Abstract
Learning and memory deficits typify patients with mild cognitive impairment (MCI) and are generally attributed to medial temporal lobe dysfunction. Although the hippocampus is perhaps the most commonly studied neuroanatomical structure in these patients, there have been few attempts to identify rehabilitative interventions that facilitate its functioning. Here, we present results from a randomized, controlled, single-blind study in which patients with MCI and healthy elderly controls (HEC) were randomized to either 3 sessions of mnemonic strategy training (MS) or a matched-exposure control group (XP). All participants underwent pre- and post-training fMRI scanning as they encoded and retrieved object-location associations. For the current report, fMRI analyses were restricted to the hippocampus, as defined anatomically. Before training, MCI patients showed reduced hippocampal activity during both encoding and retrieval, relative to HEC. Following training, the MCI MS group demonstrated increased activity during both encoding and retrieval. There were significant differences between the MCI MS and MCI XP groups during retrieval, especially within the right hippocampus. Thus, MS facilitated hippocampal functioning in a partially restorative manner. We conclude that cognitive rehabilitation techniques may help mitigate hippocampal dysfunction in MCI patients.
Mild cognitive impairment (MCI) is widely recognized as a pre-dementia state; the majority of patients convert to Alzheimer’s disease (AD) within a few years of diagnosis (Albert et al., 2011; Petersen, 2004). MCI patients demonstrate impaired learning and memory within the context of preserved global cognition and activities of daily living (Albert, et al., 2011). Because of the relatively specific memory deficits, considerable emphasis has been placed on examining the pattern of structural (Apostolova et al., 2010; Jack et al., 1997) and functional (Dickerson & Sperling, 2008) abnormalities within the medial temporal lobes, especially the hippocampus. Associative memory paradigms are commonly used in functional neuroimaging research and have proven sensitive to hippocampal dysfunction in MCI (Dickerson & Sperling, 2008; Schwindt & Black, 2009). This is consistent with the idea that the hippocampus binds the individual aspects of memories into distinct representations (Mayes, Montaldi, & Migo, 2007) thereby mediating contextually rich recollective memories (Eichenbaum, Yonelinas, & Ranganath, 2007).
The prefrontal cortex is believed to facilitate the organization and contextualization of incoming information and is known to interact with the hippocampus during normal memory functioning (Baddeley, 2003; Dickerson et al., 2007; Spaniol et al., 2009); a relationship that appears to strengthen with age (Dennis et al., 2008). However, MCI patients demonstrate reduced strategy use (Ramakers et al., 2010) and reduced prefrontal activity during learning and memory paradigms (Hampstead et al., 2011a; Machulda et al., 2009; Mandzia, McAndrews, Grady, Graham, & Black, 2009). Thus, the memory deficits in MCI may be due to dysfunction within distributed neural networks, especially those involving the prefrontal cortex, in addition to hippocampal pathology. Rehabilitative methods that increase the organization and contextualization of information may facilitate residual hippocampal functioning along a spectrum from restoration (i.e., changes that tend to normalize functioning within previously dysfunctional areas) to compensation (i.e., changes within areas that are neither abnormal at baseline nor altered by comparable interventions in healthy individuals).
Mnemonic strategies (MS), frequently used as part of comprehensive cognitive rehabilitation programs, are effective in some patient populations (Cicerone et al., 2011), and result in increased prefrontal (Kondo, 2005; Miotto, 2006) and hippocampal activity (Nyberg, 2003) in healthy participants. Similarly, we (Hampstead et al., 2011b) and others (Belleville et al., 2011) have reported increased prefrontal activity accompanying behavioral improvement after MS training in MCI patients. To our knowledge, the current study is the first to provide evidence that MS training may also facilitate hippocampal functioning and result in a partially restorative pattern of activity in MCI patients.
