein and Landman (2005) found that treated alcoholics had an average alcohol consumption over 50% higher than treatment-naive alcoholics (TNA) in the years just after they met criteria for heavy alcohol use. Findings from other recent studies reveal that treated alcohol-dependent individuals, compared to TNA, have a higher incidence of psychiatric co-morbidity (Di Sclafani et al. 2008; Fein et al. 2002; Moss et al. 2007), and greater dysfunction in intimate relationship and vocational functioning (Tucker et al. 2004). They also have more severe dependence, more emotional problems, and less engagement in everyday activities (Tucker et al. 2004). Two studies employing structural MRI (Fein et al. 2002; 2010) revealed a steeper age-related reduction of whole brain, prefrontal, and parietal gray matter volumes in TNA compared to controls, but did not find frank reductions in cortical gray matter volumes in TNA vs. controls. Such frank volume reductions are usually found in treated alcohol-dependent samples (Fein et al. 2009a). TNA also perform normally on the Iowa Gambling Task (IGT) despite active drinking (Fein et al. 2006), while long-term abstinent treated alcoholics evidence disadvantageous decision-making on the IGT (Fein et al. 2004a). Finally, in a recent investigation (Smith and Fein 2010), a younger TNA sample (19.7 to 50.4 years, mean 31.5 years of age) from Northern California had normal function on cognitive testing compared to age- and sex-comparable non-alcoholic controls, despite active moderate to heavy drinking.
In order to assess gait and balance in TNA, the present study employed the Walk-a-Line Ataxia Battery (Fregly et al. 1972), which has consistently demonstrated deficits in treated alcoholics (Smith and Fein 2011; Sullivan et al. 2000a; 2000b). The Fregly battery consists of three parts: two standing balance measures and one walking measure, each of which is performed first with eyes open (E/O) and then with eyes closed (E/C).
In addition to assessing the degree to which TNA exhibit gait and balance deficits typically found in clinical populations of alcoholics, we also examined effects of a lifetime drug codependence in TNA on Fregly performance. Degraded white matter integrity is associated with postural instability in humans (Starr et al. 2003), and diffusion tensor imaging has shown persistent white matter damage in abstinent methamphetamine abusers (A Chung et al. 2007). Additionally, neurotoxic effects of methamphetamine disrupt striatal dopaminergic pathways in both humans and animals (Davidson et al. 2001).
Like methamphetamine, chronic cocaine abuse degrades white matter integrity (Xu et al. 2010) and alters striatal dopaminergic activity, reflected in reduced striatal volumes (Barros-Loscertales et al. 2011). Furthermore, human structural imaging has shown an inverse association between years of cocaine use and cerebellar volumes (Barros-Loscertales et al. 2011), and functional MRI has shown reduced functional connectivity of dopaminergic midbrain with the cerebellum, as well as reduced cerebellar activity, in cocaine abusers (Tomasi et al. 2010). Finally, numerous animal (Dar 2000; Kalant and LeBlanc 1974) and human studies (Heishman et al. 1990; Perez-Reyes et al. 1988) have demonstrated that acute and residual effects of cannabinoid consumption include impaired motor coordination and timing, believed to result from damage to cerebellar Purkinje circuitry (Herkenham 1992; Takahashi and Linden 2000), along with altered dopaminergic activity in the striatum (Herkenham 1992; Takahashi and Linden 2000).
Methods
Participants
Participants were recruited from the community through postings at university campuses, bulletin boards, community and health centers, and through subject referrals. NSAC consisted of 39 women (mean age = 49.4) and 35 men (mean age = 47.6). Inclusion criteria for NSAC were a lifetime average of less than 30 standard drinks per month with no periods of drinking more than 60 drinks per month, and no history of alcohol or substance abuse or dependence (other than nicotine or caffeine). TNA were 25 women (mean age = 45.5) and 44 men (mean age = 44.8) who met DSM-IV (American Psychiatric Association 2000) criteria for current alcohol dependence. Within TNA, there were 21 men and 5 women with a lifetime drug dependence in addition to their alcohol dependence (ALC+DRG), and 23 men and 20 women with lifetime alcohol dependence only (ALC). Within ALC+DRG, there were 7 individuals with lifetime dependence on cocaine, 2 on methamphetamine, 1 on both cocaine and methamphetamine, 10 on marijuana, 2 on cocaine and marijuana, 2 on methamphetamine and marijuana, and 2 on marijuana, cocaine, methamphetamine and opiates. Of the 26 ALC+DRG, 7 were currently using cocaine, 2 methamphetamine, and 9 marijuana. Of these, 3 met current criteria for cocaine dependence, 2 for methamphetamine dependence, and 5 for marijuana dependence. The nine individuals reporting current use endorse using drugs in the period up to the assessment. We did not ascertain the date of last use; however an Oral Fluid Drug Screen was administered on the date of assessment, confirming abstinence for a minimum of 72 hours before testing. Table 1 reports demographic, family density of problem drinking and problem drug use, smoking status, and alcohol use and withdrawal information for NSAC, ALC, and ALC+DRG.
Table 1.
