Abstract
Although multiple biochemical pathways produce adenosine, studies suggest that the 2′,3′-cAMP-adenosine pathway (2′,3′-cAMP → 2′-AMP/3′-AMP → adenosine) contributes to adenosine production in some cells/tissues/organs. To determine whether the 2′,3′-cAMP-adenosine pathway exists in vivo in the brain, we delivered to the brain (gray matter and white matter separately) via the inflow perfusate of a microdialysis probe either 2′,3′-cAMP, 3′,5′-cAMP, 2′-AMP, 3′-AMP, or 5′-AMP and measured the recovered metabolites in the microdialysis outflow perfusate with mass spectrometry. In both gray and white matter, 2′,3′-cAMP increased 2′-AMP, 3′-AMP and adenosine, and 3′,5′-cAMP increased 5′-AMP and adenosine. In both brain regions, 2′-AMP, 3-AMP and 5′-AMP were converted to adenosine. Microdialysis experiments in 2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNPase) wild-type mice demonstrated that traumatic brain injury (TBI; controlled cortical impact model) activated the brain 2,3′-cAMP-adenosine pathway; similar experiments in CNPase knockout mice indicated that CNPase was involved in the metabolism of endogenous 2′,3′-cAMP to 2′-AMP and to adenosine. In CSF from TBI patients, 2′,3′-cAMP was significantly increased in the initial 12 hours after injury and strongly correlated with CSF levels of 2′-AMP, 3′-AMP, adenosine and inosine. We conclude that in vivo, 2′,3′-cAMP is converted to 2′-AMP/3′-AMP, and these AMPs are metabolized to adenosine. This pathway exists endogenously in both mice and humans.
Keywords: Adenosine; 2′,3′-cAMP; 3′,5′-cAMP; AMPs; Brain
Introduction
Adenosine is a purine nucleoside capable of exerting a wide array of effects in most organs of the body. Specifically in the brain, adenosine serves as both an inhibitory neurotransmitter (Stone et al. 2009) and an immunomodulator (Dare et al. 2007). Post-injury, extracellular adenosine levels become elevated and acting through specific cell-surface receptors, adenosine is neuroprotective (Stone et al. 2009). In this regard, microglia-derived adenosine may protect neurons from excitotoxic death (Lauro et al. 2008), and adenosine per se may suppress microglial migration (Haselkorn et al. 2009).
The term “cAMP” usually refers to “3′,5′-cAMP”; however, recent studies using sensitive and specific analytical methods detect both in vitro and in vivo a positional isomer of 3′,5′-cAMP, namely 2′,3′-cAMP (Jackson et al. 2009, Jackson et al. 2011b, Ren et al. 2009). Moreover, recent studies reveal that extracellular 2′,3′-cAMP is an endogenous source of adenosine in the kidney (Jackson et al. 2009, Jackson et al. 2011b). In this regard, 2′,3′-cAMP is released by the kidney in response to energy depletion, most likely due to increased mRNA turnover. The released 2′,3′-cAMP is then quickly metabolized to 2′-AMP and 3′-AMP, which are subsequently converted to adenosine in the kidney by ecto-enzymes (Jackson et al. 2009, Jackson et al. 2011b). We call this sequence of reactions the extracellular 2′,3′-cAMP-adenosine pathway (extracellular 2′,3′-cAMP → 2′-AMP/3′-AMP → adenosine). Cell culture experiments show that extracellular 2′,3′-cAMP and both extracellular 2′-AMP and 3′-AMP inhibit proliferation of human aortic and coronary vascular smooth muscle cells (Jackson et al. 2011c) and rat preglomerular vascular smooth muscle cells and glomerular mesangial cells (Jackson et al. 2010, Jackson et al. 2011a). For the most part, these anti-proliferative effects of 2′,3′-cAMP are mediated by generation of extracellular adenosine followed by activation of anti-proliferative adenosine A2B receptors. Additionally, intracellular 2′,3′-cAMP per se may lead to opening of mitochondrial permeability transition pores (Azarashvili et al. 2009), an apoptotic-inducing process, implying that the rapid metabolism of this molecule may be required in times of cellular stress.
Recent studies from our laboratory indicate that primary microglia and astrocytes metabolize extracellular 2′,3′-cAMP, but not 3′,5′-cAMP, to adenosine and suggest an important role for the extracellular 2′,3′-cAMP-adenosine pathway, but not the extracellular 3′,5′-cAMP-adenosine pathway (3′,5′-cAMP → 5′-AMP → adenosine) in these cell types (Verrier et al. 2011). However, the ability of the brain in vivo to metabolize extracellular 2′,3′-cAMP versus 3′,5′-cAMP is unknown.
In this study we first compared via microdialysis the ability of mouse brain in vivo to convert exogenous (extracellular) 2′,3′-cAMP and 3′,5′-cAMP to either 2′-AMP, 3′-AMP or 5′-AMP and ultimately to adenosine in both white and gray matter tracts. Our approach was to deliver the purine substrates to the brain parenchyma via the inflow perfusate of the microdialysis probe while measuring purine metabolites in the outflow perfusate of the probe (reverse microdialysis). Next, we examined the effects of traumatic brain injury (TBI) on endogenous interstitial brain levels of 2′,3′-cAMP, 2′-AMP, 3′-AMP, adenosine and inosine. These brain microdialysis experiments were preformed in both 2′,3′-cyclic nucleotide-3′-phosphodiesterase wild-type (CNPase +/+) and knockout (CNPase −/−) mice because CNPase is strongly expressed in brain (Sprinkle 1989, Vogel & Thompson 1988) and may be involved in the metabolism of extracellular and intracellular 2′,3′-cAMP to 2′-AMP. Finally, we investigated whether 2′,3′-cAMP and its metabolites could be detected in human cerebrospinal fluid (CSF) in patients after severe TBI and whether the levels of these purines were correlated.
Methods
Animals
All experiments were performed in male mice and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experiments in C57BL/6 Mice
Reverse microdialysis experiments were conducted in C57BL/6 mice (Taconic Farms, Germantown, NY). These experiments were performed by adapting our procedure previously described for rats (Bell et al.1998). In brief, mice were anesthetized with 2% isoflurane in a mixture of N2O:O2 (2:1) via a nose-cone and placed in a stereotatic frame (Kopf, Tujunga, CA). A small burr hole was made in the left frontal skull to allow a temperature probe to be inserted. Body temperature was monitored via rectal probe and both brain and body temperatures were maintained at 37° C for the entire procedure. Using a dental drill, a 5 mm craniotomy was performed over the left parietotemporal cortex where, via a micromanipulator arm, a microdialysis probe (CMA12, diameter 0.5 mm, length 2 mm, CMA/Microdialysis, Acton, MA) was inserted into either subcortical white matter (coordinates: bregma, −2.5 mm; ML, 0.5–0.75 mm; depth, −2.0 mm) or cortical gray matter (coordinates: bregma, −2.5 mm; ML, 1.75 mm, depth, −2.0 mm).