Methods
Participants
A total of 34 right-handed participants completed two fMRI scanning sessions as part of a randomized, controlled, single-blind study during which they learned object-location associations (OLAs) (Hampstead et al., under review). Their baseline neuropsychological and encoding-related functional magnetic resonance imaging (fMRI) data were reported earlier (Hampstead et al., 2011a). Briefly, each patient (n=18) had been diagnosed with amnestic MCI according to Petersen’s criteria (Petersen, 2004). The number of patients using cognitive enhancers (4 MS group, 3 exposure group), antidepressants (4 MS, 6 exposure), blood pressure (4 MS, 3 exposure), or cholesterol medications (5 MS, 4 exposure) was similar between intervention groups. Sixteen healthy elderly controls (HEC) were free of subjective and objective memory impairments and were independent in activities of daily living. Each participant provided informed written consent. Emory University’s Institutional Review Board and the Research and Development Committee of the Atlanta VAMC approved the study.
Stimuli
We used a 3-dimensional design program (www.Plan3d.com) to create two lists of 9 rooms that are encountered in daily life (bathroom, bedroom, dining room, garage, kitchen, laundry room, living room, office, recreational room). In each room, we identified five locations that spanned the width and, to the extent possible, height of the room and then pseudorandomly placed 5 objects within each room such that any of the objects could have reasonably appeared in any of the locations. A total of 90 objects were selected because they were highly concrete, familiar, imageable, and frequently used within everyday life. Objects were then split into two comparable lists of 45 OLAs (for additional details see Hampstead et al., under review; Hampstead et al., 2011a). Two additional OLAs were created and used as low-level perceptual controls during fMRI scanning (i.e., repeated stimuli).
Randomization and training procedures
Full details of the training procedures can be found elsewhere (Hampstead, et al., under review; also see Hampstead, Sathian, Moore, Nalisnick, & Stringer, 2008); a brief summary is provided here. MCI and HEC participants were randomized to either MS or an exposure-matched control condition (XP) on a 1:1 basis within each diagnostic group. All groups were comparable in terms of demographic variables. Within each diagnostic group (HEC or MCI), the MS and XP groups were comparable in terms of cognitive functioning (Table 1). All participants completed 5 sessions within a 2-week period of time. They underwent fMRI scanning (see below) during the 1st (pre-training) and 5th (post-training) sessions. Participants were then randomly assigned (1:1) to learn the 45 OLAs from one or other list during sessions 2–4. This list is referred to hereafter as the “trained list” with the other being the “untrained list.”
Table 1.
Demographic, baseline neuropsychological test results, and hippocampal volumes (in percent of intracranial volume – ICV; obtained via NeuroQuant©) for the HEC and MCI groups.
| HEC | MCI | Main Effect of Diagnosis F(1,30)= | Main Effect of Intervention F(1,30)= | Diagnosis × Intervention F(1,30)= | |||
|---|---|---|---|---|---|---|---|
| MS (n=8) | XP (n=8) | MS (n=9) | XP (n=9) | ||||
| Age (years) | 72.1 (7.5) | 72.1 (7.6) | 71.7 (10.2) | 70.8 (7.2) | 0.10, p=.75 | 0.03, p=.88 | 0.03, p=.88 |
| Education (years) | 15.8 (3.2) | 16.5 (2.3) | 17.4 (1.8) | 16.8 (2.4) | 1.35, p=.26 | 0.00, p=.96 | 0.70, p=.41 |
| MMSE (raw score) | 28.4 (1.6) | 27.3 (2.3) | 26.8 (2.2) | 26.7 (2.5) | 2.08, p=.16 | 0.67, p=.42 | 0.45, p=.51 |