Demographics and alcohol use measures by group: NSAC (Non-Substance Abusing Controls), TNA (Treatment-Naive Alcoholics), ALC (Alcohol-Dependent Only) and ALC+DRG (Alcohol and Drug-Dependent)
| TNA
|
NSAC
|
Effect Size
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ALC | ALC+DRG | Men (n=35) | Women (n=39) | ALC vs. NSAC | ALC+DRG vs. NSAC | ALC vs. ALC+DRG | |||
| Men (n=23) | Women (n=20) | Men (n=21) | Women (n=5) | ||||||
| Demographics |
Partial η2
|
||||||||
| Age (years) | 45.0 ± 6.7 | 45.0 ± 7.1 | 44.6 ± 7.1 | 47.2 ± 8.2 | 47.6 ± 6.8 | 49.4 ± 7.7 | 5.6** | 4.3* | 0.0 |
| Years of Education | 13.7 ± 1.7 | 14.4 ± 2.0 | 13.8 ± 2.8 | 14.8 ± 3.0 | 15.5 ± 2.4 | 16.0 ± 2.4 | 13.6*** | 9.4** | 0.0 |
| Proportion 1st Degree Relative Problem Drinkersa | 0.15 ± 0.20 | 0.25 ± 0.22 | 0.34 ± 0.29 | 0.33 ± 0.26 | 0.13 ± 0.19 | 0.19 ± 0.21 | 0.6 | 9.7** | 7.0* |
| Proportion 1st Degree Relative Problem Drug Users b | 0.05 ± 0.14 | 0.10 ± 0.15 | 0.08 ± 0.17 | 0.00 ± 0.00 | 0.04 ± 0.11 | 0.06 ± 0.14 | 0.7 | 0.2 | 0.1 |
| Alcohol Use Variables | |||||||||
| Alcohol Lifetime Use (standard drinks) | 39754 ± 30190 | 18415 ± 13027 | 51095 ± 40426 | 40453 ± 61757 | 2247 ± 2164 | 1801 ± 2245 | 42.5c | 46.6c | 7.3* |
| Duration of Alcohol Use (months) | 354.3 ± 92.7 | 319.7 ± 87.4 | 359.4 ± 108.8 | 384.6 ± 135.4 | 227.5 ± 151.3 | 227.7 ± 153.9 | 14.1c | 15.3c | 1.6 |
| Average Alcohol Consumption (standard drinks/month) | 71.5 ± 95.0 | 51.0 ± 40.4 | 99.6± 102.6 | 105.0 ± 180.4 | 8.5 ± 9.9 | 3.8 ± 5.5 | 26.3c | 33.2c | 4.0 |
| Duration of Maximum Use (months) | 144.6 ± 107.7 | 98.8 ± 68.5 | 164.8 ± 104.9 | 118.1 ± 113.9 | 86.5 ± 69.0 | 70.2 ± 69.4 | 7.3c | 15.6c | 2.6 |
| Max Average Alcohol Consumption (standard drinks/month) | 161.4 ± 127.2 | 94.6 ± 103.2 | 185.4± 115.1 | 110.2 ± 182.2 | 17.4 ± 15.6 | 15.7 ± 14.6 | 36.1c | 51.2c | 2.5 |
| Total Consumption During Maximum Use (standard drinks) | 26608 ± 26524 | 9809 ± 11017 | 36194 ± 39778 | 26747 ± 53349 | 1415 ± 1300 | 987 ± 1155 | 28.7c | 32.8c | 5.8* |
| Withdrawal Scale Mean Frequency | 15.4 ± 14.4 | 18.6 ± 15.9 | 26.9 ± 18.5 | 27.6 ± 12.9 | 4.7 ± 8.2 | 3.9 ± 5.3 | 25.1c | 47.7c | 8.9* |
| Withdrawal Scale Mean Intensity | 24.2 ± 19.0 | 24.6 ± 19.8 | 30.3 ± 19.1 | 39.9 ± 7.0 | 8.5 ± 11.1 | 8.0 ± 9.2 | 23.8c | 41.9c | 4.1 |
|
|
|||||||||
| Smoking Status |
Odds Ratios
|
||||||||
| Lifetime Smokers # (%) | 10 (43.5%) | 5 (25%) | 13 (61.9%) | 4 (80%) | 7 (20%) | 5 (12.8%) | 2.77* | 9.76*** | 3.53* |
| Current Smokers # (%) | 10 (43.5%) | 4 (20%) | 13 (61.9%) | 4 (80%) | 3 (8.6%) | 3 (7.7%) | 5.47*** | 21.41*** | 3.91** |
Effect is significant:
p ≤ .05
p ≤ .01
p ≤ .001
NSAC Men (n=34), NSAC Women (n=37), ALC Women (n=19), ALC+DRG Men (n=20) - Due to adopted participants.
NSAC Men (n=34), NSAC Women (n=37), ALC Women (n=19), ALC+DRG Men (n=19) - Due to adopted participants.
Statistical comparisons of Alcohol Use Variables between TNA and NSAC is not appropriate since the dependent variable is related to the selection criteria.
Exclusion criteria were: 1) lifetime or current diagnosis of schizophrenia or schizophreniform disorder using the computerized Diagnostic Interview Schedule (C-DIS) (American Psychiatric Association 2000; Levitan et al. 1991; Robins et al. 1995), 2) significant history of head trauma, 3) history of significant neurological disease, 4) history of diabetes, stroke, or hypertension that required an emergent medical intervention, 5) history of hepatic disease, 6) clinical evidence of Wernicke-Korsakoff syndrome, or 7) clinical evidence of a physical condition (e.g., hip, knee, ankle, foot, and/or back injuries; found in 4 NSAC, 3 ALC, and 3 ALC+DRG) that would prevent meaningful interpretation of Fregly gait and balance measures (Fregly et al. 1972).
Procedures
Participant screening was initially conducted by a phone interview assessing alcohol use/dependence, use/dependence of other drugs, medical history, and mental health history. All participants were fully informed of the study’s procedures and aims, and signed a consent form prior to their participation. Participants completed four sessions as part of a larger study. However, for the current data, only the first two sessions were considered; demographic, alcohol and drug use and withdrawal data were gathered on the first session and the gait and balance assessment on the second session. Over 90% of participants completed both sessions within a week, while the longest interval between sessions was 4.5 months for one NSAC subject and 19 days for one TNA subject. All subjects were asked to abstain from consuming alcohol for at least 24 hours prior to any lab visit and have a breathalyzer (Intoximeters, Inc., St. Louis, MO) blood alcohol concentration of 0.000. All subjects also were negative on a rapid screening test, Oral Fluid Drug Screen Device (Innovacon, Inc., San Diego, CA) for the presence of cocaine, PCP, THC, opiates, and methamphetamine or other amphetamines. Participants were compensated for their time and travel expenses, and were given a bonus upon study completion.
All participants were: 1) interviewed on their lifetime drug and alcohol use including nicotine using the timeline follow-back methodology (Skinner and Sheu 1982; Sobell et al. 1988); 2) subject to a medical history review by a trained research associate; 3) administered the Family Drinking Questionnaire based on the methodology of Mann and colleagues (1985) and Stoltenberg and colleagues (1998), which asks participants to rate family members as being alcohol abstainers, alcohol users with no problem, or problem drinkers (Family Density of Problem Drinking (FDPD) was defined as the proportion of first-degree relatives that were problem drinkers.); 4) administered the Family History Substance Use Questionnaire, similar to the Family Drinking Questionnaire, but assessing drug use problems, and the Family Density of Problem Drug Use (FDPDU) was computed; 5) administered the C-DIS which yields psychiatric diagnoses and symptom counts; 6) administered a post-alcohol withdrawal hyper-excitability (PAWH) assessment using a self-report questionnaire (Fein et al. 2004b; Fein and Allen 2005), wherein subjects estimated the frequency and general severity (on a 0–100 scale) of physical and psychological symptoms experienced during withdrawal. The symptoms were compiled from the Diagnostic Interview Schedule (DIS)(Robins et al. 1995), the alcohol dependence scale (Skinner and Allen 1982), and SSAGA interviews (Bucholz et al. 1994).
