Abstract
This study investigated the hypothesis that vascular risk factors are amyloidogenic. Participants were 43 persons, most with normal cognition or mild cognitive impairment. Vascular risk was quantified using the Framingham Coronary Risk Profile score (FCRP). Cerebral amyloid was measured by 11C-PIB PET and quantified with a Global PIB index, which is the average of distribution volume ratios in selected cortical regions of interest. In a bivariate model FCRP accounted for 16% of the variance in PIB index (p < .008) and the positive association remained significant controlling for age and sex. The effect of FCRP was independent of APOE genotype, which was also associated as expected with PIB. Carotid intima-media thickness was not associated with PIB index. Effects of individual FCRP component risk factors, cholesterol and glycemic status on PIB index were all non-significant, suggesting an aggregate effect of risk factors. Although this is a correlational observation it may represent a causal relationship as there are multiple, plausible, amyloidogenic mechanisms of vascular risk factors.
Keywords: vascular risk factors, coronary risk factors, cerebral amyloid, Mild Cognitive Impairment, Normal Aging, Alzheimer’s disease
1. Introduction
Epidemiological studies have shown that multiple risk factors for vascular disease, both cerebral and coronary, are corresponding risk factors for Alzheimer’s disease (AD) (Decarli, 2004). Among the major risk cardiovascular factors, diabetes has generally been found to confer a relative risk of 1.4-2.4 for AD (Luchsinger, 2010). Midlife hypertension, especially when untreated, substantially elevates the risk of Alzheimer’s disease decades later (Launer, et al., 1995; 2000), and correlates with neocortical plaque counts at autopsy inin aged subjects (Petrovitch, et al., 2000). The association between late life hypertension and incident AD is weaker and less clearly established (Feldstein, 2010; Qiu, et al., 2003; Skoog, et al., 1996). Similarly, studies that measure lipids at midlife have consistently reported elevated latelife risk of AD (Kivipelto, et al., 2002; Whitmer, et al., 2005; Solomon, et al., 2009) whereas studies of latelife hyperlipidemia report inconsistent or negative (protective) associations (Reitz, et al., 2004; Mielke, et al., 2005; Reitz, et al., 2010). Interestingly, it was recently reported that in middle aged persons, high total cholesterol was associated with hypometabolism in cortical regions typically affected by AD (Reiman, et al., 2010). Although the evidence on smoking is not totally consistent, a number of large, methodologically strong studies have found that midlife smoking increases the risk of AD substantially (Ott, et al., 1998; Rusanen, et al., 2011). Moreover, a number of studies show that increasing numbers of vascular risk factors are associated with increasing risk of AD (Luchsinger, et al., 2005; Whitmer, et al., 2005). The overall pattern of findings in this literature raises the possibility that there may be a common mechanism(s) behind the association between vascular risk and AD, although a particular mechanism has yet to be identified.
One potential such mechanism is that vascular risk factors lead to cerebrovascular disease which then augment the symptomatic expression of AD pathology (Snowdon, et al., 1997). Support for this view comes from community-based cohort studies that show high rates of unrecognized cerebrovascular disease in the aged (Longstreth, et al., 1998; DeCarli, et al., 2005) and also high rates of mixed AD and cerebrovascular pathology in demented cases (Schneider, et al., 2007; Neuropathology Group Medical Research Council, 2001). Alternatively, it has been proposed that vascular risk factors may promote the deposition of cerebral β-amyloid (Aβ) and thus AD (Altman and Rutledge, 2010; Bhat, 2010; Kalaria, 1999). For example, a variety of molecules important in cholesterol processing also play a role in Aβ processing (Di Paolo and Kim, 2011). There is evidence that insulin resistance can increase plasma levels of Aβ and perhaps increases cerebral deposition or retention of Aβ (Craft, 2009). Conversely, evidence from animal models show that brain clearance of amyloid occurs along vascular channels; since cerebrovascular disease leads to vascular remodeling, it is plausible that cerebrovascular disease also alters amyloid clearance (Dotti and De Strooper, 2009; Bell and Zlokovic, 2009).
This study was designed to test whether vascular risk is associated with cerebral amyloid deposition. For this initial investigation we chose the Framingham Coronary Risk Profile as a summary index of vascular risk factors. The FCRP is a weighted combination of the four major modifiable risk factors for cardiovascular disease; smoking, hypertension, diabetes, and hyperlipidemia (Wilson, et al., 1998). The FCRP is commonly used to quantify 10-year risk of incident coronary disease, is associated with elevated stroke risk (Touboul, et al., 2005), and the FCRP predicts both coronary and carotid atherosclerosis (Cho, et al., 2011; Touboul, et al., 2005). We hypothesized that higher FCRP scores would be associated with elevated cerebral Aβ measured with PET using the tracer (11)C-PIB (Pittsburg Compound B).
