Abstract
Background
TODAY (Treatment Options for type 2 Diabetes in Adolescents and Youth) is a federally-funded multi-center randomized clinical trial comparing three treatments of youth-onset type 2 diabetes.
Objective
To describe the experience of youth participating in a 2–6 month run-in period in preparation for randomization into TODAY.
Subjects
An ethnically diverse sample of 927 youth, 65.4% female, aged 13.7±2.0 years old, with type 2 diabetes for a median of 2 months (0.7–7.8 months, 25th-75th percentiles).
Methods
A run-in period was conducted to achieve HbA1c <8% with metformin monotherapy and diabetes education, and to evaluate adherence to pill taking, visit attendance, and other procedures.
Results
At entry, mean BMI and z-BMI were 35.6±7.7 and 2.3±0.4, respectively, mean HbA1c was 7.7±2.2%, only 42.5% were on a hypoglycemic treatment, and 35.6% had HbA1c ≥8%. Co-morbid conditions were common; 18.8% had hypertension, 24.2% had elevated cholesterol, and 6.5% had abnormal liver enzymes. After a median 71 days of run-in, 90.9% had HbA1c <8%, 77.9% had HbA1c <7%, and 46.4% had HbA1c <6%. Of the 772 youth achieving the target HbA1c <8%, 704 (91.2%) were randomized; non-adherence to metformin treatment was the main cause for non-randomization. Youth proceeding to randomization decreased weight by 0.68 kg and HbA1c by 1.45% compared to a weight gain of 0.71 kg and HbA1c decrease of 0.74% in the non-randomized youth (p=0.01 in both cases). Change in z-BMI was not significantly different between the two groups, however.
Conclusions
Most youth with recent onset type 2 diabetes can achieve target HbA1c <8.0% with short-term metformin monotherapy and standard diabetes education.
Keywords: type 2 diabetes, pediatric onset, clinical trial, metformin, hemoglobin A1c
Introduction
Rising rates of childhood obesity over the past two decades (1) have led to increased rates of type 2 diabetes in youth (2–5). The effectiveness of therapies used in adults with type 2 diabetes has not been well studied in youth with type 2 diabetes. Metformin monotherapy with standard diabetes education is generally recommended in adults with new onset type 2 diabetes, but limited data are available concerning this treatment approach in newly diagnosed youth. To address this, the National Institute of Diabetes and Digestive and Kidney Diseases funded a collaborative group to develop and conduct the TODAY (Treatment Options for type 2 Diabetes in Adolescents and Youth) study, a randomized clinical trial comparing three therapies. Trial methods, rationale, design, and baseline participant characteristics at randomization have been reported (6,7). Briefly, the TODAY study had a screening phase and a run-in phase, followed by the randomized controlled trial. The pre-randomization run-in phase required participants to be weaned from any non-metformin diabetes treatments and to be treated with metformin monotherapy.
The youth entering the run-in phase of the TODAY trial can provide clinically relevant experience regarding success with metformin therapy aimed at achieving target HbA1c levels <8% as well as a description of the prevalence of co-morbid conditions in this cohort. The ethnic and geographic diversity of this sample of youth with type 2 diabetes adds to its clinical relevance and the generalizability of observations for the practicing clinician.
This report describes changes in glycemic control and weight management in obese youth with recently diagnosed type 2 diabetes participating in the TODAY pre-randomization run-in period, and compares those who achieved target HbA1c values and were randomized with those who were unable to meet criteria for randomization.
Methods
Study Design
The TODAY trial consisted of a screening phase and a run-in phase followed by the clinical trial. Screening (n=1,211) was conducted from May 2004 to September 2008 and aimed to identify eligible youth with antibody negative, c-peptide positive diabetes (6,8). The TODAY clinical trial (n=704 participants), conducted from November 2004 through February 2011, was a randomized, parallel-group clinical trial designed to evaluate the relative glycemic efficacy and safety of three treatments for type 2 diabetes in youth: metformin alone, metformin plus rosiglitazone, and metformin plus an intensive lifestyle program (6,7,9). Assignment to rosiglitazone was double blind, but assignment to the lifestyle program was not. Participants were recruited from 15 clinical centers (see study group listing in on-line appendix). Eligible youth were 10–17 years of age, diagnosed with type 2 diabetes <2 years, had a BMI ≥85th percentile at diagnosis of type 2 diabetes or at screening, were accompanied by an adult caregiver who agreed to support the youth’s study participation, and were able to complete the run-in period successfully.