| RBANS Indices (Standard Scores) | |||||||
| Immediate Memory | 105.1 (12.1) | 106.5 (15.5) | 87.8 (11.6) | 86.3 (15.3) | 15.83, p<.001 | 0.00, p=.99 | 0.09, p=.77 |
| Visuospatial/construction | 95.3 (13.4) | 103.8 (15.0) | 99.0 (19.6) | 90.9 (6.6) | 0.72, p=.40 | 0.00, p=.97 | 2.39, p=.13 |
| Language | 104.6 (14.5) | 101.9 (16.8) | 93.8 (7.6) | 90.6 (6.6) | 7.33, p=.011 | 0.53, p=.47 | 0.00, p=.95 |
| Attention | 111.9 (11.1) | 108.9 (12.9) | 105.8 (13.5) | 106.0 (10.6) | 1.17, p=.29 | 0.11, p=.74 | 0.15, p=.70 |
| Delayed Memory | 104.1 (9.3) | 103.4 (9.0) | 75.9 (16.1) | 74.0 (14.9) | 41.99, p<.001 | 0.09, p=.77 | 0.02, p=.90 |
| Total Score | 105.5 (12.5) | 107.5 (16.9) | 89.9 (11.7) | 85.7 (9.0) | 18.43, p<.001 | 0.07, p=.80 | 0.51, p=.48 |
| Trails A (T-scores) | 49.0 (9.4) | 49.0 (8.5) | 42.1 (7.6) | 49.6 (14.9) | 0.76, p=.39 | 1.05, p=.31 | 1.05, p=.31 |
| Trails B (T-scores) | 48.8 (9.7) | 52.0 (8.6) | 48.6 (6.3) | 46.9 (7.4) | 0.93, p=.34 | 0.08, p=.78 | 0.80, p=.38 |
| GDS (raw scores) | 1.4 (2.4) | 0.8 (1.5) | 1.1 (1.3) | 2.0 (2.4) | 0.54, p=.47 | 0.04, p=.85 | 1.27, p=.27 |
| FAQ (raw scores) | 0.6 (0.9) | 0.0 (0) | 3.1 (3.7) | 4.1 (4.3) | 10.65, p=.003 | 0.03, p=.85 | 0.65, p=.43 |
| Hippocampal volume (% ICV) | |||||||
| Left | 0.24 (.03) | 0.23 (.03) | 0.22 (.03) | 0.23 (.03) | 1.01, p=.32 | 0.00, p=.97 | 2.16, p=.15 |
| Right | 0.26 (.03) | 0.25 (.03) | 0.22 (.04) | 0.25 (.03) | 2.46, p=.13 | 0.47, p=.50 | 2.87, p=.10 |
| OLA Improvement | |||||||
| Trained stimuli (SD) | 91.85 (11.59) | 84.92 (11.79) | 75.41 (21.57) | 54.71 (19.94) | See Results Section | ||
| Untrained stimuli (SD) | 52.49 (34.80) | 43.33 (20.19) | 12.08 (28.55) | 18.27 (15.66) | |||
Standard deviations are provided in parentheses. Analyses used a 2 (diagnostic group) × 2 (intervention group) multivariate analysis of variance. MMSE = mini-mental state exam; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; GDS = Geriatric Depression Scale; FAQ = Functional Activities Questionnaire. Statistical results for the OLA improvement scores are provided in the Results section.
The general training procedures were the same for the MS and XP groups in that both groups received an initial study period for each OLA and then 9 “test” trials in which they had to select the location of the object from among 5 choices. Corrective feedback was provided to both groups as needed. The key difference was that the MS groups were trained to use a 3-step process requiring them to identify a salient feature within the room that was close to the object, use a verbally-based “reason” that related the object and the specific feature, and then form a corresponding mental image. On each subsequent trial, MS participants recalled the feature, reason, and location in this order in an attempt to promote a specific series of steps that could be used with OLAs in general.
fMRI scanning (sessions 1 & 5)
MR scans were performed on a Siemens Trio 3T MRI scanner (Siemens Medical Solutions, Malvern, PA), using a 12-channel head coil. For blood oxygenation level-dependent (BOLD) contrast, T2*-weighted functional images were acquired using a single-shot, gradient-recalled, echo-planar imaging (EPI) sequence with the following parameters: repetition time (TR) 2000 ms, echo time (TE) 30 ms, field of view (FOV) 220 mm, flip angle (FA) 90°, 29 axial slices of 4 mm thickness, in-plane resolution 3.4×3.4 mm, and in-plane matrix 64×64. High-resolution anatomic images were acquired using a 3D MPRAGE sequence (TR 2300 ms, TE 3.9 ms, TI 1100 ms, FA 8°) consisting of 176 sagittal slices of 1 mm thickness (FOV 256 mm, in-plane resolution 1×1 mm, in-plane matrix 256×256).