2. Methods
2.1 Participants
Participants were 43 persons (31 men), mean age 79, from two longitudinal studies that recruit cognitively normal and mild cognitive impairment (MCI) patients at increased risk for vascular disease. Most participants were acquired either through community-based recruitment using a protocol designed to obtain a demographically diverse cohort, or through sources such as stroke clinics and support groups attended by people with high levels of vascular risk factors. Inclusion criteria included age 65 or older, cognitive function in the normal to mild dementia range; exclusion criteria were severe or unstable medical illness, Axis I psychopathology other than depression, head injury with significant loss of consciousness and/or cognitive sequelae, sensory or physical limitations that would preclude cognitive testing, diagnosis of dementia due to causes other than Alzheimer’s disease, vascular disease or the combination thereof. Data from 27 of these participants is included in a related paper in this issue (Marchant, et al., this issue).
Clinical diagnostic evaluations appropriate for memory disorders and dementia were done at the UC Davis Alzheimer’s Disease Center. The same group of clinicians evaluated all participants using uniform diagnostic criteria and clinical protocols that were highly similar for the two studies. Clinical Dementia Rating Scale (CDR) (Morris, 1993) scores were 0 (n=27), 0.5 (n=12), and 1-2 (n=4). Table 1 provides more details on participant characteristics.
Table 1.
Characteristcs of the sample.
| M, (SD) unless labeled otherwise | |
|---|---|
| Age | 78.9 (6.7) |
| Years Education | 14.9 (2.6) |
| Sex (M:F) | 31 male:12 female |
| MMSE | 27.9 (2.1) |
| FCRP | 16.1 (7.8) |
| Global PIB index | 1.19 (.24) (18 PIB positive) |
| CIMT (mm) | .86 (.14)** |
2.2 Vascular Risk Measure
The FCRP uses empirically derived age and gender adjusted weighting of categorical variables to predict the 10 year risk of coronary heart disease and is a weighted sum of: smoking, diabetes, hypertension, high cholesterol. Higher scores indicate greater risk.
2.3 PET imaging
Acquisition
The PIB radiotracer was synthesized at Lawrence Berkeley National Laboratory using a previously published protocol (Mathis, et al., 2003). PIB-PET imaging was conducted using a Siemens ECAT EXACT HR PET scanner in 3D acquisition mode. Ten-fifteen mCi of PIB was injected as a bolus into an antecubital vein after which dynamic acquisition frames were obtained for a total of 90 minutes: 4 × 15 sec, 8 × 30 sec, 9 × 60 sec, 2 × 180 sec, 8 × 300 sec, 3 × 600sec.
Image Analysis
PIB data were preprocessed using Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm/). Frames 6 through 34 were realigned to frame 17, and averaged to create a mean frame. The first five frames were summed and coregistered to the mean frame, with the coregistration parameters applied to these individual five frames. The coregistered frames reflecting the first 20 minutes of acquisition (frames 1-23) were then averaged to create a new image, which was used to guide coregistration with the T1-weighted MRI. Distribution volume ratio (DVR) images were generated from PIB frames corresponding to 35-90 min post-injection, and quantified using Logan graphical analysis and the participant’s grey matter cerebellar reference region (Logan, et al., 1996; Price, et al., 2005). The T1-weighted MRI was warped to MNI space using the SPM T1 template, and the warp parameters were applied to the coregistered PIB DVR image. Cerebellar ROI registration and warped images were visually inspected to ensure alignment.
Regions of interest (ROIs) were defined in MNI space using the Automated Anatomic Labeling Atlas (Tzourio-Mazoyer, et al., 2002). In order to exclude contamination from white matter and cerebrospinal fluid, ROIs were trimmed using a grey matter mask defined by each participant’s segmented MRI (Sun, et al., 2007). DVR values were extracted from ROIs vulnerable to early Aβ deposition, which include the frontal cortex (anterior to the precentral gyrus), lateral parietal cortex, lateral temporal cortex, posterior cingulate, and precuneus. A global measure of PIB uptake (Global PIB Index) was generated from the mean DVR of these ROIs (Mormino, et al., 2009; Rabinovici, et al., 2010). The occipital cortex was also examined due to its susceptibility to cerebral amyloid angiopathy. This Global PIB Index served as the primary dependent variable.
PIB positivity
A secondary, dichotomous variable defined by the presence of abnormal PIB retention was defined as follows. Eleven young adults (mean age = 24.5, SD = 3.4) underwent PIB-PET imaging using the same acquisition and processing procedures described above. PIB uptake was determined using DVR values from the Global PIB Index and the bilateral precuneus/posterior cingulate region (an area most often and earliest affected by amyloid aggregation in AD). Values 2 SDs above the young average for these two regions were established as defining values of PIB positivity. Therefore participants with a global PIB index > 1.114 or precuneus/posterior cingulate > 1.137 were determined to be PIB positive.
