Abstract
We recently discovered that inhibiting neurons in the dorsomedial hypothalamus (DMH) attenuated hyperthermia, tachycardia, hypertension, and hyperactivity evoked by the substituted amphetamine 3, 4-methylenedioxymethamphetamine (MDMA). Neurons that synthesize orexin are also found in the region of the DMH. As orexin and its receptors are involved in the regulation of heart rate and temperature, they would seem to be logical candidates as mediators of the effects evoked by amphetamines. The goal of this study was to determine if blockade of orexin-1 receptors in conscious rats would suppress cardiovascular and thermogenic responses evoked by a range of methamphetamine (METH) doses. Male Sprague-Dawley rats (n=6 per group) were implanted with telemetric transmitters measuring body temperature, heart rate, and mean arterial pressure. Animals were randomized to receive pretreatment with either the orexin-1 receptor antagonist SB-334867 (10 mg/kg) or an equal volume of vehicle. Thirty min later animals were given intraperitoneal (i.p.) injections of either saline, a low (1 mg/kg), moderate (5 mg/kg) or high (10 mg/kg) dose of METH. Pretreatment with SB-334867 significantly attenuated increases in body temperature and mean arterial pressure evoked by the moderate but not the low or high dose of METH. Furthermore, animals treated with SB-334867, compared to vehicle, had lower temperature and heart rate increases after the stress of an i.p. injection. In conclusion, temperature and cardiovascular responses to a moderate dose of METH and to stress appear to involve orexin-1 receptors. The failure to affect a low and a high dose of METH suggests a complex pharmacology dependent on dose. A better understanding of this may lead to the knowledge of how monoamines influence the orexin system and vice versa.
Keywords: Orexin, Thermoregulation, Stress, Amphetamines
1. Introduction
Methamphetamine (METH) abuse and its complications are global epidemics [1]. METH causes a number of medical problems including myocardial infarction [2], ischemic and hemorrhagic strokes [3], and in some cases fatal hyperthermia [4]; further contributing to these complications, amphetamines also increase heart rate and blood pressure [5]. Amphetamine-toxicity is thought to result from increases in extracellular concentrations of monoamines. While all amphetamines are potent releasers and reuptake inhibitors of norepinephrine, they differ in their effects on serotonin and dopamine: Amphetamine and METH predominantly affect dopamine release and reuptake while the substituted amphetamines (e.g., 3,4-methylenedioxmethamphetamine [MDMA]) have greater effects on the release and reuptake of serotonin [6, 7]. Once released, monoamines influence the release of other neurotransmitters[8, 9]), including other monoamines [7, 10]. In addition, amphetamines affect several neuropeptides (e.g., cocaine- and amphetamine-regulated transcript[11], dynorphin[12], and orexin [13, 14]). Of these, orexin holds particular promise in participating in the acute physiologic responses to amphetamines.
The orexin peptides (orexin-A and orexin-B) and their receptors have been implicated in the regulation of feeding [15], wakefulness [16], heart rate [17–19], locomotion [20], and body temperature [21, 22]—systems that are also affected by amphetamines. The orexin peptides exert their effects by binding to two G-protein-coupled receptors, OX1 and OX2: orexin-A binds to OX1 and OX2 receptors with equal affinity while orexin-B binds mainly to OX2 receptors[23]. Of the two peptides, orexin-A evokes sympathetic responses similar to those produced by amphetamines: Central injections of orexin-A increase sympathetic nerve activity to, and temperature of, interscapular brown adipose tissue (iBAT); core body temperature; plasma epinephrine; and heart rate [17, 21, 22, 24–29]. Of the two receptors, OX1 receptors have been linked to stress and thermoregulation [18, 30, 31].
A unique aspect of orexin is that is only synthesized within a discreet area of the hypothalamus—the dorsomedial hypothalamus (DMH)[32, 33]. This is a brain region that has been shown to be involved in the physiologic responses elicited by the substituted amphetamine MDMA [34]; microinjections of muscimol, a GABAA agonist, into the DMH significantly attenuated tachycardia, hyperthermia, hypertension, and hyperactivity produced by an intravenous dose of MDMA. As MDMA is structurally similar to METH, and evokes similar physiologic responses, it follows that neurons in the DMH may also be involved in these responses. It has been previously shown that METH increases the expression of c-Fos (a marker of neuronal activation) in orexin-synthesizing neurons in the region of the DMH[14].
These data collectively suggest that orexin and OX1 receptors maybe involved in the central mechanisms mediating METH-evoked responses. Therefore, we hypothesized that OX1 receptors play a critical role in hyperthermia and cardiovascular responses evoked by METH. To test this hypothesis, we examined the effects of pretreatment with SB-334867, an OX1 receptor antagonist, on physiological responses evoked by METH in conscious rats.