The same design was used for both the encoding and retrieval scans, which were separated by 1-hour delays. During each of 5 functional runs, participants viewed 9 trained, 9 untrained, and 9 repeated stimuli in a “slow” event-related design. Each 6s trial consisted of presenting the object (2s) followed immediately by the object in its location (4s). Trials were separated by 8s ISIs. Six 10s baseline periods were pseudorandomly distributed in each run to allow for signal normalization. Total run length was 7′18″. The order of runs was randomized. During encoding, participants were instructed to remember the object’s location. During retrieval, they selected the object’s location from among 3 choices, each of which was an actual target location within that room; a design intended to promote recollection over familiarity. These same procedures were repeated during the post-training session (session 5). Importantly, data analyses only included trials in which trained or untrained stimuli were successfully identified during retrieval. Additional paradigm details can be found elsewhere (Hampstead et al., 2011a).
Behavioral data analysis
Treatment efficacy was calculated using a modified change score that quantified percentage improvement relative to that possible after accounting for pre-training (session 1) performance: ((Session 5 % correct − Session 1 % correct)/(100 − Session 1 % correct))*100. This formula provided a standard metric that could be directly compared across groups and was less limited by ceiling effects than change scores using raw accuracy data. Scores for trained and untrained stimuli were analyzed separately, using a 2 (HEC vs. MCI) × 2 (MS vs. XP) analysis of variance (ANOVA).
Imaging data preprocessing
Image processing and analysis were performed using BrainVoyager QX v2.3 (Brain Innovation, Maastricht, The Netherlands). Functional runs were motion-corrected in real time using Siemens 3D-PACE (prospective acquisition motion correction). For each subject, the functional images were realigned to the first image of the series. Images were pre-processed using trilinear-sinc interpolation for intra-session alignment of functional volumes, sinc interpolation for slice scan time correction, and high-pass temporal filtering to 2 cycles/run to remove slow drifts in the data. They were then co-registered with anatomic images and transformed into Talairach space (Talairach & Tournoux, 1988). For group analysis, transformed data were spatially smoothed with an isotropic Gaussian kernel (full-width half-maximum = 4 mm) and normalized across runs and subjects using data where the predictor values are at or near zero (≤0.1; the default z-baseline normalization option in BrainVoyager).
Hippocampal masks and analyses
We created an average 3D volume using all 34 participants’ Talairach-normalized anatomic scans. We then manually traced the entire left and right hippocampi to create anatomically defined regions of interest (ROI) using vmr_segmenter (http://www.bic.uni-frankfurt.de/bv-tools/). Outlines were drawn in the coronal plane but were thoroughly cross-checked in the sagittal and axial planes to ensure precision (Malykhin et al., 2007; Pruessner et al., 2000). These ROIs were converted to functional masks and applied to the random effects, general linear models (GLMs) that examined: 1) training-induced changes in activity via the (trained correct [post > pre] > repeated [post > pre]) contrast; 2) non-specific changes in activity via the (untrained correct [post > pre] > repeated [post > pre]) contrast. These same contrasts were performed for the encoding and retrieval scans. As previously discussed (Hampstead et al., 2011b), the untrained > repeated contrast could reflect, in part, the generalization of training methods to the relatively novel untrained stimuli.
We first applied these contrasts within each of the four groups in order to identify training-related changes. Treatment-specific effects (i.e., MS vs. XP) were then examined within the HEC and MCI groups. The resulting activation maps for each contrast were corrected for multiple comparisons by imposing a cluster-volume threshold for contiguous voxels passing a voxel-wise significance threshold of p<.05, using BrainVoyager’s 3D extension of the 2D Monte Carlo simulation procedure (Forman et al., 1995). For display purposes, the activation maps were projected onto hippocampal renderings (meshes), created using the anatomic ROIs (seen in Figures 1 & 2).