2.4 Other measures
A structural MRI was obtained under a research protocol. Scans were read and rated clinically for infarcts, degree of generalized and hippocampal atrophy, and for other significant pathology. APOE genotyping and carotid ultrasound studies to measures common carotid artery intima-media thickness (CIMT) were performed under standardized research protocols (Selzer, et al., 2001). Total- , HDL-, and LDL-cholesterol, hemoglobin A1C (HbA1c), and glucose were assayed at a central laboratory from fasting blood samples.
3. Results
3.1 PIB and FCRP
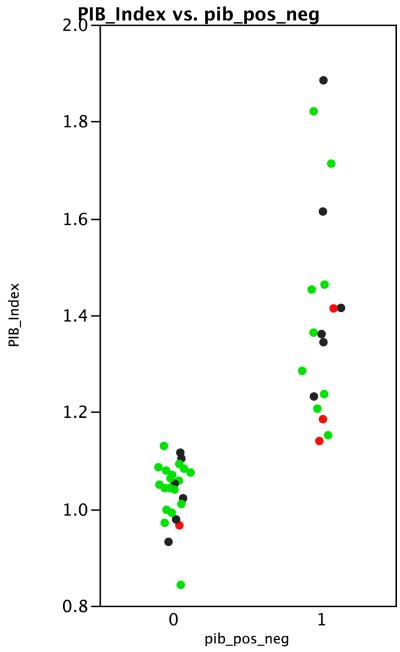
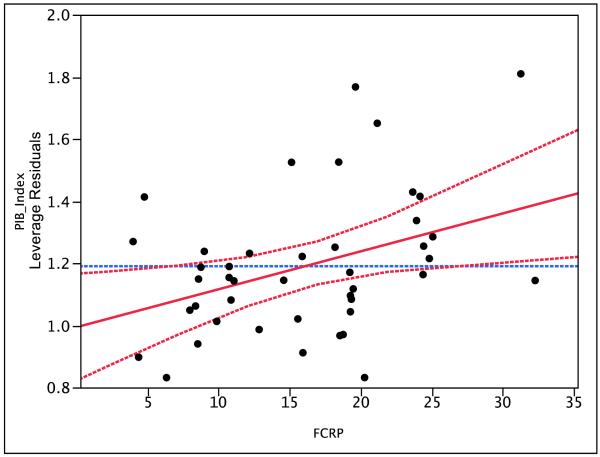
Global PIB index values were distributed rather continuously over the range of 0.8 to 1.9, with 18 (42%) cases meeting criteria for being PIB positive (Figure 1). PIB index was unrelated to age (R2 = .04, p = .22), sex (R2 = .03 , p = .23) or to CDR (R2 = .03, p = .55). In a bivariate model FCRP accounted for 16% of the variance in PIB index (p = .0075). In a multivariate model that covaried age, sex, and CDR the effect of FCRP remained significant (p = .016) and its parameter estimate was virtually identical to that obtained in the bivariate model. Figure 2 illustrates this result. Excluding the four demented cases did not reduce the size of the FCRP effect.
Figure 1.
Distribution of Global PIB index values. 0 = PIB negative, 1 = PIB positive. Green = cognitively normal; Black = MCI; Red = demented.
Figure 2.
Leverage (partial regression) plot showing the effect of FCRP on Global PIB Index controlling for Age, Gender, and CDR. Solid line indicates the estimated slope and the dotted lines mark the boundaries of the 95% confidence interval around that slope.
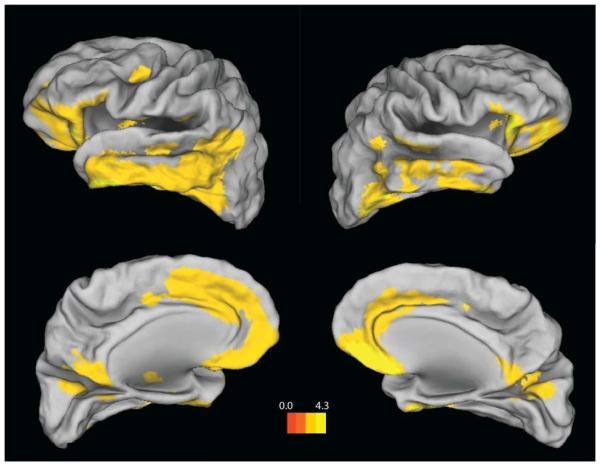
The regional distribution of PIB was explored in a set of secondary analyses. Table 2 shows mean values for DVRs in selected ROIs. Distributions of DVRs for frontal, parietal, lateral temporal, and occipital cortex, were fairly similar. Highest mean values were obtained in precuneus and posterior cingulate. Table 2 also shows the pattern of correlations between FCRP and regional DVRs. FCRP explained roughly the same proportion of variance in regional PIB in frontal, lateral temporal, and occipital cortex; lowest values were found in parietal cortex and precuneus. The pattern of association between FCRP and PIB was further explored in a voxel-wise correlational analysis (no covariates) using SPM8 with a cluster size of k > 100. The results showed a widespread positive association in temporal cortex bilaterally, and also in frontal cortex (Figure 3).