The protocol was approved by an External Evaluation Committee and by Institutional Review Boards at participating institutions. Participants provided informed consent and minors confirmed assent according to local guidelines. A Data and Safety Monitoring Board reviewed progress and safety regularly.
Run-in (Pre-Randomization) Period Objectives
After initial screening for eligibility, the run-in phase (n=927 participants) aimed to: (1) establish a relatively homogeneous study cohort regarding therapy prior to randomization; (2) determine participants’ tolerance of metformin 1000 mg twice daily but no less than 500 mg twice daily; (3) discontinue other diabetes medications; (4) ensure glycemic control (HbA1c <8%) without ketonuria for at least 2 months with metformin monotherapy; (5) provide standard diabetes education with demonstration of participant mastery; and (6) assess ability of potential participants and their families to adhere to the protocol. The run-in phase lasted from 2 to 6 months; participants who did not complete an initial run-in period successfully could make a second attempt de novo starting with screening procedures if the reason for failure had been suitably addressed in the opinion of the site clinician.
Run-in Period Procedures
Participants completed tasks similar to those required once enrolled and randomized, e.g., taking study medications (2 capsules twice daily), completing nutrition and activity diaries, and keeping appointments. The metformin dose, masked to the participant/family, was titrated to study treatment level.
A standard diabetes education (SDE) program, developed for this study (10), was delivered by a certified diabetes educator (CDE) and materials were provided in a workbook in English or Spanish. Materials described type 2 diabetes physiology, treatment, blood glucose monitoring, record keeping, glycemic targets, healthy eating, and physical activity goals, and provided progressive skill building in a developmentally appropriate and culturally sensitive manner. Delivery options included in-person, group, or telephone sessions at the study site, the participant’s home, or a mutual location such as a library, youth center, or restaurant. The education program consisted of a minimum of six sessions, each lasting 60–90 minutes and each ending with mastery activities.
Both participant and adult caregiver attended scheduled visits, which included HbA1c testing, at least every other month. Participants brought nutrition and activity logs to visits. A ‘no show’ was defined as occurring when a scheduled visit was missed without the participant/family first calling to reschedule or cancel. Data collected included demographic characteristics, physical examination, anthropometrics, laboratory tests (blood and urine), medication counts, and mastery of SDE (6). Youth participants and family members were compensated for their travel to run-in visits.
Run-in Adherence Assessment
As the run-in phase aimed to establish target HbA1c values <8% with metformin monotherapy, non-metformin treatments were weaned and metformin was titrated to a minimum of 500 mg twice daily and a maximum of 1,000 mg twice daily. Study staff assessed the ability of participants and their families to adhere to study procedures, assessments, and interventions. Satisfactory completion of behavioral tasks, including monitoring and logging of glucose levels, taking study medication, keeping nutrition and activity logs, and attending scheduled visits, were examined and tallied according to checklists by clinic study staff for a two-month period. Adherence to metformin was measured by pill counts; adherence to scheduled blood glucose monitoring was determined by meter download. Clinic study staff reviewed participant nutrition and activity records and assessed their completeness and validity.
To achieve run-in adherence criteria, participants had to take at least 80% of their pills each week for 8 out of 12 weeks, were allowed no more than one missed (‘no show’) visit, and both youth and accompanying adult caregiver had to complete SDE with demonstrated mastery scores of at least 80% on all lesson quizzes and skills tests.
Laboratory and Physical Data
Blood samples were analyzed at the Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle (6). The Adult Treatment Panel III (ATPIII) guidelines were used to characterize lipid abnormalities (11). Blood pressure percentiles were determined using a CDC program that adjusted for sex, age, and height (12).