Using an infusion pump, initially artificial CSF (NaCl 144 mM, KCl 5 mM, CaCl2 1.3 mM, MgCl2 1.3 mM) was perfused through the probe at a rate of 2 μl/min. After a 1-hour recovery period, two 30-minute outflow perfusate samples were collected and served as baseline measurements for purine levels. All samples were collected and placed in a microcentrifuge tube and quickly placed in boiling water for 90 seconds to inactivate any enzymes that might degrade the purines prior to storage at −80° C. After the baseline samples were collected, the inflow perfusate was changed to artificial CSF containing either 2′,3′-cAMP, 3′,5′-cAMP, 2′-AMP, 3′-AMP or 5′-AMP (30 micomoles/L) and four additional 30-minute outflow perfusate samples were collected. Then the inflow perfusate was changed back to purine-free CSF and an additional four 30-minute outflow perfusate samples were collected. After the last sample was collected, each mouse was decapitated and the brain was examined to ensure proper probe placement. 2′,3′-cAMP, 3′,5′-cAMP, 2′-AMP, 3′-AMP and 5′-AMP were all purchased from Sigma-Aldrich, St. Louis, MA, USA.
Experiments in CNPase −/− Mice
TBI experiments were conducted in CNPase +/+ and CNPase −/− mice. CNPase null mice were developed in Germany by Lappe-Siefke et al. (Lappe-Siefke et al. 2003) and were transferred to Dr. Rashmi Bansal’s laboratory at the University of Connecticut Health Center. CNPase knockout mice from Dr. Bansal’s colony were used by JAX® Mice and Services (The Jackson Laboratory; Bar Harbor, ME) for re-derivation of the strain by in vitro fertilization as required by University of Pittsburgh policy before importation. Breeding pairs were then shipped from JAX Mice and Services to the University of Pittsburgh where a colony is currently maintained at the Safar Center for Resuscitation Research. Genotyping was performed as described by Lappe-Siefke et al. using RT-PCR (Lappe-Siefke et al. 2003). The CNPase knockout mice make neither CNPase I nor CNPase II isoforms.
CNPase +/+ and −/− mice were prepared for brain microdialysis as described above with the probe inserted beneath the area targeted for controlled cortical impact (CCI) (coordinates: bregma, −2.5 mm; ML, 2.0 mm, depth, −2.0 mm). After a 1-hour recovery period, two 30-minute pre-injury (baseline) outflow perfusate samples were collected. Then the probe was removed and vertically directed CCI was performed as previously described (Ahmad et al. 2008) using a pneumatic cylinder (Bimba Co., Monee, IL) with a 3-mm flat-tip impounder at a velocity of 4.0 m/sec and a depth of 1 mm. Immediately after injury, the probe was repositioned beneath the contusion, and three 30-minute post-injury outflow perfusate samples were collected. In additional CNPase +/+ and −/− mice, rather than CCI reverse microdialysis experiments were conducted using 2′,3′-cAMP.
CSF Samples in TBI Patients
IRB approval from the University of Pittsburgh Medical Center was obtained for use of CSF repository samples collected from male and female adult severe TBI patients (GCS score ≤ 8) with written informed consent from legal authorized representative. All patients were treated with a standard protocol based on the Guidelines for the Management of Severe TBI in Adults (2007). These samples represented fresh, timed CSF collected from an external ventricular drain (not from a reservoir). CSF drainage was used routinely as part of standard care in the neurointensive care management of raised intracranial pressure. Samples were stored at −80°C before batch analysis.
Analytical Methods
To quantitatively measure the levels of the indicated purines, analytical methods were employed as previously described (Jackson et al. 2009). In brief, the internal standard (13C10-adenosine) was from Medical Isotopes Inc. (Pelham, NH, USA) and the purines were resolved by reverse-phase liquid chromatography (Agilent Zorbax eclipse XDB-C-18 column, 3.5 μm beads; 2.1 × 100 mm). The purines were then quantified using a triple quadrupole mass spectrometer (LC-MS/MS; TSQ Quantum-Ultra; Thermofisher Scientific) operating as previously described (Jackson et al. 2009). The limit for the detection of purines in this assay system is approximately 0.02 ng/ml.
Because 2′, ′3-cAMP could be contaminated with 2′-AMP or 3′-AMP and because 2′,3′-cAMP breakdown to 3′-AMP can occur spontaneously (Rao et al. 2010), careful quality control experiments were performed to assess these potential artifacts. In this regard, 30 micromoles/L of 2′,3′-cAMP was placed in a buffered salt solution containing magnesium and calcium for one hour at 37° C to mimic the conditions of infusing dialysate through the microdialysis probe. Subsequently, the samples were placed in boiling water for 90 seconds and then frozen for several weeks to mimic sample processing and storage conditions. Next, the samples were thawed and placed in the autosampler and analyzed for 2′-AMP and 3′-AMP by LC-MS/MS. Only 0.08% ± 0.01% (n=3) and 0.1% ± 0.02% (n=3) of the 2′,3′-cAMP gave rise to 2′-AMP and 3′-AMP, respectively. There was no difference between the amount of 2′-AMP and 3′-AMP measured. Thus, the 2′-AMP and 3′-AMP measured in our experiments was not due to contamination of 2′,3′-cAMP with these AMPs; and the 2′-AMP and 3′-AMP measured in our experiments was not due to spontaneous conversion of 2′,3′-cAMP to AMPs at any stage of the experiment (while the perfusate was sitting on the lab bench or passing through the microdialysis probe; during the brief heating process; during the sample storage time; or during the thawing and running on the LC-MS/MS).
Statistical Analysis
Data were analyzed by one-factor or two-factor analysis of variance with repeated measures, with post hoc comparisons using a Fisher’s least significance difference test or Student-Neuman-Keuls test. The criterion of significance was p<0.05. All values in the test and figures are means ± SEM.