Table 2.
Regional PIB uptake values and their correlations with FCRP.
| Mean DVR left | Mean DVR right | r left | r right | |
|---|---|---|---|---|
| frontal | 1.17 | 1.16 | .43** | .39* |
| lateral temporal | 1.22 | 1.21 | .45** | .44** |
| parietal | 1.21 | 1.16 | .31* | .25 |
| occipital | 1.21 | 1.19 | .40** | .39* |
| precuneus | 1.26 | 1.25 | .30* | .29 |
| posterior cingulate | 1.38 | 1.32 | .32* | .39* |
| striatum | 1.25 | 1.27 | .33* | .32* |
Note. The correlation coefficient (r) values represent associations in bivariate, unadjusted regressions of regional PIB DVRs on FCRP.
p < .05;
p < .01
Figure 3.
Regions showing a positive association between FCRP and Global PIB Index. Threshold at p < .005 (uncorrected), k > 100. The color scale represents T values.
3.2 APOE
APOE genotyping was available on 42 cases; 10 carried the E4 allele, 10 were 3/2 heterozygotes, 22 were E3 homozygotes. In a model that included age, sex, and CDR, both FCRP and APOE (coded E4 positive/negative) had independent effects in the expected direction on PIB index (p = .023 for FCRP; p = .040 for APOE). There was no evidence of an interaction effect between FCRP and APOE but the power of the analysis to detect interaction effects was extremely low. An analogous model found no effect of the E2 allele on Global PIB.
3.3 Carotid IMT
CIMT measurements were available on 34 subjects. CIMT was not associated with Global PIB in either bivariate or demographically adjusted models (p > .30). When CIMT was added to the demographically adjusted model of FCRP on PIB index, the significance value for FCRP fell to .076 but this was due to the loss of power with a smaller N rather than any substantial change in the parameter estimate. (Analyzing only the 34 cases with CIMT values, the beta for FCRP was .011 without CIMT in the model, and was .010 with CIMT in). An analogous model found no effect of the E2 allele on Global PIB.
3.4 Exploratory analyses
In order to test whether the effect of FRCP was mediated by cardiovascular disease, we examined whether a clinical history of either cardiac disease or stroke/TIA was associated with Global PIB index. Neither was (p = .15 for cardiac disease, p = .16 for stroke/TIA) as tested using demographically adjusted models. The effects of individual components of the FCRP on Global PIB index were also tested. Neither a clinical history of diabetes, hypertension, nor elevated lipids, considered singly, had any effect on Global PIB. History of smoking was too rare to allow testing of its effect. BMI is a vascular risk factor that correlates with other risk factors but that is not included in the FCRP. BMI did not correlate significantly with FCRP, PIB index or any of the covariates. Adding BMI to the primary statistical models did not appreciably alter the effects of FRCP or APOE.
As might be expected, HbA1c and cholesterol levels were strongly correlated with FCRP: a joint model predicting FCRP with HbA1c and the HDL:LDL ratio had an overall R2 of .51. However, we did not find evidence that these markers mediated the relationship of FCRP to amyloid. Neither HDL-, LDL- nor total cholesterol showed a significant relationship to Global PIB (p > .10). When effects of either HDL or the HDL:LDL ratio on PIB were modeled jointly with FCRP, the effect of FCRP on Global PIB remained significant whereas the parameter estimates for cholesterol fell to near zero and p >.75. HbA1C and glucose levels were available on 40 and 35 patients respectively, but neither showed a significant association with Global PIB (both p values > .30).
4. Conclusions
Elevated aggregate coronary risk, quantified by the FCRP, was associated with elevated cerebral amyloid in this elderly, and generally non-demented sample. This association was not explained by APOE, which was independently associated with increased amyloid retention, or by individual risk factors, BMI, or a history of clinical coronary or cerebrovascular disease.