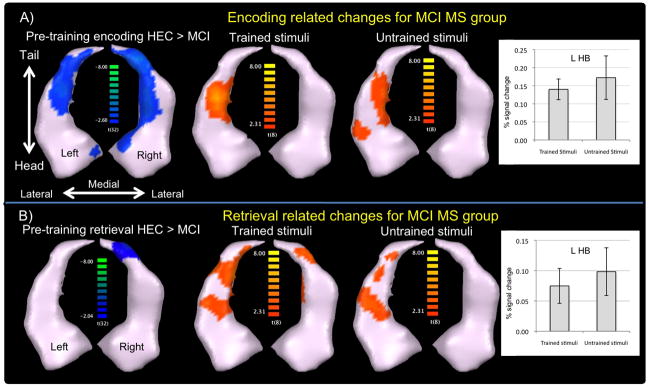
Figure 1.
A) Encoding-related activity for the MCI MS group. The left figure displays areas showing reduced activity in MCI patients relative to HEC at before training. After training, the MCI MS group demonstrated increased activity in the body of the left hippocampus for both the trained (middle) and untrained stimuli (right). Beta-weights for the left hippocampal body (L HB) are shown in the bar graphs (error bars represent the SEM). B) Retrieval-related activity for the MCI MS group, who demonstrated increased activity after training in the body and tail of the hippocampus bilaterally for trained stimuli as well as the left body and tail for untrained stimuli.
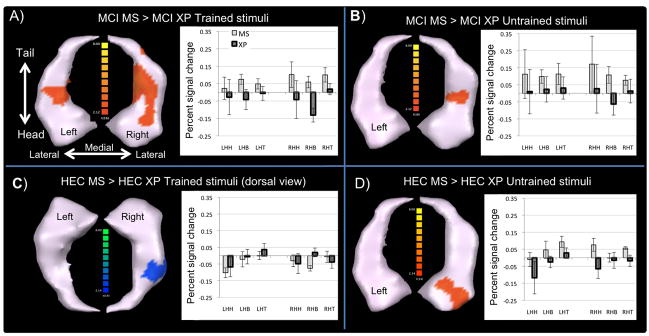
Figure 2.
Between intervention group differences in retrieval-related activity. Top row -The MCI MS group demonstrated significantly greater activity than the MCI XP group for both the trained (A) and untrained (B) stimuli. Bottom row -The HEC MS group demonstrated reduced activity relative to the HEC XP group in the body of the right hippocampus when retrieving the trained stimuli (C), but increased activity in the right hippocampal head for the untrained (novel) stimuli (D). Average beta weights for each group are shown in the corresponding graphs (error bars represent the SEM). L = left; R = right; HH = hippocampal head; HB = hippocampal body; HT = hippocampal tail
For further descriptive analysis, the hippocampal ROIs were sub-divided into head, body, and tail following anatomic boundaries established by Malykhin et al. (2007) and Pruessner et al., (2000). We extracted the subject specific beta-weights, which are the regressor coefficients used to generate the activation map, calculated using percent signal change data normalization (across runs and subjects). We then averaged the beta-weights within each hippocampal subregion. These data are shown in the bar graphs in Figures 1 and 2.
Results
Behavioral
For the trained stimuli, MS was superior to XP (F1,30 = 5.50, p=.026, pη2= .155) and HEC outperformed MCI (F1,30 = 15.68, p<.001, pη2= .343), but the interaction was not significant (F1,30 = 1.37, p=.25, pη2= .044).
For the untrained stimuli, HEC again improved more than MCI (F1,30 = 13.75, p=.001, pη2= .314) but neither the intervention effect (F1,30 = 0.28, p=.87, pη2= .001) nor the interaction (F1,30 = 0.76, p=.39, pη2= .025) were significant.