Results
Metabolism of Extracellular 2′,3′-cAMP and 3,′5′-cAMP to 2′-AMP, 3′AMP and 5′-AMP in Mouse Brain
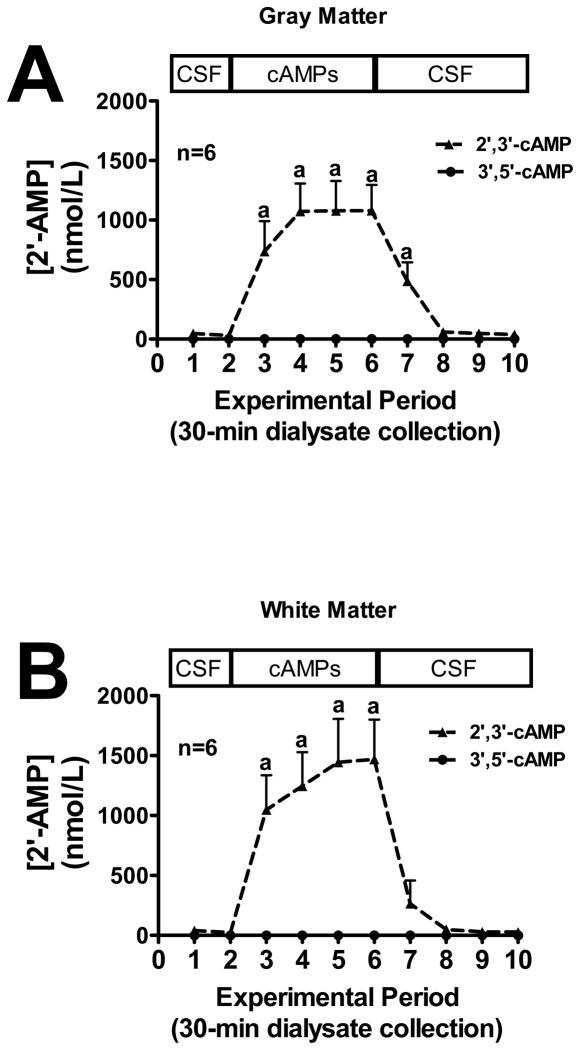
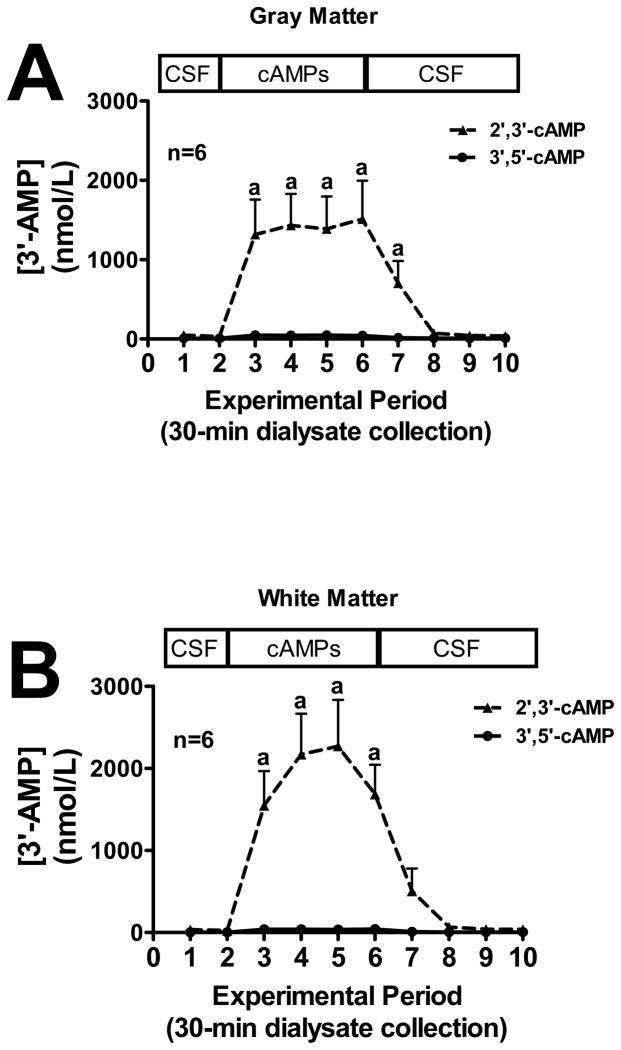
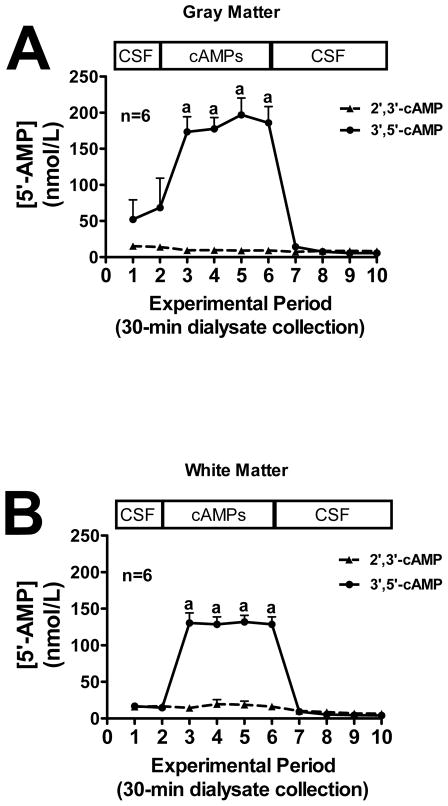
We first sought to examine the conversion of extracellular 2′,3′-cAMP versus extracellular 3′,5′-cAMP to 2′-AMP, 3′-AMP and 5′-AMP in mouse gray and white matter (see methods for location of microdialysis probes). In both gray matter (Fig. 1A) and white matter (Fig. 1B), the addition of exogenous 2′,3′-cAMP, but not 3′,5′-cAMP, to the perfusate inflow led to a significant increase in 2′-AMP levels in the perfusate outflow, whereas the addition of exogenous 3′,5′-cAMP had no effect on outflow 2′-AMP. Likewise, the addition of 2′,3′-cAMP to the perfusate inflow led to a significant increase in 3′-AMP in the perfusate outflow in both the gray matter (Fig. 2A) and white matter (Fig. 2B) while 3′,5′-cAMP had no influence on 3′-AMP levels. Administration of 3′,5′-cAMP, but not 2′,3′-cAMP, significantly increased perfusate outflow 5′-AMP levels in both gray (Fig. 3A) and white matter (Fig. 3B).
Figure 1.
Line graphs depict the effects of administering 2′,3′-cAMP or 3′,5′-cAMP via the inflow perfusate of a microdialysis probe on 2′-AMP levels in the outflow perfusate of the probe in mouse brain gray matter (A) or white matter (B) tracts. “a” indicates significantly different from experimental periods 1, 2, 8, 9 and 10 in the 2′,3′-cAMP treatment group. Values represent mean ± SEM.
Figure 2.
Line graphs depict the effects of administering 2′,3′-cAMP or 3′,5′-cAMP via the inflow perfusate of a microdialysis probe on 3′-AMP levels in the outflow perfusate of the probe in mouse brain gray matter (A) or white matter (B) tracts. “a” indicates significantly different from experimental periods 1, 2, 8, 9 and 10 in the 2′,3′-cAMP treatment group. Values represent mean ± SEM.
Figure 3.
Line graphs depict the effects of administering 2′,3′-cAMP or 3′,5′-cAMP via the inflow perfusate of a microdialysis probe on 5′-AMP levels in the outflow perfusate of the probe in mouse brain gray matter (A) or white matter (B) tracts. “a” indicates significantly different from experimental periods 1, 2, 8, 9 and 10 in the 2′,3′-cAMP treatment group. Values represent mean ± SEM.
Metabolism of Extracellular 2′,3′-cAMP and 3′,5′-cAMP to Adenosine in Mouse Brain
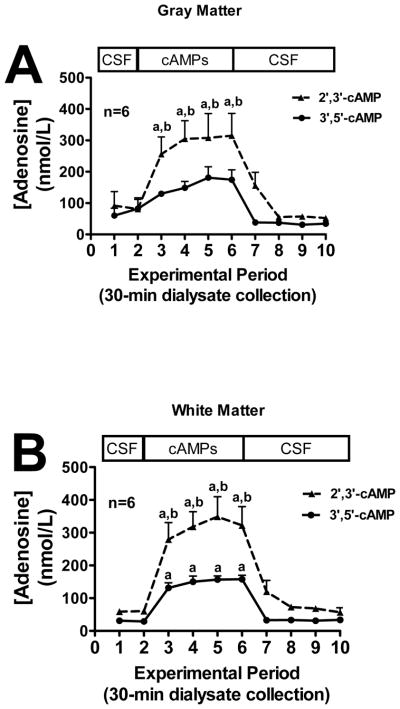
In both gray matter (Fig. 4A) and white matter (Fig. 4B), administration of exogenous 2′,3′-cAMP in the perfusate inflow led to a significant increase in adenosine levels in the perfusate outflow. In the white matter (Fig. 4B), but not gray matter (Fig. 4A), exogenous 3′,5′-cAMP significantly increased adenosine levels, although even in the gray matter 3′,5′-cAMP tended to elevate adenosine levels. In both the gray matter (Fig. 4A) and white matter (Fig. 4B) 2′,3′-cAMP increased adenosine levels in the perfusate outflow more so than 3′,5′-cAMP (gray matter: 2′,3′-cAMP > 3′,5′-cAMP by 1.7-fold; white matter: 2′,3′-cAMP > 3′,5′-cAMP by 2.2-fold).