The FCRP captures the six major risk factors for coronary heart disease: age, sex, hypertension, smoking, dyslipidemia, and diabetes. It, and similar predictive indices have proven clinically useful in cardiology because risk factors tend to cluster, and have interactive effects that limit the utility of predicting risk based on a single risk factor (D’Agostino, et al., 2008). The fact that we found a moderately strong effect of FCRP but no effect of its component measures suggests that the FCRP measures something different than the simple sum of its components. This idea is further emphasized by the fact that at least two of the FCRP components, considered individually, appear to be risk factors when elevated in midlife but not in latelife (hypertension and dyslipidemia) whereas the relationship found here is a latelife association. Other studies have shown an additive effect of vascular risk factors on risk for incident AD (Luchsinger, et al., 2005; Whitmer, et al., 2005). Together, the literature suggests that it is important to evaluate the combined presence of these risk factors with respect to cerebral amyloid deposition and raises the question of whether they converge to raise cerebral Aβ through a shared pathogenic pathway. However, there are multiple potential explanations for the observed correlation.
If the components of the FCRP together have a direct pathogenic effect on Aβ, it would be reasonable to hypothesize that the effect is vascular in nature and that persons with elevated FCRP scores have changes in cerebral vessel structure and/or function that drive amyloid deposition.
An obvious potential vascular mechanism is acceleration of amyloid deposition by cerebral infarcts. Large postmortem studies demonstrate that infarcts are substantially more common in autopsy defined cases with AD than in elderly control cases (Jellinger, 2010) but the interpretation of this correlation is complex. While there is considerable evidence that infarcts play an important comorbid role in causing the dementia associated with AD pathology (Schneider, et al., 2007; Neuropathology Group Medical Research Council, 2001), studies generally do not find a positive correlation specifically between infarcts and amyloid load. Some pathology-based studies report that vascular pathology is positively correlated with Braak staging of neurofibrillary tangles (Jellinger, 2010), but others do not find this (Strozyk, et al., 2010) and amyloid burden is not reported in these studies. There is some human (Qi, et al., 2007) and transgenic mouse (Li, et al., 2009) evidence that cerebral ischemia is associated with increased abeta deposition. The epidemiological evidence actually raises the possibility that amyloid and infarcts may be inversely related, because demented AD cases with infarcts tend to have less AD pathology than similarly impaired cases without infarcts (Snowdon, et al., 1997; Petrovitch, et al., 2001). If true, such an inverse relationship might reflect a survivor bias effect, such that persons with more severe vascular disease die younger, giving AD pathology less time to accumulate. In a companion article in this issue (Marchant, et al.) we found no evidence that Global PIB index was associated with frank cerebrovascular disease. Although it may be counterintuitive to think that vascular risk factors increase abeta deposition whereas cerebrovascular disease does not, there are several possible explanations for this pattern. Most important is the fact that vascular risk factors may have amyloidogenic effects that are not mediated by infarction.
One such potential mechanism would be risk-factor induced damage to the BBB (Kalaria, 1999), which could result in reduced clearance of Aβ from brain (Bell, et al., 2009; Bell and Zlokovic, 2009), and in increased influx of proinflammatory cytokines and other neurotoxins that could themselves amplify the amyloid cascade (Altman and Rutledge, 2010). Evidence of increased BBB permeability has also been linked to accelerated progression of AD (Bowman, et al., 2007). Microvascular damage to vascular smooth muscle cells and the endothelium with degradation of BBB function is commonly observed in AD (Kalaria, 1999) and is generally thought to arise from Aβ mediated effects. However, potential mechanisms by which primary vascular pathology could lead to reduced Aβ clearance have also been investigated. For example, recent evidence suggests that cerebral hypoxia upregulates transcription factors that suppress a low density lipoprotein that is a major transporter of Aβ across the BBB (Bell, et al., 2009;Dotti and De Strooper, 2009). In addition, cholesterol in brain, normally produced locally in the endoplasmic reticulum, can be elevated by the addition of cholesterol from plasma if the BBB becomes abnormally permeable (Di Paolo and Kim, 2011). Cholesterol processing and Aβ production are intertwined processes (Altman and Rutledge, 2010), and elevated cholesterol levels appear to increase gamma secretase levels resulting in increased Aβ (Casserly and Topol, 2004;Di Paolo and Kim, 2011).
Another potential common pathogenic pathway for the FCRP components is arteriosclerosis. The relationship between atherosclerosis (‘hardening of the arteries”) and Alzheimer’s disease is a long running controversy, scientific discussion dating back to the time of Alzheimer (Beach, et al., 2007). As coronary risk factors, the FCRP components individually have multiple pathological mechanisms but all also converge on the intermediary pathogenic step of atherosclerosis. Hypertension and hypercholesterolemia are the two major risk factors for arteriosclerosis, and smoking and diabetes also are associated with coronary atherosclerosis (Frohlich, et al., 2001). All of these factors are also independently associated with the progression of carotid atherosclerosis in older adults (van der Meer, et al., 2003), and with CIMT in young adults (Paul, et al., 2011). Several studies have reported a positive association between atherosclerosis and the pathology of Alzheimer’s disease, including a positive correlation between atherosclerosis and neuritic plaques (Sparks, et al., 1990; Beach, et al., 2007; Honig, et al., 2005; Roher, et al., 2003). There are negative reports as well (Dolan, et al., 2010).