2. Methods
2.1 Animals and surgery
All procedures conformed to guidelines set forth by the NIH and were approved by the Indiana University School of Medicine’s Animal Care and Use Committee. Male Sprague-Dawley rats (328g ± 10 g) from Harlan (Indianapolis, IN) were maintained in 12-h light–dark cycle (lights on at 7 am) in single housed cages and fed ad libitum. All experiments were performed at an ambient temperature between 24 and 25°C and at a humidity between 30% and 50%. A total of 64 animals (16 for the dose response and 48 for the orexin studies) were used for this study.
For the measurement of temperature, heart rate, and blood pressure all rats were implanted with telemetric transmitters (PXT, Transoma Med, St.Paul, MN) as previously described [31, 35]. Briefly, animals were anesthetized with a mixture of ketamine-xylazine (80 mg/kg ketamine and 11.5 mg/kg xylazine), with supplements administered as needed to maintain anesthesia. The body of the transmitter was placed in the intraabdominal cavity and the pressure transducer inserted into the abdominal aorta through the right femoral artery. The transmitter was fixed in place by suturing it to the abdominal wall musculature. All animals, for which data are reported, remained in good health throughout the course of their surgery, recovery, and prior to any experimental protocols as assessed by coat and grooming, posture, response to stimuli, and maintenance of body weight. In fact, there were no differences between the mean body weights of animals between any of the experimental groups (F7,47 =1.93, p=0.09). On the day of the experiment animals were brought to the laboratory and their home cage was placed on top of a telemetric radio receiver in an experimental room where animals were left undisturbed for at least 60 min prior to any injections. The rooms are separate from the main laboratory and are free from human traffic and extraneous noise. Animals remained alone in their home cages throughout the entire study.
2.2 Drugs
The orexin-1 receptor antagonist SB-334867 (Tocris Bioscience, Ellisville, MO) was prepared immediately before injection by dissolving 10 mg of SB-334867 in 40 µl DMSO, 60 µl of 1M HCl, and 900 µl of 10% 2-hydroxypropyl-β-cyclodextrin (Sigma–Aldrich, St. Louis, MO) in saline. This resulted in a clear yellow solution without evidence of flocculation. The vehicle was prepared the same way without drug. METH (Sigma–Aldrich) was dissolved in sterile saline.
2.3 Experimental protocol: METH dose response
Rats were randomly assigned to groups and received only one treatment. Once acclimated, rats were given an intraperitoneal (i.p) injection of either saline or one of three doses of METH: low (1mg/kg), moderate (5 mg/kg), or high (10 mg/kg). Data were recorded throughout the protocol (acclimation period to 180 min after METH injections).
2.4 Experimental protocol: The effect of SB-334867 on METH-evoked responses
Rats were randomly assigned to groups and received only one treatment. After acclimating, rats received i.p. injections of either vehicle or SB-334867 (10 mg/kg) followed 30-min later by i.p injections of either saline or METH (1 mg/kg, 5 mg/kg, or 10 mg/kg). The dose of SB-334867 used in this study is based on the paper by Sholblock et al which reported that a subcutaneous dose of 10 mg/kg resulted in an OX1 receptors occupancy of 85% [36]. Higher doses of SB-334867 have been associated with abnormal behaviors [36, 37], which are thought to be unrelated to actions at orexin receptors. As the range of METH doses that humans abuse is very wide (a single “hit” of crystal METH is ~250 mg) we likewise included a range of METH doses (1 mg/kg, 5 mg/kg, 10 mg/kg)[38]. For convenience we will refer to these doses as low, moderate, and high in this text.
Physiological parameters were recorded every minute for 60 min before injection of SB-334867 or vehicle and for at least 180 min after administration of METH or saline.
2.5. Data analysis
Statistical analyses of physiologic parameters were performed using SPSS for Windows (Version 19, IBM, Armonk, NY). The mean changes from baseline for each group following injection of METH, or saline, were analyzed by analysis of variance (ANOVA). When the F value indicated a significant difference between groups (p<0.05) post hoc comparisons were done using Tukey’s HSD test. After we analyzed the group data it was noted that, compared to vehicle alone, animals pretreated with SB334867 had lower body temperatures and heart rates prior to the injection of METH or saline. As i.p. injections represent a type of stress-induced-hyperthermia [39, 40] we decided to do a separate post hoc analysis of effects of pretreatment alone. In this analysis we compared the 30-min (between −30 min and time 0) mean temperature responses of rats pretreated with SB334867 with those pretreated with vehicle. A Student-T test was used to determine if the groups were different. Data for all these and all the analyses were plotted using Prizm (GraphPad Software Inc., La Jolla, CA).