In the Timeline Follow-Back interview for a particular substance, participants divide their use history into periods of relatively consistent use and denote the duration and the pattern and intensity of use for each period. From this information we extract the lifetime Duration of Alcohol Use (in months), Average Alcohol Consumption (in standard drinks per month) and Lifetime Use, (Average Alcohol Consumption times Duration). Duration, consumption level and use are also derived for the interval of maximum use. See Table 1.
Gait and Balance
Gait and standing balance were assessed with the Walk-a-Line Ataxia Battery (Fregly et al. 1972) which consists of three parts, each performed with eyes open (E/O) and then with eyes closed (E/C): 1) Sharpened Romberg, where participants stood with feet heel-to-toe with arms folded across the chest for a maximum of 60 seconds; 2) Stand on One Leg, wherein each leg was tested separately with a maximum score per trial of 30 seconds; and 3) Walk Heel-to-Toe wherein the participant walked in a straight line heel-to-toe with arms folded across the chest for a maximum of 10 steps. Each condition was repeated twice unless a perfect score was obtained on the first trial, in which case the participant received a perfect score.
Statistical Analyses
Because Fregly scores were highly non-normally distributed (negatively skewed for the easier tasks and positively skewed for the more difficult tasks), nonparametric statistics (Wilcoxon Mann-Whitney test) were used. The Wilcoxon Mann-Whitney odds ratios (O’Brien and Castelloe 2006) were computed as an effect size measure, indexing the likelihood of a random individual from one group having higher scores than a random individual from the other group. In Table 1, effect size for integer variables is partial η2, the proportion of dependent variable variance independently accounted for by the effect. In Table 1, the Wilcoxon Mann-Whitney Odds Ratio is the effect size measure for rates (e.g., giving the ratio of the odds of smoking for a member of one group being higher than the odds of smoking for a member of the other group).
Spearman correlations examined associations between Fregly scores and lifetime alcohol use (in standard drinks), age, family density of problem drinking and family density of problem drug use.
Results
Demographic and Alcohol Use Variables
Table 1 presents demographic and alcohol use data. NSAC were than ALC+DRG (p = .038) and ALC (p = .010). ALC and ALC+DRG did not differ in Age or Years of Education, however both ALC and ALC+DRG had less education than NSAC (p < .002). ALC+DRG had a higher family density of problem drinkers than either NSAC (p = .005) or ALC (p = .018). Groups did not differ in terms of family density of problem drug use. Finally, ALC+DRG had greater Alcohol Lifetime Use (total standard drinks) (p = .025) and Alcohol Maximum Use (total standard drinks during peak use) (p = .047) than ALC. Within TNA, men had a dramatically higher Alcohol Lifetime Use than women (p = .018), and higher Alcohol Maximum Use than women (p = .040). Frequency of smoking cigarettes was dramatically higher in alcoholics vs. controls for both lifetime and current smoking, with more smoking in ALC+DRG than ALC. Men were over twice as likely as women to be lifetime or current smokers.
The average frequency and intensity of withdrawal symptoms in TNA was dramatically greater than withdrawal symptom frequency (p < .001) and severity (p < .001) in NSAC, as would be expected since NSAC consisted entirely of light/non-drinkers. ALC+DRG had higher withdrawal symptom frequency than ALC (p = .013), with 8.9% of variance accounted for by Group differences, but there was only a weak trend for ALC+DRG to have a greater withdrawal symptom intensity than ALC (accounting for 4.1% of the variance, (p = .097). Within TNA, men and women did not differ in the frequency or average intensity of withdrawal symptoms.
Gait and Balance Measures
Table 2 shows mean Fregly scores for NSAC, ALC, and ALC+DRG.
Table 2.
Mean scores on Fregly gait and balance measures by group: NSAC, TNA ALC, and TNA ALC+DRG.
| Fregly Gait and Balance | TNA | NSAC | Wilcoxon Mann-Whitney Odds Ratios | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| ALC | ALC+DRG | Men (n=35) | Women (n=39) | NSAC vs ALC | NSAC vs ALC+DRG | ALC vs ALC+DRG | Current vs Non-Current Drug Use | |||
| Men (n=23) | Women (n=20) | Men (n=21) | Women (n=5) | |||||||
|
|
|
|||||||||
| Romberg, Eyes Open | 120.00 ± 0.00 | 118.65 ± 5.37 | 113.67 ± 15.07 | 109.00 ± 24.60 | 116.89 ± 12.90 | 117.33 ±11.64 | 0.98 | 1.30* | 1.36* | 0.46* |
| Romberg, Eyes Closed | 81.65 ± 48.73 | 87.35 ± 38.68 | 64.48 ± 47.66 | 47.80 ± 46.00 | 82.46 ± 45.45 | 89.7 ± 41.27 | 1.07 | 1.80* | 1.72 | 0.89 |
| Stand Left Leg, Eyes Open | 56.83 ± 10.76 | 58.25 ± 7.83 | 53.43 ± 16.99 | 55.60 ± 9.84 | 57.8 ± 6.37 | 53.64 ± 14.45 | 0.80 | 1.03 | 1.27 | 0.59 |
| Stand Left Leg, Eyes Closed | 19.61 ± 18.81 | 26.10 ± 22.50 | 16.14 ± 17.45 | 8.20 ± 5.63 | 21.23 ± 19.93 | 20.74 ± 17.28 | 0.94 | 1.68 | 1.74 | 1.15 |
| Stand Right Leg, Eyes Open | 59.04 ± 4.59 | 57.65 ± 10.28 | 48.29 ± 20.01 | 54.80 ± 11.63 | 56.40 ± 10.61 | 56.59 ± 11.62 | 0.92 | 1.51* | 1.66** | 0.52 |
| Stand Right Leg, Eyes Closed | 13.78 ± 11.47 | 20.40 ± 19.17 | 17.95 ± 19.32 | 12.60 ± 7.64 | 23.77 ± 19.17 | 20.97 ± 16.20 | 1.50 | 1.63 | 1.14 | 0.68 |
| Walk Floor, Eyes Open | 19.57 ± 2.09 | 20.00 ± 0.00 | 18.90 ± 2.79 | 18.20 ± 4.03 | 19.51 ± 1.90 | 19.28 ± 2.76 | 0.89 | 1.16 | 1.29 | 1.11 |
| Walk Floor, Eyes Closed | 13.09 ± 6.61 | 12.30 ± 5.40 | 10.48 ± 6.06 | 7.20 ± 7.56 | 11.69 ± 6.44 | 15.13 ± 6.09 | 1.15 | 1.99** | 1.76 | 1.20 |
Effect is significant:
p ≤ .05,
p ≤ .01
NSAC vs. ALC
There were no significant ALC vs. NSAC effects for any of the Fregly measure. There was one trend (p = .073) toward worse performance by ALC than NSAC on Stand on Right Leg E/C, (U = 1274), indicating that NSAC were 50% more likely to have a higher (better) score than ALC. Across NSAC and ALC, Spearman correlations yielded significant (one-tailed) negative associations between Age and performance on four Fregly measures: Stand on Left Leg E/O (rs = −.282, p = .001), Stand on Left Leg E/C (rs = −.340, p < .001), Stand on Right Leg E/O (rs = −.224, p = .008), and Stand on Right Leg E/C (rs = −.225, p = .007), all reflecting lower mean scores for older subjects. Multivariate ANCOVA with Group as a fixed factor and Age as a covariate showed a significant effect of Age (F (8,104) = 2.169, p = .036, partial η2 = .143), but no Group or Group by Age interaction.