We did not find direct evidence that atherosclerosis mediates the relationship of FCRP to cerebral amyloid. CIMT had only a weak relationship to Global PIB and did not moderate the effect of FCRP. However, it would be premature to discount the possibility that atherosclerosis-linked mechanisms are involved. Thickness of the vessel wall is but one measure of atherosclerosis, and CIMT is essentially a single point sampling of a distributed process. Decades ago autopsy studies demonstrated that the severity of atherosclerosis varies widely though out the vascular system (Roberts, et al., 1959,Wilkins, et al., 1959); more recently several large studies have concluded that correlations between CIMT and coronary artery atherosclerosis as measured by angiography are modest, with correlations on the order of .3 - .4 (Bots, et al., 2007). And, AD has been associated with increased intracranial (but not coronary) atherosclerosis (Roher, et al., 2011). It may also be that the FCRP components are all amyloidogenic, but through multiple, non-converging, mechanisms. If coronary risk factors increase amyloid levels through multiple pathways one would not necessarily see an association of CIMT and amyloid.
Alternatively, the association of FCRP and cerebral amyloid may reflect the shared effect of a third factor, genetic or environmental, that acts over a lifetime to increase both the FCRP and cerebral amyloid. A high fat diet (a factor beyond the scope of the present study) is an example of one such potential factor (Bhat, 2010). Another candidate is APOE, the most important genetic risk factor for late onset AD. The isoforms of APOE have a multiplicity of effects on cardiovascular system, brain, and the neurovasculature. APOE4 is a modest risk factor for coronary disease (Song, et al., 2004), while the E2 allele appears to increase the risk of diabetes (Vogelberg and Maucy, 1988; Williams, et al., 1993). In addition, APOE plays a role in cholesterol processing which at least partially account for the basic observation that the E4 allele is associated with higher levels of amyloid plaques compared to E2 or E3 (Rowe, et al., 2007). We also found that the E4 allele was associated with greater cerebral amyloid deposition. However, we found no evidence that the APOE genotype accounts for the effect of coronary risk, as the effect of FCRP was independent of that of APOE genotype.
Finally, there is the possibility that coronary atherosclerosis and cerebral amyloidosis are simply independent disorders that share pathogenic factors. That is, there may be no causal connection between coronary disease or atherosclerosis and AD, but rather these are “convergent” diseases that share risk factors and pathogenic components, such as inflammation (Casserly2003).
The major caveats regarding this study regard sampling issues. The sample is relatively small and was recruited using methods intended to increase the representation of vascular risk factors over that typically found in memory or dementia clinics. The small size limits the extent of statistical modeling that can be done, and a lack of statistical power means that smaller independent effects and, especially, interactions that may in fact exist, might not be detected. Replication of these findings is needed.
To summarize, in this elderly, generally non-demented sample with a relatively high prevalence of vascular risk factors, there was a moderately strong effect of aggregate coronary risk on cerebral amyloid deposition. This effect was independent of age and gender, and was not explained by APOE, or by laboratory measures of cholesterol, glycemic control, or blood pressure, or by clinical history of ischemic events. There are several biologically plausible mechanisms that might account for this correlation, none of which were supported in these analyses. Thus, these findings add to the literature suggesting (but in no way proving) a causal connection between coronary risk and the risk of Alzheimer’s disease and they invite further investigation of the mechanism(s) of this association.