3. Results
3.1 METH increases temperature, heart rate, and blood pressure in a dose-dependent manner
Similar to what others have reported[41], we found that the effects of METH on body temperature were dependent on the dose (Fig. 1). At the highest dose tested, METH evoked a robust and rapid hyperthermia with temperatures increasing 2°C within 60 min of administration followed by a temperature decline from 90 to 155 min, then a plateau through to the end of the recording period. A low dose of METH increased body temperature ~ 1°C with the peak occurring 90 min after injection and then declining to control levels by the end of the recording period. A moderate METH dose, similar to a low dose, increased body temperature by ~ 1°C but the peak temperature did not occur until the end of the 180 min monitoring period without evidence of decline. Similar to temperature, the effect on heart rate did not follow a linear dose-response curve. Rather, the greatest effect on heart rate was evoked by a moderate dose of METH, followed by the high and then low doses. Unlike temperature, peak heart rates for each dose occurred at approximately the same time after administration (~90 min). Meth evoked nearly identical increases in mean arterial pressure at the high and moderate doses, while the low dose had no effect compared to saline.
Fig. 1. Increases in temperature, heart rate, and blood pressure evoked by i.p. injections of saline and three doses of METH (n=4 per group).
LEFT – The graphs represent the 10 min means for the corresponding doses of METH or saline injected at time 0 (dotted vertical line). RIGHT – Each horizontal bar and number represents the 180 min mean increases from baseline for each of the listed parameters after injection of METH. The error bars represent 95% confidence intervals. A * symbol represents a significant differences (p<0.05) compared to saline. A # symbol represents a significant difference between that group and all others.
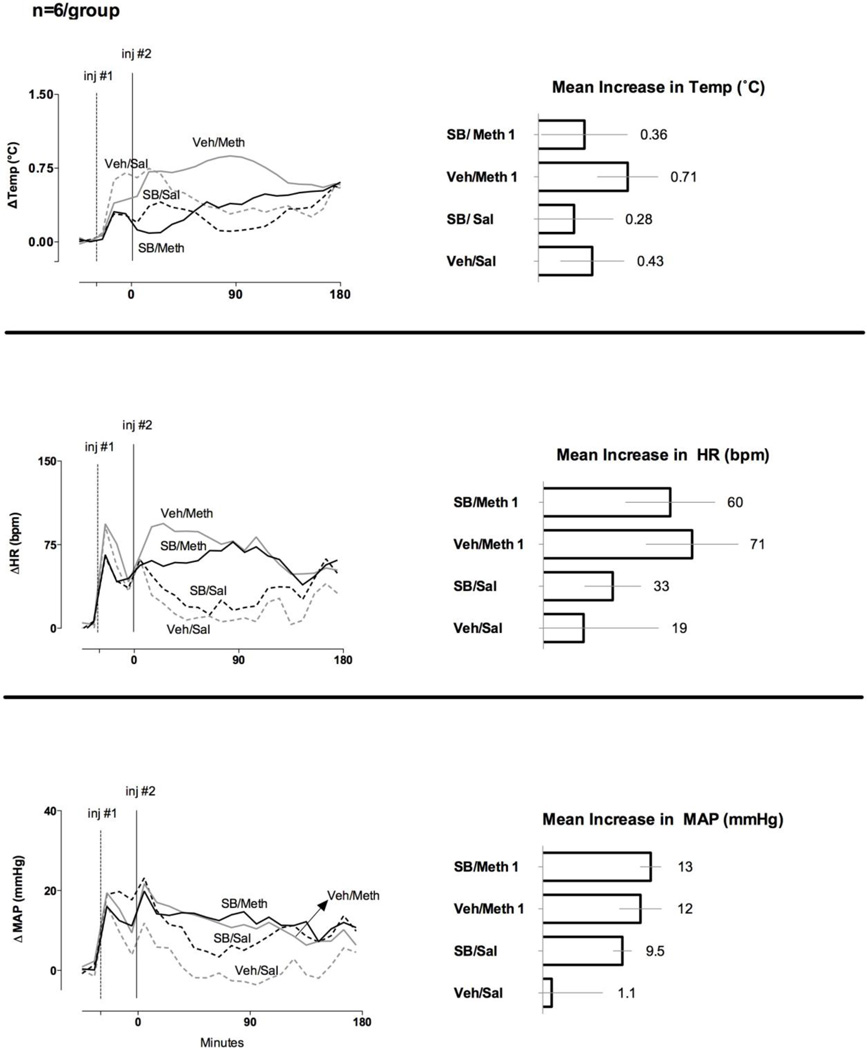
3.2 SB-334867 decreases responses to a moderate but not a low or high dose of METH
While animals treated with SB-334867 had a lower mean increase in body temperature after a low dose of METH, as compared to the vehicle-treated animals, these differences did not reach the level of statistical significance (p=0.16). Likewise, SB-334867 had no effect on tachycardia or hypertension evoked by a low dose of METH (Fig. 2).
Fig. 2. Effect of SB-334867 on the increases in body temperature and heart rate evoked by a low (1mg/kg) dose of METH (n= 6 per group).