Figure 4.
Line graphs depict the effects of administering 2′,3′-cAMP or 3′,5′-cAMP via the inflow perfusate of a microdialysis probe on adenosine levels in the outflow perfusate of the probe in mouse brain gray matter (A) or white matter (B) tracts. “a” indicates significantly different from experimental periods 1, 2, 8, 9 and 10 in the corresponding treatment group. “b” indicates significantly different from 3′,5′-cAMP at the corresponding period. Values represent mean ± SEM.
Metabolism of Extracelular 2′-AMP, 3′-AMP and 5′-AMP to Adenosine in Mouse Brain
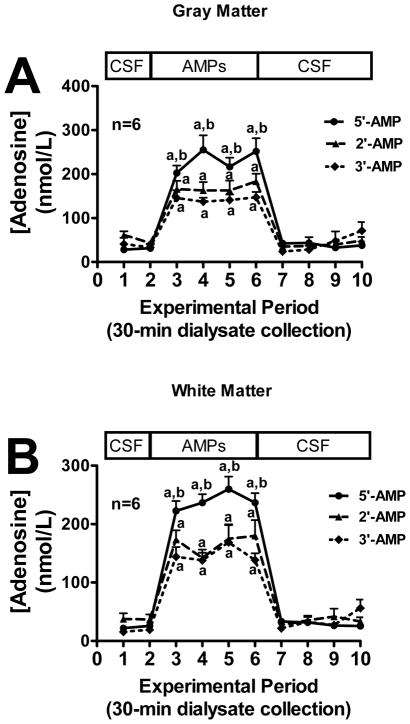
Next we examined the ability of the two regions of the brain to convert exogenous 2′-AMP, 3′-AMP, or 5′-AMP to adenosine. In both gray matter (Fig. 5A) and white matter (Fig. 5B), administration of all three AMPs in the perfusate inflow significantly increased adenosine levels in the perfusate outflow. In this regard, 2′-AMP and 3′-AMP were similarly efficacious, whereas 5′-AMP was more efficacious than 2′-AMP and 3′-AMP (gray matter: 5′-AMP > 2′-AMP by 1.4-fold and 5′-AMP > 3′-AMP by 1.7-fold; white matter: 5′-AMP > 2′-AMP by 1.4-fold and 5′-AMP > 3′-AMP by 1.5-fold).
Figure 5.
Line graphs depict the effects of administering 2′-AMP, 3′-AMP or 5′-AMP via the inflow perfusate of a microdialysis probe on adenosine levels in the outflow perfusate of the probe in mouse brain gray matter (A) or white matter (B) tracts. “a” indicates significantly different from experimental periods 1, 2, 7, 8, 9 and 10 in the corresponding treatment group. “b” indicates significantly different from 2′-AMP and 3′-AMP at the corresponding period. Values represent mean ± SEM.
Metabolism of Extracellular 2′,3′-cAMP to 2′-AMP and 3′ AMP in CNPase +/+ Versus CNPase −/− Mice
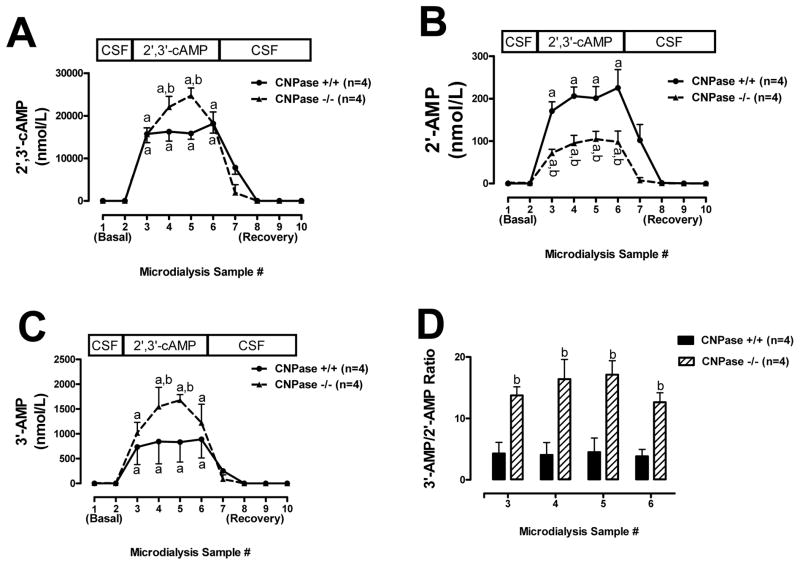
When 2′,3′-cAMP was administered via the perfusate inflow, 2′,3′-cAMP appeared in the perfusate outflow. Importantly, more 2′,3′-cAMP was recovered in the perfusate outflow in CNPase −/− compared with CNPase +/+ mice (Fig. 6A). In contrast, the levels of 2′-AMP in the perfusate outflow induced by 2′,3′-cAMP were lower in CNPase −/− mice compared to +/+ mice (Fig. 6B). Conversely, the levels of 3′-AMP in the perfusate outflow induced by 2′,3′-cAMP were much higher in the CNPase −/− mice compared to +/+ mice (Fig. 6C). Thus, the ratio of 3′-cAMP to 2′-AMP in the perfusate outflow was much greater in CNPase −/− mice compared with CNPase +/+ mice (Fig. 6D).
Figure 6.
Line graphs depict the effects of administering 2′,3′-cAMP via the inflow perfusate of a microdialysis probe on 2′,3′-cAMP (A), 2′-AMP (B) and 3′-AMP (C) in the outflow perfusate in CNPase +/+ versus CNPase −/− mice. Bar graph (D) shows ratio of 3′-AMP to 2′-AMP in outflow perfusate in CNPase +/+ versus CNPase −/− mice during administration of 2′,3′-cAMP via the inflow perfusate. “a” indicates significantly different from experimental periods 1, 2, 8, 9 and 10 in the corresponding group. “b” indicates significantly different from CNPase +/+ at the corresponding period. Values represent mean ± SEM.