NSAC vs. ALC+DRG
Comparison of NSAC vs. ALC+DRG showed poorer performance for ALC+DRG on the Sharpened Romberg E/O (U = 836.5, p = .047), Sharpened Romberg E/C (U = 688.0, p = .021), Stand on Right Leg E/O (U = 766.5, p = .016), and Walk on Floor E/C (U = 643.0, p = .010), with trends toward worse performance of ALC+DRG on Stand on Left Leg E/C (U = 718.5, p = .055), and Stand on Right Leg E/C (U = 732.5, p = .071). Across NSAC and ALC+DRG, Spearman correlations yielded significant (one-tailed) negative associations between Age and performance on three Fregly measures: Stand on Left Leg E/O (rs = −.188, p = .031), Stand on Left Leg E/C (rs = −.387, p < .001), and Stand on Right Leg E/C (rs = −.335, p < .001), and trends toward negative associations on Romberg E/O (rs = −.144, p = .077), Stand on Right Leg E/O (rs = −.144, p = .076), and Walk Floor E/C (rs = −.132, p = .095), all reflecting lower mean scores for older subjects. Multivariate ANCOVA with Group as a fixed factor and Age as a covariate yielded significant effects of Group (F (8, 87) = 2.079, p = .046, partial η2 = .161), Age (F (8, 87) = 4.852, p < .001, partial η2 = .309), and a Group by Age interaction (F (8, 87) = 2.589, p = .014, partial η2 = .192), reflecting steeper age-associated declines in Fregly performance for ALC+DRG vs. NSAC.
ALC vs. ALC+DRG
There were significant ALC vs. ALC+DRG effects on Sharpened Romberg E/O (U = 473.5, p = .043) and Stand on Right Leg E/O (U = 421.0, p = .007), with trends on Sharpened Romberg E/C (U = 410.5, p = .053), Stand on Left Leg E/C (U = 521.5, p = .061), Walk on Floor E/O (U = 488.0, p < .05) and Walk on Floor E/C (U = 405.0, p = .054), all reflecting worse performance by ALC+DRG, as indicated by odds ratios greater than 1 representing the ALC vs. ALC+DRG comparison.
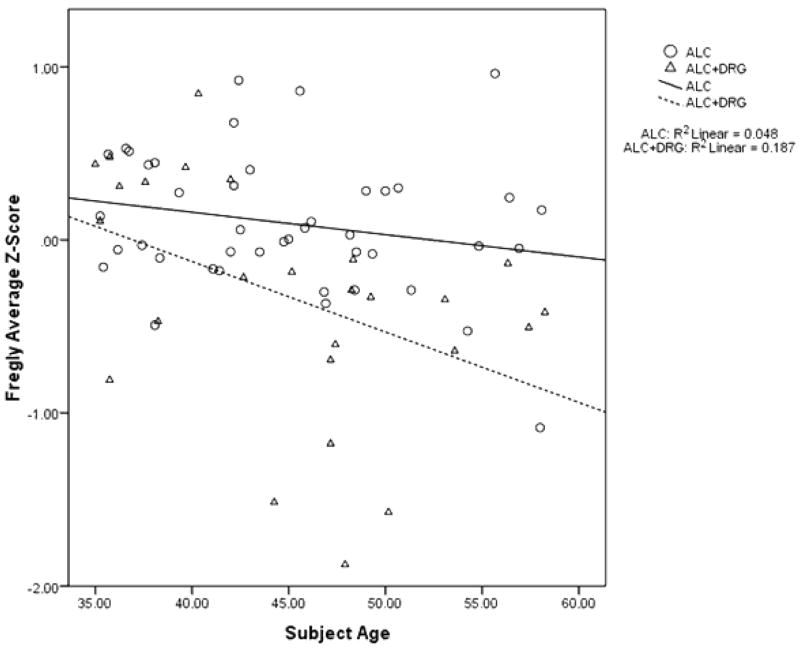
Across ALC+DRG and ALC, Spearman correlations yielded significant (one-tailed) negative correlations between Alcohol Lifetime Use and Stand on Left Leg E/C (rs = −.246, p = .022), Stand on Right Leg E/O (rs = −.260, p = .026), and Stand on Right Leg E/C (rs = −.282, p = .010). FDPD and FDPDU were not associated with Fregly scores. Multivariate ANCOVA with Fregly scores as dependent variables, Group as a fixed factor, and Age and Alcohol Lifetime Use as covariates yielded main effects for Group (F (8, 56) = 3.997, p = .001, partial η2 = .363), Age (F (8, 56) = 5.305, p < .001, partial η2 = .431), and Alcohol Lifetime Use (F (8, 56) = 2.919, p = .009, partial η2 = .294), and a Group by Age interaction (F (8, 56) = 5.123, p < .001, partial η2 = .423), reflecting a steeper age-associated decline in Fregly performance for ALC+DRG vs. ALC (See Figure 1, which shows average Fregly Z-scores, based on our entire study sample, for ALC+DRG vs. ALC as a function of Age). There was no Group by Alcohol Lifetime Use interaction, showing that although both lifetime drug codependence and Alcohol Lifetime Use make independent contributions to gait and balance disturbance in TNA, the effect of Alcohol Lifetime Use is not affected by drug codependence status.