Data Analysis
Descriptive statistics are presented for the overall sample and comparing participants who successfully completed run-in and criteria necessary for randomization versus participants who failed to reach target HbA1c values <8% or other eligibility criteria for randomization. Comparisons were performed using analysis of variance for normally distributed continuous variables, the Kruskal-Wallis test for non-normally distributed continuous variables, and the chi-square test for categorical variables. A p-value <0.05 was considered significant, with no adjustment for multiple comparisons.
Results
Reasons for non-randomization following run-in
The 223 subjects not randomized were classified according to single major reason for ineligibility. One-quarter (25.9%, n=58) did not achieve HbA1c levels <8%, 10.4% (n=23) could not be weaned from other hypoglycemic medications (e.g., insulin), and 8.5% (n= 19) could not tolerate metformin at study levels. Nearly half (45.3%, n=101) of those failing run-in were non-adherent to metformin therapy (representing 10.9% of the 927 subjects enrolled in run-in). Other reasons for failure to advance to randomization included study visit nonattendance (0.9%), inability to master SDE (0.9%), safety concerns (2.4%, e.g., abnormal LFTs), and subject refusal to proceed with study participation (5.7%).
Characteristics of run-in participants
As shown in Table 1, 65.4% of participants were female, 81.7% belonged to racial/ethnic minority groups, mean age was 13.7 years, median time from diagnosis of type 2 diabetes was 63 days, less than half (42.5%) were receiving some form of hypoglycemic treatment, and 91.9% lived with at least one biological parent. Most (86.7%) presented with acanthosis nigricans and, as expected according to eligibility criteria, were obese (average BMI 35.6 m/kg2 and z-BMI 2.3). Mean HbA1c at entry into run-in was 7.7% and almost two-thirds (64.4%) met the level required for randomization of <8%.
Table 1.
Characteristics* of Participants at Start of Run-in
| OVERALL | RANDOMIZED | |||
|---|---|---|---|---|
| (N=927) | YES (N=704) | NO (N=223) | P-value | |
| Age (years) | 13.7 (2.0) | 13.7 (2.0) | 13.9 (2.0) | 0.10 |
| Duration of diabetes (days) | <0.01 | |||
| 25th percentile | 22 | 20.5 | 31 | |
| 50th percentile | 63 | 56.5 | 86 | |
| 75th percentile | 203 | 182.5 | 286 | |
| Gender female | 65.4% | 64.9% | 66.8% | 0.60 |
| On diabetes treatment | 42.5% | 41.3% | 46.2% | 0.20 |
| BMI (m/kg2) | 35.6 (7.6) | 35.5 (7.5) | 36.0 (7.8) | 0.39 |
| z-BMI | 2.3 (0.4) | 2.3 (0.4) | 2.3 (0.4) | 0.99 |
| Acanthosis present | 86.7% | 86.6% | 87.3% | 0.77 |
| HbA1c (%) | 7.7 (2.2) | 7.5 (2.0) | 8.5 (2.6) | <0.01 |
| HbA1c (categories) | ||||
| < 8% | 64.4% | 69.1% | 49.5% | |
| 8–10% | 19.4% | 18.2% | 23.2% | |
| > 10% | 16.2% | 12.5% | 27.3% | |
| C-peptide (ng/mL) | 4.0 (2.1) | 4.1 (2.1) | 3.9 (2.1) | 0.38 |
| Race/ethnicity† | 0.02 | |||
| American Indian | 6.2% | 6.1% | 6.5% | |
| Non-Hispanic Black | 34.3% | 31.5% | 43.2% | |
| Hispanic | 39.5% | 41.1% | 34.6% | |
| Non-Hispanic White | 18.3% | 19.6% | 14.3% | |
| Asian | 1.7% | 1.7% | 1.4% | |
| Living with biological parents | 0.24 | |||
| Both | 38.1% | 38.8% | 36.1% | |
| Mother only | 49.0% | 46.9% | 55.6% | |
| Father only | 4.8% | 5.1% | 3.7% | |
| Neither | 8.1% | 9.2% | 4.6% | |
Mean (sd), percent, or percentile.