Effects of TBI on Brain Interstitial 2′,3′-cAMP, 2′-AMP, 3′-AMP, Adenosine and Inosine in CNPase +/+ Versus CNPase −/− Mice
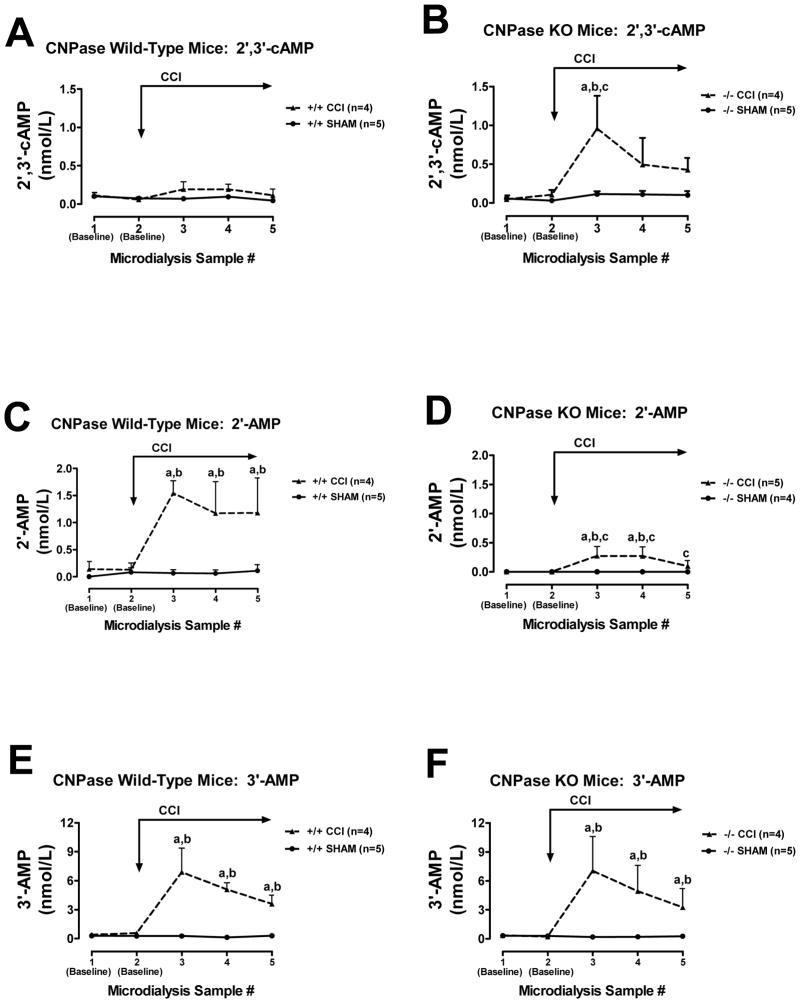
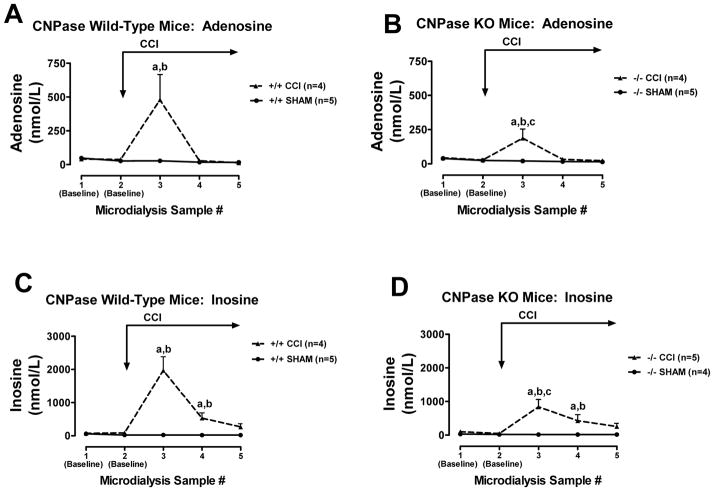
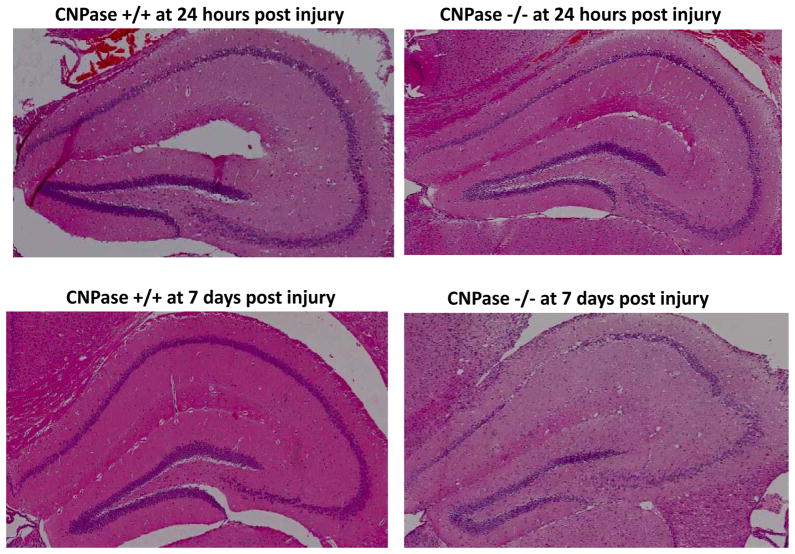
CCI did not increase interstitial 2′,3′-cAMP levels in CNPase +/+ mice (Fig. 7A), but did significantly increase interstitial 2′,3′-cAMP levels in CNPase −/− mice (Fig. 7B). In contrast, CCI increased interstitial levels of 2′-AMP in CNPase +/+ mice (Fig. 7C), and this response was nearly abolished in CNPase −/− mice (Fig. 7D). CCI increased interstitial levels of 3′-AMP similarly in both CNPase +/+ (Fig. 7E) vs. CNPase −/− mice (Fig. 7F). In CNPase +/+ mice, CCI increased interstitial adenosine levels (Fig. 8A), and this response was attenuated in CNPase −/− mice (Fig. 8B). Similarly, CCI increased interstitial inosine (metabolite of adenosine) levels in CNPase +/+ mice (Fig. 8C), and this response was also reduced in CNPase −/− mice (Fig. 8D). Fig. 9 illustrates hippocampus histology (hematoxylin and eosin stain) in four mice (Fig. 9A: CNPase +/+ mouse at 24 hours post-injury; Fig. 9B: CNPase −/− mouse at 24 hours; Fig 9C: CNPase +/+ mouse 7 days post-injury; and Fig. 9D: CNPase −/− mouse at 7 days post-injury).
Figure 7.
Line graphs depict brain interstitial levels of 2′,3′-cAMP, 2′-AMP and 3′-AMP in CNPase +/+ mice (Wild-Type) and CNPase −/− mice (KO) either subjected to controlled cortical impact (CCI) or sham impact (SHAM): (A) 2′,3′-cAMP in CNPase +/+; (B) 2′,3′-cAMP in CNPase −/−; (C) 2′-AMP in CNPase +/+; (D) 2′-AMP in CNPase −/−; (E) 3′-AMP in CNPase +/+; (F) 3′-AMP in CNPase −/−. “a” indicates significantly different from baseline in the corresponding group. “b” indicates significantly different from SHAM at the corresponding period. “c” indicates significantly different at the corresponding period from CNPase +/+ mice subjected to CCI. Values represent mean ± SEM.
Figure 8.
Line graphs depict brain interstitial levels of adenosine and inosine in CNPase +/+ mice (Wild-Type) and CNPase −/− mice (KO) either subjected to controlled cortical impact (CCI) or sham impact (SHAM): (A) adenosine in CNPase +/+; (B) adenosine in CNPase −/−; (C) inosine in CNPase +/+; (D) inosine in CNPase −/−. “a” indicates significantly different from baseline in the corresponding group. “b” indicates significantly different from SHAM at the corresponding period. “c” indicates significantly different at the corresponding period from CNPase +/+ mice subjected to CCI. Values represent mean ± SEM.
Figure 9.
This figure illustrates hippocampus histology (hematoxylin and eosin stain) in CNPase +/+ and CNPase −/− mice 24 hours and 7 days after CCI. There appeared to be more severe loss of cell bodies in the CA1, CA2 and dentate gyrus in the CNPase −/−mice, particular at 7 days after injury.