Pre-training activation differences
Baseline differences were assessed using a [“novel” (i.e. trained correct + untrained correct) > repeated] contrast since training had yet to take place and, therefore, there was no difference between “trained” and “untrained” stimuli. During encoding, MCI demonstrated significantly less activity within the hippocampal head, body, and tail bilaterally compared to HEC (Figure 1), consistent with our previous findings using whole-brain analyses (Hampstead et al., 2011a). There were no significant differences in activation between the intervention groups (MS vs. XP) within each diagnostic group (HEC or MCI).
During pre-training retrieval, MCI showed significantly reduced activity within the tail of the right hippocampus relative to HEC. A similar area showed reduced activity in the MCI MS relative to the MCI XP group. The HEC MS group demonstrated greater activity within the left hippocampal head compared to the HEC XP group.
Activation changes during encoding
Within-group
Only the MCI MS group demonstrated significant changes in activity following training, as reflected by increased activity within the left hippocampal body for both the trained and untrained stimuli (Figure 1A). These increases were almost completely confined to regions with significantly reduced activation relative to HEC at pre-training.
Between interventions
Although there were some clusters suggesting greater increases in the MCI MS than the MCI XP group, they did not survive correction. There were no significant differences as a function of intervention in the HEC.
Activation changes during retrieval
Within-group
For the trained stimuli, both the HEC MS and MCI XP groups demonstrated significantly reduced activity within the right hippocampal body, suggesting repetition suppression effects (see bar graphs in Figure 2A and C). Conversely, the MCI MS group showed increased activity with the hippocampal body and tail bilaterally (Figure 1B). There were no significant changes in the HEC XP group.
Both MS groups demonstrated increased activity for the untrained stimuli. In the HEC MS group, increased activity was evident in the left hippocampal tail and right hippocampal head (see bar graphs in Figure 2D). Increases in the MCI MS group were restricted to the left body and tail (Figure 1B). There were no significant changes in either XP group.
Between interventions
In the MCI patients, there were significant differences between the MS and XP groups within the left hippocampal body and throughout the right hippocampus for the trained stimuli (Figure 2A). For the untrained stimuli (Figure 2B), significant differences were evident within the right hippocampal body. The bar charts indicate that these differences resulted from increased activity in the MS compared to reduced or stable activity in the XP group.
As the HEC groups retrieved trained stimuli (Figure 2C), significant differences were evident in the body of the right hippocampus due to increased/stable activity in the XP but reduced activity in the MS group. For the untrained stimuli (Figure 2D), significant differences were evident in the right hippocampal head due to increased activity in the MS group but decreased activity in the XP group.
Discussion
The current study is the first, to our knowledge, to specifically examine changes in hippocampal activity following a randomized, controlled, single-blind intervention study in patients with MCI. From a behavioral standpoint, MS-trained participants demonstrated significantly greater improvement for trained stimuli relative to those in the XP groups. Although there were no significant intervention effects for the untrained stimuli, applying MS is time-intensive and our current design allowed only 2s exposure to the object and an additional 4s to the object in its location. While this design is sufficient for measuring changes in activation that presumably reflect underlying mechanisms, the available time was likely insufficient for participants to fully develop and/or rehearse strategies for new stimuli. Our ongoing studies are designed to determine whether participants can, in fact, generalize the trained strategies. Importantly, the behavioral findings with the current subset of participants are analogous to those from the entire sample who completed the behavioral intervention study (Hampstead et al., under review).
During encoding, only the MCI MS group showed significant changes in activation, which were increases within the left hippocampal body for both trained and untrained stimuli. These changes were almost completely confined to areas that had deficient activity during the pre-training scan, suggesting that MS worked in a partially restorative manner. The encoding-related changes did not differ significantly between the MCI MS and MCI XP groups, so additional work is needed to determine their implications. However, HEC show a significant relationship between activation in this area and OLA memory test performance (Hampstead et al., 2011a), and a quantitative meta-analysis including both verbal and visuospatial stimuli found that left hippocampal activity is critical for successful encoding (Spaniol, et al., 2009).