LEFT – The graphs represent the 10 min means for the treatment groups after administration of SB-334867 or vehicle at t = −30 min (inj #1, dotted line) followed at t = 0 min (inj #2, solid line) by METH or saline. RIGHT – Each horizontal bar and number represents the 180 min mean increases from baseline for each of the listed parameters after injection of METH or saline. The error bars represent 95% confidence intervals.
SB-334867 significantly attenuated temperature and blood pressure responses to a moderate dose of METH (Fig. 3). Compared to vehicle-treated animals, SB-334867 did not significantly attenuate METH-induced increases in heart rate (p=0.16). Although not specifically noted in Fig. 3, heart rate increases were significantly greater in the Vehicle/Meth group compared to the Vehicle/Saline group (p=0.017).
Fig. 3. Effect of SB-334867 on the increases in body temperature and heart rate evoked by a moderate (5 mg/kg) dose of METH (n=6 per group).
LEFT – The graphs represent the 10 min means for the treatment groups after administration of SB-334867 or vehicle at t = −30 min (inj #1, dotted line) followed at t = 0 min (inj #2, solid line) by METH or saline. RIGHT - Each horizontal bar and number represents the 180 min mean increases from baseline for each of the listed parameters after injection of METH or saline. The error bars represent 95% confidence intervals. The corresponding p-values are given for those post-hoc analyses that showed significant differences between the SB-3348678 and vehicle-treated METH groups.
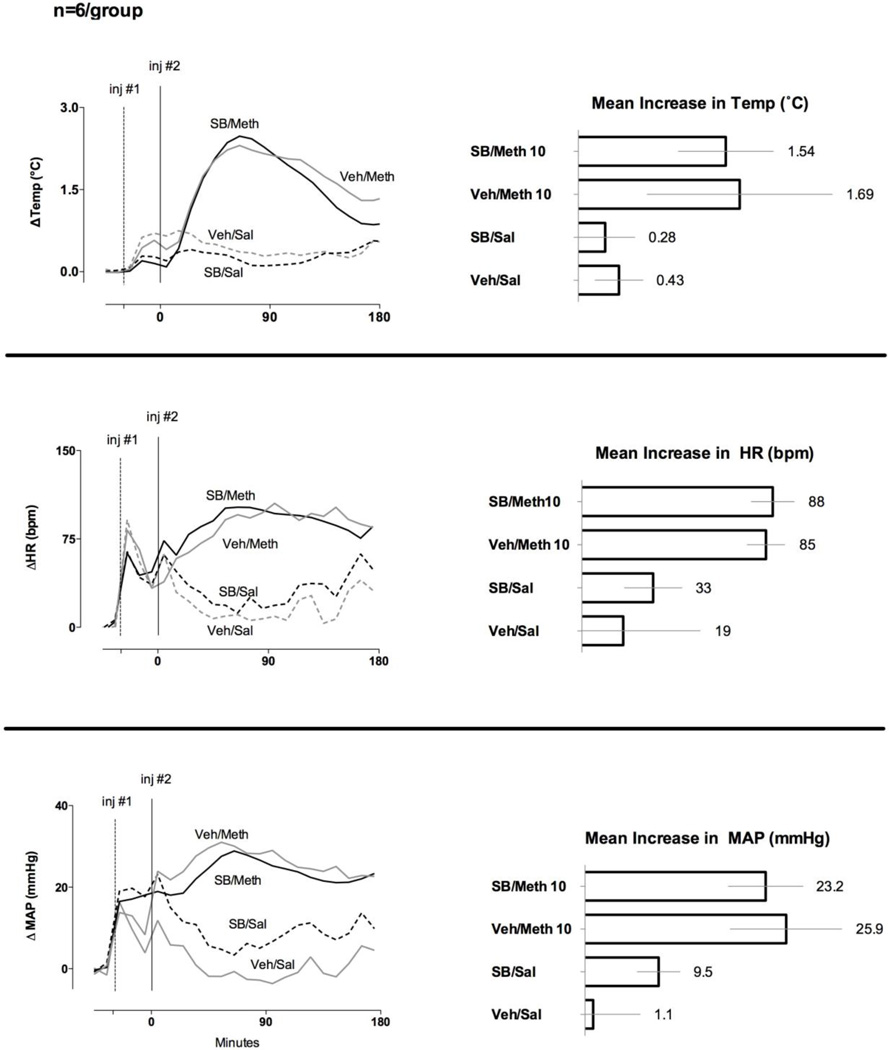
Increases in temperature, heart rate, and mean arterial pressure evoked by the high dose of METH were nearly identical between rats pretreated with vehicle or SB-334867 (Fig. 4).
Fig. 4. Effect of SB-334867 on the increases in body temperature and heart rate evoked by a high (10 mg/kg) dose of METH (n=6 per group).