Race/ethnicity was determined by self report on two separate items. For data analysis, 25 (3.6%) who reported belonging to more than one racial group were assigned to a racial/ethnic group according to the following priority of risk for type 2 diabetes in youth: AI > Hispanic > NHB > NHW (5).
Glycemic control with metformin monotherapy during run-in
Of the 927 run-in participants, 90.9% achieved HbA1c <8%, 77.9% achieved HbA1c <7%, and 46.4% achieved HbA1c <6%. Of the 772 who reached target HbA1c <8%, only 704 (91.2%) also met all randomization criteria for enrollment into the TODAY clinical trial. Of the 223 participants who did not meet inclusion criteria for randomization at the end of run-in, 105 were unable to achieve HbA1c <8% on metformin monotherapy. Only a few individuals who failed the initial run-in made a second attempt starting de novo with screening and a second run-in period; 10 (1.4%) of the 704 participants subsequently randomized underwent a second run-in period compared with 15 of the 223 who did not qualify for randomization (6.7%).
In addition to the difference in glycemic control between those proceeding to randomization and those not, the non-randomized sugroup had a longer duration of type 2 diabetes compared to those randomized (median 86 and 56 days, respectively, p<0.01). There was also a modest difference in the racial distribution between groups, with a slightly higher percentage of non-Hispanic Black participants and a slightly lower percentage of Hispanic and non-Hispanic White participants in the non-randomized group compared with the randomized group (p=0.02).
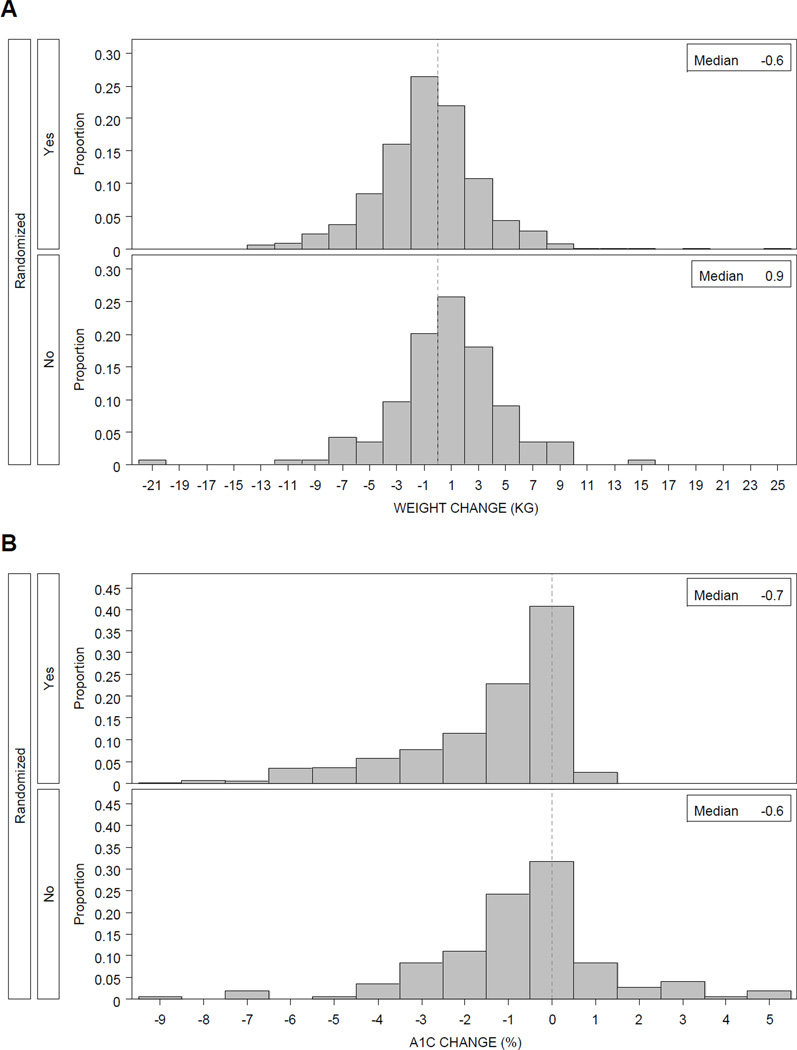
During run-in, weight decreased by a mean of 0.68 kg in participants meeting the randomization criteria, while there was a mean weight gain of 0.71 kg in the non-randomized group (Table 2, p<0.01). Figure 1A shows the distribution of weight loss in 2 kg increments in the two groups. The change in weight did not translate into significant change in z-BMI during the run-in interval.