While retrieving trained stimuli, HEC XP demonstrated no significant change while HEC MS and MCI XP showed a pattern consistent with repetition suppression effects within the right hippocampal body. Conversely, the MCI MS group demonstrated significant increases that appeared to be a mixture of restorative (right tail) and compensatory processes (left tail and bilaterally within the body). These retrieval-related changes differed significantly between the MCI MS and MCI XP groups. The bilateral findings suggest that MS-trained patients were able to utilize both categorical (i.e., relational) and coordinate (i.e., exact) processing mechanisms, which are believed to be mediated by the left and right hemispheres, respectively (Postma, Kessels, & van Asselen, 2008). The significant increases within the right hippocampus for the untrained stimuli raise the possibility that MS was especially effective at facilitating coordinate processing.
The theoretical implications of these findings are intriguing. One possibility is that MS training facilitates a more interactive and efficient neocortical network that helps stabilize dysfunctional hippocampal processing (Yassa et al., 2010). This possibility is supported by findings of increased prefrontal-hippocampal connectivity with aging (Dennis, et al., 2008) as well as evidence of increased prefrontal activity (Kondo, 2005; Miotto, 2006) and neocortical connectivity following MS training (Hampstead et al., 2011b). Thus, connectivity analyses will be critical for understanding such changes in future studies. Our demonstration of increased hippocampal activity in MS-trained patients may be related to findings of hippocampal hyperactivation in MCI (Dickerson & Sperling, 2008) and suggests that future studies could profitably consider the manner in which patients learn information.
While the current findings are based on small sample sizes and clearly require replication with larger groups, they provide preliminary evidence that cognitive interventions, like MS, may partially mitigate dysfunctional medial temporal lobe processing in patients with MCI.
Acknowledgments
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Rehabilitation Research and Development Service through grants B6366W to BMH and VA Merit B6662R to KS and by the Emory Alzheimer’s Disease Research Center (NIA: 2P50AG025688). Support to KS from the National Institutes of Health (NIH) grant K24 EY017332 is also acknowledged. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government. We wish to thank Dr. Pamela Phillips for her assistance with participant recruitment and testing.
Footnotes
Conflicts of Interest
No author has any conflict of interest.
Author Contributions
Each author provided significant intellectual contribution to warrant authorship and declares that he/she has seen and approved this manuscript. Dr. Benjamin M. Hampstead had full access to all the data in the study; he and Dr. K. Sathian had final responsibility for the decision to submit for publication.
Works Cited
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging and Alzheimer’s Association workgroup. Alzheimer’s & Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Thompson PM, Green AE, Hwang KS, Zoumalan C, Jack CR, et al. 3D Comparison of Low, Intermediate, and Advanced Hippocampal Atrophy in MCI. Human Brain Mapping. 2010;31(5):786–797. doi: 10.1002/hbm.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory: Looking back and looking forward. Nature Reviews Neuroscience. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Belleville S, Clement F, Mellah S, Gilbert B, Fontaine F, Gauthier S. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain. 2011;134:1623–1634. doi: 10.1093/brain/awr037. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence-Based Cognitive Rehabilitation: Updated Review of the Literature From 2003 Through 2008. Archives of Physical Medicine and Rehabilitation. 2011;92(4):519–530. doi: 10.1016/j.apmr.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettell SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(4):791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Miller SL, Greve DN, Dale AM, Albert MS, Schacter DL, et al. Prefrontal-hippocampal-fusiform activity during encoding predicts intraindividual differences in free recall ability: An event-related functional-anatomic MRI study. Hippocampus. 2007;17:1060–1070. doi: 10.1002/hipo.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: Insights from functional MRI studies. Neuropsychologia. 2008;46(6):1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas A, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI) - Use of a cluster size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Hampstead BM, Sathian K, Moore AB, Nalisnick C, Stringer AY. Explicit memory training leads to improved memory for face-name pairs in patients with mild cognitive impairment: results of a pilot investigation. Journal of the International Neuropsychological Society. 2008;14(5):883–889. doi: 10.1017/S1355617708081009. [DOI] [PubMed] [Google Scholar]