LEFT – The graphs represent the 10 min means for the treatment groups after administration of SB-334867 or vehicle at t = −30 min (inj #1, dotted line) followed at t = 0 min (inj #2, solid line) by METH or saline. RIGHT - Each horizontal bar and number represent the 180 min mean increases from baseline for each of the listed parameters after injection of METH or saline. The error bars represent 95% confidence intervals.
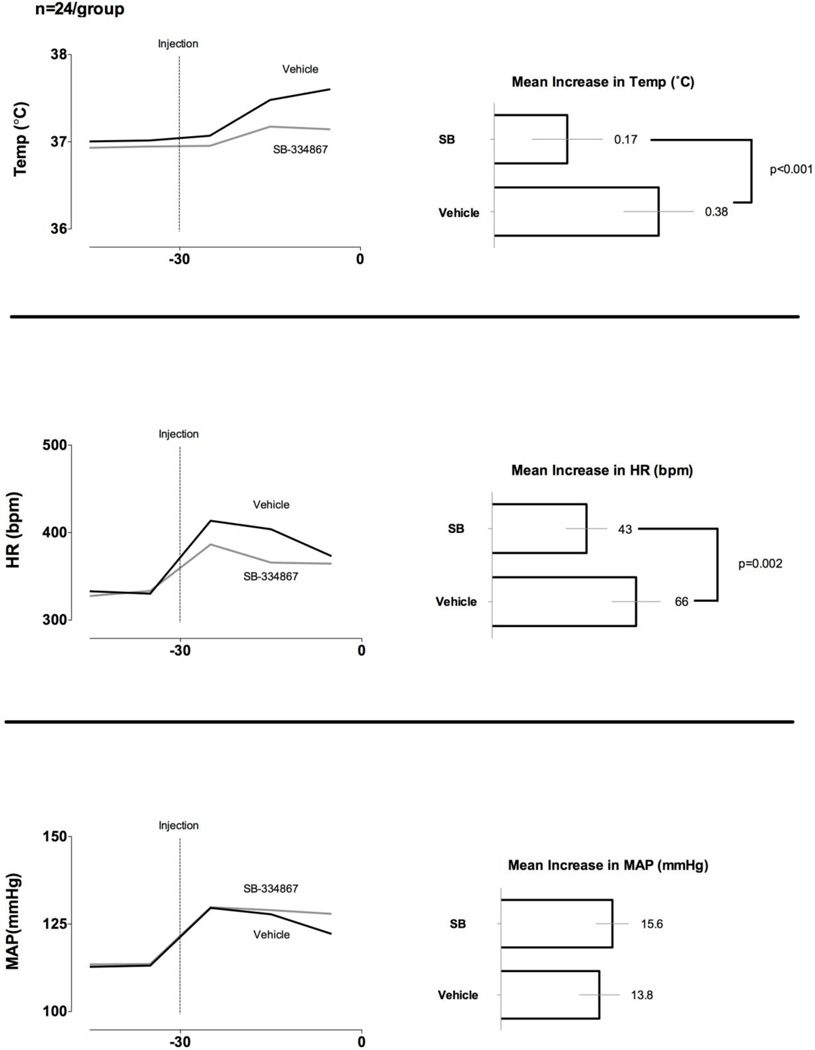
3.3 SB-334867 prevents stress-evoked hyperthermia and tachycardia
The design of our experiment allowed for an additional post hoc analysis beyond that addressing the primary hypothesis. Since prior to the injection of METH or saline each animal had received either SB-334867 or vehicle we were able to investigate the role of OX1 receptors on the “stress” responses evoked by the procedure involved in administering an i.p. injection (i.e., picking the animal up by the tail followed by the injection itself). As can be seen in Fig. 5, SB-334867 significantly attenuated the increases in both temperature and heart rate, but not mean arterial pressure, seen after i.p. injections.
Fig. 5. Effect of SB-334867 on body temperature and heart rate associated with i.p. injections (n=24 per group).
Each horizontal bar and number represent the mean increases in the measured parameter for the 30 min period after an i.p. injection. The error bars represent 95% confidence intervals. The p-values for t-tests between groups are listed when <0.05.
4. Discussion
The current findings demonstrate that the orexin-1 receptor antagonist SB-334867 attenuates temperature and blood pressure responses produced by a moderate but not a low or high dose of METH. Furthermore, we show that SB-334867 decreases temperature and heart rate response provoked by an i.p. injection. When viewed in the light of previous work, our data highlights the complex systems involved in mediating responses to amphetamines and stress. Furthermore, our data illustrates the importance of studying a range of doses when conducting experiments with METH.