CSF 2′,3′-cAMP and Its Metabolites in Patients with Severe TBI
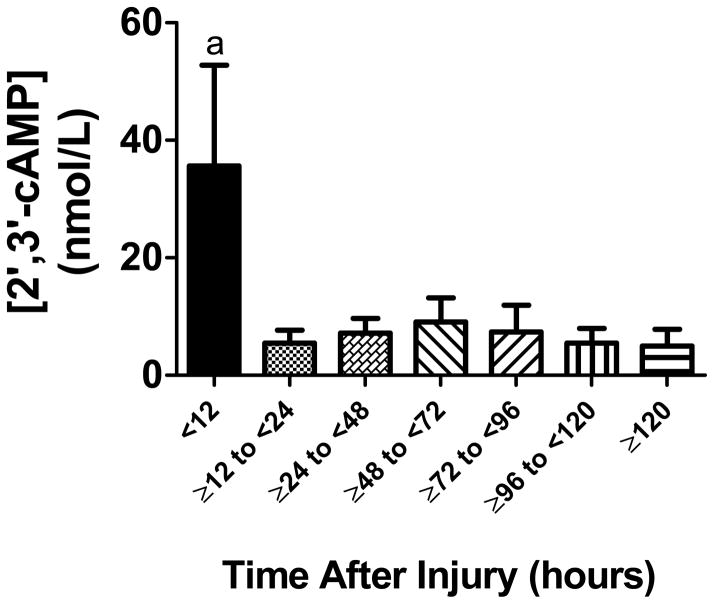
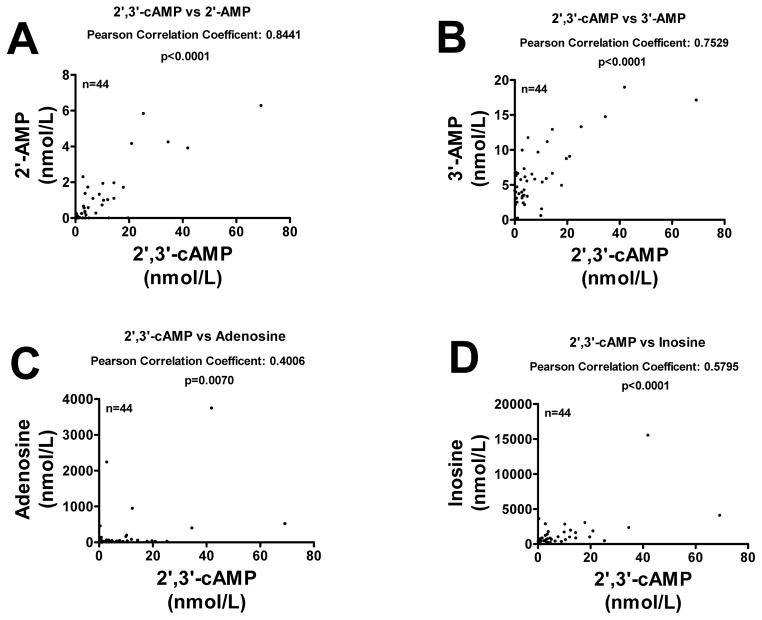
CSF samples (n=44) were obtained from 13 patients with severe TBI. Patient age was 25.92 ± 3.00 years (mean ± SEM). Glasgow coma scale scores ranged between 3 and 8 (median 7). There were 9 males and 4 females. Mean time after injury for CSF sampling was 65.24 ± 5.97 hours. 12-month Glasgow outcome scale scores ranged between 2 and 5 (median 3). In the CSF samples, we detected 2′,3′-cAMP, 2′-AMP, 3′-AMP, adenosine and inosine. CSF 2′,3′-cAMP levels were significantly increased in the initial 12 hours versus all other time points after TBI (Fig. 10). The levels of 2′-AMP (Fig. 11A) and 3′-AMP (Fig. 11B) were highly and significantly correlated with the level of 2′,3′-cAMP. The correlation between 2′,3′-cAMP and adenosine (Fig. 11C) was significant but weak; however, 2′,3′-cAMP and inosine (Fig. 11D) were highly and significantly correlated.
Figure 10.
Time course of CSF levels of 2′,3′-cAMP in patients with severe TBI. 2′,3′-cAMP levels were significantly increased early (< 12 hours) after injury. “a” indicates significantly different versus all other time points.
Figure 11.
Graphs show correlations between CSF levels of 2′,3′-cAMP versus 2′-AMP (A), 3′-AMP (B), adenosine (C) and inosine (D) in patients with severe traumatic brain injury.
Discussion
The current findings indicate that the brain in vivo can convert extracellular 2′,3′-cAMP to 2′-AMP and 3′-AMP and convert extracellular 2′-AMP and 3′-AMP to adenosine. Thus, our experiments establish that the intact brain in vivo expresses the extracellular 2′,3′-cAMP-adenosine pathway.
Our previous studies show that in primary microglia and astrocytes in culture, extracellular 2′,3′-cAMP is efficiently converted to 2′-AMP and 3′-AMP, whereas extracellular 3′,5′-cAMP is poorly metabolized to 5′-AMP (Verrier et al. 2011). The present findings confirm that in the brain in vivo the extracellular 2′,3′-cAMP-adenosine pathway is more efficient than the extracellular 3′,5′-cAMP-adenosine pathway because of the greater conversion of extracellular 2′,3′-cAMP to its respective AMPs (2′-AMP and 3′-AMP). For example, in gray matter 2′,3′-cAMP increases adenosine by 1.7-fold more than does 3′,5′-cAMP, and in white matter 2′,3′-cAMP increases adenosine by 2.2-fold more than does 3′,5′-cAMP. Nevertheless, the present findings indicate that the mouse brain in vivo can convert extracellular 3′,5′-cAMP to 5′-AMP and then to adenosine, and these finding are in full agreement with the results of several other published studies that demonstrate the existence of the extracellular 3′,5′-cAMP-adenosine pathway in the brain (Brundege et al. 1997, Rosenberg & Dichter 1989, Rosenberg et al. 1994, Rosenberg & Li 1995, Rosenberg & Li 1996).
What enzymes participate in the metabolism of 2′,3′-cAMP to 2′-AMP in the brain? One possibility answer to this question is CNPase, which is a highly abundant protein in the brain (Sprinkle 1989, Thompson 1992). Although neuroscientists have long viewed this enzyme as a structural protein and convenient marker for oligodendrocytes, CNPase, at least in vitro, converts 2′,3′-cAMP to 2′-AMP and is the only known enzyme that promotes this reaction (Schmidt 1999, Thompson 1992, Vogel & Thompson 1988). Importantly, the present study demonstrates using reverse microdialysis that the conversion of extracellular 2′,3′-cAMP to 2′-AMP is markedly suppressed in CNPase −/− mice compared with CNPase +/+ mice. These data indicate that indeed CNPase is an important enzyme in the brain that converts extracellular 2′,3′-cAMP to 2′-AMP in vivo. The present findings also indicate that CNPase is not involved in the metabolism of extracellular 2′,3′-cAMP to 3′-AMP. Indeed, in CNPase −/− mice, exogenous 2′,3′-cAMP is shunted down the 3′-AMP pathway such that the ratio of 3′-AMP to 2′-AMP increases.