- Hampstead BM, Sathian K, Phillips PA, Amaraneni A, Delaune WR, Stringer AY. Mnemonic strategy training improves memory for object location associations in both healthy elderly and patients with amnestic mild cognitive impairment: a randomized, single-blind study. doi: 10.1037/a0027545. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead BM, Stringer AY, Stilla RF, Amaraneni A, Sathian K. Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object-location associations. Neuropsychologia. 2011a;49:2349–2361. doi: 10.1016/j.neuropsychologia.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead BM, Stringer AY, Stilla RF, Deshpande G, Hu XP, Moore AB, et al. Activation and Effective Connectivity Changes Following Explicit-Memory Training for Face-Name Pairs in Patients With Mild Cognitive Impairment: A Pilot Study. Neurorehabilitation and Neural Repair. 2011b;25(3):210–222. doi: 10.1177/1545968310382424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, Waring SC, OBrien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49(3):786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Suzuki M, Mugikura S, Abe N, Takahashi S, Iijima T, Fujii T. Changes in brain activation associated with use of a memory strategy: a functional MRI study. Neuroimage. 2005;24:1154–1163. doi: 10.1016/j.neuroimage.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Senjem ML, Weigand SD, Smith GE, Ivnik RJ, Boeve BF, et al. Functional magnetic resonance imaging changes in amnestic and nonamnestic mild cognitive impairment during encoding and recognition tasks. Journal of the International Neuropsychological Society. 2009;15(3):372–382. doi: 10.1017/S1355617709090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin NV, Bouchard TP, Ogilvie CJ, Coupland NJ, Seres P, Camicioli R. Three dimensional volumetric analysis and reconstruction of the amygdala and hippocampal head, body, and tail. Psychiatry Research. 2007;155(2):155–165. doi: 10.1016/j.pscychresns.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Mandzia JL, McAndrews MP, Grady CL, Graham SJ, Black SE. Neural correlates of incidental memory in mild cognitive impairment: An fMRI study. Neurobiology of Aging. 2009;30(5):717–730. doi: 10.1016/j.neurobiolaging.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;11(3):126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Miotto EC, Savage CR, Evans JJ, Wilson BA, Martins MGM, Iaki S, Amaro E. Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Human Brain Mapping. 2006;27:288–295. doi: 10.1002/hbm.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Sandblom J, Jones S, Neely AS, Petersson KM, Ingvar M, Backman L. Neural correlates of training-related memory improvement in adulthood and aging. Proceedings of the National Academy of Sciences. 2003;100:13728–13733. doi: 10.1073/pnas.1735487100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of International Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Postma A, Kessels RPC, van Asselen M. How the brain remembers and forgets where things are: The neurocognition of object-location memory. Neuroscience and Biobehavioral Reviews. 2008;32(8):1339–1345. doi: 10.1016/j.neubiorev.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Series W, Pruessner M, Collins DL, Kabani N, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cerebral Cortex. 2000;10(4):433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Ramakers IHGB, Visser PJ, Aalten P, Maes HL, Lansdaal HGM, Meijs CJC, et al. The predictive value of memory strrategies for Alzheimer’s disease in subjects with mild cognitive impairment. Archives of Clinical Neuropsychology. 2010;25(1):71–77. doi: 10.1093/arclin/acp093. [DOI] [PubMed] [Google Scholar]
- Schwindt GC, Black SE. Functional imaging studies of episodic memory in Alzheimer’s disease: a quantitative meta-analysis. Neuroimage. 2009;45(1):181–190. doi: 10.1016/j.neuroimage.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47(8–9):1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL. High resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuro Image. 2010;51(3):1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]