Acknowledgements
This work was supported by grants from the National Institutes on Health AG12435, AG10129, and AG031563.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman R, Rutledge JC. The vascular contribution to Alzheimer’s disease. Clin Sci (Lond) 2010;119(10):407–21. doi: 10.1042/CS20100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, Pandya Y, Esh C, Connor DJ, Sabbagh M, Walker DG, Roher AE. Circle of Willis atherosclerosis: association with Alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113(1):13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, Miano JM, Zlokovic BV. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11(2):143–53. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118(1):103–13. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat NR. Linking cardiometabolic disorders to sporadic Alzheimer’s disease: a perspective on potential mechanisms and mediators. J Neurochem. 2010;115(3):551–62. doi: 10.1111/j.1471-4159.2010.06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bots ML, Baldassarre D, Simon A, de Groot E, O’Leary DH, Riley W, Kastelein JJ, Grobbee DE. Carotid intima-media thickness and coronary atherosclerosis: weak or strong relations? Eur Heart J. 2007;28(4):398–406. doi: 10.1093/eurheartj/ehl482. [DOI] [PubMed] [Google Scholar]
- Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68(21):1809–14. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casserly IP, Topol EJ. Convergence of atherosclerosis and alzheimer’s disease: Cholesterol, inflammation, and misfolded proteins. Discov Med. 2004;4(22):149–56. [PubMed] [Google Scholar]
- Cho HJ, Lee JH, Kim YJ, Moon Y, Ko SM, Kim HY. Comprehensive evaluation of coronary artery disease and aortic atherosclerosis in acute ischemic stroke patients: usefulness based on framingham risk score and stroke subtype. Cerebrovasc Dis. 2011;31(6):592–600. doi: 10.1159/000326075. [DOI] [PubMed] [Google Scholar]
- Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66(3):300–5. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino RB, Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- Decarli C. Vascular factors in dementia: an overview. J Neurol Sci. 2004;226(1-2):19–23. doi: 10.1016/j.jns.2004.09.005. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Kim T-W. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12(5):284–96. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68(2):231–40. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, De Strooper B. Alzheimer’s dementia by circulation disorders: when trees hide the forest. Nat Cell Biol. 2009;11(2):114–6. doi: 10.1038/ncb0209-114. [DOI] [PubMed] [Google Scholar]
- Feldstein CA. Effects of blood pressure changes on Alzheimer’s disease. Neuroepidemiology. 2010;35(3):202–12. doi: 10.1159/000316872. [DOI] [PubMed] [Google Scholar]
- Frohlich J, Dobiasova M, Lear S, Lee KW. The role of risk factors in the development of atherosclerosis. Crit Rev Clin Lab Sci. 2001;38(5):401–40. doi: 10.1080/20014091084245. [DOI] [PubMed] [Google Scholar]
- Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: Analysis of data from the US National Alzheimer’s Coordinating Center. Neurology. 2005;64(3):494–500. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Prevalence and impact of cerebrovascular lesions in Alzheimer and lewy body diseases. Neurodegener Dis. 2010;7(1-3):112–5. doi: 10.1159/000285518. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. The blood-brain barrier and cerebrovascular pathology in Alzheimer’s disease. Ann N Y Acad Sci. 1999;893:113–25. doi: 10.1111/j.1749-6632.1999.tb07821.x. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137(3):149–55. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Solomon A. Cholesterol as a risk factor for Alzheimer’s disease - epidemiological evidence. Acta Neurol Scand Suppl. 2006;185:50–7. doi: 10.1111/j.1600-0404.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- Launer L, Masaki K, Petrovich H, Foley D, Havlik R. The association between mid-life blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–51. [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21(1):49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang X, Yang D, Luo G, Chen S, Le W. Hypoxia increases Abeta generation by altering beta- and gamma-cleavage of APP. Neurobiol Aging. 2009;30(7):1091–8. doi: 10.1016/j.neurobiolaging.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834–40. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr., Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55(9):1217–25. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA. Diabetes, related conditions, and dementia. J Neurol Sci. 2010;299(1-2):35–8. doi: 10.1016/j.jns.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–51. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NL, Reed BR, DeCarli CS, Madison CM, Weiner MW, Chui HC, Jagust WJ. Cerebrovascular disease, beta-amyloid and cognition in aging. under review. [DOI] [PMC free article] [PubMed]
- Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46(13):2740–54. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Zandi PP, Sjogren M, Gustafson D, Ostling S, Steen B, Skoog I. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64(10):1689–95. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–23. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–1414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Neuropathology Group Medical Research Council Cognitive Function and Aging Study Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357(9251):169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- Ott A, Slooter AJ, Hofman A, van Harskamp F, Witteman JC, Van Broeckhoven C, van Duijn CM, Breteler MM. Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: the Rotterdam Study [see comments] Lancet. 1998;351(9119):1840–3. doi: 10.1016/s0140-6736(97)07541-7. [DOI] [PubMed] [Google Scholar]