What enzymes participate in the metabolism of 2′,3′-cAMP to 3′-AMP in the brain? The present study does not address this question; however, there exist a number of 2′,3′-cyclic nucleotide-2′-phosphodiesterases that can mediate this reaction. For example researchers have identified a large array of such enzymes in microorganisms (for listing see http://www.brenda-enzymes.org/php/result_flat.php4?ecno=3.1.4.16). Moreover, although these 2′,3′-cyclic nucleotide-2′-phosphodiesterases were identified using in vitro assay systems with synthetic substrates, important recent experiments by Rao et al. identify six phosphohydrolases from microogranisms that metabolize 2′,3′-cAMP to mostly 3′-AMP (Rao et al. 2010). Most likely organisms express a wide array of 2′,3′-cyclic nucleotide-2′-phosphodiesterases that can serve as ecto- and/or endo-2′,3′-cAMP-2′-phosphodiesterases that metabolize extracellular and intracellular 2′,3′-cAMP to 3′-AMP.
Currently, little is known about the molecular identity of ecto-2′-AMPases and ecto-3′-AMPases. What is known is that ecto-2′-AMPases and ecto-3′-AMPases are distinct from CD73 (also called ecto-5′-nucleotidase) that functions as an ecto-5′-AMPase (i.e., converts extracellular 5′-AMP to adenosine). This conclusion is based on the observations that pharmacological inhibition of CD73 does not alter the metabolism of extracellular 2′-AMP and 3′-AMP to adenosine in preglomerular vascular smooth muscle cells (Jackson et al. 2010), glomerular mesangial cells (Jackson et al. 2010), aortic vascular smooth muscle cells (Jackson et al. 2011c), coronary artery vascular smooth muscle cells (Jackson et al. 2011c), microglia (Verrier et al. 2011) or astrocytes (Verrier et al. 2011). Also, the fact that the metabolism of extracellular 2′,3′-cAMP to adenosine is similar in kidneys from CD73 knockout versus wild-type mice (Jackson et al. 2011b) indicates no role for CD73 in the metabolism of 2′-AMP or 3′-AMP.
To address whether in vivo the brain manufactures endogenous extracellular 2′,3′-cAMP, we employed a CCI model of TBI with the idea that injury would activate the production and release of endogenous 2′,3′-cAMP into the brain interstitial space. Also, we performed these experiments in both CNPase +/+ and −/− mice with the hypothesis that deleting CNPase would impede the rapid metabolism of endogenous 2′,3′-cAMP and thus augment its detection. Indeed this approach of performing CCI in CNPase −/−mice allowed the detection of endogenous 2′,3′-cAMP in the brain interstitium following TBI. To our knowledge, this is the first detection of endogenous 2′,3′-cAMP in the brain.
In CNPase +/+ mice, CCI has little effect on extracellular 2′,3′-cAMP levels. In contrast, in these same wild-type mice, CCI induces an increase in both extracellular 2′-AMP and 3′-AMP, suggesting that normally any 2′,3′-cAMP formed by brain injury is rapidly converted to AMPs. Importantly, deleting CNPase diminishes the ability of CCI to increase extracellular 2′-AMP, but not 3′-AMP, and augments the ability of CCI to increase extracellular 2′,3′-cAMP. These data strongly support the concept that CNPase is a critical biochemical mechanism regulating the endogenous levels of 2′,3′-cAMP and for generating endogenous 2′-AMP. Moreover, the fact that CCI-induced increases in interstitial levels of adenosine and inosine are attenuated in CNPase −/−mice suggests that the 2′,3′-cAMP-adenosine pathway modulates endogenous adenosine.
Brain injury could activate an extracellular 2′,3′-cAMP-adenosine pathway (i.e., efflux of 2′,3′-cAMP, followed by conversion of extracellular 2′,3′-cAMP to 2′-AMP/3′-AMP and the extracellular AMPs to adenosine), an intracellular 2′,3′-cAMP-adenosine pathway (i.e., conversion of intracellular 2′,3′-cAMP to intracellular 2′-AMP/3′-AMP and the intracellular AMPs to adenosine, followed by efflux of adenosine) or a transcellular 2′,3′-cAMP-adenosine pathway (i.e., conversion of intracellular 2′,3′-cAMP to intracellular 2′-AMP/3′-AMP, followed by efflux of the AMPs and their conversion to adenosine in the extracellular compartment). It is not possible to infer from the present data whether following brain injury CNPase regulates the extracellular levels of endogenous 2′,3′-cAMP, 2′-AMP and adenosine via an extracellular, intracellular or transcellular mechanism. The reverse microdialysis experiments confirm that CNPase can metabolize extracellular 2′,3′-cAMP to 2′-AMP, which suggests that CNPase regulates the 2′,3′-cAMP-adenosine pathway in least in part by acting extracellularly. However, it is conceivable that following injury intracellular CNPase metabolizes intracellular 2′,3′-cAMP which increases intracellular levels of 2′-AMP and adenosine which then exit the cells via nucleoside/nucleotide transporters. Most likely, CNPase participates in regulating all three mechanisms following brain injury.
2′,3′-cAMP is formed in cells from mRNA degradation (Thompson et al. 1994), and therefore its biosynthesis is stimulated by cell injury which stimulates mRNA breakdown (Jackson et al. 2009, Jackson et al. 2011b). Because intracellular accumulation of 2′,3′-cAMP is a pro-apoptotic signal via activation of mitochondrial permeability transition pores (Azarashvili et al. 2009) and because adenosine is neuroprotective, the 2′,3′-cAMP-adenosine pathway may be neuroprotective [i.e., the metabolism of 2′,3′-cAMP in times of cell injury may not only be a source of extracellular adenosine (neuroprotectant) but also a critical mechanism to rid the cell of intracellular 2′,3′-cAMP (an intracellular toxin)]. If this hypothesis is correct, brain injury should be worse in CNPase −/− mice. Although determining the role of CNPase in brain injury was beyond the scope and intent of the current study, we did assess brain histology in a few mice at 1 and 7 days post-injury (CCI). In this regard, preliminary observations suggest that histopathology of the hippocampus is worse in CNPase −/− versus CNPase +/+ mice; however, these preliminary studies must be confirmed by a more comprehensive investigation involving large numbers of mice and including not only histopathology but functional outcome as well.
Another critical issue is whether the 2′,3′-cAMP-adenosine pathway exists in human brains. For ethical reasons, this hypothesis is difficult to address. However, the observations that 2′,3′-cAMP, 2′-AMP and 3′-AMP exist in CSF from TBI patients and that 2′,3′-cAMP levels in CSF correlate strongly with 2′-AMP, 3′-AMP and inosine (adenosine metabolite) are supportive evidence that the 2′,3′-cAMP-adenosine pathway exists in human brains. Additional supportive evidence is that CNPase activity is present in CSF and CNPase activity is increased in patients with multiple sclerosis (Suda et al. 1984, Banik et al. 1979). Our finding of elevated levels of 2′,3′-cAMP in CSF from patients after TBI is also novel and corroborates the findings in our mouse TBI model. Our human data also suggest that a comprehensive study in a larger sample size is warranted to characterize fully the time course and magnitude of the 2′,3′-cAMP response in human TBI, and define its relationship to injury severity and clinical outcome. This finding could be quite important given the fact that the CNPase −/− mouse has been shown to develop a neurodegenerative phenotype (Lappe-Siefke C. et al. 2003).
In conclusion, our results support the conclusion that the 2′,3′-cAMP-adenosine pathway exists in the brain in vivo. However, this pathway is not normally active in the brain but rather is activated by brain injury and may play a role to limit brain damage following TBI.
Acknowledgments
This work was supported by NIH grants NS070003 (PMK/EKJ), DK068575 (EKJ), DK079307 (EKJ), NS38087 (PMK), NS30318 (PMK, DOO), NS38878 (RB) and NS41078 (RB).
Footnotes
No author had a conflict of interest.