Table 2.
Participant Characteristics and Outcomes during Run-in
| OVERALL | RANDOMIZED | ||||
|---|---|---|---|---|---|
| (N=927) | YES (N=704) | NO (N=223) | |||
| 25th, 50th, 75th percentiles | |||||
| Days from screening to run-in | 18, 25, 36 | 18, 25, 35 | 19, 27, 45 | ||
| Run-in length (days) | 59, 70, 91 | 62, 71, 89 | 23, 57, 109 | ||
| mean (SD) or % | P-value | ||||
| Weight change* (kg) | −0.43 (4.01) | −0.68 (3.91) | 0.71 (4.27) | <0.01 | |
| z-BMI change* | −0.046 (0.123) | −0.049 (0.108) | −0.033 (0.177) | 0.15 | |
| HbA1c† change* | −1.33 (1.91) | −1.45 (1.84) | −0.74 (2.14) | <0.01 | |
| Last HbA1c | < 8% | 90.9% | 100.0% | 46.9% | |
| 8–10% | 6.0% | -- | 35.2% | ||
| > 10% | 3.1% | -- | 17.9% | ||
| Co-morbid conditions‡ | |||||
| Total cholesterol (mg/dL) | 159.3 (43.5) | 156.3 (36.8) | 168.7 (59.0) | <0.01 | |
| HDL cholesterol (mg/dL) | 40.2 (9.9) | 39.8 (9.4) | 41.6 (11.4) | 0.02 | |
| Triglycerides (mg/dL) | 143.5 (332.7) | 127.0 (90.9) | 195.4 (657.0) | <0.01 | |
Change from screening visit to final run-in visit.
HbA1c sample size N=704 for randomized and N=145 for non-randomized for an overall N=849.
Only statistically significant variables are given; those tested but not significant were systolic blood pressure, diastolic blood pressure, LDL cholesterol, and liver function tests.
Figure 1. Distribution of Change in Weight and HbA1c of Participants during Run-in (Pre-Randomization Phase) of TODAY.
Figure 1A. The 704 participants who met all randomization criteria, including HbA1c <8% on metformin monotherapy, experienced significantly more weight loss than the 223 who did not meet criteria. Six participants lost >10 kg (5 among the successes and 1 among the failures).
Figure 1B. A greater proportion of the 704 participants who met all randomization criteria, including HbA1c <8% on metformin monotherapy, experienced a decline in HbA1c compared with the 223 who did not meet criteria. Eleven participants had HbA1c decline by >7% during run-in (8 among the successes and 3 among the failures).
Initial and final run-in HbA1c levels differed significantly between the randomized and the non-randomized participants with means of 7.5% and 8.5%, respectively (p<0.0001), at screening and 6.0% and 8.0%, respectively (p<0.01), at the end of the run-in period. Over two-thirds of those who proceeded to randomization had acceptable HbA1c levels <8% initially compared to only half of those who did not (Table 1). There was a significantly greater improvement in glycemic control in those randomized, with an HbA1c decrement of 1.45% versus 0.74% in those non-randomized (p<0.01). Figure 1B shows the distribution of change in HbA1c. There were 11 participants (8 who proceeded to randomization) who reduced their HbA1c by >7%; all began run-in with values >12%. C-peptide levels were not different between those who were randomized and those who were not (Table 1).