- Paul TK, Chen W, Srinivasan SR, He J, Berenson GS. Contrast of the impact of multiple cardiovascular risk factors on the femoral and carotid intima-media thickness in asymptomatic young adults: The Bogalusa Heart Study. Atherosclerosis. 2011;216(2):359–64. doi: 10.1016/j.atherosclerosis.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiology of Aging. 2000;21(1):57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, White LR, Ross GW, Steinhorn SC, Li CY, Masaki KH, Davis DG, Nelson J, Hardman J, Curb JD, Blanchette PL, Launer LJ, Yano K, Markesbery WR. Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology. 2001;57(2):226–34. doi: 10.1212/wnl.57.2.226. [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. 2005;25(11):1528–47. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Qi JP, Wu H, Yang Y, Wang DD, Chen YX, Gu YH, Liu T. Cerebral ischemia and Alzheimer’s disease: the expression of amyloid-beta and apolipoprotein E in human hippocampus. J Alzheimers Dis. 2007;12(4):335–41. doi: 10.3233/jad-2007-12406. [DOI] [PubMed] [Google Scholar]
- Qiu C, von Strauss E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch Neurol. 2003;60(2):223–8. doi: 10.1001/archneur.60.2.223. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Furst AJ, Alkalay A, Racine CA, O’Neil JP, Janabi M, Baker SL, Agarwal N, Bonasera SJ, Mormino EC, Weiner MW, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ. Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain. 2010;133(Pt 2):512–28. doi: 10.1093/brain/awp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Langbaum JB, Lee W, Reschke C, Bandy D, Alexander GE, Caselli RJ. Higher serum total cholesterol levels in late middle age are associated with glucose hypometabolism in brain regions affected by Alzheimer’s disease and normal aging. Neuroimage. 2010;49(1):169–76. doi: 10.1016/j.neuroimage.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61(5):705–14. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol. 2010;67(12):1491–7. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JC, Moses C, Wilkins RH. Autopsy Studies in Atherosclerosis I. Distribution and Severity of Atherosclerosis in Patients Dying without Morphologic Evidence of Atherosclerotic Catastrophe. Circulation. 1959;20:511–9. [Google Scholar]
- Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, Sue LI, Beach TG. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol. 2003;23(11):2055–62. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- Roher AE, Tyas SL, Maarouf CL, Daugs ID, Kokjohn TA, Emmerling MR, Garami Z, Belohlavek M, Sabbagh MN, Sue LI, Beach TG. Intracranial atherosclerosis as a contributing factor to Alzheimer’s disease dementia. Alzheimers Dement. 2011;7(4):436–44. doi: 10.1016/j.jalz.2010.08.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–25. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Rusanen M, Kivipelto M, Quesenberry CP, Jr., Zhou J, Whitmer RA. Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med. 2011;171(4):333–9. doi: 10.1001/archinternmed.2010.393. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154(1):185–93. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Oden A, Svanborg A. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141–5. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277(10):813–7. [PubMed] [Google Scholar]
- Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004;141(2):137–47. doi: 10.7326/0003-4819-141-2-200407200-00013. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hunsaker JC, 3rd, Scheff SW, Kryscio RJ, Henson JL, Markesbery WR. Cortical senile plaques in coronary artery disease, aging and Alzheimer’s disease. Neurobiol Aging. 1990;11(6):601–7. doi: 10.1016/0197-4580(90)90024-t. [DOI] [PubMed] [Google Scholar]
- Strozyk D, Dickson DW, Lipton RB, Katz M, Derby CA, Lee S, Wang C, Verghese J, Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Sun FT, Schriber RA, Greenia JM, He J, Gitcho A, Jagust WJ. Automated template-based PET region of interest analyses in the aging brain. Neuroimage. 2007;34(2):608–17. doi: 10.1016/j.neuroimage.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touboul PJ, Labreuche J, Vicaut E, Amarenco P. Carotid intima-media thickness, plaques, and Framingham risk score as independent determinants of stroke risk. Stroke. 2005;36(8):1741–5. doi: 10.1161/01.STR.0000174490.23495.57. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van der Meer IM, del Sol A. Iglesias, Hak AE, Bots ML, Hofman A, Witteman JC. Risk factors for progression of atherosclerosis measured at multiple sites in the arterial tree: the Rotterdam Study. Stroke. 2003;34(10):2374–9. doi: 10.1161/01.STR.0000088643.07108.19. [DOI] [PubMed] [Google Scholar]
- Vogelberg KH, Maucy E. Apo E 2 phenotypes in type II diabetics with and without insulin therapy. Klin Wochenschr. 1988;66(15):690–3. doi: 10.1007/BF01726928. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Williams RR, Hunt SC, Hopkins PN, Wu LL, Hasstedt SJ, Berry TD, Barlow GK, Stults BM, Schumacher MC, Ludwig EH, et al. Genetic basis of familial dyslipidemia and hypertension: 15-year results from Utah. Am J Hypertens. 1993;6(11 Pt 2):319S–27S. doi: 10.1093/ajh/6.11.319s. [DOI] [PubMed] [Google Scholar]
- Wilkins RH, Roberts JC, Moses C. Autopsy Studies in Atherosclerosis III. Distribution and Severity of Atherosclerosis in the Presence of Obesity, Hypertension, Nephrosclerosis, and Rheumatic Heart Disease. Circulation. 1959;20:527–36. [Google Scholar]
- Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]