Recently, Phelps et al. conducted a comprehensive dose response study on the changes in temperature and behavior produced by METH [41]. In this study they demonstrated that temperature responses do not follow a simple linear relationship. In regard to onset of temperature increases, they showed that high doses caused an immediate rise, low doses a more delayed rise, and intermediate doses the most delayed rise. We showed similar responses to the three doses of METH tested (Fig. 1). We also showed that cardiovascular responses to METH do not follow a simple pattern; this has been previously reported although with lower doses (0.1 to 3.0 mg) [42]. Our results suggest that METH’s pharmacology is multifaceted, and the role that orexin plays in temperature and cardiovascular responses is dependent on the dose.
While studies employing c-fos have shown that METH activates orexin neurons [14], no pharmacologic studies to date have demonstrated that orexin is involved in mediating responses to METH. Our findings, that SB-334867 only affected responses elicited by a moderate dose of METH points to a complex interaction between monoamines and orexin neurons. While amphetamines increase the release of monoamines at nerve terminals, orexin neurons project to, and excite monoaminergic synthesizing neurons—noradrenergic, serotonergic, and dopaminergic [43–47]. In addition, monoamines have been shown to inhibit orexin-synthesizing neurons thereby providing a putative feedback loop [48–52]. Further complicating matters, norepinephrine and dopamine can, under certain conditions, excite orexin neurons [49, 53–55]. The pharmacologic conditions produced by a moderate dose of METH may reflect another unique condition in which orexin neurons are excited by monoamines. It is of particular interest that SB-334867 seemed to preferentially affect METH responses occurring after 60 min (Fig. 4), a time point well after extracellular levels of monoamines have peaked from a moderate doses of METH [56, 57]. As peptides such as orexin have relatively long half-lives and may diffuse throughout the CNS [58], it is plausible that orexin-dependent effects may not be seen until after monamine levels have peaked. If true, responses to amphetamines may involve at least two distinct pharmacologic phases: an initial phase corresponding with the rise and peak of extracellular monoamines, and a later phase involving orexin. If true, it is not clear why SB-334867 failed to decrease temperature responses evoked by a high dose of METH. One possibility is that orexin-mediated effects at these high doses of METH occur at time-points when serum levels of SB-334867 have fallen below those needed to be effective; the half-life of SB334867 is reported to be 2–4 hr[37]. It is also possible that high doses of amphetamines contribute to hyperthermia by working through non-monoaminergic or orexinergic mediated pathways (e.g., binding directly to peripheral adrenergic receptors). Further research is needed to explore these possibilities.
In addition to affecting METH responses, SB-334867 reduced temperature and heart rate responses seen after i.p. injections. As the vehicle in this study may have effects on body temperature independent of those elicited by a simple i.p. injection of saline (an accepted model of stress-induced hyperthermia[39, 40]), we can cannot definitely say that reductions in temperature and heart rate in the SB-334867 treated group, compared to vehicle, were the result of decreasing the stress of the injection procedure or by reducing a previously unstudied thermogenic response mediated by vehicle itself. Previous work by others, however, supports our contention that SB-334867 reduces experimental stress. Inhibiting the DMH, where many orexin neurons reside, decreases the acute physiologic and endocrine responses to a variety of stressors [59, 60]. That some of the relevant neurons in the DMH may be orexinergic is also supported by immunohistochemical studies showing that immobilization and foot-shock stressors increase c-Fos expression in orexin neurons [61, 62]. In addition, the administration of an orexin-1 receptor antagonist blocks the increases in plasma concentrations of ACTH resulting from immobilization stress [18]. Emotional stressors also appear to involve orexin neurons as orexin-knockout mice have decreased blood pressure and heart rate responses to an intruder (intruder stress) [63]. Despite these findings, cardiovascular responses to a tail pinch do not appear to involve orexin receptors; compared to wild-type, orexin-knockout mice have similar increases in heart rate and blood pressure after a tail pinch [63]. These data suggest that orexin neurons in the region of the DMH play an important role in the cardiovascular and temperature response to a variety of, but not all, stressors.
In conclusion, orexin-1 receptors appear to play a role in temperature and cardiovascular responses evoked by a moderate dose of METH and by stress. A better understanding of when and how the orexin system is activated by these conditions may help direct future therapies targeting the deleterious effects of amphetamines and stress.
Highlights.