Figure 1.
Average Fregly Z-scores, based on our entire study sample, for TNA, ALC+DRG and ALC as a function of Age.
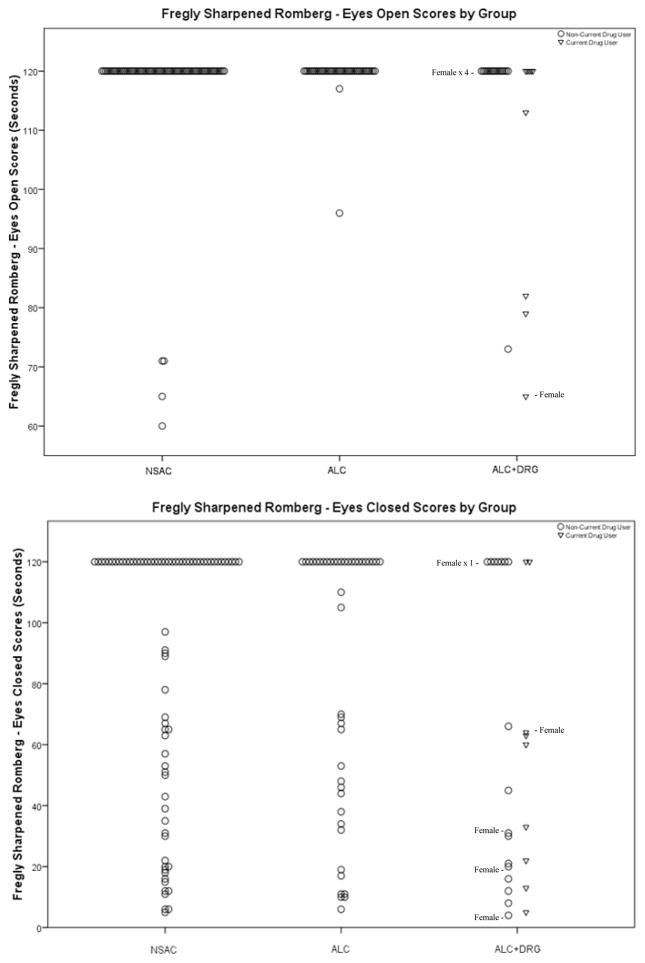
Table 2 also shows the comparison within ALC+DRG between current and non-current drug users. As noted above, there were only 9 current drug users (including one woman). Current drug users had significantly poorer Fregly performance than non-current drug users only on the Romberg E/O test, but these comparisons had very limited power given the small sample sizes.
Figures 2 through 5 display scores for each Fregly measure by Group (and by current drug use status within ALC+DRG). Since ALC+DRG was primarily male, we identified all ALC+DRG females on figure 2–5 to show the degree to which differences between ALC+DRG and the other groups was present in females. All Fregly measures exhibit a ceiling effect that was more prevalent on E/O than E/C measures. Figure 2 shows the scores for Sharpened Romberg E/O and E/C measures. NSAC and ALC scored similarly on E/O. Both groups had nearly 95% of participants with perfect scores, while ALC+DRG performed considerably worse, especially current drug users. Non-current drug users were more than twice as likely to perform better than current drug users (OR = 2.19). The E/C measure, like most all E/C measures, has more variability than the E/O measure, but showed the same pattern as did E/O. NSAC and ALC had similar distributions, with better performance than ALC+DRG; however there was no difference between current and non-current drug users for the Romberg E/C measure.
Figure 2.
Fregly Sharpened Romberg
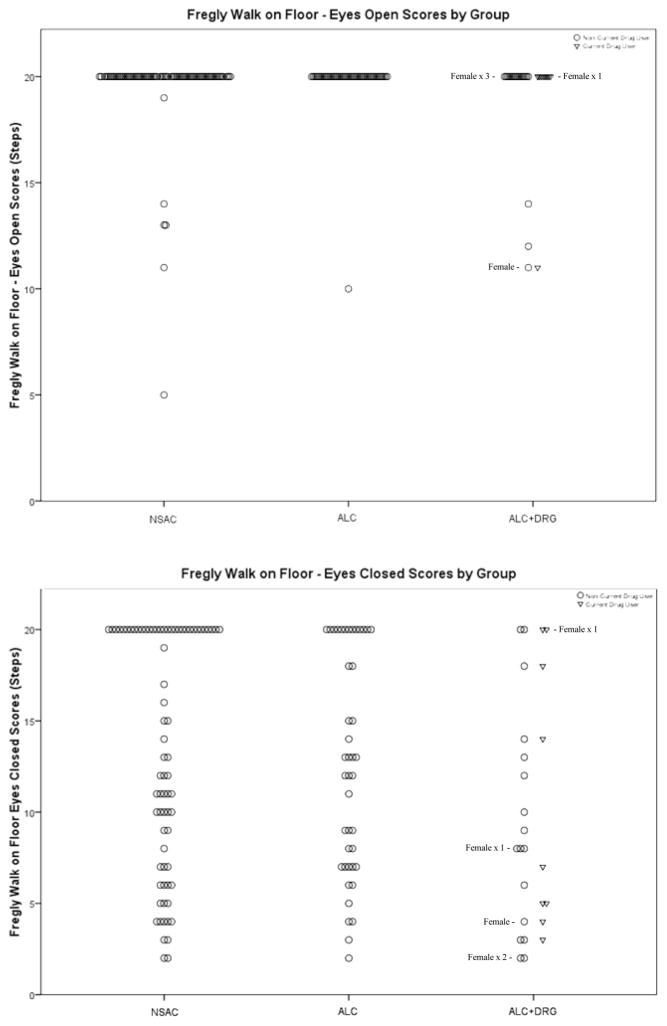
Figure 5.
Frcgly Walk on Floor
Figure 3 displays the scores for the Stand on Left Leg E/O and E/C. The E/O measure shows a ceiling effect for most participants. The Left Leg E/C measure has a negative skew and is more evenly distributed across groups than E/O.