References
- Guidelines for the management of severe head injury. Introduction. J Neurotraum. 2007;24:S1–S106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Rose ME, Vagni V, Griffith RP, Dixon CE, Kochanek PM, Hickey RW, Graham SH. Genetic disruption of cyclooxygenase-2 does not improve histological or behavioral outcome after traumatic brain injury in mice. J Neurosci Res. 2008;86:3605–3612. doi: 10.1002/jnr.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarashvili T, Krestinina O, Galvita A, Grachev D, Baburina Y, Stricker R, Evtodienko Y, Reiser G. Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2′,3′-cyclic nucleotides and 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Am J Physiol Cell Physiol. 2009;296:1428–1439. doi: 10.1152/ajpcell.00006.2009. [DOI] [PubMed] [Google Scholar]
- Banik NL, Mauldin LB, Hogan EL. Activity of 2′,3′-cyclic nucleotide 3′-phosphohydrolase in human cerebrospinal fluid. Annals of Neurology. 1979;5:539–541. doi: 10.1002/ana.410050608. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Kochanek PM, Carcillo JA, et al. Interstitial adenosine, inosine, and hypoxanthine are increased after experimental traumatic brain injury in the rat. J Neurotraum. 1998;15:163–170. doi: 10.1089/neu.1998.15.163. [DOI] [PubMed] [Google Scholar]
- Brundege JM, Diao L, Proctor WR, Dunwiddie TV. The role of cyclic AMP as a precursor of extracellular adenosine in the rat hippocampus. Neuropharmacology. 1997;36:1201–1210. doi: 10.1016/s0028-3908(97)00102-0. [DOI] [PubMed] [Google Scholar]
- Dare E, Schulte G, Karovic O, Hammarberg C, Fredholm BB. Modulation of glial cell functions by adenosine receptors. Physiol Behav. 2007;92:15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Haselkorn ML, Shellington D, Jackson E, et al. Adenosine A1 receptor activation as a brake on the microglial response after experimental traumatic brain injury in mice. J Neurotraum. 2009;27:901–910. doi: 10.1089/neu.2009.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Gillespie DG, Dubey RK. 2′-AMP and 3′-AMP inhibit proliferation of preglomerular vascular smooth muscle cells and glomerular mesangial cells via A2B receptors. J Pharmacol Exp Ther. 2011a;337:444–450. doi: 10.1124/jpet.110.178137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Cheng D, Mi Z. Extracellular cAMP-adenosine pathways in the mouse kidney. Am J Physiol Renal Physiol. 2011b;301:F565–F573. doi: 10.1152/ajprenal.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Gillespie DG. 2′,3′-cAMP, 3′-AMP and 2′-AMP inhibit human aortic and coronary vascular smooth muscle cell proliferation via A2B receptors. Am J Physiol Heart Circ Physiol. 2011c;301:H391–H401. doi: 10.1152/ajpheart.00336.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Gillespie DG, Dubey RK. Extracellular 2′,3′-cyclic adenosine 5′-monophosphate is a potent inhibitor of preglomerular vascular smooth muscle cell and mesangial cell growth. Hypertension. 2010;56:151–158. doi: 10.1161/HYPERTENSIONAHA.110.152454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem. 2009;284:33097–33106. doi: 10.1074/jbc.M109.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave K-A. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination.[see comment] Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Lauro C, Di Angelantonio S, Cipriani R, Sobrero F, Antonilli L, Brusadin V, Ragozzino D, Limatola C. Activity of adenosine receptors type 1 Is required for CX3CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. J Immunol. 2008;180:7590–7596. doi: 10.4049/jimmunol.180.11.7590. [DOI] [PubMed] [Google Scholar]
- Rao F, Qi Y, Murugan E, Pasunooti S, Ji Q. 2′,3′-cAMP hydrolysis by metal-dependent phosphodiesterases containing DHH, EAL, and HD domains is non-specific: Implications for PDE screening. Biochem Biophys Res Commun. 2010;398:500–505. doi: 10.1016/j.bbrc.2010.06.107. [DOI] [PubMed] [Google Scholar]
- Ren J, Mi Z, Stewart NA, Jackson EK. Identification and quantification of 2′,3′-cAMP release by the kidney. J Pharmacol Exp Ther. 2009;328:855–865. doi: 10.1124/jpet.108.146712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PA, Dichter MA. Extracellular cAMP accumulation and degradation in rat cerebral cortex in dissociated cell culture. J Neurosci. 1989;9:2654–2663. doi: 10.1523/JNEUROSCI.09-08-02654.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PA, Knowles R, Knowles KP, Li Y. Beta-adrenergic receptor-mediated regulation of extracellular adenosine in cerebral cortex in culture. J Neurosci. 1994;14:2953. doi: 10.1523/JNEUROSCI.14-05-02953.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PA, Li Y. Adenylyl cyclase activation underlies intracellular cyclic AMP accumulation, cyclic AMP transport, and extracellular adenosine accumulation evoked by beta-adrenergic receptor stimulation in mixed cultures of neurons and astrocytes derived from rat cerebral cortex. Brain Res. 1995;692:227–232. doi: 10.1016/0006-8993(95)00668-g. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA, Li Y. Forskolin evokes extracellular adenosine accumulation in rat cortical cultures. Neurosci Lett. 1996;211:49. doi: 10.1016/0304-3940(96)12718-x. [DOI] [PubMed] [Google Scholar]
- Schmidt S. Candidate autoantigens in multiple sclerosis. Mult Scler. 1999;5:147–160. doi: 10.1177/135245859900500303. [DOI] [PubMed] [Google Scholar]
- Sprinkle TJ. 2′,3′-cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol. 1989;4:235–301. [PubMed] [Google Scholar]
- Stone TW, Ceruti S, Abbracchio MP. Adenosine receptors and neurological disease: neuroprotection and neurodegeneration. Handb Exp Pharm. 2009;193:535–587. doi: 10.1007/978-3-540-89615-9_17. [DOI] [PubMed] [Google Scholar]
- Suda H, Hosokawa T, Ohno R, Hamaguchi K, Tsukada Y. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase activity in the cerebrospinal fluid of patients with demyelinating diseases. Neurochemical Pathology. 1984;2:85–102. doi: 10.1007/BF02834248. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Venegas FD, Raines RT. Energetics of catalysis by ribonucleases: fate of the 2′,3′-cyclic phosphodiester intermediate. Biochemistry. 1994;33:7408–7414. doi: 10.1021/bi00189a047. [DOI] [PubMed] [Google Scholar]
- Thompson RJ. 2′,3′-cyclic nucleotide-3′-phosphohydrolase and signal transduction in central nervous system myelin. Biochem Soc Trans. 1992;20:621–626. doi: 10.1042/bst0200621. [DOI] [PubMed] [Google Scholar]
- Verrier JD, Exo JL, Jackson TC, Ren J, Gillespie DG, Dubey RK, Kochanek PM, Jackson EK. Expression of the 2′,3′-cAMP-adenosine pathway in astrocytes and microglia. J Neurochem. 2011;118:979–987. doi: 10.1111/j.1471-4159.2011.07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel US, Thompson RJ. Molecular structure, localization, and possible functions of the myelin-associated enzyme 2′,3′-cyclic nucleotide 3′-phosphodiesterase. J Neurochem. 1988;50:1667–1677. doi: 10.1111/j.1471-4159.1988.tb02461.x. [DOI] [PubMed] [Google Scholar]