METH has complex dose dependent temperature and cardiovascular responses
SB-334867 attenuated temperature and blood pressure responses to a moderate dose of METH
SB-334867 had no effect on responses evoked by a low or high dose of METH
SB-334867 attenuated temperature and heart rate responses to the stress of an i.p. injection
Acknowledgements
This research was supported by Public Health Service grants DA020484 and DA026867
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNDOC. Annual Report 2008. 2008
- 2.Westover AN, Nakonezny PA, Haley RW. Acute myocardial infarction in young adults who abuse amphetamines. Drug Alcohol Depend. 2008;96:49–56. doi: 10.1016/j.drugalcdep.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Connor AD, Rusyniak DE, Bruno A. Cerebrovascular and cardiovascular complications of alcohol and sympathomimetic drug abuse. Med Clin North Am. 2005;89:1343–1358. doi: 10.1016/j.mcna.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Rusyniak DE, Sprague JE. Hyperthermic syndromes induced by toxins. Clin Lab Med. 2006;26:165–184. doi: 10.1016/j.cll.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Deussen A. Hyperthermia and hypothermia. Effects on the cardiovascular system. Der Anaesthesist. 2007;56:907–911. doi: 10.1007/s00101-007-1219-4. [DOI] [PubMed] [Google Scholar]
- 6.Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 7.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Mark KA, Quinton MS, Russek SJ, Yamamoto BK. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J Neurosci. 2007;27:6823–6831. doi: 10.1523/JNEUROSCI.0013-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taguchi K, Atobe J, Kato M, Chuma T, Chikuma T, Shigenaga T, et al. The effect of methamphetamine on the release of acetylcholine in the rat striatum. Eur J Pharmacol. 1998;360:131–137. doi: 10.1016/s0014-2999(98)00653-0. [DOI] [PubMed] [Google Scholar]
- 10.Bankson MG, Cunningham KA. 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin-dopamine interactions. J Pharmacol Exp Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- 11.Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romualdi P, Donatini A, Capobianco A, Ferri S. Methamphetamine alters prodynorphin gene expression and dynorphin A levels in rat hypothalamus. Eur J Pharmacol. 1999;365:183–186. doi: 10.1016/s0014-2999(98)00905-4. [DOI] [PubMed] [Google Scholar]
- 13.Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22:6742–6746. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 16.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 17.Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res. 1999;831:248–253. doi: 10.1016/s0006-8993(99)01457-2. [DOI] [PubMed] [Google Scholar]
- 18.Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. Am J Physiol. 2007;292:R382–R387. doi: 10.1152/ajpregu.00496.2006. [DOI] [PubMed] [Google Scholar]
- 19.Shirasaka T, Takasaki M, Kannan H. Cardiovascular effects of leptin and orexins. Am J Physiol Regul Integr Comp Physiol. 2003;284:R639–R651. doi: 10.1152/ajpregu.00359.2002. [DOI] [PubMed] [Google Scholar]
- 20.Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286:E551–E559. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- 21.Monda M, Viggiano AN, Viggiano A, Viggiano E, Lanza A, De Luca V. Hyperthermic reactions induced by orexin A: role of the ventromedial hypothalamus. Eur J Neurosci. 2005;22:1169–1175. doi: 10.1111/j.1460-9568.2005.04309.x. [DOI] [PubMed] [Google Scholar]
- 22.Monda M, Viggiano A, Viggiano A, Viggiano E, Messina G, Tafuri D, et al. Sympathetic and hyperthermic reactions by orexin A: Role of cerebral catecholaminergic neurons. Regul Pept. 2007;139:39–44. doi: 10.1016/j.regpep.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Langmead CJ, Jerman JC, Brough SJ, Scott C, Porter RA, Herdon HJ. Characterisation of the binding of [3H]-SB-674042, a novel nonpeptide antagonist, to the human orexin-1 receptor. Br J Pharmacol. 2004;141:340–346. doi: 10.1038/sj.bjp.0705610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, et al. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport. 2000;11:1977–1980. doi: 10.1097/00001756-200006260-00034. [DOI] [PubMed] [Google Scholar]
- 25.Russell SH, Small CJ, Dakin CL, Abbott CR, Morgan DG, Ghatei MA, et al. The central effects of orexin-A in the hypothalamic-pituitary-adrenal axis in vivo and in vitro in male rats. J Neuroendocrinol. 2001;13:561–566. doi: 10.1046/j.1365-2826.2001.00672.x. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimichi G, Yoshimatsu H, Masaki T, Sakata T. Orexin-A regulates body temperature in coordination with arousal status. Exp Biol Med. 2001;226:468–476. doi: 10.1177/153537020122600513. [DOI] [PubMed] [Google Scholar]
- 27.Monda M, Viggiano A, Mondola P, De Luca V. Inhibition of prostaglandin synthesis reduces hyperthermic reactions induced by hypocretin-1/orexin A. Brain Res. 2001;909:68–74. doi: 10.1016/s0006-8993(01)02606-3. [DOI] [PubMed] [Google Scholar]
- 28.Shirasaka T, Kunitake T, Takasaki M, Kannan H. Neuronal effects of orexins: relevant to sympathetic and cardiovascular functions. Regul Pept. 2002;104:91–95. doi: 10.1016/s0167-0115(01)00352-4. [DOI] [PubMed] [Google Scholar]