Co-morbid Conditions
There was a substantial presence of co-morbid indicators in this cohort (see bottom of Table 2). Overall, 18.8% of participants had a blood pressure >95th percentile according to age, gender, and height, 37.6% had LDL-cholesterol ≥100 mg/dL, 24.2% had total cholesterol ≥180 mg/dL, and 6.5% had abnormal liver function tests. There was no difference in blood pressure, LDL-cholesterol, or liver function tests between those randomized and those not. Mean total cholesterol was significantly lower in the former group (156.3 mg/dL) compared to the latter failure group (168.7 mg/dL, p<0.01). Average triglycerides were significantly higher in non-randomized participants (195.4 mg/dL and 127.0 mg/dL, respectively, p<0.01) and correlated with their HbA1c (r=0.07, p=0.03). Higher mean HDL-cholesterol was evident in the non-randomized group (41.6 mg/dL) versus the randomized group (39.8 mg/dL) and related to higher total cholesterol.
Discussion
Reports of the early management of type 2 diabetes in youth is limited. It is unclear if response to therapy in this age group is similar to that observed in adults or if the course of the disease in youth is more aggressive. The TODAY study is the largest clinical trial of treatment regimens for pediatric type 2 diabetes and addresses some of these issues. Examination of the pre-randomization period of this trial provided the opportunity to understand better the short-term responsiveness of youth early in the course of their type 2 diabetes to metformin monotherapy, the initial therapy generally recommended for adults with type 2 diabetes. The finding that 90.9% of the 927 participants who entered run-in achieved or maintained HbA1c <8% suggests that metformin monotherapy can be effective in youth with type 2 diabetes, at least early in the course of the disease.
There were differences between those who did and did not meet randomization criteria. Not surprisingly, a greater proportion of those who successfully completed the run-in period had HbA1c levels <8% at the screening visit. Duration of type 2 diabetes was about one month longer in the non-randomized group. There were more non-Hispanic Blacks and fewer Hispanics and non-Hispanic Whites in the group that did not meet randomization criteria. All youth in the TODAY study were required to enroll with a committed adult caregiver who would also participate. Family support has consistently been associated with improved youth outcomes (13–15). Very few (<1%) failed run-in due to visit nonattendance.
The primary reasons for failure to randomize were related to diabetes treatments and included inability to adhere to metformin dosing schedule. While ~45% of the failure group was unable to demonstrate medication adherence, this represents only about 11% of all those who entered run-in. As for the main trial, the run-in period required twice daily treatment with a short-acting metformin preparation. Adherence might have been improved if long-acting metformin preparations had been available. The demographics of the TODAY run-in participants are similar to those reported by the population-based cohort in the SEARCH study (16,17). Co-morbid conditions were similarly prevalent in this sample. Triglycerides were the only co-morbid condition associated with randomization status; however, we cannot infer a causal relationship as triglyceride levels may have been a proxy for glycemic control.
In conclusion, the TODAY pre-randomization experience supports the current practice of using metformin as initial oral therapy in youth with new onset type 2 diabetes along with standard diabetes education. Knowledge gained about co-morbidities, family support, etc. may prove useful to other researchers and clinicians dealing with this patient population. The 704 youth randomized to the 3 treatment intervention arms ended participation in TODAY after 2–6 years of treatment. Future findings should provide additional needed guidance concerning treatment approaches for youth with type 2 diabetes.
Supplementary Material
Acknowledgments
This work was completed with funding from NIDDK/NIH grant numbers U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254; from the National Center for Research Resources General Clinical Research Centers Program grant numbers M01-RR00036 (Washington University School of Medicine), M01-RR00043-45 (Childrens Hospital Los Angeles), M01-RR00069 (University of Colorado Denver), M01-RR00084 (Children’s Hospital of Pittsburgh), M01-RR01066 (Massachusetts General Hospital), M01-RR00125 (Yale University), and M01-RR14467 (University of Oklahoma Health Sciences Center); and from the NCRR Clinical and Translational Science Awards grant numbers UL1-RR024134 (Children’s Hospital of Philadelphia), UL1-RR024139 (Yale University), UL1-RR024153 (Children’s Hospital of Pittsburgh), UL1-RR024989 (Case Western Reserve University), UL1-RR024992 (Washington University), UL1-RR025758 (Massachusetts General Hospital), and UL1-RR025780 (University of Colorado Denver).