Figure 3.
Fregly Stand on Left Leg
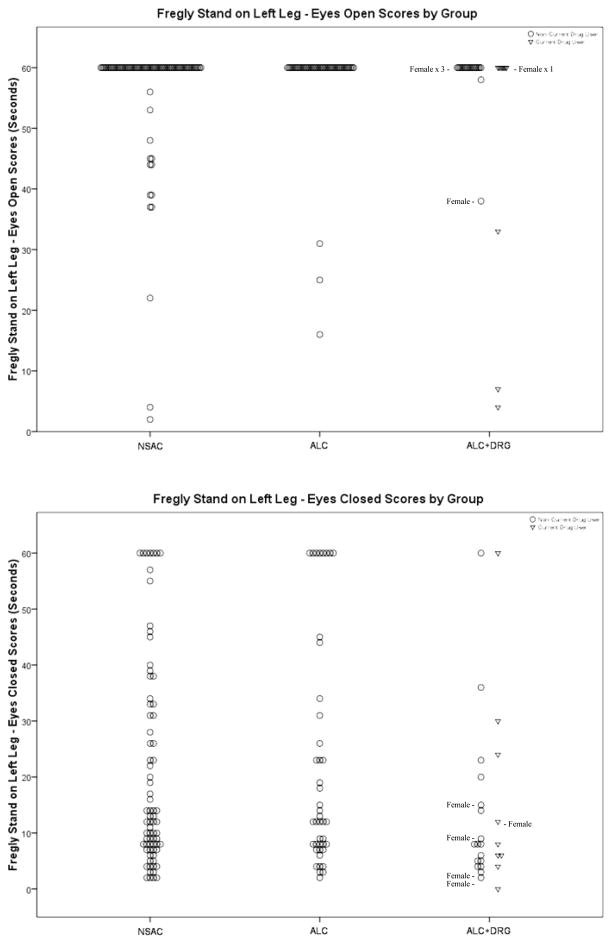
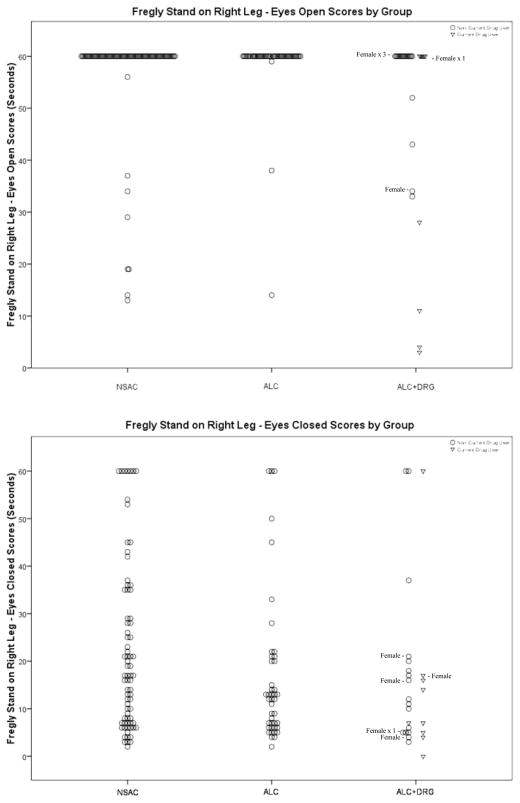
Figure 4 shows the scores for Stand on Right Leg E/O and E/C. NSAC and ALC scored significantly better than ALC+DRG (OR = 1.51 and 1.66) with nearly 90% of NSAC and ALC achieving perfect scores, while the ALC+DRG group achieved perfect scores only two-thirds of the time. The Stand on Right Leg E/C measure had many scores at both ends of the distribution and few in the middle, across all groups. ALC and ALC+DRG scores were similarly distributed.
Figure 4.
Fregly Stand on Right Leg
Figure 5 displays the scores for Walk on Floor E/O and E/C measures. NSAC, ALC and ALC+DRG all had similar score distributions for the E/O measure. Both NSAC and ALC groups exhibited a ceiling effect with nearly half of NSAC and a third of ALC participants scoring a maximum of 20 steps for the Eyes Closed measure. The ALC+DRG group was noticeably less successful at this task with only 4 of the 26 members scoring a perfect 20 steps. Within the ALC+DRG group, both current drug and non-current drug users were distributed across the full range of performance and did not differ (as also indicated by an odds ratio of close to 1).
Effect of Smoking Status on Fregly Scores
Nonparametric comparison of TNA smokers vs. non-smokers found no difference on any Fregly measure, whether examining ever smoked (all cases, p > 0.51) or currently smoking (all cases, p > 0.33).
Effects of Withdrawal Symptoms on Fregly Scores
Across TNA, we computed Spearman correlations of each Fregly measure with the average frequency and intensity of withdrawal symptoms. The only significant associations were for the Sharpened Romberg E/O measure where increasing frequency (r =−.27, p = 0.25) and severity of withdrawal symptoms (r = −0.25, p = .038; p’s uncorrected for multiple comparisons) was associated with poorer performance.
Discussion
Our central findings were 1) no evidence of disturbed gait and balance in ALC compared to NSAC, and 2) disturbed gait and balance in ALC+DRG relative to both NSAC and ALC, along with a steeper age-associated decline in Fregly performance in ALC+DRG than in the other groups. We noted that NSAC were older than either TNA sample, biasing our results against demonstrating gait and balance disturbance in TNA. Given this age difference, our results likely underestimate gait and balance impairments in TNA.
ALC were not significantly impaired relative to NSAC on any of the Fregly measures, with only a trend toward worse performance on Stand on Right Leg E/C, a measure of balance without visual support that is among the most susceptible to impairment. The lack of gait and balance disturbance in ALC (who are actively drinking) is in contrast to that typically found in samples of treated alcoholics, particularly those with relatively short abstinence durations (Smith and Fein 2011; Sullivan et al. 2000a; 2000b), for whom impairment on multiple gait and balance measures is commonly reported. This finding is consistent with the contention that TNA samples represent a population with less severe disease than treated alcoholics (Fein and Landman 2005), including less brain structural damage (Fein et al. 2002; Fein et al. 2010), less impairment on other performance measures that include decision making in a simulated gambling task (Fein et al. 2006), and neuropsychological performance in a broad range of cognitive domains (Smith and Fein 2010).