- 29.Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277:R1780–R1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- 30.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nat Med. 16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rusyniak DE, Zaretsky DV, Zaretskaia MV, DiMicco JA. The role of orexin-1 receptors in physiologic responses evoked by microinjection of PgE2 or muscimol into the medial preoptic area. Neurosci Lett. 2011;498:162–166. doi: 10.1016/j.neulet.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 33.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Rusyniak DE, Zaretskaia MV, Zaretsky DV, DiMicco JA. Microinjection of muscimol into the dorsomedial hypothalamus suppresses MDMA-evoked sympathetic and behavioral responses. Brain Res. 2008;1226:116–123. doi: 10.1016/j.brainres.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaretsky DV, Zaretskaia MV, Rusyniak DE, DiMicco JA. Stress-free microinjections in conscious rats. J Neurosci Methods. 2011;199:199–207. doi: 10.1016/j.jneumeth.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, et al. Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology (Berl) 2011;215:191–203. doi: 10.1007/s00213-010-2127-x. [DOI] [PubMed] [Google Scholar]
- 37.Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 39.Adriaan Bouwknecht J, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Dilsaver SC, Majchrzak MJ. Effects of placebo (saline) injections on core temperature in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:417–422. doi: 10.1016/0278-5846(90)90029-g. [DOI] [PubMed] [Google Scholar]
- 41.Phelps G, Speaker HA, Sabol KE. Relationship between methamphetamine-induced behavioral activation and hyperthermia. Brain Res. 2010;1357:41–52. doi: 10.1016/j.brainres.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 42.Arora H, Owens SM, Gentry WB. Intravenous (+)-methamphetamine causes complex dose-dependent physiologic changes in awake rats. Eur J Pharmacol. 2001;426:81–87. doi: 10.1016/s0014-2999(01)01202-x. [DOI] [PubMed] [Google Scholar]
- 43.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 44.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 47.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J Neurosci. 2004;24:7159–7166. doi: 10.1523/JNEUROSCI.1027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alberto CO, Trask RB, Quinlan ME, Hirasawa M. Bidirectional dopaminergic modulation of excitatory synaptic transmission in orexin neurons. J Neurosci. 2006;26:10043–10050. doi: 10.1523/JNEUROSCI.1819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. Eur J Neurosci. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, van den Pol AN. Direct and indirect inhibition by catecholamines of hypocretin/orexin neurons. J Neurosci. 2005;25:173–183. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamanaka A, Muraki Y, Tsujino N, Goto K, Sakurai T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003;303:120–129. doi: 10.1016/s0006-291x(03)00299-7. [DOI] [PubMed] [Google Scholar]
- 53.Yamanaka A, Muraki Y, Ichiki K, Tsujino N, Kilduff TS, Goto K, et al. Orexin neurons are directly and indirectly regulated by catecholamines in a complex manner. J Neurophysiol. 2006;96:284–298. doi: 10.1152/jn.01361.2005. [DOI] [PubMed] [Google Scholar]
- 54.Uschakov A, Grivel J, Cvetkovic-Lopes V, Bayer L, Bernheim L, Jones BE, et al. Sleep-deprivation regulates alpha-2 adrenergic responses of rat hypocretin/orexin neurons. PloS one. 2011;6:e16672. doi: 10.1371/journal.pone.0016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grivel J, Cvetkovic V, Bayer L, Machard D, Tobler I, Mühlethaler M, et al. The wake-promoting hypocretin/orexin neurons change their response to noradrenaline after sleep deprivation. J Neurosci. 2005;25:4127–4130. doi: 10.1523/JNEUROSCI.0666-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pereira FC, Lourenco E, Milhazes N, Morgadinho T, Ribeiro CF, Ali SF, et al. Methamphetamine, morphine, and their combination: acute changes in striatal dopaminergic transmission evaluated by microdialysis in awake rats. Ann N Y Acad Sci. 2006;1074:160–173. doi: 10.1196/annals.1369.016. [DOI] [PubMed] [Google Scholar]
- 57.Gough B, Imam SZ, Blough B, Slikker W, Jr., Ali SF. Comparative effects of substituted amphetamines (PMA, MDMA, and METH) on monoamines in rat caudate: a microdialysis study. Ann N Y Acad Sci. 2002;965:410–420. doi: 10.1111/j.1749-6632.2002.tb04182.x. [DOI] [PubMed] [Google Scholar]
- 58.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature reviews Neuroscience. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 59.DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 60.DiMicco JA, Sarkar S, Zaretskaia MV, Zaretsky DV. Stress-induced cardiac stimulation and fever: Common hypothalamic origins and brainstem mechanisms. Auton Neurosci. 2006:106–119. doi: 10.1016/j.autneu.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 61.Zhu L, Onaka T, Sakurai T, Yada T. Activation of orexin neurones after noxious but not conditioned fear stimuli in rats. Neuroreport. 2002;13:1351–1353. doi: 10.1097/00001756-200207190-00027. [DOI] [PubMed] [Google Scholar]
- 62.Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, et al. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): A novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–R593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]