The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; Sanofi-Aventis.
Abbreviations
- ATPIII
Adult Treatment Panel III
- BMI
Body Mass Index
- CDE
Certified Diabetes Educator
- LFT
Liver Function Tests
- SDE
Standard Diabetes Education
- TODAY
Treatment Options for type 2 Diabetes in Adolescents and Youth
Footnotes
Members of the writing group have no conflicts of interest or financial arrangements to disclose.
Members of the TODAY Study Group are listed in the on-line appendix.
Contributor Information
Lori Laffel, Joslin Diabetes Center, Boston MA 02215, USA.
Nancy Chang, Children’s Hospital Los Angeles, Los Angeles CA 90027, USA.
Margaret Grey, Yale University, New Haven CT 06519, USA.
Dan Hale, University of Texas Health Sciences Center, San Antonio TX 78229, USA.
Laurie Higgins, Joslin Diabetes Center, Boston MA 02215, USA.
Kathryn Hirst, George Washington University, Rockville MD 20852, USA.
Roberto Izquierdo, State University of New York Upstate Medical University, Syracuse NY 13214, USA.
Mary Larkin, Massachusetts General Hospital, Boston MA 02114, USA.
Christina Macha, University of Oklahoma Health Sciences Center, Oklahoma City OK 73104, USA.
Trang Pham, George Washington University, Rockville MD 20852, USA.
Aimee Wauters, University of Texas Health Sciences Center, San Antonio TX, 78229, USA.
Ruth S. Weinstock, State University of New York Upstate Medical University, Syracuse NY 13214, USA
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136:664–672. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 4.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 5.SEARCH for Diabetes in Youth Study Group. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 6.TODAY Study Group. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copeland KC, Zeitler P, Geffner M, et al. for TODAY Study Group. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96:159–167. doi: 10.1210/jc.2010-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klingensmith GJ, Pyle L, Arslanian S, et al. for TODAY Study Group. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care. 2010;33:1970–1975. doi: 10.2337/dc10-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.TODAY Study Group. Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. International Journal of Obesity. 2010;34:217–226. doi: 10.1038/ijo.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grey M, Schreiner B, Pyle L. Development of a diabetes education program for youth with type 2 diabetes. Diabetes Educ. 2009;35:108–116. doi: 10.1177/0145721708325156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cholesterol Education Program. Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. NIH Pub. No. 02-5215. Bethesda, MD: National Heart, Lung, and Blood Institute; 2002. [Google Scholar]
- 12.Centers for Disease Control and Prevention, National Center for Health Statistics. 2000 CDC growth charts for the United States: methods and development, Vital and Health Statistics Series 11, Number 246. 2002 ( http://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf), CDC growth charts ( http://www.cdc.gov/growthcharts/), and a SAS program for the CDC growth charts ( http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm) [PubMed]
- 13.Laffel LM, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. J Pediatr. 2003;142:409–416. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- 14.Anderson BJ, Cullen K, McKay S. Quality of life, family behavior, and health outcomes in children with type 2 diabetes. Pediatr Ann. 2005;34:722–729. doi: 10.3928/0090-4481-20050901-12. [DOI] [PubMed] [Google Scholar]
- 15.Anderson BJ, McKay SV. Barriers to glycemic control in youth with type 1 diabetes and type 2 diabetes. Pediatr Diabetes. 2011;12:197–205. doi: 10.1111/j.1399-5448.2010.00667.x. [DOI] [PubMed] [Google Scholar]
- 16.Kershnar AK, Daniels SR, Imperatore G, et al. Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr. 2006;149:314–319. doi: 10.1016/j.jpeds.2006.04.065. [DOI] [PubMed] [Google Scholar]
- 17.Petitti DB, Klingensmith GJ, Bell RA, et al. for the SEARCH for Diabetes in Youth Study Group. Glycemic control in youth with diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr. 2009;155:668–672. doi: 10.1016/j.jpeds.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.