Second, in contrast to ALC, ALC+DRG had significantly impaired gait and balance relative to both NSAC and ALC. Odds ratios indicate that ALC were at least 66% more likely to score better than ALC+DRG on four of the eight Fregly measures and at least 27% more likely to score better on three others. Figures 2–5 show that this effect was not due to more men than women being in ALC+DRG – the effect was clearly present in the ALC+DRG women. In many ways, ALC+DRG look like treated alcoholics. Our prior research on TNA was limited to individuals with lifetime alcohol dependence, but without lifetime dependence on other drugs of abuse. ALC+DRG have a family density of alcohol problems that is higher than ALC, and similar to that found in our Honolulu sample of treated alcoholics (Smith and Fein 2011). Moreover, the higher average alcohol consumption in ALC+DRG vs. ALC is also consistent with what we find in treated samples. We have shown in California data that an ALC sample does not have the P300 event-related potential amplitude reduction present in treated alcoholics (Fein and Andrew 2011), suggesting that they do not have the inherited genetic vulnerability to alcoholism which may be related to brain structural and functional abnormalities that are additive to the effects of actual alcohol abuse. Examination of P3b amplitude in ALC+DRG could prove invaluable in determining whether ALC+DRG are more similar to treated alcoholics vs. TNA in their inherited genetic vulnerabilities. We also note the higher family density of alcohol problems in TNA women than men. This could reflect a selection bias for a higher drinking threshold (relative to their body weight) for women compared to men, when volunteering for a study of heavy drinkers. It has been shown that women have a higher threshold for drinking-related problems before seeking treatment (Dawson 1996; Schober and Annis 1996), leading to an over-representation of the most severely affected women in treatment samples.
The multivariate analysis shows that lifetime drug codependence and greater lifetime alcohol consumption contribute independently to the gait and balance disturbance in ALC+DRG vs. ALC. Were the difference between groups a mere consequence of the higher alcohol consumption in ALC+DRG vs. ALC, then there would not have been a group effect on Fregly measures in addition to the effect of alcohol consumption. The independent contribution of drug codependence and greater alcohol use to more severe ataxia in ALC+DRG is consistent with reports of additive detrimental effects of chronic abuse of ethanol and of other drugs (e.g., marijuana) on cognitive and motor performance (Esplin and Capek 1976; Perez-Reyes et al. 1988). Furthermore, our finding of a steeper age-associated decline in Fregly performance in ALC+DRG vs. ALC, suggests that chronic alcohol abuse and drug codependence may accelerate age-related functional decline and anatomical degeneration of the neural underpinnings of gait and balance. This is consistent with evidence from Volkow and colleagues that both methamphetamine (Volkow et al. 2001) and alcohol abuse (Volkow et al. 2007) damage the nigrostriatal dopamine system. The same investigators have proposed that methamphetamine abuse, even at relatively young ages, may increase risk for development of parkinsonian-like motor deficits with age (Volkow et al. 2001). This proposal is supported by findings of abnormalities in regional cerebral blood flow (rCBF) in the striatum, thalamus, cingulum, mesiodorsal prefrontal cortex, and pons that resemble patterns reported for persons with atypical Parkinson’s disease in chronic methamphetamine abusers (YA Chung et al. 2010). Other investigations (Eisch and Harburg 2006; Hernandez-Rabaza et al. 2006) have provided evidence that opiates and psychostimulants, as well as alcohol, can accelerate aging effects on the brain by diminishing neural progenitors and/or by impairing the long-term survival of neural precursors.
In conclusion, our finding of no disturbance of gait and balance in ALC is consistent with previous findings of minimal neuroanatomical and neuropsychological impairment in TNA, providing further evidence that TNA (without a drug codependence) represents a population that is different, and less impaired, than treated alcoholics. Furthermore, our results suggest that ALC+DRG may represent a subset of TNA, who, in addition to having a lifetime drug codependence, may differ from ALC along other population parameters. A limitation of the present study was that small sample sizes within drug dependence categories did not allow for meaningful comparisons of gait and balance for individuals with lifetime abuse of different drugs (i.e., cocaine vs. methamphetamine vs. marijuana vs. various poly-drug abuse combinations). Larger samples will be needed to disentangle effects on gait and balance in alcoholics that are associated with lifetime codependence on different drugs or combinations of drugs. Further, our study did not include a drug only group, making it difficult to dissociate the potential contributions of alcohol vs. drugs.
Acknowledgments
This work was supported by National Institutes for Health, NIH Grants #AA013659 and #AA011311.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 2000. [Google Scholar]
- Baker KG, Harding AJ, Halliday GM, Kril JJ, Harper CG. Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience. 1999;91:429–438. doi: 10.1016/s0306-4522(98)90664-9. [DOI] [PubMed] [Google Scholar]
- Barros-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, Parcet MA, Avila C. Reduced striatal volume in cocaine-dependent patients. Neuroimage. 2011;56:1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Cham R, Studenski SA, Perera S, Bohnen NI. Striatal dopaminergic denervation and gait in healthy adults. Exp Brain Res. 2008;185:391–398. doi: 10.1007/s00221-007-1161-3. [DOI] [PubMed] [Google Scholar]
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, Sim ME, Song IC, Kim J, Chang KH, Renshaw PF. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10:765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- Chung YA, Peterson BS, Yoon SJ, Cho SN, Chai S, Jeong J, Kim DJ. In vivo evidence for long-term CNS toxicity, associated with chronic binge use of methamphetamine. Drug Alcohol Depend. 2010;111:155–160. doi: 10.1016/j.drugalcdep.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: Findings from the national epidemiologic survey on alcohol and related conditions. Drug Alcohol Depend. 2007;86:214–221. doi: 10.1016/j.drugalcdep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Dar MS. Cerebellar cb(1) receptor mediation of delta(9)-THC-induced motor incoordination and its potentiation by ethanol and modulation by the cerebellar adenosinergic a(1) receptor in the mouse. Brain Res. 2000;864:186–194. doi: 10.1016/s0006-8993(00)02103-x. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: Necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Gender differences in the probability of alcohol treatment. J Subst Abuse. 1996;8:211–225. doi: 10.1016/s0899-3289(96)90260-6. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Finn P, Fein G. Treatment-naive active alcoholics have greater psychiatric comorbidity than normal controls but less than treated abstinent alcoholics. Drug Alcohol Depend. 2008;98:115–122. doi: 10.1016/j.drugalcdep.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- Esplin B, Capek R. Quantitative characterization of THC and ethanol interaction. Res Commun Chem Pathol Pharmacol. 1976;15:199–202. [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2004a;28:1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, McGillivray S, Finn P. Mismatch negativity: No difference between treatment-naive alcoholics and controls. Alcohol Clin Exp Res. 2004b;28:1861–1866. doi: 10.1097/01.alc.0000148109.79230.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Allen J. EEG spectral changes in treatment-naive, actively drinking alcoholics. Alcohol Clin Exp Res. 2005;29:538–546. doi: 10.1097/01.alc.0000159107.08471.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B. Treated and treatment-naive alcoholics come from different populations. Alcohol Clin Exp Res. 2005;35:19–26. doi: 10.1016/j.alcohol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Fein G, McGillivray S, Finn P. Normal performance on a simulated gambling task in treatment-naive alcohol-dependent individuals. Alcohol Clin Exp Res. 2006;30:959–966. doi: 10.1111/j.1530-0277.2006.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Chu R, Barakos J. Parietal gray matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic men. Alcohol Clin Exp Res. 2009a;33:1806–1814. doi: 10.1111/j.1530-0277.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Di Sclafani V, Barakos J, Harper C. Increased white matter signal hyperintensities in long-term abstinent alcoholics compared with nonalcoholic controls. Alcohol Clin Exp Res. 2009b;33:70–78. doi: 10.1111/j.1530-0277.2008.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Barakos J. Age related gray matter shrinkage in a treatment naïve actively drinking alcohol dependent sample. Alcohol Clin Exp Res. 2010;34:175–182. doi: 10.1111/j.1530-0277.2009.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Andrew C. Event-related potentials during visual target detection in treatment-naive active alcoholics. Alcohol Clin Exp Res. 2011;35:1171–1179. doi: 10.1111/j.1530-0277.2011.01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregly AR, Graybiel A, Smith MJ. Walk on floor eyes closed (wofec): A new addition to an ataxia test battery. Alcohol Clin Exp Res. 1972;43:395–399. [PubMed] [Google Scholar]
- Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: Profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav. 1990;37:561–565. doi: 10.1016/0091-3057(90)90028-g. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Cannabinoid receptor localization in brain: Relationship to motor and reward systems. Alcohol Clin Exp Res. 1992;654:19–32. doi: 10.1111/j.1749-6632.1992.tb25953.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Dominguez-Escriba L, Barcia JA, Rosel JF, Romero FJ, Garcia-Verdugo JM, Canales JJ. Binge administration of 3,4-methylenedioxymethamphetamine (“ecstasy“) impairs the survival of neural precursors in adult rat dentate gyrus. Neuropharmacology. 2006;51:967–973. doi: 10.1016/j.neuropharm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Jaatinen P, Rintala J. Mechanisms of ethanol-induced degeneration in the developing, mature, and aging cerebellum. Cerebellum. 2008;7:332–347. doi: 10.1007/s12311-008-0034-z. [DOI] [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE. Effect of acute and chronic pretreatment with delta1-tetrahydrocannabinol on motor impairment by ethanol in the rat. Can J Physiol Pharmacol. 1974;52:291–297. doi: 10.1139/y74-040. [DOI] [PubMed] [Google Scholar]
- Levitan RD, Blouin AG, Navarro JR, Hill J. Validity of the computerized DIS for diagnosing psychiatric inpatients. Can J Psychiatry. 1991;36:728–731. [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Melgaard B, Ahlgren P. Ataxia and cerebellar atrophy in chronic alcoholics. J Neurol. 1986;233:13–15. doi: 10.1007/BF00313983. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien R, Castelloe J. Exploiting the link between the Wilcoxon-Mann-Whitney test and a simple odds statistic. SAS Institute Inc; San Francisco, CA: 2006. pp. 209–231. [Google Scholar]
- Perez-Reyes M, Hicks RE, Bumberry J, Jeffcoat AR, Cook CE. Interaction between marihuana and ethanol: Effects on psychomotor performance. Alcohol Clin Exp Res. 1988;12:268–276. doi: 10.1111/j.1530-0277.1988.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging. 2006;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. Diagnostic interview schedule for DSM-IV (DIS-IV) 1995. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Rohlfing T, O’Reilly AW, Sassoon SA, Pfefferbaum A, Sullivan EV. Improvement in memory and static balance with abstinence in alcoholic men and women: Selective relations with change in brain structure. Psychiatry Res. 2007;155:91–102. doi: 10.1016/j.pscychresns.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober R, Annis HM. Barriers to help-seeking for change in drinking: A gender-focused review of the literature. Alcohol Clin Exp Res. 1996;21:81–92. doi: 10.1016/0306-4603(95)00041-0. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: Measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The lifetime drinking history and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith S, Fein G. Cognitive performance in treatment-naive active alcoholics. Alcohol Clin Exp Res. 2010;34:2097–2105. doi: 10.1111/j.1530-0277.2010.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Fein G. Persistent but less severe ataxia in long-term versus short-term abstinent alcoholic men and women: A cross-sectional analysis. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. Studies of Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Starr JM, Leaper SA, Murray AD, Lemmon HA, Staff RT, Deary IJ, Whalley LJ. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: Density versus dichotomy. Alcohol Clin Exp Res. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: Relation to ataxia. Neuropsychology. 2000a;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: Relationships to changes in brain structure. Neuropsychology. 2000b;14:178–188. [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A. Striatal and forebrain nuclei volumes: Contribution to motor function and working memory deficits in alcoholism. Biol Psychiatry. 2005;57:768–776. doi: 10.1016/j.biopsych.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: A quantitative physiological and MRI study. Cerebral Cortex. 2006;16:1077–1086. doi: 10.1093/cercor/bhj048. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Linden DJ. Cannabinoid receptor modulation of synapses received by cerebellar Purkinje cells. J Neurophysiol. 2000;83:1167–1180. doi: 10.1152/jn.2000.83.3.1167. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS ONE. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Rippens PD. Different variables are associated with help-seeking patterns and long-term outcomes among problem drinkers. Alcohol Clin Exp Res. 2004;29:433–439. doi: 10.1016/j.addbeh.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH, editors. The Wernicke-Korsakoff syndrome and related neurological disorders due to alcoholism and malnutrition. F.A. Davis Co; Philadephia: 1989. [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Alcohol Clin Exp Res. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: Possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35:1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]